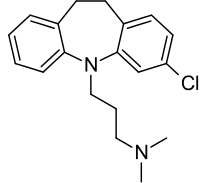
Clomipramine
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Pratik Bahekar, MBBS [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
Suicidality and Antidepressant Drug
See full prescribing information for complete Boxed Warning.
Condition Name:
Antidepressants increased the risk compared to placebo of suicidal thinking and behavior (suicidality) in children, adolescents, and young adults in short-term studies of major depressive disorder (MDD) and other psychiatric disorders. Anyone considering the use of clomipramine hydrochloride capsules or any other antidepressant in a child, adolescent, or young adult must balance this risk with the clinical need. Short-term studies did not show an increase in the risk of suicidality with antidepressants compared to placebo in adults beyond age 24; there was a reduction in risk with antidepressants compared to placebo in adults aged 65 and older. Depression and certain other psychiatric disorders are themselves associated with increases in the risk of suicide. Patients of all ages who are started on antidepressant therapy should be monitored appropriately and observed closely for clinical worsening, suicidality, or unusual changes in behavior. Families and caregivers should be advised of the need for close observation and communication with the prescriber. Clomipramine hydrochloride capsules are not approved for use in pediatric patients except for patients with obsessive compulsive disorder (OCD) (see WARNINGS: Clinical Worsening and Suicide Risk, PRECAUTIONS: Information for Patients, and PRECAUTIONS: Pediatric Use).
|
Overview
Clomipramine is a Tricyclic antidepressant that is FDA approved for the {{{indicationType}}} of obsessive-compulsive disorder. There is a Black Box Warning for this drug as shown here. Common adverse reactions include diaphoresis, weight increased, constipation, diarrhea, indigestion, loss of appetite, nausea, xerostomia, myalgia, dizziness, feeling nervous, headache, insomnia, myoclonus, somnolence, tremor, abnormal vision, disorder of the urinary system, disorder of ejaculation, impotence, pharyngitis fatigue..
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Obsessive-compulsive disorder
- Monotherapy
- Maximum, 250 mg/day[1]
- Combination Therapy
- Initial dose, 25 mg/day; weekly titration up to 75 mg/day; mean dose, 55 mg/day) plus fluoxetine (maximum dose, 40 mg/day), quetiapine initial dose, 50 mg/day; weekly titration up to 200 mg/day; mean dose, 142 mg/day
Off-Label Use and Dosage (Adult)
Non–Guideline-Supported Use
Delusional disorder
- Initial, 25 mg/day ORALLY, may increase dosage to 100 mg/day during the first 2 weeks (MAX dose 250 mg/day, mean dose 140 mg/day)
Depression
- Initial, 75 mg/day ORALLY (3 divided doses); may increase dosage slowly as needed and tolerated to a range of 100-250 mg/day (3 divided doses)
Disorder of ejaculation
Obsessive-compulsive disorder, Intravenous therapy
- Initial, 25 mg/day ORALLY, may increase dosage to 100 mg/day ORALLY during the first 2 weeks; MAX dose 250 mg/day
Pain, chronic
Panic disorder
- 25-75 mg/day ORALLY
Pervasive developmental disorder
- There is limited information about Off-Label Non–Guideline-Supported Use of Clomipramine in adult patients.
- There is limited information about Off-Label Non–Guideline-Supported Use of Clomipramine in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Obsessive-compulsive disorder
- 10 years and older, initial, 25 mg/day ORALLY, may increase dosage to 3 mg/kg or 100 mg/day (whichever is less) ORALLY during the first 2 weeks; MAX dose 200 mg/day OR 3 mg/kg/day (whichever is less)
Off-Label Use and Dosage (Pediatric)
Non–Guideline-Supported Use
Depression
- Safety and effectiveness in children less than 10 years of age have not been established
- 20-30 mg/day ORALLY; may increase dosage by 10 mg/day at 4-5 day intervals as needed and tolerated
- There is limited information about Off-Label Non–Guideline-Supported Use of Clomipramine in pediatric patients.
Contraindications
- Contraindicated in patients with a history of hypersensitivity to clomipramine hydrochloride or other tricyclic antidepressants
- Monoamine Oxidase Inhibitors (MAOIs)-The use of MAOIs intended to treat psychiatric disorders with clomipramine or within 14 days of stopping treatment with clomipramine is contraindicated because of an increased risk of serotonin syndrome.
- The use of clomipramine within 14 days of stopping an MAOI intended to treat psychiatric disorders is also contraindicated.
- Starting clomipramine in a patient who is being treated with linezolid or intravenous methylene blue is also contraindicated because of an increased risk of serotonin syndrome.
- Myocardial Infarction- Clomipramine is contraindicated during the acute recovery period after a myocardial infarction.
Warnings
|
Suicidality and Antidepressant Drug
See full prescribing information for complete Boxed Warning.
Condition Name:
Antidepressants increased the risk compared to placebo of suicidal thinking and behavior (suicidality) in children, adolescents, and young adults in short-term studies of major depressive disorder (MDD) and other psychiatric disorders. Anyone considering the use of clomipramine hydrochloride capsules or any other antidepressant in a child, adolescent, or young adult must balance this risk with the clinical need. Short-term studies did not show an increase in the risk of suicidality with antidepressants compared to placebo in adults beyond age 24; there was a reduction in risk with antidepressants compared to placebo in adults aged 65 and older. Depression and certain other psychiatric disorders are themselves associated with increases in the risk of suicide. Patients of all ages who are started on antidepressant therapy should be monitored appropriately and observed closely for clinical worsening, suicidality, or unusual changes in behavior. Families and caregivers should be advised of the need for close observation and communication with the prescriber. Clomipramine hydrochloride capsules are not approved for use in pediatric patients except for patients with obsessive compulsive disorder (OCD) (see WARNINGS: Clinical Worsening and Suicide Risk, PRECAUTIONS: Information for Patients, and PRECAUTIONS: Pediatric Use).
|
Clinical Worsening and Suicide Risk
- Patients with major depressive disorder (MDD), both adult and pediatric, may experience worsening of their depression and/or the emergence of suicidal ideation and behavior (suicidality) or unusual changes in behavior, whether or not they are taking antidepressant medications, and this risk may persist until significant remission occurs. Suicide is a known risk of depression and certain other psychiatric disorders, and these disorders themselves are the strongest predictors of suicide. There has been a long-standing concern, however, that antidepressants may have a role in inducing worsening of depression and the emergence of suicidality in certain patients during the early phases of treatment. Pooled analyses of short-term placebo-controlled trials of antidepressant drugs (SSRIs and others) showed that these drugs increase the risk of suicidal thinking and behavior (suicidality) in children, adolescents, and young adults (ages 18-24) with major depressive disorder (MDD) and other psychiatric disorders. Short-term studies did not show an increase in the risk of suicidality with antidepressants compared to placebo in adults beyond age 24; there was a reduction with antidepressants compared to placebo in adults aged 65 and older.
- The pooled analyses of placebo-controlled trials in children and adolescents with MDD, obsessive compulsive disorder (OCD), or other psychiatric disorders included a total of 24 short-term trials of 9 antidepressant drugs in over 4400 patients. The pooled analyses of placebo-controlled trials in adults with MDD or other psychiatric disorders included a total of 295 short-term trials (median duration of 2 months) of 11 antidepressant drugs in over 77,000 patients. There was considerable variation in risk of suicidality among drugs, but a tendency toward an increase in the younger patients for almost all drugs studied. There were differences in absolute risk of suicidality across the different indications, with the highest incidence in MDD. The risk differences (drug vs placebo), however, were relatively stable within age strata and across indications.

- No suicides occurred in any of the pediatric trials. There were suicides in the adult trials, but the number was not sufficient to reach any conclusion about drug effect on suicide.
- It is unknown whether the suicidality risk extends to longer-term use, i.e., beyond several months. However, there is substantial evidence from placebo-controlled maintenance trials in adults with depression that the use of antidepressants can delay the recurrence of depression.
- All patients being treated with antidepressants for any indication should be monitored appropriately and observed closely for clinical worsening, suicidality, and unusual changes in behavior, especially during the initial few months of a course of drug therapy, or at times of dose changes, either increases or decreases.
- The following symptoms, anxiety, agitation, panic attacks, insomnia, irritability, hostility, aggressiveness, impulsivity, akathisia (psychomotor restlessness), hypomania, and mania, have been reported in adult and pediatric patients being treated with antidepressants for major depressive disorder as well as for other indications, both psychiatric and nonpsychiatric. Although a causal link between the emergence of such symptoms and either the worsening of depression and/or the emergence of suicidal impulses has not been established, there is concern that such symptoms may represent precursors to emerging suicidality.
- Consideration should be given to changing the therapeutic regimen, including possibly discontinuing the medication, in patients whose depression is persistently worse, or who are experiencing emergent suicidality or symptoms that might be precursors to worsening depression or suicidality, especially if these symptoms are severe, abrupt in onset, or were not part of the patient’s presenting symptoms.
- Families and caregivers of patients being treated with antidepressants for major depressive disorder or other indications, both psychiatric and nonpsychiatric, should be alerted about the need to monitor patients for the emergence of agitation, irritability, unusual changes in behavior, and the other symptoms described above, as well as the emergence of suicidality, and to report such symptoms immediately to health care providers. Such monitoring should include daily observation by families and caregivers. Prescriptions for clomipramine hydrochloride should be written for the smallest quantity of capsules consistent with good patient management, in order to reduce the risk of overdose.
Screening Patients for Bipolar Disorder
- A major depressive episode may be the initial presentation of bipolar disorder. It is generally believed (though not established in controlled trials) that treating such an episode with an antidepressant alone may increase the likelihood of precipitation of a mixed/manic episode in patients at risk for bipolar disorder. Whether any of the symptoms described above represent such a conversion is unknown. However, prior to initiating treatment with an antidepressant, patients with depressive symptoms should be adequately screened to determine if they are at risk for bipolar disorder; such screening should include a detailed psychiatric history, including a family history of suicide, bipolar disorder, and depression. It should be noted that clomipramine hydrochloride is not approved for use in treating bipolar depression.
Serotonin Syndrome
- The development of a potentially life-threatening serotonin syndrome has been reported with SNRIs and SSRIs, including clomipramine alone but particularly with concomitant use of other serotonergic drugs (including triptans, tricyclic antidepressants, fentanyl, lithium, tramadol, tryptophan, buspirone, and St. John's Wort) and with drugs that impair metabolism of serotonin (in particular, MAOIs, both those intended to treat psychiatric disorders and also others, such as linezolid and intravenous methylene blue).
- Serotonin syndrome symptoms may include mental status changes (e.g., agitation, hallucinations, delirium, and coma), autonomic instability (e.g., tachycardia, labile blood pressure, dizziness, diaphoresis, flushing, hyperthermia), neuromuscular changes (e.g., tremor, rigidity, myoclonus, hyperreflexia, incoordination), seizures, and/or gastrointestinal symptoms (e.g., nausea, vomiting, diarrhea). Patients should be monitored for the emergence of serotonin syndrome.
- The concomitant use of clomipramine with MAOIs intended to treat psychiatric disorders is contraindicated. Clomipramine should also not be started in a patient who is being treated with MAOIs such as linezolid or intravenous methylene blue. All reports with methylene blue that provided information on the route of administration involved intravenous administration in the dose range of 1 mg/kg to 8 mg/kg. No reports involved the administration of methylene blue by other routes (such as oral tablets or local tissue injection) or at lower doses. There may be circumstances when it is necessary to initiate treatment with an MAOI such as linezolid or intravenous methylene blue in a patient taking clomipramine. Clomipramine should be discontinued before initiating treatment with the MAOI.
- If concomitant use of clomipramine with other serotonergic drugs, including triptans, tricyclic antidepressants, fentanyl, lithium, tramadol, buspirone, tryptophan, and St. John’s Wort is clinically warranted, patients should be made aware of a potential increased risk for serotonin syndrome, particularly during treatment initiation and dose increases.
- Treatment with clomipramine and any concomitant serotonergic agents should be discontinued immediately if the above events occur and supportive symptomatic treatment should be initiated.
Seizures
- During premarket evaluation, seizure was identified as the most significant risk of clomipramine hydrochloride use.
- The observed cumulative incidence of seizures among patients exposed to clomipramine hydrochloride at doses up to 300 mg/day was 0.64% at 90 days, 1.12% at 180 days, and 1.45% at 365 days. The cumulative rates correct the crude rate of 0.7% (25 of 3519 patients) for the variable duration of exposure in clinical trials.
- Although dose appears to be a predictor of seizure, there is a confounding of dose and duration of exposure, making it difficult to assess independently the effect of either factor alone. The ability to predict the occurrence of seizures in subjects exposed to doses of clomipramine greater than 250 mg is limited, given that the plasma concentration of clomipramine may be dose-dependent and may vary among subjects given the same dose. Nevertheless, prescribers are advised to limit the daily dose to a maximum of 250 mg in adults and 3 mg/kg (or 200 mg) in children and adolescents.
- Caution should be used in administering clomipramine to patients with a history of seizures or other predisposing factors, e.g., brain damage of varying etiology, alcoholism, and concomitant use with other drugs that lower the seizure threshold.
- Rare reports of fatalities in association with seizures have been reported by foreign postmarketing surveillance, but not in U.S. clinical trials. In some of these cases, clomipramine had been administered with other epileptogenic agents; in others, the patients involved had possibly predisposing medical conditions. Thus a causal association between clomipramine treatment and these fatalities has not been established.
- Physicians should discuss with patients the risk of taking clomipramine while engaging in activities in which sudden loss of consciousness could result in serious injury to the patient or others, e.g., the operation of complex machinery, driving, swimming, climbing.
Adverse Reactions
Clinical Trials Experience
Central Nervous System
- Frequent – abnormal thinking, vertigo. Infrequent – abnormal coordination, abnormal EEG, abnormal gait, apathy, ataxia, coma, convulsions, delirium, delusion, dyskinesia, dysphonia, encephalopathy, euphoria, extrapyramidal disorder, hallucinations, hostility, hyperkinesia, hypnagogic hallucinations, hypokinesia, leg cramps, manic reaction, neuralgia, paranoia, phobic disorder, psychosis, sensory disturbance, somnambulism, stimulation, suicidal ideation, suicide attempt, teeth-grinding. Rare – anticholinergic syndrome, aphasia, apraxia, catalepsy, cholinergic syndrome, choreoathetosis, generalized spasm, hemiparesis, hyperesthesia, hyperreflexia, hypoesthesia, illusion, impaired impulse control, indecisiveness, mutism, neuropathy, nystagmus, oculogyric crisis, oculomotor nerve paralysis, schizophrenic reaction, stupor, suicide.
Cardiovascular
- Infrequent – abnormal ECG, arrhythmia, bradycardia, cardiac arrest, extrasystoles, pallor. Rare – aneurysm, atrial flutter, bundle branch block, cardiac failure, cerebral hemorrhage, heart block, myocardial infarction, myocardial ischemia, peripheral ischemia, thrombophlebitis, vasospasm, ventricular tachycardia.
Respiratory
- Infrequent – bronchitis, hyperventilation, increased sputum, pneumonia. Rare – cyanosis, hemoptysis, hypoventilation, laryngismus.
Digestive System
- Infrequent – abnormal hepatic function, blood in stool, colitis, duodenitis, gastric ulcer, gastritis, gastroesophageal reflux, gingivitis, glossitis, hemorrhoids, hepatitis, increased saliva, irritable bowel syndrome, peptic ulcer, rectal hemorrhage, tongue ulceration, tooth caries. Rare – cheilitis, chronic enteritis, discolored feces, gastric dilatation, gingival bleeding, hiccup, intestinal obstruction, oral/pharyngeal edema, paralytic ileus, salivary gland enlargement.
Hypersensitive Reactions
- Infrequent – alopecia, cellulitis, cyst, eczema, erythematous rash, genital pruritus, maculopapular rash, photosensitivity reaction, psoriasis, pustular rash, skin discoloration. Rare – chloasma, folliculitis, hypertrichosis, piloerection, seborrhea, skin hypertrophy, skin ulceration.
Urogenital System
- Infrequent – endometriosis, epididymitis, hematuria, nocturia, oliguria, ovarian cyst, perineal pain, polyuria, prostatic disorder, renal calculus, renal pain, urethral disorder, urinary incontinence, uterine hemorrhage, vaginal hemorrhage. Rare – albuminuria, anorgasmy, breast engorgement, breast fibroadenosis, cervical dysplasia, endometrial hyperplasia, premature ejaculation, pyelonephritis, pyuria, renal cyst, uterine inflammation, vulvar disorder.
Endocrine System
- Infrequent – hypothyroidism. Rare – goiter, gynecomastia, hyperthyroidism.
Hemic and Lymphatic System
- Infrequent – lymphadenopathy. Rare – leukemoid reaction, lymphoma-like disorder, marrow depression.
Metabolic and Nutritional Disorder
- Infrequent – dehydration, diabetes mellitus, gout, hypercholesterolemia, hyperglycemia, hyperuricemia, hypokalemia. Rare – fat intolerance, glycosuria.
Musculoskeletal System
- Infrequent – arthrosis. Rare – dystonia, exostosis, lupus erythematosus rash, bruising, myopathy, myositis, polyarteritis nodosa, torticollis.
Miscellaneous
- The most commonly observed adverse events associated with the use of clomipramine and not seen at an equivalent incidence among placebo-treated patients were gastrointestinal complaints, including dry mouth, constipation, nausea, dyspepsia, and anorexia; nervous system complaints, including somnolence, tremor, dizziness, nervousness, and myoclonus; genitourinary complaints, including changed libido, ejaculatory failure, impotence, and micturition disorder; and other miscellaneous complaints, including fatigue, sweating, increased appetite, weight gain, and visual changes.
Postmarketing Experience
There is limited information regarding Clomipramine Postmarketing Experience in the drug label.
Drug Interactions
- The risks of using clomipramine in combination with other drugs have not been systematically evaluated. Given the primary CNS effects of clomipramine, caution is advised in using it concomitantly with other CNS-active drugs. Clomipramine should not be used with MAO inhibitors.
- Close supervision and careful adjustment of dosage are required when clomipramine is administered with anticholinergic or sympathomimetic drugs.
- Several tricyclic antidepressants have been reported to block the pharmacologic effects of guanethidine, clonidine, or similar agents, and such an effect may be anticipated with clomipramine because of its structural similarity to other tricyclic antidepressants.
- The plasma concentration of clomipramine has been reported to be increased by the concomitant administration of haloperidol; plasma levels of several closely related tricyclic antidepressants have been reported to be increased by the concomitant administration of methylphenidate or hepatic enzyme inhibitors (e.g., cimetidine, fluoxetine) and decreased by the concomitant administration of hepatic enzyme inducers (e.g., barbiturates, phenytoin), and such an effect may be anticipated with clomipramine as well. Administration of clomipramine has been reported to increase the plasma levels of phenobarbital, if given concomitantly.
- Drugs Metabolized by P450 2D6-The biochemical activity of the drug metabolizing isozyme cytochrome P450 2D6 (debrisoquin hydroxylase) is reduced in a subset of the Caucasian population (about 7% to 10% of Caucasians are so-called “poor metabolizers”); reliable estimates of the prevalence of reduced P450 2D6 isozyme activity among Asian, African and other populations are not yet available. Poor metabolizers have higher than expected plasma concentrations of tricyclic antidepressants (TCAs) when given usual doses. Depending on the fraction of drug metabolized by P450 2D6, the increase in plasma concentration may be small, or quite large (8 fold increase in plasma AUC of the TCA). In addition, certain drugs inhibit the activity of this isozyme and make normal metabolizers resemble poor metabolizers. An individual who is stable on a given dose of TCA may become abruptly toxic when given one of these inhibiting drugs as concomitant therapy. The drugs that inhibit cytochrome P450 2D6 include some that are not metabolized by the enzyme (quinidine; cimetidine) and many that are substrates for P450 2D6 (many other antidepressants, phenothiazines, and the Type 1C antiarrhythmics propafenone and flecainide). While all the selective serotonin reuptake inhibitors (SSRIs), e.g., fluoxetine, sertraline, paroxetine, and fluvoxamine, inhibit P450 2D6, they may vary in the extent of inhibition. Fluvoxamine has also been shown to inhibit P450 1A2, an isoform also involved in TCA metabolism. The extent to which SSRI-TCA interactions may pose clinical problems will depend on the degree of inhibition and the pharmacokinetics of the SSRI involved. Nevertheless, caution is indicated in the co-administration of TCAs with any of the SSRIs and also in switching from one class to the other. Of particular importance, sufficient time must elapse before initiating TCA treatment in a patient being withdrawn from fluoxetine, given the long half-life of the parent and active metabolite (at least 5 weeks may be necessary). Concomitant use of agents in the tricyclic antidepressant class (which includes clomipramine hydrochloride) with drugs that can inhibit cytochrome P450 2D6 may require lower doses than usually prescribed for either the tricyclic antidepressant agent or the other drug. Furthermore, whenever one of these drugs is withdrawn from co-therapy, an increased dose of tricyclic antidepressant agent may be required. It is desirable to monitor TCA plasma levels whenever an agent of the tricyclic antidepressant class including clomipramine hydrochloride is going to be co-administered with another drug known to be an inhibitor of P450 2D6 (and/or P450 1A2).
- Because clomipramine hydrochloride is highly bound to serum protein, the administration of clomipramine hydrochloride to patients taking other drugs that are highly bound to protein (e.g., warfarin, digoxin) may cause an increase in plasma concentrations of these drugs, potentially resulting in adverse effects. Conversely, adverse effects may result from displacement of protein-bound clomipramine by other highly bound drugs.
Use in Specific Populations
Pregnancy
- No teratogenic effects were observed in studies performed in rats and mice at doses up to 100 mg/kg, which is 24 times the maximum recommended human daily dose (MRHD) on a mg/kg basis and 4 times (rats) and 2 times (mice) the MRHD on a mg/m2 basis. Slight nonspecific embryo/fetotoxic effects were seen in the offspring of treated rats given 50 and 100 mg/kg and of treated mice given 100 mg/kg.
- There are no adequate or well-controlled studies in pregnant women. Withdrawal symptoms, including jitteriness, tremor, and seizures, have been reported in neonates whose mothers had taken clomipramine hydrochloride until delivery. Clomipramine should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Clomipramine in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Clomipramine during labor and delivery.
Nursing Mothers
- Clomipramine has been found in human milk. Because of the potential for adverse reactions, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
- Safety and effectiveness in the pediatric population other than pediatric patients with OCD have not been established . Anyone considering the use of clomipramine hydrochloride in a child or adolescent must balance the potential risks with the clinical need.
- In a controlled clinical trial in children and adolescents (10 to 17 years of age), 46 outpatients received clomipramine for up to 8 weeks. In addition, 150 adolescent patients have received clomipramine in open-label protocols for periods of several months to several years. Of the 196 adolescents studied, 50 were 13 years of age or less and 146 were 14 to 17 years of age. The adverse reaction profile in this age group is similar to that observed in adults.
- The risks, if any, that may be associated with clomipramine’s extended use in children and adolescents with OCD have not been systematically assessed. The evidence supporting the conclusion that clomipramine is safe for use in children and adolescents is derived from relatively short term clinical studies and from extrapolation of experience gained with adult patients. In particular, there are no studies that directly evaluate the effects of long term clomipramine use on the growth, development, and maturation of children and adolescents. Although there is no evidence to suggest that clomipramine adversely affects growth, development or maturation, the absence of such findings is not adequate to rule out a potential for such effects in chronic use.
- The safety and effectiveness in pediatric patients below the age of 10 have not been established. Therefore, specific recommendations cannot be made for the use of clomipramine in pediatric patients under the age of 10.
Geriatic Use
- Clinical studies of clomipramine hydrochloride did not include sufficient numbers of subjects age 65 and over to determine whether they respond differently from younger subjects; 152 patients at least 60 years of age participating in various U.S. clinical trials received clomipramine hydrochloride for periods of several months to several years. No unusual age-related adverse events were identified in this population. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function and of concomitant disease or other drug therapy.
Gender
There is no FDA guidance on the use of Clomipramine with respect to specific gender populations.
Race
There is no FDA guidance on the use of Clomipramine with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Clomipramine in patients with renal impairment.
Hepatic Impairment
- During premarketing testing, clomipramine hydrochloride was occasionally associated with elevations in SGOT and SGPT (pooled incidence of approximately 1% and 3%, respectively) of potential clinical importance (i.e., values greater than 3 times the upper limit of normal). In the vast majority of instances, these enzyme increases were not associated with other clinical findings suggestive of hepatic injury; moreover, none were jaundiced. Rare reports of more severe liver injury, some fatal, have been recorded in foreign postmarketing experience. Caution is indicated in treating patients with known liver disease, and periodic monitoring of hepatic enzyme levels is recommended in such patients.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Clomipramine in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Clomipramine in patients who are immunocompromised.
Administration and Monitoring
Administration
- The treatment regimens described below are based on those used in controlled clinical trials of clomipramine in 520 adults, and 91 children and adolescents with OCD. During initial titration, clomipramine should be given in divided doses with meals to reduce gastrointestinal side effects. The goal of this initial titration phase is to minimize side effects by permitting tolerance to side effects to develop or allowing the patient time to adapt if tolerance does not develop.
- Because both clomipramine and its active metabolite, desmethylclomipramine, have long elimination half-lives, the prescriber should take into consideration the fact that steady-state plasma levels may not be achieved until 2 to 3 weeks after dosage change. Therefore, after initial titration, it may be appropriate to wait 2 to 3 weeks between further dosage adjustments.
- Initial Treatment/Dose Adjustment (Adults)
Treatment with clomipramine hydrochloride should be initiated at a dosage of 25 mg daily and gradually increased, as tolerated, to approximately 100 mg during the first 2 weeks. During initial titration, clomipramine should be given in divided doses with meals to reduce gastrointestinal side effects. Thereafter, the dosage may be increased gradually over the next several weeks, up to a maximum of 250 mg daily. After titration, the total daily dose may be given once daily at bedtime to minimize daytime sedation.
- Initial Treatment/Dose Adjustment (Children and Adolescents)
As with adults, the starting dose is 25 mg daily and should be gradually increased (also given in divided doses with meals to reduce gastrointestinal side effects) during the first 2 weeks, as tolerated, up to a daily maximum of 3 mg/kg or 100 mg, whichever is smaller. Thereafter, the dosage may be increased gradually over the next several weeks up to a daily maximum of 3 mg/kg or 200 mg, whichever is smaller. As with adults, after titration, the total daily dose may be given once daily at bedtime to minimize daytime sedation.
Monitoring
- Maintenance/Continuation Treatment (Adults, Children, and Adolescents)
While there are no systematic studies that answer the question of how long to continue clomipramine, OCD is a chronic condition and it is reasonable to consider continuation for a responding patient. Although the efficacy of clomipramine after 10 weeks has not been documented in controlled trials, patients have been continued in therapy under double-blind conditions for up to 1 year without loss of benefit. However, dosage adjustments should be made to maintain the patient on the lowest effective dosage, and patients should be periodically reassessed to determine the need for treatment. During maintenance, the total daily dose may be given once daily at bedtime.
- Switching a Patient To or From a Monoamine Oxidase Inhibitor (MAOI) Intended to Treat Psychiatric Disorders
At least 14 days should elapse between discontinuation of an MAOI intended to treat psychiatric disorders and initiation of therapy with clomipramine. Conversely, at least 14 days should be allowed after stopping Anafranil before starting an MAOI intended to treat psychiatric disorders.
- Use of Clomipramine With Other MAOIs, Such as Linezolid or Methylene Blue
- Do not start clomipramine in a patient who is being treated with linezolid or intravenous methylene blue because there is increased risk of serotonin syndrome. In a patient who requires more urgent treatment of a psychiatric condition, other interventions, including hospitalization, should be considered (see CONTRAINDICATIONS).
In some cases, a patient already receiving clomipramine therapy may require urgent treatment with linezolid or intravenous methylene blue. If acceptable alternatives to linezolid or intravenous methylene blue treatment are not available and the potential benefits of linezolid or intravenous methylene blue treatment are judged to outweigh the risks of serotonin syndrome in a particular patient, clomipramine should be stopped promptly, and linezolid or intravenous methylene blue can be administered. The patient should be monitored for symptoms of serotonin syndrome for two weeks or until 24 hours after the last dose of linezolid or intravenous methylene blue, whichever comes first. Therapy with clomipramine may be resumed 24 hours after the last dose of linezolid or intravenous methylene blue (see WARNINGS). The risk of administering methylene blue by non-intravenous routes (such as oral tablets or by local injection) or in intravenous doses much lower than 1 mg/kg with clomipramine is unclear. The clinician should, nevertheless, be aware of the possibility of emergent symptoms of serotonin syndrome with such use.
IV Compatibility
There is limited information regarding the compatibility of Clomipramine and IV administrations.
Overdosage
- Deaths may occur from overdosage with this class of drugs. Multiple drug ingestion (including alcohol) is common in deliberate tricyclic overdose. As the management is complex and changing, it is recommended that the physician contact a poison control center for current information on treatment. Signs and symptoms of toxicity develop rapidly after tricyclic overdose. Therefore, hospital monitoring is required as soon as possible.
- Human Experience
In U.S. clinical trials, 2 deaths occurred in 12 reported cases of acute overdosage with clomipramine either alone or in combination with other drugs. One death involved a patient suspected of ingesting a dose of 7000 mg. The second death involved a patient suspected of ingesting a dose of 5750 mg. The 10 nonfatal cases involved doses of up to 5000 mg, accompanied by plasma levels of up to 1010 ng/mL. All 10 patients completely recovered. Among reports from other countries of clomipramine overdose, the lowest dose associated with a fatality was 750 mg. Based upon postmarketing reports in the United Kingdom, clomipramine’s lethality in overdose is considered to be similar to that reported for closely related tricyclic compounds marketed as antidepressants.
- Manifestations
Signs and symptoms vary in severity depending upon factors such as the amount of drug absorbed, the age of the patient, and the time elapsed since drug ingestion. Critical manifestations of overdose include cardiac dysrhythmias, severe hypotension, convulsions, and CNS depression including coma. Changes in the electrocardiogram, particularly in QRS axis or width, are clinically significant indicators of tricyclic toxicity. Other CNS manifestations may include drowsiness, stupor, ataxia, restlessness, agitation, delirium, severe perspiration, hyperactive reflexes, muscle rigidity, and athetoid and choreiform movements. Cardiac abnormalities may include tachycardia, signs of congestive heart failure, and in very rare cases, cardiac arrest. Respiratory depression, cyanosis, shock, vomiting, hyperpyrexia, mydriasis, and oliguria or anuria may also be present.
- Management
Obtain an ECG and immediately initiate cardiac monitoring. Protect the patient’s airway, establish an intravenous line, and initiate gastric decontamination. A minimum of 6 hours of observation with cardiac monitoring and observation for signs of CNS or respiratory depression, hypotension, cardiac dysrhythmias and/or conduction blocks, and seizures is necessary. If signs of toxicity occur at any time during this period, extended monitoring is required. There are case reports of patients succumbing to fatal dysrhythmias late after overdose; these patients had clinical evidence of significant poisoning prior to death and most received inadequate gastrointestinal decontamination. Monitoring of plasma drug levels should not guide management of the patient. Gastrointestinal Decontamination All patients suspected of tricyclic overdose should receive gastrointestinal decontamination. This should include large volume gastric lavage followed by activated charcoal. If consciousness is impaired, the airway should be secured prior to lavage. Emesis is contraindicated.
- Cardiovascular
A maximal limb-lead QRS duration of ≥ 0.10 seconds may be the best indication of the severity of the overdose. Intravenous sodium bicarbonate should be used to maintain the serum pH in the range of 7.45 to 7.55. If the pH response is inadequate, hyperventilation may also be used. Concomitant use of hyperventilation and sodium bicarbonate should be done with extreme caution, with frequent pH monitoring. A pH >7.60 or a pCO2 < 20 mmHg is undesirable. Dysrhythmias unresponsive to sodium bicarbonate therapy/hyperventilation may respond to lidocaine, bretylium, or phenytoin. Type 1A and 1C antiarrhythmics are generally contraindicated (e.g., quinidine, disopyramide, and procainamide). In rare instances, hemoperfusion may be beneficial in acute refractory cardiovascular instability in patients with acute toxicity. However, hemodialysis, peritoneal dialysis, exchange transfusions, and forced diuresis generally have been reported as ineffective in tricyclic poisoning.
- CNS
In patients with CNS depression, early intubation is advised because of the potential for abrupt deterioration. Seizures should be controlled with benzodiazepines, or if these are ineffective, other anticonvulsants (e.g., phenobarbital, phenytoin). Physostigmine is not recommended except to treat life-threatening symptoms that have been unresponsive to other therapies, and then only in consultation with a poison control center.
- Psychiatric Follow-up
Since overdosage is often deliberate, patients may attempt suicide by other means during the recovery phase. Psychiatric referral may be appropriate.
- Pediatric Management
The principles of management of child and adult overdosages are similar. It is strongly recommended that the physician contact the local poison control center for specific pediatric treatment.
Pharmacology
Mechanism of Action
There is limited information regarding Clomipramine Mechanism of Action in the drug label.
Structure
Pharmacodynamics
Clomipramine is presumed to influence obsessive and compulsive behaviors through its effects on serotonergic neuronal transmission. The actual neurochemical mechanism is unknown, but clomipramine’s capacity to inhibit the reuptake of serotonin (5-HT) is thought to be important.
Pharmacokinetics
- Absorption/Bioavailability
Clomipramine from a capsule is as bioavailable as clomipramine from a solution. The bioavailability of clomipramine hydrochloride capsules is not significantly affected by food. In a dose proportionality study involving multiple clomipramine doses, steady-state plasma concentrations (Css) and area-under-plasma-concentration-time curves (AUC) of clomipramine and clomipramine’s major active metabolite, desmethylclomipramine, were not proportional to dose over the ranges evaluated, i.e., between 25 and 100 mg/day and between 25 and 150 mg/day, although Css and AUC are approximately linearly related to dose between 100 and 150 mg/day. The relationship between dose and clomipramine/desmethylclomipramine concentrations at higher daily doses has not been systematically assessed, but if there is significant dose dependency at doses above 150 mg/day, there is the potential for dramatically higher Css and AUC even for patients dosed within the recommended range. This may pose a potential risk to some patients (see WARNINGS and PRECAUTIONS: Drug Interactions). After a single 50 mg oral dose, maximum plasma concentrations of clomipramine occur within 2 to 6 hours (mean, 4.7 hr) and range from 56 ng/mL to 154 ng/mL (mean, 92 ng/mL). After multiple daily doses of 150 mg of clomipramine hydrochloride, steady-state maximum plasma concentrations range from 94 ng/mL to 339 ng/mL (mean, 218 ng/mL) for clomipramine and from 134 ng/mL to 532 ng/mL (mean, 274 ng/mL) for desmethylclomipramine. Additional information from a rising dose study of doses up to 250 mg suggests that desmethylclomipramine may exhibit nonlinear pharmacokinetics over the usual dosing range. At a dose of clomipramine hydrochloride capsules 200 mg, subjects who had a single blood sample taken approximately 9 to 22 hours, (median 16 hours), after the dose had plasma concentrations of up to 605 ng/mL for clomipramine, 781 ng/mL for desmethylclomipramine, and 1386 ng/mL for both.
- Distribution
Clomipramine distributes into cerebrospinal fluid (CSF) and brain and into breast milk. Desmethylclomipramine also distributes into CSF, with a mean CSF/plasma ratio of 2.6. The protein binding of clomipramine is approximately 97%, principally to albumin, and is independent of clomipramine concentration. The interaction between clomipramine and other highly protein-bound drugs has not been fully evaluated, but may be important (see PRECAUTIONS: Drug Interactions).
- Metabolism
Clomipramine is extensively biotransformed to desmethylclomipramine and other metabolites and their glucuronide conjugates. Desmethylclomipramine is pharmacologically active, but its effects on Obsessive-Compulsive Disorder (OCD) behaviors are unknown. These metabolites are excreted in urine and feces, following biliary elimination. After a 25 mg radiolabeled dose of clomipramine in two subjects, 60% and 51%, respectively, of the dose were recovered in the urine and 32% and 24%, respectively, in feces. In the same study, the combined urinary recoveries of clomipramine and desmethylclomipramine were only about 0.8 to 1.3% of the dose administered. Clomipramine does not induce drug-metabolizing enzymes, as measured by antipyrine half-life.
- Elimination
Evidence that the Css and AUC for clomipramine and desmethylclomipramine may increase disproportionately with increasing oral doses suggests that the metabolism of clomipramine and desmethylclomipramine may be capacity limited. This fact must be considered in assessing the estimates of the pharmacokinetic parameters presented below, as these were obtained in individuals exposed to doses of 150 mg. If the pharmacokinetics of clomipramine and desmethylclomipramine are nonlinear at doses above 150 mg, their elimination half-lives may be considerably lengthened at doses near the upper end of the recommended dosing range (i.e., 200 mg/day to 250 mg/day). Consequently, clomipramine and desmethylclomipramine may accumulate, and this accumulation may increase the incidence of any dose- or plasma-concentration-dependent adverse reactions, in particular seizures. After a 150 mg dose, the half-life of clomipramine ranges from 19 hours to 37 hours (mean, 32 hr) and that of desmethylclomipramine ranges from 54 hours to 77 hours (mean, 69 hr). Steady-state levels after multiple dosing are typically reached within 7 to 14 days for clomipramine. Plasma concentrations of the metabolite exceed the parent drug on multiple dosing. After multiple dosing with 150 mg/day, the accumulation factor for clomipramine is approximately 2.5 and for desmethylclomipramine is 4.6. Importantly, it may take two weeks or longer to achieve this extent of accumulation at constant dosing because of the relatively long elimination half-lives of clomipramine and desmethylclomipramine (see DOSAGE AND ADMINISTRATION). The effects of hepatic and renal impairment on the disposition of clomipramine have not been determined.
Nonclinical Toxicology
ANIMAL TOXICOLOGY Phospholipidosis and testicular changes, commonly associated with tricyclic compounds, have been observed with clomipramine hydrochloride. In chronic rat studies, changes related to clomipramine hydrochloride consisted of systemic phospholipidosis, alterations in the testes (atrophy, mineralization) and secondary changes in other tissues. In addition cardiac thrombosis and dermatitis/keratitis were observed in rats treated for 2 years at doses which were 24 and 10 times the maximum recommended human daily dose (MRHD), respectively, on a mg/kg basis, and 4 and 1.5 times the MRHD, respectively, on a mg/m2 basis.
Clinical Studies
There is limited information regarding Clomipramine Clinical Studies in the drug label.
How Supplied
Clomipramine hydrochloride capsules USP for oral administration are available as: 25 mg: White capsules imprinted GG 822 with orange and yellow ink bands, filled with white powder and supplied as: NDC 0781-2027-31 bottles of 30 NDC 0781-2027-01 bottles of 100 NDC 0781-2027-10 bottles of 1000 50 mg: White capsules imprinted GG 823 with blue and yellow ink bands, filled with white powder and supplied as: NDC 0781-2037-31 bottles of 30 NDC 0781-2037-01 bottles of 100 NDC 0781-2037-10 bottles of 1000 75 mg: White capsules imprinted GG 824 with dark yellow and yellow ink bands, filled with white powder and supplied as: NDC 0781-2047-31 bottles of 30 NDC 0781-2047-01 bottles of 100 NDC 0781-2047-10 bottles of 1000
Storage
Store at 20º to 25ºC (68º to 77ºF) [see USP Controlled Room Temperature]. Protect from moisture. Dispense in a tight, light-resistant container.
Images
Drug Images
{{#ask: Page Name::Clomipramine |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Clomipramine |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Prescribers or other health professionals should inform patients, their families, and their caregivers about the benefits and risks associated with treatment with clomipramine hydrochloride and should counsel them in its appropriate use.
- A patient Medication Guide about “Antidepressant Medicines, Depression and other Serious Mental Illnesses, and Suicidal Thoughts or Actions” is available for clomipramine hydrochloride.
- The prescriber or health professional should instruct patients, their families, and their caregivers to read the Medication Guide and should assist them in understanding its contents. Patients should be given the opportunity to discuss the contents of the Medication Guide and to obtain answers to any questions they may have. The complete text of the Medication Guide is reprinted at the end of this document.
- Patients should be advised of the following issues and asked to alert their prescriber if these occur while taking clomipramine hydrochloride.
- Clinical Worsening and Suicide Risk
Patients, their families, and their caregivers should be encouraged to be alert to the emergence of anxiety, agitation, panic attacks, insomnia, irritability, hostility, aggressiveness, impulsivity, akathisia (psychomotor restlessness), hypomania, mania, other unusual changes in behavior, worsening of depression, and suicidal ideation, especially early during antidepressant treatment and when the dose is adjusted up or down. Families and caregivers of patients should be advised to look for the emergence of such symptoms on a day-to-day basis, since changes may be abrupt. Such symptoms should be reported to the patient’s prescriber or health professional, especially if they are severe, abrupt in onset, or were not part of the patient’s presenting symptoms. Symptoms such as these may be associated with an increased risk for suicidal thinking and behavior and indicate a need for very close monitoring and possibly changes in the medication. Physicians are advised to discuss the following issues with patients for whom they prescribe clomipramine hydrochloride:
- The risk of seizure;
- The relatively high incidence of sexual dysfunction among males (see PRECAUTIONS:Sexual Dysfunction);
- Since clomipramine may impair the mental and/or physical abilities required for the performance of complex tasks, and since clomipramine is associated with a risk of seizures, patients should be cautioned about the performance of complex and hazardous tasks (see WARNINGS);
- Patients should be cautioned about using alcohol, barbiturates, or other CNS depressants concurrently, since clomipramine may exaggerate their response to these drugs;
- Patients should notify their physician if they become pregnant or intend to become pregnant during therapy;
- Patients should notify their physician if they are breast-feeding.
Precautions with Alcohol
Alcohol-Clomipramine interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Clomipramine Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Clomipramine Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Flament MF, Rapoport JL, Berg CJ, Sceery W, Kilts C, Mellström B et al. (1985) Clomipramine treatment of childhood obsessive-compulsive disorder. A double-blind controlled study. Arch Gen Psychiatry 42 (10):977-83. PMID: 3899048
- ↑ Template:Cite isbn
{{#subobject:
|Page Name=Clomipramine |Pill Name=007812027.jpg |Drug Name=Clomipramine Hydrochloride 25 MG Oral Capsule |Pill Ingred=silicon dioxide, starch, corn, d&c red no. 30, d&c yellow no. 10, fd&c blue no. 1, fd&c blue no. 2, fd&c red no. 40, fd&c yellow no. 6, gelatin, lactose monohydrate, magnesium stearate, sodium lauryl sulfate, titanium dioxide|+sep=; |Pill Imprint=GG822 |Pill Dosage=25 mg |Pill Color=White|+sep=; |Pill Shape=Capsule |Pill Size (mm)=18.00 |Pill Scoring=1 |Pill Image= |Drug Author=Sandoz Inc |NDC=0781-2027-01
}}
{{#subobject:
|Page Name=Clomipramine |Pill Name=007812037.jpg |Drug Name=Clomipramine Hydrochloride 50 MG Oral Capsule |Pill Ingred=silicon dioxide, starch, corn, d&c yellow no. 10, fd&c blue no. 1, fd&c blue no. 2, fd&c red no. 40, fd&c yellow no. 6, gelatin, lactose monohydrate, magnesium stearate, sodium lauryl sulfate, titanium dioxide|+sep=; |Pill Imprint=GG823 |Pill Dosage=50 mg |Pill Color=White|+sep=; |Pill Shape=Capsule |Pill Size (mm)=19.00 |Pill Scoring=1 |Pill Image= |Drug Author=Sandoz Inc |NDC=0781-2037-01
}}
{{#subobject:
|Page Name=Clomipramine |Pill Name=007812047.jpg |Drug Name=Clomipramine Hydrochloride 75 MG Oral Capsule |Pill Ingred=|+sep=; |Pill Imprint=GG824 |Pill Dosage=silicon dioxide, starch, corn, d&c yellow no. 10, fd&c blue no. 1, fd&c blue no. 2, fd&c red no. 40, fd&c yellow no. 6, gelatin, lactose monohydrate, magnesium stearate, sodium lauryl sulfate, titanium dioxide |Pill Color=White|+sep=; |Pill Shape=Capsule |Pill Size (mm)=19.00 |Pill Scoring=1 |Pill Image= |Drug Author=Sandoz Inc |NDC=0781-2047-01
}}
{{#subobject:
|Label Page=Clomipramine |Label Name=clomipramine2.png.jpg
}}
{{#subobject:
|Label Page=Clomipramine |Label Name=clomipramine3.png.jpg
}}
{{#subobject:
|Label Page=Clomipramine |Label Name=Clomipramine.png.jpg
}}
{{#subobject:
|Label Page=Clomipramine |Label Name=CLOMIPRAMINE33.PNG
}}