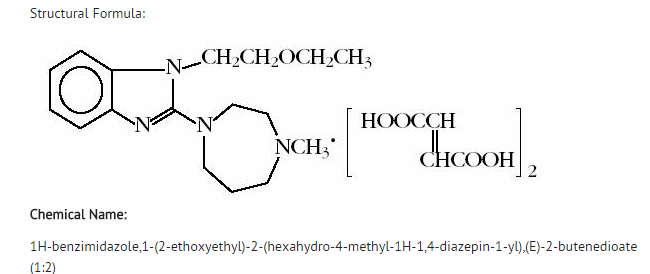
Emedastine
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Ammu Susheela, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Emedastine is an antihistamine that is FDA approved for the treatment of signs and symptoms of allergic conjunctivitis. Common adverse reactions include headache.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- emedastine is indicated for the temporary relief of the signs and symptoms of allergic conjunctivitis.
- Emedastineis indicated for the temporary relief of the signs and symptoms of allergic conjunctivitis.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Emedastine in adult patients.
Non–Guideline-Supported Use
- Asthma; Adjunct
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Emedastine in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Emedastine in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Emedastine in pediatric patients.
Contraindications
- EMADINE® (emedastine difumarate ophthalmic solution) is contraindicated in persons with a known hypersensitivity to emedastine difumarate or any of its components.
Warnings
- FOR TOPICAL OPHTHALMIC USE ONLY - NOT FOR INJECTION OR ORAL USE.
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Emedastine Clinical Trials Experience in the drug label.
Postmarketing Experience
- In controlled clinical studies of emedastine lasting for 42 days, the most frequent adverse reaction was headache 11%. The following adverse experiences were reported in less than 5% of patients: Abnormal dreams, asthenia, bad taste, blurred vision, burning or stinging, corneal infiltrates, corneal staining, dermatitis, discomfort, dry eye, foreign body sensation, hyperemia, keratitis, pruritus, rhinitis, sinusitis and tearing. Some of these events were similar to the underlying disease being studied.
Drug Interactions
There is limited information regarding Emedastine Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
- Teratology and peri- and post-natal studies have been conducted with emedastine difumarate in rats and rabbits. At 15,000 times the maximum recommended ocular human use level, emedastine difumarate was shown not to be teratogenic in rats and rabbits and no effects on peri/post-natal development were observed in rats. However, at 70,000 times the maximum recommended ocular human use level, emedastine difumarate was shown to increase the incidence of external, visceral and skeletal anomalies in rats. There are, however, no adequate and well controlled studies in pregnant women. Because animal studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Emedastine in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Emedastine during labor and delivery.
Nursing Mothers
- Emedastine has been identified in breast milk in rats following oral administration. It is not known whether topical ocular administration could result in sufficient systemic absorption to produce detectable quantities in breast milk. Nevertheless, caution should be exercised when emedastine is administered to a nursing mother.
Pediatric Use
Safety and effectiveness in pediatric patients below the age of 3 years have not been established.
Geriatic Use
No overall differences in safety or effectiveness have been observed between elderly and younger patients.
Gender
There is no FDA guidance on the use of Emedastine with respect to specific gender populations.
Race
There is no FDA guidance on the use of Emedastine with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Emedastine in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Emedastine in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Emedastine in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Emedastine in patients who are immunocompromised.
Administration and Monitoring
Administration
Monitoring
There is limited information regarding Monitoring of Emedastine in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Emedastine in the drug label.
Overdosage
- Somnolence and malaise have been reported following daily oral administration. Oral ingestion of the contents of a 15 mL DROP-TAINER® dispenser would be equivalent to 7.5 mg. In case of overdosage, treatment is symptomatic and supportive.
Pharmacology
| |
Emedastine
| |
| Systematic (IUPAC) name | |
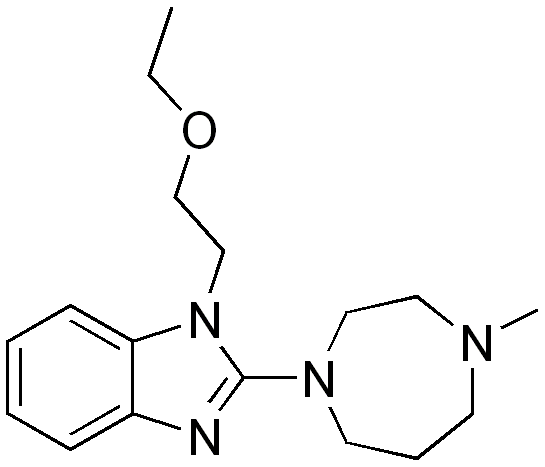
| 1-(2-ethoxyethyl)-2- (4-methyl-1,4-diazepan-1-yl)- benzoimidazole | |
| Identifiers | |
| CAS number | |
| ATC code | S01 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 302.415 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status | |
| Routes | ? |
Mechanism of Action
- Emedastine is a relatively selective, histamine H1 antagonist. In vitro examinations of emedastine's affinity for histamine receptors (H1: Ki=1.3 nM, H2: Ki=49,067 nM, and H3: Ki=12,430 nM) demonstrate relative selectivity for the H1 histamine receptor.
- In vivo studies have shown concentration-dependent inhibition of histamine-stimulated vascular permeability in the conjunctiva following topical ocular administration. Emedastine appears to be devoid of effects on adrenergic, dopaminergic and serotonin receptors.
Structure
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Emedastine in the drug label.
Pharmacokinetics
- Following topical administration in man, emedastine was shown to have low systemic exposure. In a study involving 10 normal volunteers dosed bilaterally twice daily for 15 days with emedastine ophthalmic solution 0.05%, plasma concentrations of the parent compound were generally below the quantitation limit of the assay (<0.3 ng/mL). Samples in which emedastine was quantifiable ranged from 0.30 to 0.49 ng/mL. The elimination half-life of oral emedastine in plasma is 3-4 hours. Approximately 44% of the oral dose is recovered in the urine over 24 hours with only 3.6% of the dose excreted as parent drug. Two primary metabolites, 5- and 6-hydroxyemedastine, are excreted in the urine as both free and conjugated forms. The 5'-oxoanalogs of 5- and 6-hydroxyemedastine and the N-oxide are also formed as minor metabolites.
- In an environmental study, patients with allergic conjunctivitis were treated with emedastine for six weeks. The results demonstrated that emedastine provides relief of the signs and symptoms of allergic conjunctivitis. In conjunctival antigen challenge studies, in which subjects were challenged with antigen both initially and up to four hours after dosing, emedastine was demonstrated to be significantly more effective than placebo in preventing ocular itching associated with allergic conjunctivitis.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Emedastine in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of Emedastine in the drug label.
How Supplied
- emedastine is supplied as follows:
- 5 mL in opaque, plastic DROP-TAINER® dispenser.
- 5 mL: NDC 0065-0325-05
Storage
- STORAGE: Store at 4° - 30°C (39° - 86°F).
Images
Drug Images
{{#ask: Page Name::Emedastine |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Emedastine |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Emedastine in the drug label.
Precautions with Alcohol
- Alcohol-Emedastine interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- EMADINE®[1]
Look-Alike Drug Names
There is limited information regarding Emedastine Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Label Page=Emedastine |Label Name=E 02.jpg
}}
{{#subobject:
|Label Page=Emedastine |Label Name=E 03.jpg
}}
{{#subobject:
|Label Page=Emedastine |Label Name=DailyMed - EMADINE- emedastine difumarate solution .png
}}