Clotiapine: Difference between revisions
(Blanked the page) |
No edit summary |
||
| Line 1: | Line 1: | ||
{{Drugbox | |||
| Verifiedfields = changed | |||
| Watchedfields = changed | |||
| verifiedrevid = 460045350 | |||
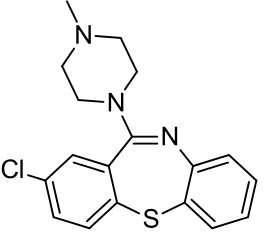
| IUPAC_name = 8-chloro-6-(4-methylpiperazin-1-yl)benzo[b][1,5]benzothiazepine | |||
| image = Clotiapine.png | |||
| width = 200 | |||
<!--Clinical data--> | |||
| tradename = Etumina, Etumine, Entumin, Etomine, Entumine | |||
| Drugs.com = {{drugs.com|international|clotiapine}} | |||
| pregnancy_category = | |||
| legal_status = Rx-only | |||
| routes_of_administration = Oral, Intravenous and [[Intramuscular]] | |||
<!--Pharmacokinetic data--> | |||
| bioavailability = | |||
| protein_bound = | |||
| metabolism = | |||
| elimination_half-life = | |||
| excretion = | |||
<!--Identifiers--> | |||
| CAS_number_Ref = {{cascite|changed|??}} | |||
| CAS_number = 2058-52-8 | |||
| ATC_prefix = N05 | |||
| ATC_suffix = AH06 | |||
| ChEMBL_Ref = {{ebicite|correct|EBI}} | |||
| ChEMBL = 304902 | |||
| PubChem = 16351 | |||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| ChemSpiderID = 15510 | |||
| UNII_Ref = {{fdacite|correct|FDA}} | |||
| UNII = Z05HCY0X1T | |||
| KEGG_Ref = {{keggcite|correct|kegg}} | |||
| KEGG = D01597 | |||
<!--Chemical data--> | |||
| C=18 | H=18 | Cl=1 | N=3 | S=1 | |||
| molecular_weight = 343.87 g/mol | |||
| smiles = Clc2ccc1Sc4c(/N=C(\c1c2)N3CCN(C)CC3)cccc4 | |||
| InChI = 1/C18H18ClN3S/c1-21-8-10-22(11-9-21)18-14-12-13(19)6-7-16(14)23-17-5-3-2-4-15(17)20-18/h2-7,12H,8-11H2,1H3 | |||
| InChIKey = KAAZGXDPUNNEFN-UHFFFAOYAF | |||
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChI = 1S/C18H18ClN3S/c1-21-8-10-22(11-9-21)18-14-12-13(19)6-7-16(14)23-17-5-3-2-4-15(17)20-18/h2-7,12H,8-11H2,1H3 | |||
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChIKey = KAAZGXDPUNNEFN-UHFFFAOYSA-N | |||
}} | |||
__NOTOC__ | |||
{{SI}} | |||
{{CMG}} | |||
==Overview== | |||
'''Clotiapine''' ('''Entumine''') is an [[atypical antipsychotic]]<ref>{{cite pmid|8105359}}</ref> of the [[dibenzothiazepine]] [[chemical class]].<ref name="Schmutz">{{cite doi|10.1002/hlca.19670500131}}</ref> It was first introduced in a few European countries (namely, [[Belgium]], [[Italy]], [[Spain]] and [[Switzerland]]), [[Argentina]], [[Taiwan]] and [[Israel]] in 1970.<ref name = forg/> | |||
Some sources regard clotiapine as a [[typical antipsychotic]] rather than [[Atypical antipsychotic|atypical]] due to its high incidence of [[extrapyramidal side effects]] compared to the atypicals like clozapine and quetiapine, to which it is structurally related.<ref name = ClotChlor>{{cite journal|title=Clotiapine compared with chlorpromazine in chronic schizophrenia|journal=Schizophrenia Research|volume=80|issue=2-3|pages=343-347|author=Geller, V; Gorzaltsan, I; Shleifer, T; Belmaker, RH; Bersudsky, Y|pmid=16126373|date=December 2005|doi=10.1016/j.schres.2005.07.007}}</ref> Despite its profile of a relatively high incidence of extrapyramidal side effects it has demonstrated efficacy in treatment-resistant schizophrenic patients according to a number of psychiatrists with clinical experience with it, some weak clinical evidence supports this view too.<ref name = ClotChlor/><ref>{{cite journal|title=chlorpromazine, clotiapine and thioridazine--a comparative clinical trial on Bantu psychotic patients|journal=South African Medical Journal|date=August 1971|volume=45|issue=34|pages=945-947|pmid=4939661|url=http://archive.samj.org.za/1971%20VOL%20XLV%20Jul-Dec/Articles/08%20August/4.3%20CHLORPROMOZAINE,%20CLOTIAPINE%20AND%20THIORIDAZINE%20-%20A%20COMPARATIVE%20CLINICAL%20TRIAL%20AND%20BANTU%20PSYCHOT.pdf|format=PDF|author=Van Wyk, AJ; Marais, GF}}</ref><ref name = forg>{{cite journal|title=Clotiapine: Another forgotten treasure in psychiatry?|journal=European Neuropsychopharmacology|volume=7|issue=Suppl 2|pages=S217|doi=10.1016/S0924-977X(97)88712-3|date=September 1997|author=Lokshin, P; Kotler, M; Belmaker, RH}}</ref> | |||
==Synthesis== | |||
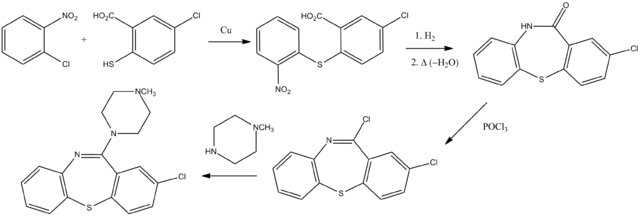
[[Ullmann condensation]] of the substituted thiosalyciclic acid () with ortho-chloronitrobenzene results in the displacement | |||
of chlorine by thiophenoxide and the formation of the thioether (). The nitro group in this last intermediate is then reduced to an aniline (); the resulting amino acid is then cyclized thermally to the lactam (). Treatment of that with [[phosphorus oxychloride]] gives the imino chloride (). Reaction with N-methylpiperazine leads to the replacement of chlorine by nitrogen and the formation of clothiapine ().<ref name="Schmutz" /> | |||
[[File:Clothiapine0.png|400px]] | |||
==References== | |||
{{reflist|2}} | |||
{{Antipsychotics}} | |||
{{Adrenergics}} | |||
{{Cholinergics}} | |||
{{Dopaminergics}} | |||
{{Histaminergics}} | |||
{{Serotonergics}} | |||
{{Piperazines}} | |||
{{Tricyclics}} | |||
[[Category:Atypical antipsychotics]] | |||
[[Category:Piperazines]] | |||
[[Category:Drug]] | |||
Revision as of 17:58, 9 April 2015
 | |
| Clinical data | |
|---|---|
| Trade names | Etumina, Etumine, Entumin, Etomine, Entumine |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral, Intravenous and Intramuscular |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C18H18ClN3S |
| Molar mass | 343.87 g/mol |
| 3D model (JSmol) | |
| |
| |
| | |
|
WikiDoc Resources for Clotiapine |
|
Articles |
|---|
|
Most recent articles on Clotiapine |
|
Media |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Clotiapine at Clinical Trials.gov Clinical Trials on Clotiapine at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Clotiapine
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Clotiapine Discussion groups on Clotiapine Patient Handouts on Clotiapine Directions to Hospitals Treating Clotiapine Risk calculators and risk factors for Clotiapine
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Clotiapine |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Clotiapine (Entumine) is an atypical antipsychotic[1] of the dibenzothiazepine chemical class.[2] It was first introduced in a few European countries (namely, Belgium, Italy, Spain and Switzerland), Argentina, Taiwan and Israel in 1970.[3]
Some sources regard clotiapine as a typical antipsychotic rather than atypical due to its high incidence of extrapyramidal side effects compared to the atypicals like clozapine and quetiapine, to which it is structurally related.[4] Despite its profile of a relatively high incidence of extrapyramidal side effects it has demonstrated efficacy in treatment-resistant schizophrenic patients according to a number of psychiatrists with clinical experience with it, some weak clinical evidence supports this view too.[4][5][3]
Synthesis
Ullmann condensation of the substituted thiosalyciclic acid () with ortho-chloronitrobenzene results in the displacement of chlorine by thiophenoxide and the formation of the thioether (). The nitro group in this last intermediate is then reduced to an aniline (); the resulting amino acid is then cyclized thermally to the lactam (). Treatment of that with phosphorus oxychloride gives the imino chloride (). Reaction with N-methylpiperazine leads to the replacement of chlorine by nitrogen and the formation of clothiapine ().[2]
References
- ↑ PMID 8105359 (PMID 8105359)
Citation will be completed automatically in a few minutes. Jump the queue or expand by hand - ↑ 2.0 2.1 Template:Cite doi
- ↑ 3.0 3.1 Lokshin, P; Kotler, M; Belmaker, RH (September 1997). "Clotiapine: Another forgotten treasure in psychiatry?". European Neuropsychopharmacology. 7 (Suppl 2): S217. doi:10.1016/S0924-977X(97)88712-3.
- ↑ 4.0 4.1 Geller, V; Gorzaltsan, I; Shleifer, T; Belmaker, RH; Bersudsky, Y (December 2005). "Clotiapine compared with chlorpromazine in chronic schizophrenia". Schizophrenia Research. 80 (2–3): 343–347. doi:10.1016/j.schres.2005.07.007. PMID 16126373.
- ↑ Van Wyk, AJ; Marais, GF (August 1971). "chlorpromazine, clotiapine and thioridazine--a comparative clinical trial on Bantu psychotic patients" (PDF). South African Medical Journal. 45 (34): 945–947. PMID 4939661.
- Pages with script errors
- Pages with incomplete PMID references
- CS1 maint: Multiple names: authors list
- Template:drugs.com link with non-standard subpage
- Drugs with non-standard legal status
- Articles with changed CASNo identifier
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Infobox drug
- Chemical pages without DrugBank identifier
- Drugboxes which contain changes to verified fields
- Drugboxes which contain changes to watched fields
- Atypical antipsychotics
- Piperazines
- Drug