Vilazodone
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Alberto Plate [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Vilazodone is a antidepressant, serotonine agonist that is FDA approved for the treatment of major depressive disorder (MDD). Common adverse reactions include diarrhea, nausea, vomiting, xerostomia, dizziness, insomnia.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Initial Treatment of Major Depressive Disorder
- The recommended dose for Vilazodone is 40 mg once daily. Vilazodone should be titrated, starting with an initial dose of 10 mg once daily for 7 days, followed by 20 mg once daily for an additional 7 days, and then an increase to 40 mg once daily. Vilazodone should be taken with food. Vilazodone blood concentrations (AUC) in the fasted state can be decreased by approximately 50% compared to the fed state, and may result in diminished effectiveness in some patients.
Maintenance/Continuation/Extended Treatment
- The efficacy of Vilazodone has not been systematically studied beyond 8 weeks. It is generally agreed that acute episodes of major depressive disorder require several months or longer of sustained pharmacologic therapy. Patients should be reassessed periodically to determine the need for maintenance treatment and the appropriate dose for treatment.
Concomitant Use of CYP3A4 Inhibitors or CYP3A4 Inducers
Patients receiving concomitant CYP3A4 inhibitors
- Reduce the Vilazodone dose to 20 mg if co-administered with a strong inhibitor of CYP3A4 (e.g., ketoconazole). During co-administration with moderate inhibitors of CYP3A4 (e.g., erythromycin), the Vilazodone dose should be reduced to 20 mg for patients with intolerable adverse events. The Vilazodone dose should be readjusted to the original level when CYP3A4 inhibitors are discontinued.
Patients receiving concomitant CYP3A4 inducers
- Based on clinical response, consider increasing the dose of Vilazodone up to 2-fold when concomitantly used with strong CYP3A4 inducers (e.g., carbamazepine) for greater than 14 days. The maximum daily dose should not exceed 80 mg. If CYP3A4 inducers are discontinued, reduce the Vilazodone dose to the original level in 14 days.
Discontinuing Treatment
- Discontinuation symptoms have been reported with discontinuation of serotonergic drugs such as Vilazodone Gradual dose reduction is recommended, instead of abrupt discontinuation, whenever possible. Monitor patients for these symptoms when discontinuing Vilazodone If intolerable symptoms occur following a dose decrease or upon discontinuation of treatment, consider resuming the previously prescribed dose and decreasing the dose at a more gradual rate.
Switching a Patient To or From a Monoamine Oxidase Inhibitor (MAOI) Intended to Treat Psychiatric Disorders
- At least 14 days should elapse between discontinuation of an MAOI intended to treat psychiatric disorders and initiation of therapy with Vilazodone Conversely, at least 14 days should be allowed after stopping Vilazodone before starting an MAOI intended to treat psychiatric disorders.
Use of Vilazodone with Other MAOIs such as Linezolid or Methylene Blue
- Do not start Vilazodone in a patient who is being treated with linezolid or intravenous methylene blue because there is an increased risk of serotonin syndrome. In a patient who requires more urgent treatment of a psychiatric condition, other interventions, including hospitalization, should be considered.
- In some cases, a patient already receiving Vilazodone therapy may require urgent treatment with linezolid or intravenous methylene blue. If acceptable alternatives to linezolid or intravenous methylene blue treatment are not available and the potential benefits of linezolid or intravenous methylene blue treatment are judged to outweigh the risks of serotonin syndrome in a particular patient, Vilazodone should be stopped promptly, and linezolid or intravenous methylene blue can be administered. The patient should be monitored for symptoms of serotonin syndrome for 2 weeks or until 24 hours after the last dose of linezolid or intravenous methylene blue, whichever comes first. Therapy with Vilazodone may be resumed 24 hours after the last dose of linezolid or intravenous methylene blue.
- The risk of administering methylene blue by non-intravenous routes (such as oral tablets or by local injection) or in intravenous doses much lower than 1 mg/kg with Vilazodone is unclear. The clinician should, nevertheless, be aware of the possibility of emergent symptoms of serotonin syndrome with such use.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Vilazodone in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Vilazodone in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Vilazodone FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Vilazodone in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Vilazodone in pediatric patients.
Contraindications
Monoamine Oxidase Inhibitors (MAOIs)
- The use of MAOIs intended to treat psychiatric disorders with Vilazodone or within 14 days of stopping treatment with Vilazodone is contraindicated because of an increased risk of serotonin syndrome. The use of Vilazodone within 14 days of stopping an MAOI intended to treat psychiatric disorders is also contraindicated.
- Starting Vilazodone in a patient who is being treated with MAOIs such as linezolid or intravenous methylene blue is also contraindicated because of an increased risk of serotonin syndrome.
Warnings
Suicidal Thoughts and Behaviors in Children, Adolescents, and Young Adults
- Patients with major depressive disorder (MDD), both adult and pediatric, may experience worsening of their depression and/or the emergence of suicidal ideation and behavior (suicidality) or unusual changes in behavior, whether or not they are taking antidepressant medications, and this risk may persist until significant remission occurs. Suicide is a known risk of depression and certain other psychiatric disorders, and these disorders themselves are the strongest predictors of suicide. There has been a long-standing concern, however, that antidepressants may have a role in inducing worsening of depression and the emergence of suicidality in certain patients during the early phases of treatment. Pooled analyses of short-term placebo-controlled studies of antidepressant drugs (selective serotonin reuptake inhibitors (SSRIs] and others) showed that these drugs increase the risk of suicidal thinking and behavior (suicidality) in children, adolescents, and young adults (ages 18-24) with MDD and other psychiatric disorders. Short-term studies did not show an increase in the risk of suicidality with antidepressants compared to placebo in adults beyond age 24; there was a reduction with antidepressants compared to placebo in adults aged 65 and older.
- The pooled analyses of placebo-controlled studies in children and adolescents with MDD, obsessive compulsive disorder (OCD), or other psychiatric disorders included a total of 24 short-term studies of 9 antidepressant drugs in over 4,400 patients. The pooled analyses of placebo-controlled studies in adults with MDD or other psychiatric disorders included a total of 295 short-term studies (median duration of 2 months) of 11 antidepressant drugs in over 77,000 patients. There was considerable variation in risk of suicidality among drugs, but a tendency toward an increase in the younger patients for almost all drugs studied. There were differences in absolute risk of suicidality across the different indications, with the highest incidence in MDD. The risk differences (drug vs. placebo), however, were relatively stable within age strata and across indications. These risk differences (drug-placebo difference in the number of cases of suicidality per 1000 patients treated) are provided in Table 1.

- No suicides occurred in any of the pediatric studies. There were suicides in the adult studies, but the number was not sufficient to reach any conclusion about drug effect on suicide.
- It is unknown whether the suicidality risk extends to longer-term use, i.e., beyond several months. However, there is substantial evidence from placebo-controlled maintenance studies in adults with depression that the use of antidepressants can delay the recurrence of depression.
- All patients being treated with antidepressants for any indication should be monitored appropriately and observed closely for clinical worsening, suicidality, and unusual changes in behavior, especially during the initial few months of a course of drug therapy, or at times of dose changes, either increases or decreases.
- The following symptoms, anxiety, agitation, panic attacks, insomnia, irritability, hostility, aggressiveness, impulsivity, akathisia (psychomotor restlessness), hypomania, and mania, have been reported in adult and pediatric patients being treated with antidepressants for major depressive disorder as well as for other indications, both psychiatric and nonpsychiatric. Although a causal link between the emergence of such symptoms and either the worsening of depression and/or the emergence of suicidal impulses has not been established, there is concern that such symptoms may represent precursors to emerging suicidality.
- Consideration should be given to changing the therapeutic regimen, including possibly discontinuing the medication, in patients whose depression is persistently worse, or who are experiencing emergent suicidality or symptoms that might be precursors to worsening depression or suicidality, especially if these symptoms are severe, abrupt in onset, or were not part of the patient's presenting symptoms.
- If the decision has been made to discontinue treatment, medication should be tapered, as rapidly as is feasible, but with recognition that abrupt discontinuation can be associated with certain symptoms
- Families and caregivers of patients being treated with antidepressants for major depressive disorder or other indications, both psychiatric and nonpsychiatric, should be alerted about the need to monitor patients for the emergence of agitation, irritability, unusual changes in behavior, and the other symptoms described above, as well as the emergence of suicidality, and to report such symptoms immediately to healthcare providers. Such monitoring should include daily observation by families and caregivers. Prescriptions for Vilazodone should be written for the smallest quantity of tablets consistent with good patient management, in order to reduce the risk of overdose.
Screening patients for bipolar disorder
- A major depressive episode may be the initial presentation of bipolar disorder. It is generally believed (though not established in controlled studies) that treating such an episode with an antidepressant alone may increase the likelihood of precipitation of a mixed/manic episode in patients at risk for bipolar disorder. Whether any of the symptoms described above represent such a conversion is unknown. However, prior to initiating treatment with an antidepressant, patients with depressive symptoms should be adequately screened to determine if they are at risk for bipolar disorder; such screening should include a detailed psychiatric history, including a family history of suicide, bipolar disorder, and depression. It should be noted that Vilazodone is not approved for use in treating bipolar depression.
Serotonin Syndrome
- The development of a potentially life-threatening serotonin syndrome has been reported with SNRIs and SSRIs, including Vilazodone alone but particularly with concomitant use of other serotonergic drugs (including triptans, tricyclic antidepressants, fentanyl, lithium, tramadol, tryptophan, buspirone, and St. John's Wort) and with drugs that impair metabolism of serotonin (in particular, MAOIs, both those intended to treat psychiatric disorders and also others, such as linezolid and intravenous methylene blue). Symptoms of serotonin syndrome were noted in 0.1% of MDD patients treated with Vilazodone in premarketing clinical trials.
- Serotonin syndrome symptoms may include mental status changes (e.g., agitation, hallucinations, delirium, and coma), autonomic instability (e.g., tachycardia, labile blood pressure, dizziness, diaphoresis, flushing, hyperthermia), neuromuscular symptoms (e.g., tremor, rigidity, myoclonus, hyperreflexia, incoordination), seizures, and/or gastrointestinal symptoms (e.g., nausea, vomiting, diarrhea). Patients should be monitored for the emergence of serotonin syndrome.
- The concomitant use of Vilazodone with MAOIs intended to treat psychiatric disorders is contraindicated. Vilazodone should also not be started in a patient who is being treated with MAOIs such as linezolid or intravenous methylene blue. All reports with methylene blue that provided information on the route of administration involved intravenous administration in the dose range of 1 mg/kg to 8 mg/kg. No reports involved the administration of methylene blue by other routes (such as oral tablets or local tissue injection) or at lower doses. There may be circumstances when it is necessary to initiate treatment with an MAOI such as linezolid or intravenous methylene blue in a patient taking Vilazodone Vilazodone should be discontinued before initiating treatment with the MAOI and Dosage and Administration.
- If concomitant use of Vilazodone with other serotonergic drugs including, triptans, tricyclic antidepressants, fentanyl, lithium, tramadol, buspirone, tryptophan and St. John's Wort is clinically warranted, patients should be made aware of a potential increased risk for serotonin syndrome, particularly during treatment initiation and dose increases.
- Treatment with Vilazodone and any concomitant serotonergic agents, should be discontinued immediately if the above events occur and supportive symptomatic treatment should be initiated.
Seizures
- Vilazodone has not been systematically evaluated in patients with a seizure disorder. Patients with a history of seizures were excluded from clinical studies. Like other antidepressants, Vilazodone should be prescribed with caution in patients with a seizure disorder.
Abnormal Bleeding
- The use of drugs that interfere with serotonin reuptake inhibition, including Vilazodone, may increase the risk of bleeding events. Concomitant use of aspirin, nonsteroidal anti-inflammatory drugs (NSAIDs), warfarin, and other anticoagulants may add to this risk. Case reports and epidemiological studies (case-control and cohort design) have demonstrated an association between use of drugs that interfere with serotonin reuptake and the occurrence of gastrointestinal bleeding. Bleeding events related to SSRIs have ranged from ecchymosis, hematoma, epistaxis, and petechiae to life-threatening hemorrhages.
- Patients should be cautioned about the risk of bleeding associated with the concomitant use of Vilazodone and NSAIDs, aspirin, or other drugs that affect coagulation or bleeding.
Activation of Mania/Hypomania
- Symptoms of mania/hypomania were reported in 0.1% of patients treated with Vilazodone in clinical studies. Activation of mania/hypomania has also been reported in a small proportion of patients with major affective disorder who were treated with other antidepressants. As with all antidepressants, use Vilazodone cautiously in patients with a history or family history of bipolar disorder, mania, or hypomania.
Discontinuation of Treatment with Vilazodone
- There have been reports of adverse events occurring upon discontinuation of serotonergic antidepressants, particularly when discontinuation is abrupt, including the following: dysphoric mood, irritability, agitation, dizziness, sensory disturbances (e.g., paresthesia, such as electric shock sensations), anxiety, confusion, headache, lethargy, emotional lability, insomnia, hypomania, tinnitus, and seizures. While these events are generally self-limiting, there have been reports of serious discontinuation symptoms.
- Monitor patients for these symptoms when discontinuing Vilazodone Reduce the dose gradually whenever possible. If intolerable symptoms occur following a decrease in the dose or upon discontinuation of treatment, consider resuming the previously prescribed dose. Subsequently, the dose may be decreased, but at a more gradual rate.
Hyponatremia
- Although no cases of hyponatremia resulting from Vilazodone treatment were reported in the clinical studies, hyponatremia has occurred as a result of treatment with SSRIs and SNRIs. In many cases, hyponatremia appears to be the result of the syndrome of inappropriate antidiuretic hormone secretion (SIADH). Cases with serum sodium lower than 110 mmol/L have been reported. Elderly patients may be at greater risk of developing hyponatremia with SSRIs. Also, patients taking diuretics or who are otherwise volume depleted can be at greater risk. Discontinuation of Vilazodone in patients with symptomatic hyponatremia and appropriate medical intervention should be instituted. Signs and symptoms of hyponatremia include headache, difficulty concentrating, memory impairment, confusion, weakness, and unsteadiness, which can lead to falls. Signs and symptoms associated with more severe and/or acute cases have included hallucination, syncope, seizure, coma, respiratory arrest, and death.
Adverse Reactions
Clinical Trials Experience
The most commonly observed adverse reactions in Vilazodone-treated MDD patients in placebo-controlled studies (incidence ≥ 5% and at least twice the rate of placebo) were: diarrhea, nausea, vomiting, and insomnia.
Patient Exposure
- The safety of Vilazodone was evaluated in 2,177 patients (18-70 years of age) diagnosed with MDD who participated in clinical studies, representing 552 patient-years of exposure. In an open-label 52 week study at 40 mg daily, 599 patients were exposed to Vilazodone for a total of 348 patient-years. The information presented in these sections was derived from studies of Vilazodone 40 mg daily in major depressive disorder including: 1) 2 placebo-controlled 8-week studies in 861 patients, including 436 receiving vilazodone; and 2) an open-label 52-week study of 599 patients. These studies included a titration period of 10 mg daily for 7 days followed by 20 mg daily for 7 days. In these clinical trials, Vilazodone was administered with food. Because clinical trials are conducted under widely varying conditions and varying lengths of time, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect rates observed in practice.
Adverse reactions reported as reasons for discontinuation of treatment
- In the placebo-controlled studies of MDD there was no single adverse reaction leading to discontinuation in > 1% of the patients. Overall, 7.1% of the patients who received Vilazodone discontinued treatment due to an adverse reaction, compared with 3.2% of placebo-treated patients in these studies.
Common adverse reactions in placebo-controlled MDD studies
- Table 2 shows the incidence of common adverse reactions that occurred in ≥ 2% of Vilazodone-treated MDD patients (and greater than in placebo-treated patients) in the placebo-controlled studies.


Laboratory Tests
- Vilazodone has not been associated with any clinically important changes in laboratory test parameters in serum chemistry (including liver function tests), hematology and urinalysis, as measured in placebo-controlled studies. These studies include analysis of mean change from baseline and the proportion of patients meeting criteria for potentially clinically significant changes from baseline. Results from a 52-week open-label study were consistent with the findings from the placebo-controlled studies.
ECG
- Vilazodone has not been associated with any clinically significant effect on ECG parameters, including QT, QTc, PR interval and QRS interval, or with any arrhythmogenic potential. ECGs were evaluated in a thorough QTc study at doses up to 80 mg daily with food and in the placebo-controlled studies.
Vital Signs
- Vilazodone has not been associated with any clinically significant effect on vital signs, including systolic and diastolic blood pressure and heart rate, as measured in placebo-controlled studies. These studies included analyses of (1) change from baseline, and (2) the proportion of patients meeting criteria for potentially clinically significant changes from baseline. Results from a 52-week open-label study were consistent with the findings from the placebo-controlled studies.
Weight
- Vilazodone had no effect on body weight as measured by the mean change from baseline in the 8-week, placebo-controlled studies. The mean changes in weight were +0.16 kg in the Vilazodone group and +0.18 kg in the placebo group. The proportions of patients with a weight gain ≥ 7% were 0.9% in the Vilazodone group and 1.2% in the placebo group. The proportions of patients with a weight decrease ≥ 7% were 1.4% in the Vilazodone group and 1.4% in the placebo group.
Other adverse reactions observed in clinical studies
The following listing does not include reactions:
1) already listed in previous tables or elsewhere in labeling
2) for which a drug cause was remote
3) which were so general as to be uninformative
4) which were not considered to have significant clinical implications
5) which occurred at a rate equal to or less than placebo
Reactions are categorized by body system according to the following definitions: frequent adverse reactions are those occurring in at least 1/100 patients; infrequent adverse reactions are those occurring in 1/100 to 1/1000 patients; rare reactions are those occurring in fewer than 1/1000 patients:
Cardiac Disorders
- Infrequent: Ventricular extrasystoles
Eye Disorders
- Frequent: vision blurred, dry eye.
- Infrequent: cataracts.
General disorders
- Infrequent: feeling abnormal
Metabolism and Nutrition Disorders
- Frequent: decreased appetite
Nervous System
Psychiatric disorders
- Infrequent: Panic attack, mania
Renal and Urinary Disorder
Infrequent: pollakiuria
Skin and subcutaneous tissue disorders
- Frequent: hyperhidrosis, night sweats
Postmarketing Experience
The following adverse reactions have been identified during postapproval use of Vilazodone. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to estimate their frequency or establish a causal relationship to drug exposure. These events include:
General Disorders and administrative site conditions
Psychiatric Disorders
Drug Interactions
Central Nervous System (CNS)-Active Agents
- The risk of using Vilazodone in combination with other CNS-active drugs has not been systematically evaluated. Consequently, use caution when Vilazodone is prescribed in combination with other CNS-active drugs.
Monoamine Oxidase Inhibitors (MAOIs)
Serotonergic Drugs
Drugs that Interfere with Hemostasis (e.g., NSAIDs, Aspirin, and Warfarin)
- Serotonin release by platelets plays an important role in hemostasis. Epidemiological studies of case-control and cohort design have demonstrated an association between use of psychotropic drugs that interfere with serotonin reuptake and the occurrence of upper gastrointestinal bleeding. These studies have also shown that concurrent use of an NSAID or aspirin may potentiate this risk of bleeding. Altered anticoagulant effects, including increased bleeding, have been reported when SSRIs and SNRIs are co-administered with warfarin. Patients receiving warfarin therapy should be carefully monitored when Vilazodone is initiated or discontinued.
Potential for Other Drugs to Affect Vilazodone

Inhibitors of CYP3A4
- Metabolism by CYP3A4 is a major elimination pathway for vilazodone. Concomitant use of Vilazodone and strong inhibitors of CYP3A4 (e.g., ketoconazole) can increase vilazodone plasma concentrations by approximately 50%. The Vilazodone dose should be reduced to 20 mg if co-administered with a strong inhibitor of CYP3A4. During co-administration with moderate inhibitors of CYP3A4 (e.g., erythromycin), the Vilazodone dose should be reduced to 20 mg for patients with intolerable adverse events. No dose adjustment is recommended when Vilazodone is co-administered with mild inhibitors of CYP3A4 (e.g., cimetidine).
Inducers of CYP3A4
- Based on clinical response, consider increasing the dose of Vilazodone up to 2-fold when concomitantly used with strong CYP3A4 inducers (e.g., carbamazepine) for greater than 14 days. The maximum daily dose should not exceed 80 mg. Concomitant use of Vilazodone with strong inducers of CYP3A4 (e.g., carbamazepine) can decrease vilazodone systemic exposure by approximately 45%. If CYP3A4 inducers are discontinued, reduce the Vilazodone dose to the original level in 14 days.
Inhibitors of other CYP enzymes
- Concomitant administration of Vilazodone with inhibitors of CYP2C19 and CYP2D6 is not expected to alter plasma concentrations of vilazodone. These isoforms are minor elimination pathways in the metabolism of vilazodone. In vitro studies have shown that CYP1A2, CYP2A6, CYP2C9 and CYP2E1 have minimal contribution to the metabolism of vilazodone.
Potential for Vilazodone to Affect Other Drugs
- Drugs metabolized by CYP1A2, CYP2C9, CYP2D6, CYP3A4 or CYP2C19. Co-administration of Vilazodone with substrates for CYP1A2, CYP2C9, CYP3A4, or CYP2D6 is unlikely to result in clinically significant changes in the concentrations of the CYP substrates. A study in healthy subjects found that Vilazodone (20 mg/day for 8-10 days) had no effect on the pharmacokinetics of caffeine, flurbiprofen, nifedipine or debrisoquine, probes for CYP1A2, CYP2C9, CYP3A4, and CYP2D6, respectively. Vilazodone co-administration with mephenytoin to healthy subjects resulted in a small (11%) increase in mephenytoin biotransformation, suggestive of a minor induction of CYP2C19. In vitro studies have shown that Vilazodone is a moderate inhibitor of CYP2C19 and CYP2D6.
Drugs metabolized by CYP2C8
- Co-administration of Vilazodone with a CYP2C8 substrate may lead to an increase in concentration of the other drug. In vitro studies suggest that Vilazodone may inhibit the biotransformation of substrates of CYP2C8. The effect of Vilazodone on CYP2C8 activity has not been tested in vivo.
Induction of CYP isoforms
- Vilazodone did not induce CYP1A1, CYP1A2, CYP2A6, CYP2B6, CYP2C9, CYP2C19, CYP2D6, CYP2E1, CYP3A4 or CYP3A5 in an in vitro study in cultured human hepatocytes. Chronic administration of vilazodone is unlikely to induce the metabolism of drugs metabolized by these major CYP isoforms.
Drugs Highly Bound to Plasma Protein
- The interaction between vilazodone and other highly protein-bound drugs has not been evaluated. Because vilazodone is highly bound to plasma protein, administration of Vilazodone to a patient taking another drug that is highly protein bound may cause increased free concentrations of the other drug.
Triptans
- There are postmarketing reports of serotonin syndrome with concomitant use of a serotonergic antidepressant and a triptan. If concomitant treatment with Vilazodone and a triptan is clinically warranted, careful observation of the patient is advised, particularly during treatment initiation and dose increases.
Alcohol
- As with other psychotropic medications, the use of alcohol by patients taking Vilazodone is not recommended, because of the potential for pharmacodynamic interactions.
Use in Specific Populations
Pregnancy
Teratogenic Effects
- Vilazodone caused some developmental toxicity in rats, but was not teratogenic in rats or rabbits. There are no adequate and well-controlled studies of Vilazodone in pregnant women. When treating pregnant women with Vilazodone, carefully consider whether the potential benefits outweigh the potential risks of treatment.
- No teratogenic effects were observed when vilazodone was given to pregnant rats or rabbits during the period of organogenesis at oral doses up to 200 and 36 mg/kg/day, respectively. These doses are 48 and 17 times, in rats and rabbits, respectively, the maximum recommended human dose (MRHD) of 40 mg on a mg/m2 basis. Fetal body weight gain was reduced, and skeletal ossification was delayed in both rats and rabbits at these doses; these effects were not observed at doses up to 10 times the MRHD in rats or 4 times the MRHD in rabbits.
- When vilazodone was administered to pregnant rats at an oral dose of 30 times the MRHD during the period of organogenesis and throughout pregnancy and lactation, the number of live born pups was decreased. There was an increase in early postnatal pup mortality, and among surviving pups there was decreased body weight, delayed maturation, and decreased fertility in adulthood. There was some maternal toxicity at this dose. These effects were not seen at 6 times the MRHD.
Nonteratogenic Effects
- Neonates exposed to Vilazodone and other SSRIs or serotonin and norepinephrine reuptake inhibitors (SNRIs), late in the third trimester have developed complications requiring prolonged hospitalization, respiratory support, and tube feeding. Such complications can arise immediately upon delivery. Reported clinical findings have included respiratory distress, cyanosis, apnea, seizures, temperature instability, feeding difficulty, vomiting, hypoglycemia, hypotonia, hypertonia, hyperreflexia, tremor, jitteriness, irritability, and constant crying. These features are consistent with either a direct toxic effect of SSRIs and SNRIs or, possibly, a drug discontinuation syndrome. It should be noted that, in some cases, the clinical picture is consistent with serotonin syndrome.
- Infants exposed to SSRIs in pregnancy may have an increased risk for persistent pulmonary hypertension of the newborn (PPHN). PPHN occurs in 1-2 per 1,000 live births in the general population and is associated with substantial neonatal morbidity and mortality. Several recent epidemiologic studies suggest a positive statistical association between SSRI use (including Vilazodone) in pregnancy and PPHN. Other studies do not show a significant statistical association.
- Physicians should also note the results of a prospective longitudinal study of 201 pregnant women with a history of major depression, who were either on antidepressants or had received antidepressants less than 12 weeks prior to their last menstrual period, and were in remission. Women who discontinued antidepressant medication during pregnancy showed a significant increase in relapse of their major depression compared to those women who remained on antidepressant medication throughout pregnancy.
- When treating a pregnant woman with Vilazodone the physician should carefully consider both the potential risks of taking an SSRI, along with the established benefits of treating depression with an antidepressant. This decision can only be made on a case by case basis.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Vilazodone in women who are pregnant.
Labor and Delivery
- The effect of Vilazodone on labor and delivery in humans is unknown. Vilazodone should be used during labor and delivery only if the potential benefit outweighs the potential risk.
Nursing Mothers
- Vilazodone is excreted into the milk of lactating rats. The effect of Vilazodone on lactation and nursing in humans is unknown. Breast feeding in women treated with Vilazodone should be considered only if the potential benefit outweighs the potential risk to the child.
Pediatric Use
- Clinical studies on the use of Vilazodone in pediatric patients have not been conducted; therefore, the safety and effectiveness of Vilazodone in the pediatric population have not been established. Vilazodone is not approved for use in pediatric patients.
Geriatic Use
- No dose adjustment is recommended on the basis of age. Results from a single-dose (20 mg) pharmacokinetic study in elderly (> 65 years-old) vs. young (24-55 years-old) subjects demonstrated that the pharmacokinetics were generally similar between the two age groups. Of the 2177 patients in clinical studies with Vilazodone 37 (1.7%) were 65 years of age or older, and 272 (12.5%) were 55 to 64 years of age. Greater sensitivity of some older individuals cannot be ruled out.
- Serotonergic antidepressants have been associated with cases of clinically significant hyponatremia in elderly patients, who may be at greater risk for this adverse event.
Gender
- After adjustment for body weight, the systemic exposures between males and females are similar.

Race
There is no FDA guidance on the use of Vilazodone with respect to specific racial populations.
Renal Impairment
- In mild, moderate, and severe renal impairment, no dose adjustment is necessary
Hepatic Impairment
- Vilazodone is eliminated primarily by hepatic metabolism. In mild, moderate, and severe hepatic impairment, no dose adjustment is necessary.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Vilazodone in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Vilazodone in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Vilazodone Administration in the drug label.
Monitoring
There is limited information regarding Vilazodone Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Vilazodone and IV administrations.
Overdosage
Human Experience
- There is limited clinical experience regarding human overdosage with Vilazodone Four patients and 1 patient's child experienced an overdose of Vilazodone all recovered. The adverse reactions associated with overdose of Vilazodone at doses of 200-280 mg as observed in clinical trials included serotonin syndrome, lethargy, restlessness, hallucinations, and disorientation.
Management of Overdose
- Consult a Certified Poison Control Center for up-to-date guidance and advice. Telephone numbers for certified poison control centers are listed in the Physicians' Desk Reference® (PDR). No specific antidotes for vilazodone are known. In case of an overdose, provide supportive care, including close medical supervision and monitoring. Treatment should consist of those general measures employed in the management of overdosage with any drug. Consider the possibility of multiple drug overdose. Ensure an adequate airway, oxygenation, and ventilation. Monitor cardiac rhythm and vital signs. General supportive and symptomatic measures are also recommended. Gastric lavage with a large-bore orogastric tube with appropriate airway protection, if needed, may be considered. Removal of vilazodone by dialysis has not been studied; however, the high volume of distribution of vilazodone suggests that dialysis will not be effective in reducing vilazodone plasma concentrations.
Pharmacology
| |
Vilazodone
| |
| Systematic (IUPAC) name | |
| 5-(4-[4-(5-Cyano-1H-indol-3-yl)butyl]piperazin-1-yl)benzofuran-2-carboxamide | |
| Identifiers | |
| CAS number | |
| ATC code | N06 |
| PubChem | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 441.524 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | 72% (Oral, with food)[1] |
| Metabolism | Hepatic via CYP3A4[1] |
| Half life | 25 hours[1] |
| Excretion | Faecal and renal[1] |
| Therapeutic considerations | |
| Licence data |
|
| Pregnancy cat. |
C(US) |
| Legal status |
[[Prescription drug|Template:Unicode-only]](US) |
| Routes | Oral |
Mechanism of Action
- The mechanism of the antidepressant effect of vilazodone is not fully understood but is thought to be related to its enhancement of serotonergic activity in the CNS through selective inhibition of serotonin reuptake. Vilazodone is also a partial agonist at serotonergic 5-HT1A receptors; however, the net result of this action on serotonergic transmission and its role in vilazodone's antidepressant effect are unknown.
Structure
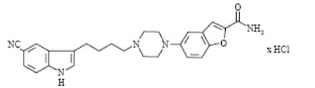
- Vilazodone HCl is 2-benzofurancarboxamide, 5-4-4-(5-cyano-1H-indol-3-yl)butyl-1-piperazinyl-, hydrochloride (1:1). Its molecular weight is 477.99. The structural formula is:

Pharmacodynamics
- Vilazodone binds with high affinity to the serotonin reuptake site (Ki= 0.1 nM), but not to the norepinephrine (Ki=56 nM) or dopamine (Ki=37 nM) reuptake sites. Vilazodone potently and selectively inhibits reuptake of serotonin (IC50= 1.6 nM). Vilazodone also binds selectively with high affinity to 5-HT1A receptors (IC50=2.1 nM) and is a 5-HT1A receptor partial agonist.
- Thorough QT Study: Treatment with Vilazodone did not prolong the QTc interval. The effect of vilazodone (20, 40, 60, and 80 mg) on the QTc interval was evaluated in a randomized, placebo-, and active-controlled (moxifloxacin 400 mg), parallel-group, thorough QTc study in 157 healthy subjects. The study demonstrated an ability to detect small effects. The upper bound of the 90% confidence interval for the largest placebo-adjusted, baseline-corrected QTc interval was below 10 msec, based on the individual correction method (QTcI). This is below the threshold for clinical concern. However, it is unknown whether 80 mg is adequate to represent a high clinical exposure condition.
Pharmacokinetics
- Vilazodone activity is due primarily to the parent drug. The pharmacokinetics of vilazodone (5 mg – 80 mg) are dose-proportional. Accumulation of vilazodone is predictable from single dose data, does not vary with dose, and steady-state is achieved in about 3 days. Elimination of vilazodone is primarily by hepatic metabolism with a terminal half-life of approximately 25 hours. At steady-state, after daily dosing of Vilazodone 40 mg under fed conditions, the mean Cmax value is 156 ng/mL, and the mean AUC (0-24 hours) value is 1645 ng•h/mL.
Absorption
- Vilazodone concentrations peak at a median of 4-5 hours (Tmax) after administration and decline with a terminal half-life of approximately 25 hours. The absolute bioavailability of vilazodone is 72% with food. Administration of Vilazodone with food (high fat or light meal) increases oral bioavailability (Cmax increased by approximately 147-160%, and AUC increased by approximately 64-85%).
- Co-administration of Vilazodone with ethanol or with a proton pump inhibitor (pantoprazole) did not affect the rate or extent of vilazodone absorption. In addition, neither the Tmax nor terminal elimination rate of vilazodone was altered by co-administration with either pantoprazole or ethanol. Absorption is decreased by approximately 25% if vomiting occurs within 7 hours of ingestion; no replacement dose is needed.
Distribution
- Vilazodone is widely distributed and approximately 96-99% protein-bound
Metabolism and Elimination
- Vilazodone is extensively metabolized through CYP and non-CYP pathways (possibly by carboxylesterase), with only 1% of the dose recovered in the urine and 2% of the dose recovered in the feces as unchanged vilazodone. CYP3A4 is primarily responsible for its metabolism among CYP pathways, with minor contributions from CYP2C19 and CYP2D6. In vitro studies with human microsomes and human hepatocytes indicate that vilazodone is unlikely to inhibit or induce the metabolism of other CYP (except for CYP2C8) substrates; and an in vivo study with probe substrates for CYP2C19, CYP2D6 and CYP3A4 showed vilazodone did not alter the pharmacokinetics of the probe substrates. However, an in vivo study with probe substrate for CYP2C19 demonstrated a minor induction of CYP2C19. Strong inhibitors of CYP3A4 (e.g., ketoconazole) can reduce the metabolism of vilazodone in vivo and increase exposure. Conversely, strong inducers of CYP3A4 (e.g., carbamazepine) can decrease vilazodone exposure.
- The presence of mild or moderate renal impairment, or mild, moderate, or severe hepatic impairment did not affect the apparent clearance of vilazodone.
Nonclinical Toxicology
There is limited information regarding Vilazodone Nonclinical Toxicology in the drug label.
Clinical Studies
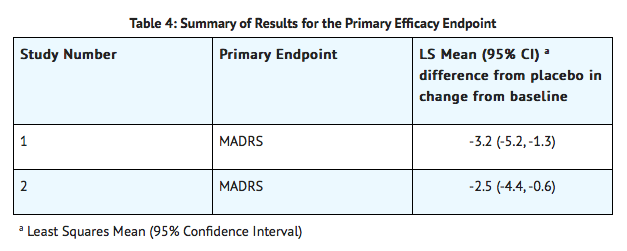
- The efficacy of Vilazodone as a treatment for major depressive disorder was established in two 8-week, multicenter, randomized, double-blind, placebo-controlled studies in adult (18-70 years of age) outpatients who met the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR) criteria for MDD. In these studies, patients were titrated over 2 weeks to a dose of 40 mg of Vilazodone with food (n=436) or placebo (n = 433) once daily. Vilazodone was superior to placebo in the improvement of depressive symptoms as measured by the mean change from baseline to Week 8 in the Montgomery-Asberg Depression Rating Scale (MADRS) total score. Examination of population subgroups based on age (there were few patients over 65), gender, and race did not reveal any clear evidence of differential responsiveness.
How Supplied
- 10 mg, pink, oval tablet, debossed with 10 on one side
- 0456-1110-30: 30-count bottles
- 20 mg, orange, oval tablet, debossed with 20 on one side
- 0456-1120-30: 30-count bottles
- 40 mg, blue, oval tablet, debossed with 40 on one side
- 0456-1140-30: 30-count bottles
- Patient Starter Kit
- 0456-1100-31: blister card containing 30 tablets:
- 10 mg, pink, oval, debossed with 10 on one side: 7 tablets
- 20 mg, orange, oval, debossed with 20 on one side: 7 tablets
- 40 mg, blue, oval, debossed with 40 on one side: 16 tablets
- 0456-1100-31: blister card containing 30 tablets:
Storage
- Stored at 25°C (77°F) with excursions permitted to 15°C - 30°C (59°F - 86°F)
Images
Drug Images
{{#ask: Page Name::Vilazodone |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel

{{#ask: Label Page::Vilazodone |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Vilazodone Patient Counseling Information in the drug label.
Precautions with Alcohol
- Alcohol-Vilazodone interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
Look-Alike Drug Names
There is limited information regarding Vilazodone Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ 1.0 1.1 1.2 1.3 "Vilazodone (vilazodone hydrochloride) tablet Vilazodone (vilazodone hydrochloride) kit [Forest Laboratories, Inc.]". DailyMed. Forest Laboratories, Inc. December 2012. Retrieved 28 October 2013.
{{#subobject:
|Label Page=Vilazodone |Label Name=Vilazodone 40mg2.png
}}
{{#subobject:
|Label Page=Vilazodone |Label Name=Vilazodone 40mg.png
}}