Alosetron
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Vignesh Ponnusamy, M.B.B.S. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING
See full prescribing information for complete Boxed Warning.
SERIOUS GASTROINTESTINAL ADVERSE REACTIONS:
|
Overview
Alosetron is a selective serotonin 5-HT3 antagonist that is FDA approved for the {{{indicationType}}} of severe diarrhea-predominant irritable bowel syndrome (IBS). There is a Black Box Warning for this drug as shown here. Common adverse reactions include constipation, abdominal pain, nausea, and gastrointestinal discomfort and pain.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Diarrhea-Predominant Irritable Bowel Syndrome
- LOTRONEX is indicated only for women with severe diarrhea-predominant irritable bowel syndrome (IBS) who have:
- chronic IBS symptoms (generally lasting 6 months or longer),
- had anatomic or biochemical abnormalities of the gastrointestinal tract excluded, and
- not responded adequately to conventional therapy.
- Diarrhea-predominant IBS is severe if it includes diarrhea and one or more of the following:
- frequent and severe abdominal pain/discomfort,
- frequent bowel urgency or fecal incontinence,
- disability or restriction of daily activities due to IBS.
- Because of infrequent but serious gastrointestinal adverse reactions associated with LOTRONEX, the indication is restricted to those patients for whom the benefit-to-risk balance is most favorable.
- Dosing Information
- To lower the risk of constipation, LOTRONEX should be started at a dosage of 0.5 mg twice a day. Patients who become constipated at this dosage should stop taking LOTRONEX until the constipation resolves. They may be restarted at 0.5 mg once a day. If constipation recurs at the lower dose, LOTRONEX should be discontinued immediately.
- Patients well controlled on 0.5 mg once or twice a day may be maintained on this regimen. If after 4 weeks the dosage is well tolerated but does not adequately control IBS symptoms, then the dosage can be increased to up to 1 mg twice a day. LOTRONEX should be discontinued in patients who have not had adequate control of IBS symptoms after 4 weeks of treatment with 1 mg twice a day.
- LOTRONEX can be taken with or without food.
- LOTRONEX should be discontinued immediately in patients who develop constipation or signs of ischemic colitis. LOTRONEX should not be restarted in patients who develop ischemic colitis.
- Clinical trial and postmarketing experience suggest that debilitated patients or patients taking additional medications that decrease gastrointestinal motility may be at greater risk of serious complications of constipation. Therefore, appropriate caution and follow-up should be exercised if LOTRONEX is prescribed for these patients.
- Postmarketing experience suggests that elderly patients may be at greater risk for complications of constipation; therefore, appropriate caution and follow-up should be exercised if LOTRONEX is prescribed for these patients.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Alosetron in adult patients.
Non–Guideline-Supported Use
Indigestion, Non-ulcer
- Alosetron 0.5 mg.[1]
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Alosetron in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Alosetron in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Alosetron in pediatric patients.
Contraindications
- Constipation
- LOTRONEX should not be initiated in patients with constipation.
- History of Severe Bowel or Hepatic Disorders
- LOTRONEX is contraindicated in patients with a history of the following:
- chronic or severe constipation or sequelae from constipation
- intestinal obstruction, stricture, toxic megacolon, gastrointestinal perforation, and/or adhesions
- ischemic colitis, impaired intestinal circulation, thrombophlebitis, or hypercoagulable state
- Crohn's disease or ulcerative colitis
- diverticulitis
- severe hepatic impairment
- LOTRONEX is contraindicated in patients with a history of the following:
- Lack of Understanding of Patient Acknowledgement Form
- LOTRONEX should not be used by patients who are unable to understand or comply with the Patient Acknowledgement Form for LOTRONEX.
- Concomitant Use of Fluvoxamine
- Concomitant administration of LOTRONEX with fluvoxamine is contraindicated. Fluvoxamine, a known strong inhibitor of CYP1A2, has been shown to increase mean alosetron plasma concentrations (AUC) approximately 6-fold and prolong the half-life by approximately 3-fold.
Warnings
|
WARNING
See full prescribing information for complete Boxed Warning.
SERIOUS GASTROINTESTINAL ADVERSE REACTIONS:
|
Precautions
- Serious Complications of Constipation
- Some patients have experienced serious complications of constipation without warning.
- Serious complications of constipation, including obstruction, ileus, impaction, toxic megacolon, and secondary bowel ischemia, have been reported with use of LOTRONEX during clinical trials. Complications of constipation have been reported with use of 1 mg twice daily and with lower doses. A dose response relationship has not been established for serious complications of constipation. The incidence of serious complications of constipation was approximately 0.1% (1 per 1,000 patients) in women receiving either LOTRONEX or placebo. In addition, rare cases of perforation and death have been reported from postmarketing clinical practice. In some cases, complications of constipation required intestinal surgery, including colectomy. Patients who are elderly, debilitated, or taking additional medications that decrease gastrointestinal motility may be at greater risk for complications of constipation.
- LOTRONEX should be discontinued immediately in patients who develop constipation [see Boxed Warning].
- Ischemic Colitis
- Some patients have experienced ischemic colitis without warning.
- Ischemic colitis has been reported in patients receiving LOTRONEX in clinical trials as well as during marketed use of the drug. In IBS clinical trials, the cumulative incidence of ischemic colitis in women receiving LOTRONEX was 0.2% (2 per 1,000 patients, 95% confidence interval 1 to 3) through 3 months and was 0.3% (3 per 1,000 patients, 95% confidence interval 1 to 4) through 6 months. Ischemic colitis has been reported with use of 1 mg twice daily and with lower doses. A dose-response relationship has not been established. Ischemic colitis was reported in one patient receiving placebo. The patient experience in controlled clinical trials is insufficient to estimate the incidence of ischemic colitis in patients taking LOTRONEX for longer than 6 months.
- LOTRONEX should be discontinued immediately in patients with signs of ischemic colitis such as rectal bleeding, bloody diarrhea, or new or worsening abdominal pain. Because ischemic colitis can be life-threatening, patients with signs or symptoms of ischemic colitis should be evaluated promptly and have appropriate diagnostic testing performed. Treatment with LOTRONEX should not be resumed in patients who develop ischemic colitis.
- Prescribing Program for LOTRONEX
- To prescribe LOTRONEX, the prescriber must be enrolled in the Prescribing Program for LOTRONEX. To enroll, prescribers must understand the benefits and risks of treatment with LOTRONEX for severe diarrhea-predominant IBS, including the information in the Prescribing Information, Medication Guide, and Patient Acknowledgement Form for LOTRONEX.
- To enroll in the Prescribing Program for LOTRONEX, call 1-888-423-5227 or visit www.lotronexppl.com to complete the Prescriber Enrollment Form.
Adverse Reactions
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
- Patients With Irritable Bowel Syndrome: Table 1 summarizes adverse reactions from 22 repeat-dose studies in patients with IBS who were treated with 1 mg of LOTRONEX twice daily for 8 to 24 weeks. The adverse reactions in Table 1 were reported in 1% or more of patients who received LOTRONEX and occurred more frequently on LOTRONEX than on placebo. A statistically significant difference was observed for constipation in patients treated with LOTRONEX compared to placebo (p<0.0001).
- Gastrointestinal: Constipation is a frequent and dose-related side effect of treatment with LOTRONEX. In clinical studies constipation was reported in approximately 29% of patients with IBS treated with LOTRONEX 1 mg twice daily (n = 9,316). This effect was statistically significant compared to placebo (p<;<0.0001). Eleven percent (11%) of patients treated with LOTRONEX 1 mg twice daily withdrew from the studies due to constipation. Although the number of patients with IBS treated with LOTRONEX 0.5 mg twice daily is relatively small (n = 243), only 11% of those patients reported constipation and 4% withdrew from clinical studies due to constipation. Among the patients treated with LOTRONEX 1 mg twice daily who reported constipation, 75% reported a single episode and most reports of constipation (70%) occurred during the first month of treatment, with the median time to first report of constipation onset of 8 days. Occurrences of constipation in clinical trials were generally mild to moderate in intensity, transient in nature, and resolved either spontaneously with continued treatment or with an interruption of treatment. However, serious complications of constipation have been reported in clinical studies and in postmarketing experience. In Studies 1 and 2, 9% of patients treated with LOTRONEX reported constipation and 4 consecutive days with no bowel movement. Following interruption of treatment, 78% of the affected patients resumed bowel movements within a 2-day period and were able to re-initiate treatment with LOTRONEX.
- Hepatic: A similar incidence in elevation of ALT (>2-fold) was seen in patients receiving LOTRONEX or placebo (1.0% vs. 1.2%). A single case of hepatitis (elevated ALT, AST, alkaline phosphatase, and bilirubin) without jaundice in a patient receiving LOTRONEX was reported in a 12-week study. A causal association with LOTRONEX has not been established.
- Long-Term Safety: Patient experience in controlled clinical trials is insufficient to estimate the incidence of ischemic colitis in patients taking LOTRONEX for longer than 6 months.
- Women With Severe Diarrhea-Predominant Irritable Bowel Syndrome: Table 2 summarizes the gastrointestinal adverse reactions from 1 repeat-dose study in female patients with severe diarrhea-predominant IBS who were treated for 12 weeks. The adverse reactions in Table 2 were reported in 3% or more of patients who received LOTRONEX and occurred more frequently with LOTRONEX than with placebo. Other events reported in 3% or more of patients who received LOTRONEX and occurring more frequently with LOTRONEX than with placebo included upper respiratory tract infection, viral gastroenteritis, muscle spasms, headaches, and fatigue.
- Adverse reactions reported in another study of 701 women with severe diarrhea-predominant IBS were similar to those shown in Table 2. Gastrointestinal adverse reactions reported in 3% or more of patients who received LOTRONEX and occurring more frequently with LOTRONEX than with placebo included constipation (14% and 10% of patients taking LOTRONEX 1 mg twice daily or 0.5 mg as needed, respectively, compared with 2% taking placebo), abdominal pain, nausea, vomiting, and flatulence. Other events reported in 3% or more of patients who received LOTRONEX and occurring more frequently with LOTRONEX than with placebo included nasopharyngitis, sinusitis, upper respiratory tract infection, urinary tract infection, viral gastroenteritis, and cough.
- Constipation: Constipation was the most frequent adverse reaction among women with severe diarrhea-predominant IBS represented in Table 2. There was a dose response in the groups treated with LOTRONEX in the number of patients withdrawn due to constipation (2% on placebo, 5% on 0.5 mg once daily, 8% on 1 mg once daily, and 11% on 1 mg twice daily). Among these patients with severe diarrhea-predominant IBS treated with LOTRONEX who reported constipation most (75%) reported one episode which occurred within the first 15 days of treatment and persisted for 4 to 5 days.
- Other Events Observed During Clinical Evaluation of LOTRONEX: During its assessment in clinical trials, multiple and single doses of LOTRONEX were administered, resulting in 11,874 subject exposures in 86 completed clinical studies. The conditions, dosages, and duration of exposure to LOTRONEX varied between trials, and the studies included healthy male and female volunteers as well as male and female patients with IBS and other indications.
- In the listing that follows, reported adverse reactions were classified using a standardized coding dictionary. Only those events that an investigator believed were possibly related to LOTRONEX, occurred in at least 2 patients, and occurred at a greater frequency during treatment with LOTRONEX than during placebo administration are presented. Serious adverse reactions occurring in at least 1 patient for whom an investigator believed there was reasonable possibility that the event was related to treatment with LOTRONEX and occurring at a greater frequency in patients treated with LOTRONEX than placebo-treated patients are also presented.
- In the following listing, events are categorized by body system. Within each body system, events are presented in descending order of frequency. The following definitions are used: infrequent adverse reactions are those occurring on one or more occasion in 1/100 to 1/1,000 patients; rare adverse reactions are those occurring on one or more occasion in fewer than 1/1,000 patients.
- Although the events reported occurred during treatment with LOTRONEX, they were not necessarily caused by it.
Blood and Lymphatic
Rare: Quantitative red cell or hemoglobin defects, and hemorrhage.
Cardiovascular
Infrequent: Tachyarrhythmias. Rare: Arrhythmias, increased blood pressure, and extrasystoles.
Drug Interaction, Overdose, and Trauma
Rare: Contusions and hematomas.
Ear, Nose, and Throat
Rare: Ear, nose, and throat infections; viral ear, nose, and throat infections; and laryngitis.
Endocrine and Metabolic
Rare: Disorders of calcium and phosphate metabolism, hyperglycemia, hypothalamus/pituitary hypofunction, hypoglycemia, and fluid disturbances.
Eye
Rare: Light sensitivity of eyes.
Gastrointestinal
Infrequent: Hyposalivation, dyspeptic symptoms, gastrointestinal spasms, ischemic colitis, and gastrointestinal lesions. Rare: Abnormal tenderness, colitis, gastrointestinal signs and symptoms, proctitis, diverticulitis, positive fecal occult blood, hyperacidity, decreased gastrointestinal motility and ileus, gastrointestinal obstructions, oral symptoms, gastrointestinal intussusception, gastritis, gastroduodenitis, gastroenteritis, and ulcerative colitis.
Hepatobiliary Tract and Pancreas
Rare: Abnormal bilirubin levels and cholecystitis.
Lower Respiratory
Infrequent: Breathing disorders.
Musculoskeletal
Rare: Muscle pain; muscle stiffness, tightness and rigidity; and bone and skeletal pain.
Neurological
Infrequent: Hypnagogic effects. Rare: Memory effects, tremors, dreams, cognitive function disorders, disturbances of sense of taste, disorders of equilibrium, confusion, sedation, and hypoesthesia.
Non-Site Specific
Infrequent: Malaise and fatigue, cramps, pain, temperature regulation disturbances. Rare: Burning sensations, hot and cold sensations, cold sensations, and fungal infections.
Psychiatry
Infrequent: Anxiety. Rare: Depressive moods.
Reproduction
Rare: Sexual function disorders, female reproductive tract bleeding and hemorrhage, reproductive infections, and fungal reproductive infections.
Skin
Infrequent: Sweating and urticaria. Rare: Hair loss and alopecia; acne and folliculitis; disorders of sweat and sebum; allergic skin reaction; eczema; skin infections; dermatitis and dermatosis; and nail disorders.
Urology
Infrequent: Urinary frequency. Rare: Bladder inflammation; polyuria and diuresis; and urinary tract hemorrhage.
Postmarketing Experience
- In addition to events reported in clinical trials, the following events have been identified during use of LOTRONEX in clinical practice. Because they were reported voluntarily from a population of unknown size, estimates of frequency cannot be made. These events have been chosen for inclusion due to a combination of their seriousness, frequency of reporting, or potential causal connection to LOTRONEX.
Gastrointestinal
Impaction, perforation, ulceration, small bowel mesenteric ischemia.
Neurological
Skin
Rash.
Drug Interactions
- In vivo data suggest that alosetron is primarily metabolized by cytochrome P450 (CYP) 1A2, with minor contributions from CYP3A4 and CYP2C9. Therefore, inducers or inhibitors of these enzymes may change the clearance of alosetron.
- CYP1A2 Inhibitors
- Fluvoxamine is a known strong inhibitor of CYP1A2 and also inhibits CYP3A4, CYP2C9, and CYP2C19. In a pharmacokinetic study, 40 healthy female subjects received fluvoxamine in escalating doses from 50 to 200 mg/ day for 16 days, with coadministration of alosetron 1 mg on the last day. Fluvoxamine increased mean alosetron plasma concentrations (AUC) approximately 6-fold and prolonged the half-life by approximately 3-fold. Concomitant administration of alosetron and fluvoxamine is contraindicated [see Contraindications (4.3)].
- Concomitant administration of alosetron and moderate CYP1A2 inhibitors, including quinolone antibiotics and cimetidine, has not been evaluated, but should be avoided unless clinically necessary because of similar potential drug interactions.
- CYP3A4 Inhibitors
- Ketoconazole is a known strong inhibitor of CYP3A4. In a pharmacokinetic study, 38 healthy female subjects received ketoconazole 200 mg twice daily for 7 days, with coadministration of alosetron 1 mg on the last day. Ketoconazole increased mean alosetron plasma concentrations (AUC) by 29%. Caution should be used when alosetron and ketoconazole are administered concomitantly. Coadministration of alosetron and strong CYP3A4 inhibitors such as clarithromycin, telithromycin, protease inhibitors, voriconazole, and itraconazole has not been evaluated but should be undertaken with caution because of similar potential drug interactions. The effect of induction or inhibition of other pathways on exposure to alosetron and its metabolites is not known.
- Other CYP Enzymes
- In vitro human liver microsome studies and an in vivo metabolic probe study demonstrated that alosetron did not inhibit CYP enzymes 3A4, 2C9, or 2C19. In vitro at total drug concentrations 27-fold higher than peak plasma concentrations observed with the 1 mg dose, alosetron inhibited CYP enzymes 1A2 (60%) and 2E1 (50%). In an in vivo metabolic probe study, alosetron did not inhibit CYP2E1 but did produce 30% inhibition of both CYP1A2 and N-acetyltransferase. Although not studied with alosetron, inhibition of N-acetyltransferase may have clinically relevant consequences for drugs such as isoniazid, procainamide, and hydralazine. The effect on CYP1A2 was explored further in a clinical interaction study with theophylline and no effect on metabolism was observed. Another study showed that alosetron had no clinically significant effect on plasma concentrations of the oral contraceptive agents ethinyl estradiol and levonorgestrel (CYP3A4 substrates). A clinical interaction study was also conducted with alosetron and the CYP3A4 substrate cisapride. No significant effects on cisapride metabolism or QT interval were noted. The effects of alosetron on monoamine oxidases and on intestinal first pass secondary to high intraluminal concentrations have not been examined. Based on the above data from in vitro and in vivo studies, it is unlikely that alosetron will inhibit the hepatic metabolic clearance of drugs metabolized by the CYP enzymes 2C9, 2C19, or 2E1.
- Alosetron does not appear to induce the major cytochrome P450 drug-metabolizing enzyme 3A. Alosetron also does not appear to induce CYP enzymes 2E1 or 2C19. It is not known whether alosetron might induce other enzymes.
Use in Specific Populations
Pregnancy
- Pregnancy Category B
- Reproduction studies have been performed in rats at doses up to 40 mg/kg/day (about 160 times the recommended human dose based on body surface area) and rabbits at oral doses up to 30 mg/kg/day (about 240 times the recommended daily human dose based on body surface area). These studies have revealed no evidence of impaired fertility or harm to the fetus due to alosetron. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, LOTRONEX should be used during pregnancy only if clearly needed
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Alosetron in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Alosetron during labor and delivery.
Nursing Mothers
- Alosetron and/or metabolites of alosetron are excreted in the breast milk of lactating rats. It is not known whether alosetron is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when LOTRONEX is administered to a nursing woman.
Pediatric Use
- Safety and effectiveness in pediatric patients have not been established. Use of LOTRONEX is not recommended in the pediatric population, based upon the risk of serious complications of constipation and ischemic colitis in adults.
Geriatic Use
- In some studies in healthy men or women, plasma concentrations were elevated by approximately 40% in individuals 65 years and older compared to young adults. However, this effect was not consistently observed in men.
- Postmarketing experience suggests that elderly patients may be at greater risk for complications of constipation therefore, appropriate caution and follow-up should be exercised if LOTRONEX is prescribed for these patients.
Gender
There is no FDA guidance on the use of Alosetron with respect to specific gender populations.
Race
There is no FDA guidance on the use of Alosetron with respect to specific racial populations.
Renal Impairment
- Renal impairment (creatinine clearance 4 to 56 mL/min) has no effect on the renal elimination of alosetron due to the minor contribution of this pathway to elimination. The effect of renal impairment on metabolite pharmacokinetics and the effect of end-stage renal disease have not been assessed.
Hepatic Impairment
- Due to the extensive hepatic metabolism of alosetron, increased exposure to alosetron and/or its metabolites is likely to occur in patients with hepatic impairment. Alosetron should not be used in patients with severe hepatic impairment and should be used with caution in patients with mild or moderate hepatic impairment.
- A single 1 mg oral dose of alosetron was administered to 1 female and 5 male patients with moderate hepatic impairment (Child-Pugh score of 7 to 9) and to 1 female and 2 male patients with severe hepatic impairment (Child-Pugh score of >9). In comparison with historical data from healthy subjects, patients with severe hepatic impairment displayed higher systemic exposure to alosetron. The female with severe hepatic impairment displayed approximately 14-fold higher exposure, while the female with moderate hepatic impairment displayed approximately 1.6-fold higher exposure, than healthy females. Due to the small number of subjects and high intersubject variability in the pharmacokinetic findings, no definitive quantitative conclusions can be made. However, due to the greater exposure to alosetron in the female with severe hepatic impairment, alosetron should not be used in females with severe hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Alosetron in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Alosetron in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
Monitoring
There is limited information regarding Monitoring of Alosetron in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Alosetron in the drug label.
Overdosage
Acute Overdose
- There is no specific antidote for overdose of LOTRONEX. Patients should be managed with appropriate supportive therapy. Individual oral doses as large as 16 mg have been administered in clinical studies without significant adverse reactions. This dose is 8 times higher than the recommended total daily dose. Inhibition of the metabolic elimination and reduced first pass of other drugs might occur with overdoses of LOTRONEX.
Chronic Overdose
There is limited information regarding Chronic Overdose of Alosetron in the drug label.
Pharmacology
| |
Alosetron
| |
| Systematic (IUPAC) name | |
| 5-methyl-2-[(4-methyl-1H-imidazol-5-yl)methyl]-2,3,4,5-tetrahydro-1H-pyrido[4,3-b]indol-1-one | |
| Identifiers | |
| CAS number | |
| ATC code | A03 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 294.351 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | 50–60% |
| Protein binding | 82% |
| Metabolism | Hepatic (including CYP2C9, CYP3A4 and CYP1A2) |
| Half life | 1.5–1.7 hours |
| Excretion | Renal 73%, faecal 24% |
| Therapeutic considerations | |
| Pregnancy cat. |
B (U.S.) |
| Legal status |
℞-only (U.S.) |
| Routes | Oral |
Mechanism of Action
- Alosetron is a potent and selective 5-HT3 receptor antagonist. 5-HT3 receptors are ligand-gated cation channels that are extensively distributed on enteric neurons in the human gastrointestinal tract, as well as other peripheral and central locations. Activation of these channels and the resulting neuronal depolarization affect the regulation of visceral pain, colonic transit, and gastrointestinal secretions, processes that relate to the pathophysiology of IBS. 5-HT3 receptor antagonists such as alosetron inhibit activation of non-selective cation channels, which results in the modulation of the enteric nervous system.
- The cause of IBS is unknown. IBS is characterized by visceral hypersensitivity and hyperactivity of the gastrointestinal tract, which lead to abnormal sensations of pain and motor activity. Following distention of the rectum, patients with IBS exhibit pain and discomfort at lower volumes than healthy volunteers. Following such distention, alosetron reduced pain and exaggerated motor responses, possibly due to blockade of 5-HT3 receptors.
Structure
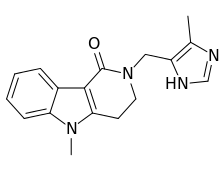
- The active ingredient in LOTRONEX Tablets is alosetron hydrochloride (HCl), a potent and selective antagonist of the serotonin 5-HT3 receptor type. Chemically, alosetron is designated as 2,3,4,5-tetrahydro-5-methyl-2-[(5-methyl-1H-imidazol-4-yl)methyl]-1H-pyrido[4,3-b]indol-1-one, monohydrochloride. Alosetron is achiral and has the empirical formula C17H18N4O•HCl, representing a molecular weight of 330.8. Alosetron is a white to beige solid that has a solubility of 61 mg/mL in water, 42 mg/mL in 0.1M hydrochloric acid, 0.3 mg/mL in pH 6 phosphate buffer, and <;<0.1 mg/mL in pH 8 phosphate buffer. The chemical structure of alosetron is:
- LOTRONEX Tablets are supplied for oral administration as 0.5 mg (white) and 1 mg (blue) tablets. The 0.5 mg tablet contains 0.562 mg alosetron HCl equivalent to 0.5 mg alosetron, and the 1 mg tablet contains 1.124 mg alosetron HCl equivalent to 1 mg of alosetron. Each tablet also contains the inactive ingredients lactose (anhydrous), magnesium stearate, microcrystalline cellulose, and pregelatinized starch. The white film coat for the 0.5 mg tablet contains hypromellose, titanium dioxide, and triacetin. The blue film coat for the 1 mg tablet contains hypromellose, titanium dioxide, triacetin, and indigo carmine.
Pharmacodynamics
- In healthy volunteers and patients with IBS, alosetron (2 mg orally, twice daily for 8 days) increased colonic transit time without affecting orocecal transit time. In healthy volunteers, alosetron also increased basal jejunal water and sodium absorption after a single 4 mg dose. In patients with IBS, multiple oral dosages of alosetron (4 mg twice daily for 6.5 days) significantly increased colonic compliance.
- Single oral doses of alosetron administered to healthy men produced a dose-dependent reduction in the flare response seen after intradermal injection of serotonin. Urinary 6-β-hydroxycortisol excretion decreased by 52% in elderly subjects after 27.5 days of alosetron 2 mg administered orally twice daily. This decrease was not statistically significant. In another study utilizing alosetron 1 mg administered orally twice daily for 4 days, there was a significant decrease in urinary 6-β-hydroxycortisol excretion. However, there was no change in the ratio of 6-β-hydroxycortisol to cortisol, indicating a possible decrease in cortisol production. The clinical significance of these findings is unknown.
Pharmacokinetics
- The pharmacokinetics of alosetron have been studied after single oral doses ranging from 0.05 to 16 mg in healthy men. The pharmacokinetics of alosetron have also been evaluated in healthy women and men and in patients with IBS after repeated oral dosages ranging from 1 mg twice daily to 8 mg twice daily.
- Absorption: Alosetron was rapidly absorbed after oral administration with a mean absolute bioavailability of approximately 50% to 60% (approximate range, 30% to >90%). After administration of radiolabeled alosetron, only 1% of the dose was recovered in the feces as unchanged drug. Following oral administration of a 1 mg alosetron dose to young men, a peak plasma concentration of approximately 5 ng/mL occurred at 1 hour. In young women, the mean peak plasma concentration was approximately 9 ng/mL, with a similar time to peak.
- Plasma concentrations were 30% to 50% lower and less variable in men compared to women given the same oral dose. Population pharmacokinetic analysis in IBS patients confirmed that alosetron concentrations were influenced by gender (27% lower in men).
- Food Effects: Alosetron absorption is decreased by approximately 25% by co-administration with food, with a mean delay in time to peak concentration of 15 minutes.
- Distribution: Alosetron demonstrates a volume of distribution of approximately 65 to 95 L. Plasma protein binding is 82% over a concentration range of 20 to 4,000 ng/mL.
- Metabolism and Elimination: Plasma concentrations of alosetron increase proportionately with increasing single oral doses up to 8 mg and more than proportionately at a single oral dose of 16 mg. Twice-daily oral dosing of alosetron does not result in accumulation. The terminal elimination half-life of alosetron is approximately 1.5 hours (plasma clearance is approximately 600 mL/min). Population pharmacokinetic analysis in patients with IBS confirmed that alosetron clearance is minimally influenced by doses up to 8 mg.
- Renal elimination of unchanged alosetron accounts for only 13% of the dose. Renal clearance is approximately 112 mL/min.
- A study with 14C-labeled alosetron in Caucasian males (n = 3) and females (n = 3) and an Asian male (n = 1) showed similar serum metabolite profiles. Unchanged alosetron was the major component in serum, with other metabolites being present at low concentrations, none amounting to more than 15% of the unmetabolized alosetron concentration. The circulating metabolites were identified as 6-hydroxy glucuronide, 6-hydroxy sulphate, 7-hydroxy sulphate, hydroxymethyl imidazole, and mono- and bis-oxygenated imidazole derivatives of alosetron. The metabolites are unlikely to contribute to the biological activity of alosetron. Of the circulating Phase I metabolites, only the hydroxymethyl imidazole has weak pharmacological activity, around 10-fold less potent than alosetron. Total recovery of radioactivity in the excreta was 85 ± 6%. The majority of the radiolabeled dose is excreted in the urine (74 ± 5%). The major urinary metabolites were the 6-hydroxy glucuronide and the mono- and bis-oxygenated imidazole derivatives of alosetron. 11 ± 4% of the radiolabeled dose was excreted in the feces with less than 1% of the dose being excreted as the unchanged alosetron.
- Alosetron is metabolized by human microsomal cytochrome P450 (CYP), shown in vitro to involve enzymes 2C9 (30%), 3A4 (18%), and 1A2 (10%). Non–CYP-mediated Phase I metabolic conversion also contributes to an extent of about 11%. However, in vivo data suggest that CYP1A2 plays a more prominent role in alosetron metabolism (62 to 97% of alosetron clearance) based on correlation of alosetron clearance with in vivo CYP1A2 activity measured by probe substrate, increased clearance induced by smoking, and inhibition of clearance by fluvoxamine.
Nonclinical Toxicology
- In 2-year oral studies, alosetron was not carcinogenic in mice at doses up to 30 mg/kg/day or in rats at doses up to 40 mg/kg/day. These doses are about 60 to 160 times, respectively, the recommended human dose of alosetron of 2 mg/day (1 mg twice daily) based on body surface area. Alosetron was not genotoxic in the Ames tests, the mouse lymphoma cell (L5178Y/TK±) forward gene mutation test, the human lymphocyte chromosome aberration test, the ex vivo rat hepatocyte unscheduled DNA synthesis (UDS) test, or the in vivo rat micronucleus test for mutagenicity. Alosetron at oral doses up to 40 mg/kg/day (about 160 times the recommended daily human dose based on body surface area) was found to have no effect on fertility and reproductive performance of male or female rats.
Clinical Studies
- Dose-Ranging Study
- Data from a dose-ranging study of women (n = 85) who received LOTRONEX 0.5 mg twice daily indicated that the incidence of constipation (14%) was lower than that experienced by women receiving 1 mg twice daily (29%). Therefore, to lower the risk of constipation, LOTRONEX should be started at a dosage of 0.5 mg twice a day. The efficacy of the 0.5 mg twice-daily dosage in treating severe diarrhea-predominant IBS has not been adequately evaluated in clinical trials.
- Efficacy Studies
- LOTRONEX has been studied in women with IBS in five 12-week US multicenter, randomized, double-blind, placebo-controlled clinical studies.
- Studies in Non-Constipated Women with Irritable Bowel Syndrome: Studies 1 and 2 were conducted in non-constipated women with IBS meeting the Rome Criteria1 for at least 6 months. Women with severe pain or a history of severe constipation were excluded. A 2-week run-in period established baseline IBS symptoms.
- About two thirds of the women had diarrhea-predominant IBS. Compared with placebo, 10% to 19% more women with diarrhea-predominant IBS who received LOTRONEX had adequate relief of IBS abdominal pain and discomfort during each month of the study.
- Studies in Women With Severe Diarrhea-Predominant Irritable Bowel Syndrome: LOTRONEX is indicated only for women with severe diarrhea-predominant IBS [see Indications and Usage (1)]. The efficacy of LOTRONEX in this subset of the women studied in clinical trials is supported by prospective and retrospective analyses.
- Prospective Analyses: Studies 3 and 4 were conducted in women with diarrhea-predominant IBS and bowel urgency on at least 50% of days at entry. Women receiving LOTRONEX had significant increases over placebo (13% to 16%) in the median percentage of days with urgency control.
- The lower gastrointestinal functions of stool consistency, stool frequency, and sense of incomplete evacuation were also evaluated by patients' daily reports. Stool consistency was evaluated on a scale of 1 to 5 (1 = very hard, 2 = hard, 3 = formed, 4 = loose, and 5 = watery). At baseline, average stool consistency was approximately 4 (loose) for both treatment groups. During the 12 weeks of treatment, the average stool consistency decreased to approximately 3.0 (formed) for patients who received LOTRONEX and 3.5 for the patients who received placebo in the 2 studies.
- At baseline, average stool frequency was approximately 3.2 per day for both treatment groups. During the 12 weeks of treatment, the average daily stool frequency decreased to approximately 2.1 and 2.2 for patients receiving LOTRONEX and 2.7 and 2.8 for patients receiving placebo in the 2 studies.
- There was no consistent effect upon the sense of incomplete evacuation during the 12 weeks of treatment for patients receiving LOTRONEX as compared to patients receiving placebo in either study.
- Study 5 was conducted in women with severe diarrhea-predominant IBS and 1 or more of the following: frequent and severe abdominal pain or discomfort, frequent bowel urgency or fecal incontinence, disability or restriction of daily activities due to IBS. To evaluate the proportion of patients who responded to treatment, patients were asked every 4 weeks to compare their IBS symptoms during the previous month of treatment with how they usually felt during the 3 months prior to the study using an ordered 7-point scale (substantially worse to substantially improved). A responder was defined as a subject who reported moderate or substantial improvement on this global improvement scale (GIS). At Week 12, all three groups receiving LOTRONEX had significantly greater percentages of GIS responders compared to the placebo group (43% to 51% vs. 31%) using a Last Observation Carried Forward (LOCF) analysis. It should be noted that approximately 4% of subjects in each LOTRONEX dose group who were classified as responders using this approach were observed only through week 4. At each of the 4 week intervals of the treatment phase, all three dosages of LOTRONEX provided improvement in the average adequate relief rate of IBS pain and discomfort, stool consistency, stool frequency, and sense of urgency compared with placebo.
- Retrospective Analyses: In analyses of patients from Studies 1 and 2 who had diarrhea-predominant IBS and indicated their baseline run-in IBS symptoms were severe at the start of the trial, LOTRONEX provided greater adequate relief of IBS pain and discomfort than placebo. In further analyses of Studies 1 and 2, 57% of patients had urgency at baseline on 5 or more days per week. In this subset, 32% of patients on LOTRONEX had urgency no more than 1 day in the last week of the trial, compared with 19% of patients on placebo.
- In Studies 3 and 4, 66% of patients had urgency at baseline on 5 or more days per week. In this subset, 50% of patients on LOTRONEX had urgency no more than 1 day in the last week of the trial, compared with 29% of patients on placebo. Moreover, in the same subset, 12% on LOTRONEX had urgency no more than 2 days per week in any of the 12 weeks on treatment compared with 1% of placebo patients.
- In Studies 1 and 2, patient-reported subjective outcomes related to IBS were assessed by questionnaires obtained at baseline and week 12. Patients in the more severe subset who received LOTRONEX reported less difficulty sleeping, less tiredness, fewer eating problems, and less interference with social activities and work/main activities due to IBS symptoms or problems compared to those who received placebo. Change in the impact of IBS symptoms and problems on emotional and mental distress and on physical and sexual activity in women who received LOTRONEX were not statistically different from those reported by women who received placebo.
- Long-Term Use
- In a 48-week multinational, double-blind, placebo-controlled study, LOTRONEX 1 mg twice daily was evaluated in 714 women with non-constipated IBS. A retrospective analysis of the subset of women with severe diarrhea-predominant IBS (urgency on at least 10 days during the 2-week baseline period) was performed. Of the 417 patients with severe diarrhea-predominant IBS, 62% completed the trial.
- LOTRONEX (n = 198) provided a greater average rate of adequate relief of IBS pain and discomfort (52% vs. 41%) and a greater average rate of satisfactory control of bowel urgency (60% vs. 48%) compared with placebo (n = 219). Significant improvement of these symptoms occurred for most of the 48-week treatment period with no evidence of tachyphylaxis.
How Supplied
- LOTRONEX Tablets, 0.5 mg (0.562 mg alosetron HCl equivalent to 0.5 mg alosetron) are white, oval, film-coated tablets debossed with GX EX1 on one face.
- Bottles of 30 (NDC 65483-894-03) with child-resistant closures.
- LOTRONEX Tablets, 1 mg (1.124 mg alosetron HCl equivalent to 1 mg alosetron), are blue, oval, film-coated tablets debossed with GX CT1 on one face.
- Bottles of 30 (NDC 65483-895-03) with child-resistant closures.
- Store at 20-25°C (68-77°F). Protect from light and moisture.
Storage
There is limited information regarding Alosetron Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Alosetron |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Alosetron |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Prescriber and Patient Responsibilities
- Patients should be fully counseled on and understand the risks and benefits of LOTRONEX before an initial prescription is written. The patient may be educated by the enrolled prescriber or a healthcare provider under a prescriber's direction.
- Prescribers must:
- counsel patients for whom LOTRONEX is appropriate about the benefits and risks of LOTRONEX and discuss the impact of IBS symptoms on the patient's life.
- give the patient a copy of the Medication Guide, which outlines the benefits and risks of LOTRONEX, and instruct the patient to read it carefully. Answer all questions the patient may have about LOTRONEX. The complete text of the Medication Guide is printed at the end of this document.
- review the Patient Acknowledgement Form for LOTRONEX with the patient, answer all questions, and give a copy of the signed Patient Acknowledgement Form to the patient.
- provide each patient with appropriate instructions for taking LOTRONEX.
- Copies of the Patient Acknowledgement Form for LOTRONEX and additional copies of the Medication Guide are available by contacting Prometheus at 1-888-423-5227 or visiting www.lotronexppl.com.
- Patients who are prescribed LOTRONEX should be instructed to:
- read the Medication Guide before starting LOTRONEX and each time they refill their prescription.
- not start taking LOTRONEX if they are constipated.
- immediately discontinue LOTRONEX and contact their prescriber if they become constipated, or have symptoms of ischemic colitis such as new or worsening abdominal pain, bloody diarrhea, or blood in the stool. Contact their prescriber again if their constipation does not resolve after discontinuation of LOTRONEX. Resume LOTRONEX only if their constipation has resolved and after discussion with and the agreement of their treating prescriber.
- stop taking LOTRONEX and contact their prescriber if LOTRONEX does not adequately control IBS symptoms after 4 weeks of taking 1 mg twice a day.
Precautions with Alcohol
- Alcohol-Alosetron interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- LOTRONEX®[2]
Look-Alike Drug Names
- Lotronex® — Protonix®[3]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Talley NJ, Van Zanten SV, Saez LR, Dukes G, Perschy T, Heath M; et al. (2001). "A dose-ranging, placebo-controlled, randomized trial of alosetron in patients with functional dyspepsia". Aliment Pharmacol Ther. 15 (4): 525–37. PMID 11284782.
- ↑ "LOTRONEX alosetron hydrochloride tablet".
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Alosetron |Pill Name=No image.jpg |Drug Name= |Pill Ingred=|+sep=; |Pill Imprint= |Pill Dosage= |Pill Color=|+sep=; |Pill Shape= |Pill Size (mm)= |Pill Scoring= |Pill Image= |Drug Author= |NDC=
}}
{{#subobject:
|Label Page=Alosetron |Label Name=Alosetron05.png
}}
{{#subobject:
|Label Page=Alosetron |Label Name=Alosetron06.png
}}
{{#subobject:
|Label Page=Alosetron |Label Name=Alosetron07.png
}}
{{#subobject:
|Label Page=Alosetron |Label Name=Alosetron08.png
}}
{{#subobject:
|Label Page=Alosetron |Label Name=Alosetron09.png
}}





