Tramadol
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Ammu Susheela, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Tramadol is an opioid analgesic that is FDA approved for the treatment of management of moderate to moderately severe chronic pain in adults who require around-the-clock treatment of their pain for an extended period of time. Common adverse reactions include seizure risk, suicide risk, serotonin syndrome, anaphylactoid and allergic reactions, respiratory depression, withdrawal symptoms.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- It is indicated for the management of moderate to moderately severe chronic pain in adults who require around-the-clock treatment of their pain for an extended period of time.
- 100 mg Capsules: White capsule imprinted with blue ink “G 252” on cap and “100” between lines on the body
- 200 mg Capsules: White capsule imprinted with violet ink “G 253” on cap and “200” between lines on the body
- 300 mg Capsules: White capsule imprinted with red ink “G 254” on cap and “300” between lines on the body
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
] There is limited information regarding Off-Label Guideline-Supported Use of Tramadol in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Tramadol in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Tramadol in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Tramadol in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Tramadol in pediatric patients.
Contraindications
- Tramadol is contraindicated in patients who have previously demonstrated hypersensitivity to tramadol, any other component of ConZip™, or opioids. Reactions range from pruritis to fatal anaphylactoid reactions.
- ConZip™ is contraindicated in patients with significant respiratory depression in unmonitored settings or the absence of resuscitative equipment.
- ConZip™ is contraindicated in patients with acute or severe bronchial asthma or hypercapnia in unmonitored settings or the absence of resuscitative equipment.
Warnings
Seizure Risk
- Seizures have been reported in patients receiving tramadol within the recommended dosage range. Spontaneous post-marketing reports indicate that seizure risk is increased with doses of tramadol above the recommended range. * Concomitant use of tramadol increases the seizure risk in patients taking:
- Selective serotonin reuptake inhibitors and serotonin-norepinephrine reuptake inhibitors (SNRIs) antidepressants or anorectics,
Tricyclic antidepressants (TCAs), and other tricyclic compounds (e.g., cyclobenzaprine, promethazine, etc.), other opioids, MAO inhibitors, neuroleptics, or other drugs that reduce the seizure threshold.
- Risk of seizures may also increase in patients with epilepsy, those with a history of seizures, or in patients with a recognized risk for seizure (such as head trauma, metabolic disorders, alcohol and drug withdrawal, CNS infections).
- In tramadol overdose, naloxone administration may increase the risk of seizure.
Suicide Risk
- Do not prescribe ConZip™ for patients who are suicidal or addiction-prone. Consideration should be given to the use of non-narcotic analgesics in patients who are suicidal or depressed.
- Prescribe ConZip™ with caution for patients with a history of misuse and/or are taking CNS-active drugs including tranquilizers or antidepressant drugs, or alcohol in excess, and patients who suffer from emotional disturbance or depression.
- Tell your patients not to exceed the recommended dose and to limit their intake of alcohol.
Serotonin Syndrome Risk
- The development of a potentially life-threatening serotonin syndrome may occur with use of tramadol products, including ConZip™ , particularly with concomitant use of serotonergic drugs such as SSRIs, SNRIs, TCAs, MAOIs and triptans, with drugs which impair metabolism of serotonin (including MAOIs) and with drugs which impair metabolism of tramadol (CYP2D6 and CYP3A4 inhibitors).
- This may occur within the recommended dose.
- Serotonin syndrome may include mental-status changes (e.g., agitation, hallucinations, coma), autonomic instability (e.g., tachycardia, labile blood pressure, hyperthermia), neuromuscular aberrations (e.g., hyperreflexia, incoordination) and/or gastrointestinal symptoms (e.g., nausea, vomiting, diarrhea).
Anaphylactoid Reactions
- Serious and rarely fatal anaphylactoid reactions have been reported in patients receiving therapy with tramadol. When these events do occur it is often following the first dose.
- Other reported allergic reactions include pruritus, hives, bronchospasm, angioedema, toxic epidermal necrolysis and Stevens-Johnson syndrome. Patients with a history of anaphylactoid reactions to codeine and other opioids may be at increased risk and therefore should not receive ConZip™.
Respiratory Depression
- Administer ConZip™ cautiously in patients at risk for respiratory depression.
- In these patients alternative non-opioid analgesics should be considered. If large doses of tramadol are administered with anesthetic medications or alcohol, respiratory depression may result. Respiratory depression should be treated as an overdose.
- If naloxone is to be administered, use cautiously because it may precipitate seizures.
Interaction With Central Nervous System (CNS) Depressants, Including Alcohol and Drugs of Abuse
- Tramadol may be expected to have additive effects when used in conjunction with alcohol, other opioids, or illicit drugs that cause central nervous system depression. Use ConZip™ with caution and in reduced dosages when administered to patients receiving CNS depressants such as alcohol, opioids, anesthetic agents, narcotics, phenothiazines, tranquilizers or sedative hypnotics.
- ConZip™ increases the risk of CNS and respiratory depression in these patients. Alcohol-containing beverages should not be consumed by patients using ConZip™.
Patients with Increased Intracranial Pressure or Head Trauma
- Use ConZip™ with caution in patients with increased intracranial pressure or head injury.
- The respiratory depressant effects of opioids include carbon dioxide retention and secondary elevation of cerebrospinal fluid pressure, and may be markedly exaggerated in these patients.
- Additionally, pupillary changes (miosis) from tramadol may obscure the existence, extent, or course of intracranial pathology. Clinicians should also maintain a high index of suspicion for adverse drug reaction when evaluating altered mental status in these patients if they are receiving ConZip™.
Use in Ambulatory Patients
- ConZip™ may impair the mental and or physical abilities required for the performance of potentially hazardous tasks such as driving a car or operating machinery.
- Caution patients initiating therapy with ConZip™ or those whose dose has been increased to refrain from potentially hazardous activities until it is established that their mental and physical abilities are not significantly impaired.
Use With MAO Inhibitors and SSRIs
- Use ConZip™ with great caution in patients taking monoamine oxidase inhibitors.
- Animal studies have shown increased deaths with combined administration. Concomitant use of ConZip™ with MAO inhibitors or SSRI's increases the risk of adverse reactions, including seizure and serotonin syndrome.
Withdrawal Symptoms
- Withdrawal symptoms may occur if ConZip™ is discontinued abruptly. These symptoms may include: anxiety, sweating, insomnia, rigors, pain, nausea, tremors, diarrhea, upper respiratory symptoms, piloerection, and rarely hallucinations.
- Clinical experience with other formulations of tramadol suggests that withdrawal symptoms may be reduced by tapering ConZip™ when discontinuing tramadol therapy.
Misuse, Abuse and Diversion of Opioids
- ConZip™ contains tramadol, an opioid agonist of the morphine-type. Such drugs are sought by drug abusers and people with addiction disorders and are subject to criminal diversion.
- Tramadol can be abused in a manner similar to other opioid agonists, legal or illicit. This should be considered when prescribing or dispensing ConZip™ in situations where the physician or pharmacist is concerned about an increased risk of misuse, abuse, or diversion.
- ConZip™ could be abused by crushing, chewing, snorting, or injecting the dissolved product. These practices will result in the uncontrolled delivery of the opioid and pose a significant risk to the abuser that could result in overdose and death.
- Concerns about abuse, addiction, and diversion should not prevent the proper management of pain. The development of addiction to opioid analgesics in properly managed patients with pain has been reported to be rare. However, data are not available to establish the true incidence of addiction in chronic pain patients.
- Healthcare professionals should contact their State Professional Licensing Board, or State Controlled Substances Authority for information on how to prevent and detect abuse or diversion of this product.
Risk of Overdosage
- Serious potential consequences of overdosage with ConZip™ are central nervous system depression, respiratory depression and death. In treating an overdose, primary attention should be given to maintaining adequate ventilation along with general supportive treatment.
Acute Abdominal Conditions
- The administration of ConZip™ may complicate the clinical assessment of patients with acute abdominal conditions.
Adverse Reactions
Clinical Trials Experience
- The serious or otherwise important adverse reactions are following.
- Seizure risk
- Suicide risk
- Serotonin syndrome
- Anaphylactoid and allergic reactions
- Respiratory depression
- Withdrawal symptoms
Clinical Studies Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
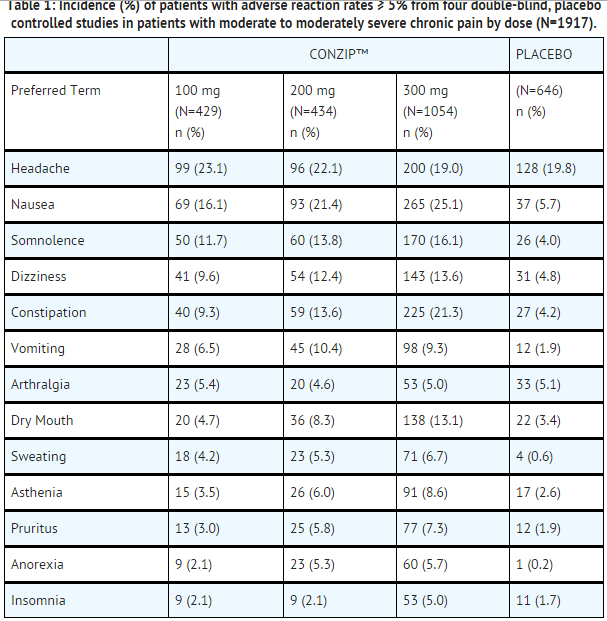
- ConZip™ capsules were administered to a total of 1987 patients in clinical trials. These included four double-blind and one long-term, open-label study in patients with osteoarthritis of the hip and knee. A total of 812 patients were 65 years or older.
- Adverse reactions with doses from 100 mg to 300 mg in the four pooled, randomized, double-blind, placebo-controlled studies in patients with chronic non-malignant pain are presented in the following table.
Adverse Reactions with Incidence Rates of 1.0% to <5.0%
- Cardiac disorders: hypertension
- Gastrointestinal disorders: dyspepsia, flatulence
- General disorders: abdominal pain, accidental injury, chills, fever, flu syndrome, neck pain, pelvic pain
- Investigations: hyperglycemia, urine abnormality
- Metabolism and nutrition disorders: peripheral edema, weight loss
- Musculoskeletal, connective tissue and bone disorders: myalgia
- Nervous system disorders: paresthesia, tremor, withdrawal syndrome
- Psychiatric disorders: agitation, anxiety, apathy, confusion, depersonalization, depression, euphoria, nervousness
- Respiratory, thoracic and mediastinal disorders: bronchitis, pharyngitis, rhinitis, sinusitis
- Skin and subcutaneous tissue disorders: rash
- Urogenital disorders: prostatic disorder, urinary tract infection
- Vascular disorders: vasodilatation
Adverse Reactions with Incidence Rates of 0.5% to <1.0% at Any Dose and Serious Adverse Reactions Reported in at least Two Patients
- Cardiac disorders: EKG abnormal, hypotension, tachycardia
- Gastrointestinal disorders: gastroenteritis
- General disorders: neck rigidity, viral infection
- Hematologic/Lymphatic disorders; anemia, ecchymoses
- Metabolism and nutrition disorders: blood urea nitrogen increased, GGT increased, gout, SGPT increased
- Musculoskeletal disorders: arthritis, arthrosis, joint disorder, leg cramps
- Nervous system disorders: emotional lability, hyperkinesia, hypertonia, thinking abnormal, twitching, vertigo
- Respiratory disorders: pneumonia
- Skin and subcutaneous tissue disorders: hair disorder, skin disorder, urticaria
- Special Senses: eye disorder, lacrimation disorder
- Urogenital disorders: cystitis, dysuria, sexual function abnormality, urinary retention
Postmarketing Experience
There is limited information regarding Tramadol Postmarketing Experience in the drug label.
Drug Interactions
Drugs Affecting Seizure Threshold
- Concomitant use of tramadol increases the seizure risk in patients taking SSRI/SNRI antidepressants or anorectics, TCA antidepressants and other tricyclic compounds, other opioids, MAOIs, neuroleptics or other drugs that lower the seizure threshold.
CYP2D6 and/or CYP3A4 inhibitors
- Tramadol is metabolized by CYP2D6 to form the active metabolite, O-desmethyl tramadol (M1). In vitro drug interaction studies in human liver microsomes indicate that concomitant administration with inhibitors of CYP2D6 such as fluoxetine, paroxetine, and amitriptyline could result in some inhibition of the metabolism of tramadol.
- Tramadol is also metabolized by CYP3A4. Administration of CYP3A4 inhibitors, such as ketoconazole and erythromycin with ConZip™ may affect the metabolism of tramadol leading to altered tramadol exposure.
- Concomitant administration of CYP2D6 and/or CYP3A4 inhibitors, such as quinidine, fluoxetine, paroxetine and amitriptyline (CYP2D6 inhibitors), and ketoconazole and erythromycin (CYP3A4 inhibitors), may reduce metabolic clearance of tramadol increasing the risk for serious adverse events including seizures and serotonin syndrome.
Serotonergic Drugs
- There have been post-marketing reports of serotonin syndrome with use of tramadol and SSRIs/SNRIs or MAOIs and α2-adrenergic blockers. Caution is advised when ConZip™ is co-administered with other drugs that may affect the serotonergic neurotransmitter systems, such as SSRIs, MAOIs, triptans, linezolid (an antibiotic which is a reversible non-selective MAOI), lithium, or St. John's Wort. If concomitant treatment of ConZip™ with a drug affecting the serotonergic neurotransmitter system is clinically warranted, careful observation of the patient is advised, particularly during treatment initiation and dose increases.
Triptans
- Based on the mechanism of action of tramadol and the potential for serotonin syndrome, caution is advised when ConZip™ is co-administered with a triptan.
- If concomitant treatment of ConZip™ with a triptan is clinically warranted, careful observation of the patient is advised, particularly during treatment initiation and dose increases.
Interaction With Central Nervous System (CNS) Depressants
- ConZip™ should be used with caution and in reduced dosages when administered to patients receiving CNS depressants such as opioids, anesthetic agents, narcotics, phenothiazines, tranquilizers or sedative hypnotics. ConZip™ increases the risk of CNS and respiratory depression in these patients.
Quinidine
- Quinidine is a strong inhibitor of CYP2D6. Coadministration of quinidine with an extended-release tramadol product resulted in a 50-60% increase in tramadol exposure and a 50-60% decrease in M1 exposure. The clinical consequences of these findings are unknown.
- In vitro drug interaction studies in human liver microsomes indicate that tramadol has no effect on quinidine metabolism.
Digoxin and Warfarins
- Post-marketing surveillance of tramadol has revealed rare reports of digoxin toxicity and alteration of warfarin effect, including elevation of prothrombin times.
CYP3A4 Inducers
- Administration of CYP3A4 inducers, such as carbamazepine, rifampin and St. John's Wort, with ConZip™ may affect the metabolism of tramadol leading to reduced tramadol exposure.
- Patients taking carbamazepine, a CYP3A4 inducer, may have a significantly reduced analgesic effect of tramadol. Because carbamazepine increases tramadol metabolism and because of the seizure risk associated with tramadol, concomitant administration of ConZip™ and carbamazepine is not recommended.
Use in Specific Populations
Pregnancy
Teratogenic Effects
- There are no adequate and well-controlled studies in pregnant women. Use ConZip™ during pregnancy only if the potential benefit justifies the potential risk to the fetus.
- Neonatal seizures, neonatal withdrawal syndrome, fetal death and still birth have been reported during post-marketing reports with tramadol HCl immediate-release products. ConZip™ should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
- Tramadol was not teratogenic at oral dose levels up to 50 mg/kg/day (1.6-fold the maximum daily human dose (MDHD)) in rats and 100 mg/kg (approximately 6.5-fold MDHD) in rabbits during organogenesis. However, embryo-fetal lethality, reductions in fetal weight and skeletal ossification, and increased supernumerary ribs were observed at a maternal toxic dose of 140 mg/kg in mice (approximately 2.3-fold MDHD), 80 mg/kg in rats (2.6-fold MDHD) or 300 mg/kg in rabbits (approximately 19-fold MDHD).
Non-teratogenic Effects
- Tramadol caused a reduction in neonatal body weight at a dose of 50 mg/kg (1.6-fold the MDHD) and reduced pup survival at an oral dose of 80 mg/kg (approximately 2.6-fold MDHD) when rats were treated during late gestation throughout lactation period.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Tramadol in women who are pregnant.
Labor and Delivery
- ConZip™ should not be used in pregnant women prior to or during labor unless the potential benefits outweigh the risks.
- Safe use in pregnancy has not been established. Chronic use during pregnancy may lead to physical dependence and post-partum withdrawal symptoms in the newborn.
- Tramadol has been shown to cross the placenta. The mean ratio of serum tramadol in the umbilical veins compared to maternal veins was 0.83 for 40 women given tramadol during labor.
- The effect of ConZip™, if any, on the later growth, development, and functional maturation of the child is unknown.
Nursing Mothers
- ConZip™ is not recommended for obstetrical preoperative medication or for post-delivery analgesia in nursing mothers because its safety in infants and newborns has not been studied.
- Following a single IV 100 mg dose of tramadol, the cumulative excretion in breast milk within 16 hours post-dose was 100 μg of tramadol (0.1% of the maternal dose) and 27 μg of M1. It is not known whether this drug is excreted in human milk following an oral dose.
Pediatric Use
- The safety and efficacy of ConZip™ in patients under 18 years of age have not been established. The use of ConZip™ in the pediatric population is not recommended.
Geriatic Use
- In general, caution should be used when selecting the dose for an elderly patient. Usually, dose administration should start at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal or cardiac function and of concomitant disease or other drug therapy.
- Eight hundred and twelve elderly (65 years of age or older) subjects were exposed to ConZip™ in clinical trials. Of those subjects, two hundred and forty were 75 years of age and older. In general, higher incidence rates of adverse events were observed for patients older than 65 years of age compared with patients 65 years and younger, particularly for the following adverse events: nausea, constipation, somnolence, dizziness, dry mouth, vomiting, asthenia, pruritus, anorexia sweating, fatigue, weakness, postural hypotension and dyspepsia.
- For this reason, ConZip™ should be used with great caution in patients older than 75 years of age.
Gender
There is no FDA guidance on the use of Tramadol with respect to specific gender populations.
Race
There is no FDA guidance on the use of Tramadol with respect to specific racial populations.
Renal Impairment
- ConZip™ has not been studied in patients with renal impairment. Impaired renal function results in a decreased rate and extent of excretion of tramadol and its active metabolite, M1.
- The limited availability of dose strengths of ConZip™ does not permit the dosing flexibility required for safe use in patients with severe renal impairment. Therefore, ConZip™ should not be used in patients with severe renal impairment.
Hepatic Impairment
- ConZip™ has not been studied in patients with hepatic impairment. The limited availability of dose strengths of ConZip™ does not permit the dosing flexibility required for safe use in patients with severe hepatic impairment. Therefore, ConZip™ should not be used in patients with severe hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Tramadol in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Tramadol in patients who are immunocompromised.
Administration and Monitoring
Administration
Monitoring
General Dosing Considerations
- ConZip™ is an extended-release formulation intended for once a day dosing in adults aged 18 years and older. The tablets must be swallowed whole with liquid and must not be split, chewed, dissolved or crushed.
- Chewing, crushing or splitting the tablet could result in the uncontrolled delivery of tramadol, in overdose and death.
- Do not administer ConZip™ at a dose exceeding 300 mg per day. Do not use ConZip™ more than once daily or concomitantly with other tramadol products.
Patients Not Currently on Tramadol Immediate-Release Products
- Initiate treatment with ConZip™ at a dose of 100 mg once daily and titrated up as necessary by 100 mg increments every five days to achieve a balance between relief of pain and tolerability.
Patients Currently on Tramadol Immediate-Release Products
- Calculate the 24-hour tramadol IR dose and initiate a total daily dose of ConZip™ rounded down to the next lowest 100 mg increment. The dose may subsequently be individualized according to patient need. Due to limitations in flexibility of dose selection with ConZip™, some patients maintained on tramadol IR products may not be able to convert to ConZip™.
Patients 65 Years of Age and Older
- Initiate dosing of an elderly patient (over 65 years of age) should be initiated cautiously, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal or cardiac function and of concomitant disease or other drug therapy.
- ConZip™ should be administered with even greater caution in patients over 75 years, due to the greater frequency of adverse events seen in this population.
Patients with Renal Impairment
- The limited availability of dose strengths and once daily dosing of ConZip™ do not permit the dosing flexibility required for safe use in patients with severe renal impairment. Do not use ConZip™ in patients with creatinine clearance less than 30 mL/min.
Patients with Hepatic Impairment
- The limited availability of dose strengths and once daily dosing of tramadol hydrochloride extended-release capsules do not permit the dosing flexibility required for safe use in patients with severe hepatic impairment. * Do not use ConZip™ in patients with severe hepatic impairment (Child-Pugh Class C).
Discontinuation of Treatment
- Withdrawal symptoms may occur if ConZip™ is discontinued abruptly. Clinical experience with tramadol suggests that withdrawal symptoms may be reduced by tapering ConZip™.
Food Effects
- ConZip™ may be taken without regard to food.
IV Compatibility
There is limited information regarding IV Compatibility of Tramadol in the drug label.
Overdosage
Human Experience
- Acute overdosage with tramadol can be manifested by respiratory depression, somnolence progressing to stupor or coma, skeletal muscle flaccidity, cold and clammy skin, constricted pupils, bradycardia, hypotension, and death.
- Deaths due to overdose have been reported with abuse and misuse of tramadol, by ingesting, inhaling, or injecting the crushed tablets. Review of case reports has indicated that the risk of fatal overdose is further increased when tramadol is abused concurrently with alcohol or other CNS depressants, including other opioids.
Management of Overdose
- In the treatment of tramadol overdosage, primary attention should be given to the reestablishment of a patent airway and institution of assisted or controlled ventilation. Supportive measures (including oxygen and vasopressors) should be employed in the management of circulatory shock and pulmonary edema accompanying overdose as indicated.
- Cardiac arrest or arrhythmias may require cardiac massage or defibrillation.
- While naloxone will reverse some, but not all, symptoms caused by overdosage with tramadol, the risk of seizures is also increased with naloxone administration.
- In animals, convulsions following the administration of toxic doses of ConZip™ could be suppressed with barbiturates or benzodiazepines but were increased with naloxone. Naloxone administration did not change the lethality of an overdose in mice.
- Hemodialysis is not expected to be helpful in an overdose because it removes less than 7% of the administered dose in a 4-hour dialysis period.
Pharmacology
Mechanism of Action
- ConZip™ contains tramadol, a centrally acting synthetic opioid analgesic. Although its mode of action is not completely understood, from animal tests, at least two complementary mechanisms appear applicable: binding of parent and M1 metabolite to μ-opioid receptors and weak inhibition of reuptake of norepinephrine and serotonin.
- Opioid activity is due to both low affinity binding of the parent compound and higher affinity binding of the O-demethylated metabolite M1 to μ-opioid receptors. In animal models, M1 is up to 6 times more potent than tramadol in producing analgesia and 200 times more potent in μ-opioid binding. Tramadol-induced analgesia is only partially antagonized by the opiate antagonist naloxone in several animal tests.
- The relative contribution of both tramadol and M1 to human analgesia is dependent upon the plasma concentrations of each compound.
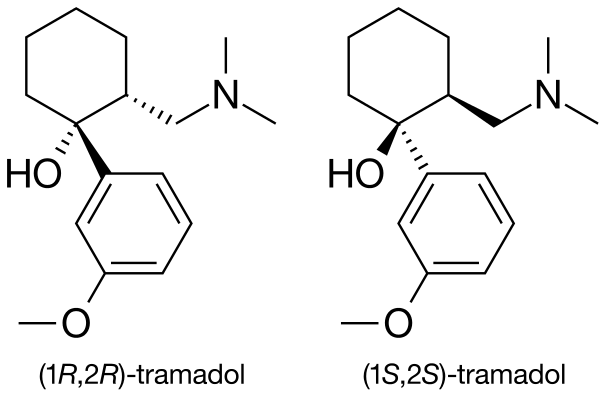
Structure
Pharmacodynamics
- Tramadol has been shown to inhibit reuptake of norepinephrine and serotonin in vitro, as have some other opioid analgesics. These mechanisms may contribute independently to the overall analgesic profile of tramadol.
- The relationship between exposure of tramadol and M1 and efficacy has not been evaluated in clinical studies.
- Apart from analgesia, tramadol administration may produce a constellation of symptoms (including dizziness, somnolence, nausea, constipation, sweating and pruritus) similar to that of other opioids.
- In contrast to morphine, tramadol has not been shown to cause histamine release. At therapeutic doses, tramadol has no effect on heart rate, left ventricular function or cardiac index.
- Orthostatic hypotension has been observed.
Pharmacokinetics
- The analgesic activity of tramadol is due to both parent drug and the M1 metabolite. ConZip™ is administered as a racemate and both tramadol and M1 are detected in the circulation.
- The Cmax and AUC of ConZip™ capsules have been observed to be dose-proportional over an oral dose range of 100 to 300 mg in healthy subjects.
Absorption
- After a single dose administration of ConZip™, Tmax occurs around 10-12 hours.
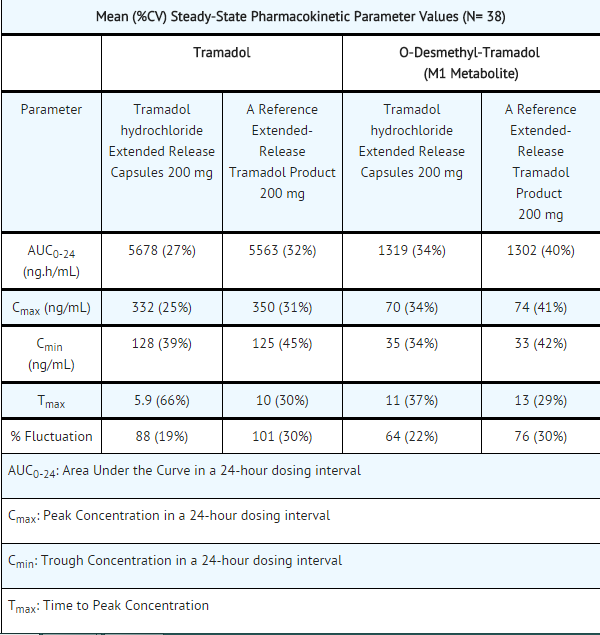
- The mean Cmax and AUC of ConZip™ capsules after a 300 mg single dose was 308 ng/mL and 6777 ng*hr/mL, respectively under fasting conditions. ConZip™ is bioequivalent to a reference extended-release tramadol product following a single 300 mg dose under fasting conditions.
- At steady-state, ConZip™ at 200 mg has been observed to be bioequivalent to a reference extended-release tramadol product at 200 mg under fasting conditions.
- Following administration of ConZip™ 200 mg capsules, steady-state plasma concentrations of both tramadol and M1 are achieved within four days of once daily dosing.
Food Effects
- The rate and extent of absorption of ConZip™ capsules (300 mg) are similar following oral administration with or without food. Therefore, ConZip™ capsules can be administered without regard to meals.
Distribution
- The volume of distribution of tramadol was 2.6 and 2.9 liters/kg in male and female subjects, respectively, following a 100 mg intravenous tramadol dose.
- The binding of tramadol to human plasma proteins is approximately 20% and binding also appears to be independent of concentration up to 10 μg/mL. Saturation of plasma protein binding occurs only at concentrations outside the clinically relevant range.
Metabolism
- Tramadol is extensively metabolized after oral administration. The major metabolic pathways appear to be N – (mediated by CYP3A4 and CYP2B6) and O – (mediated by CYP2D6) demethylation and glucuronidation or sulfation in the liver.
- One metabolite (O-desmethyl tramadol, denoted M1) is pharmacologically active in animal models. Formation of M1 is dependent on CYP2D6 and as such is subject to inhibition and polymorphism, which may affect the therapeutic response.
Elimination
- Tramadol is eliminated primarily through metabolism by the liver and the metabolites are eliminated primarily by the kidneys.
- Approximately 30% of the dose is excreted in the urine as unchanged drug, whereas 60% of the dose is excreted as metabolites. The remainder is excreted either as unidentified or as unextractable metabolites.
- The mean plasma elimination half-lives of racemic tramadol and racemic M1 after administration of ConZip™ capsules are approximately 10 and 11 hours, respectively.
Special Populations
Renal Impairment
- Impaired renal function results in a decreased rate and extent of excretion of tramadol and its active metabolite, M1. The pharmacokinetics of tramadol was studied in patients with mild or moderate renal impairment after receiving multiple doses of an extended-release tramadol product at 100 mg.
- There is no consistent trend observed for tramadol exposure related to renal function in patients with mild (CLcr: 50-80 mL/min) or moderate (CLcr: 30-50 mL/min) renal impairment in comparison to patients with normal renal function (CLcr > 80 mL/min).
- However, exposure of M1 increased 20-40% with increased severity of the renal impairment (from normal to mild and moderate). The pharmacokinetics of tramadol has not been studied in patients with severe renal impairment (CLcr < 30 mL/min).
- The limited availability of dose strengths of ConZip™ does not permit the dosing flexibility required for safe use in patients with severe renal impairment. Therefore, ConZip™ should not be used in patients with severe renal impairment.
- The total amount of tramadol and M1 removed during a 4-hour dialysis period is less than 7% of the administered dose.
Hepatic Impairment
- Pharmacokinetics of tramadol was studied in patients with mild or moderate hepatic impairment after receiving multiple doses of an extended-release tramadol product at 100 mg.
- The exposure of (+)- and (-)-tramadol was similar in mild and moderate hepatic impairment patients in comparison to patients with normal hepatic function. However, exposure of (+)- and (-)-M1 decreased ~50% with increased severity of the hepatic impairment (from normal to mild and moderate).
- The pharmacokinetics of tramadol has not been studied in patients with severe hepatic impairment. After the administration of tramadol immediate-release tablets to patients with advanced cirrhosis of the liver, tramadol area under the plasma concentration time curve was larger and the tramadol and M1 half-lives were longer than subjects with normal hepatic function.
- The limited availability of dose strengths of ConZip™ does not permit the dosing flexibility required for safe use in patients with severe hepatic impairment. Therefore, ConZip™ should not be used in patients with severe hepatic impairment.
Gender
- Based on pooled multiple-dose pharmacokinetics studies for an extended-release tramadol product in 166 healthy subjects (111 males and 55 females), the dose-normalized AUC values for tramadol were somewhat higher in females than in males.
- There was a considerable degree of overlap in values between male and female groups. Dosage adjustment based on gender is not recommended.
Age
- The effect of age on pharmacokinetics of ConZip™ has not been studied. Healthy elderly subjects aged 65 to 75 years administered an immediate-release formulation of tramadol, have plasma concentrations and elimination half-lives comparable to those observed in healthy subjects less than 65 years of age.
- In subjects over 75 years, mean maximum plasma concentrations are elevated (208 vs. 162 ng/mL) and the mean elimination half-life is prolonged (7 vs. 6 hours) compared to subjects 65 to 75 years of age.
- Adjustment of the daily dose is recommended for patients older than 75 years.
Drug Interactions
Poor / Extensive Metabolizers, CYP2D6
- The formation of the active metabolite, M1, is mediated by CYP2D6, a polymorphic enzyme. Approximately 7% of the population has reduced activity of the CYP2D6 isoenzyme of cytochrome P-450 metabolizing enzyme system. These individuals are “poor metabolizers” of debrisoquine, dextromethorphan and tricyclic antidepressants, among other drugs. Based on a population PK analysis of Phase 1 studies with IR tablets in healthy subjects, concentrations of tramadol were approximately 20% higher in "poor metabolizers" versus "extensive metabolizers," while M1 concentrations were 40% lower.
CYP2D6 Inhibitors
- In vitro drug interaction studies in human liver microsomes indicate that concomitant administration with inhibitors of CYP2D6 such as fluoxetine, paroxetine, and amitriptyline could result in some inhibition of the metabolism of tramadol.
Quinidine
- Tramadol is metabolized to active metabolite M1 by CYP2D6. Coadministration of quinidine, a selective inhibitor of CYP2D6, with tramadol ER resulted in a 50-60% increase in tramadol exposure and a 50-60% decrease in M1 exposure. The clinical consequences of these findings are unknown.
- To evaluate the effect of tramadol, a CYP2D6 substrate on quinidine, an in vitro drug interaction study in human liver microsomes was conducted. The results from this study indicate that tramadol has no effect on quinidine metabolism.
CYP3A4 Inhibitors and Inducers
- Since tramadol is also metabolized by CYP3A4, administration of CYP3A4 inhibitors, such as ketoconazole and erythromycin, or CYP3A4 inducers, such as rifampin and St. John's Wort, with ConZip™ may affect the metabolism of tramadol leading to altered tramadol exposure
Cimetidine
- Concomitant administration of tramadol immediate-release tablets with cimetidine, a weak CPY3A4 inhibitor, does not result in clinically significant changes in tramadol pharmacokinetics. No alteration of the ConZip™ dosage regimen with cimetidine is recommended.
Carbamazepine
- Carbamazepine, a CYP3A4 inducer, increases tramadol metabolism. Patients taking carbamazepine may have a significantly reduced analgesic effect of tramadol. Concomitant administration of ConZip™ and carbamazepine is not recommended.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- Carcinogenicity assessment has been conducted in mice, rats and p53(+/-) heterozygous mice. A slight, but statistically significant, increase in two common murine tumors, pulmonary and hepatic, was observed in a mouse carcinogenicity study, particularly in aged mice. Mice were dosed orally up to 30 mg/kg (90 mg/m2 or 0.5 times the maximum daily human dosage of 185 mg/m2) for approximately two years, although the study was not done with the Maximum Tolerated Dose. This finding is not believed to suggest risk in humans.
- No treatment-related tumors were noted in a rat carcinogenicity study (dosing orally up to 30 mg/kg, 180 mg/m2, or equivalent to the maximum daily human dosage) or in a second study where rats were treated with up to 75 mg/kg/day for males and 100 mg/kg/day for females (approximately 2.4 and 3.2-fold MDHD, respectively) for two years. However, the excessive decrease in body weight gain observed in the rat study might have reduced their sensitivity to any potential carcinogenic effect of the drug.
- No carcinogenic effect of tramadol was observed in p53(+/–)-heterozygous mice at oral doses up to 150 mg/kg/day (approximately 2.4-fold maximum daily human dose [MDHD] of 300 mg/day for a 60 kg adult based on body surface conversion) for 26 weeks.
- Tramadol was not mutagenic in the following assays: a bacterial reverse mutation assay using Salmonella and E. coli, a mouse lymphoma assay (in the absence of metabolic activation), chromosomal aberration test in Chinese hamsters, a bone marrow micronucleus test in mice and Chinese hamsters, and a dominant lethal mutation test in mice. Mutagenic results occurred in the presence of metabolic activation in the mouse lymphoma assay and micronucleus test in rats. Overall, the weight of evidence from these tests indicates that tramadol does not pose a genotoxic risk to humans.
- No effects on fertility were observed for tramadol at oral dose levels up to 50 mg/kg/day in male and female rats (1.6-fold the MDHD).
Clinical Studies
- ConZip™ is bioequivalent under fasting conditions to another extended-release tramadol product which did demonstrate efficacy in two of four clinical trials of patients with chronic pain.
- To qualify for inclusion into these studies, patients were required to have moderate to moderately severe pain as defined by a pain intensity score of ≥40 mm, off previous medications, on a 0 – 100 mm visual analog scale (VAS).
- In one 12-week randomized, double-blind, placebo-controlled study, patients with moderate to moderately severe pain due to osteoarthritis of the knee and/or hip were administered doses from 100 mg to 400 mg daily. Treatment with the extended-release tramadol product was initiated at 100 mg once daily for four days then increased by 100 mg per day increments every five days to the randomized fixed dose. Between 51% and 59% of patients in active treatment groups completed the study and 56% of patients in the placebo group completed the study.
- Discontinuations due to adverse events were more common in the extended-release tramadol product 200 mg, 300 mg and 400 mg treatment groups (20%, 27%, and 30% of discontinuations, respectively) compared to 14% of the patients treated with the extended-release tramadol product 100 mg and 10% of patients treated with placebo.
- Pain, as assessed by the WOMAC Pain subscale, was measured at 1, 2, 3, 6, 9, and 12 weeks and change from baseline assessed. A responder analysis based on the percent change in WOMAC Pain subscale demonstrated a statistically significant improvement in pain for the 100 mg and 200 mg treatment groups compared to placebo.
- In one 12-week randomized, double-blind, placebo-controlled flexible-dosing trial of the extended-release tramadol product in patients with osteoarthritis of the knee, patients titrated to an average daily dose of approximately 270 mg/day.
- Forty-nine percent of patients randomized to the active treatment group completed the study, while 52% of patients randomized to placebo completed the study. Most of the early discontinuations in the active treatment group were due to adverse events, accounting for 27% of the early discontinuations in contrast to 7% of the discontinuations from the placebo group.
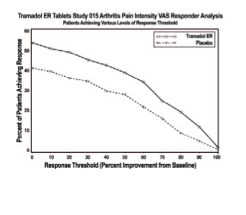
- Thirty-seven percent of the placebo-treated patients discontinued the study due to lack of efficacy compared to 15% of active-treated patients. The active treatment group demonstrated a statistically significant decrease in the mean Visual Analog Scale (VAS) score, and a statistically significant difference in the responder rate, based on the percent change from baseline in the VAS score, measured at 1, 2, 4, 8, and 12 weeks, between patients receiving the extended-release tramadol product and placebo
- Four randomized, placebo-controlled clinical trials of ConZip™ were conducted, none of which demonstrated efficacy but which differed in design from the preceding clinical studies described.
- Two trials were 12-week randomized placebo-controlled trials of ConZip™ 100 mg/day, 200 mg/day, and 300 mg/day versus placebo in patients with moderate to moderately severe osteoarthritis pain of the hip and knee. The other two 12 week trials were similar in design, but only studied ConZip™ 300 mg/day.
- In this fixed-dose design, subjects were required to titrate to a fixed dose, even if their pain responded to a lower titration dose
How Supplied
- ConZip™ capsules (tramadol hydrochloride) are supplied as opaque white hard gelatin capsules, imprinted as follows.
- 100 mg Capsules: White capsule imprinted with blue ink “G 252” on cap and “100” between lines on the body
- Bottle of 30 capsules: NDC 68025-053-30
- 200 mg Capsules: White capsule imprinted with violet ink “G 253” on cap and “200” between lines on the body
- Bottle of 30 capsules: NDC 68025-055-30
- 300 mg Capsules: White capsule imprinted with red ink “G 254” on cap and “300” between lines on the body
- Bottle of 30 capsules: NDC 68025-056-30
Storage
- Dispense in a tight container. Store at 25°C; excursions permitted to 15°C to 30°C (59°F to 86°F). Keep out of reach of children.
Images
Drug Images
{{#ask: Page Name::Tramadol |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Tramadol |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Inform patients that:
- ConZip™ is for oral use only and should be swallowed whole. The capsule should not be chewed, dissolved, crushed or split.
- ConZip™ may cause seizures and/or serotonin syndrome with concomitant use of serotonergic agents (including SRIs, NRIs, and triptans) or drugs that significantly reduce the metabolic clearance of tramadol.
- Not to change the prescribed single-dose or 24-hour dosing regimen of ConZip™, and that exceeding the prescribed dose can result in respiratory depression, seizures or death.
- ConZip™ may impair mental or physical abilities required for the performance of potentially hazardous tasks such as driving a car or operating machinery.
- ConZip™ should not be taken with alcohol containing beverages.
ConZip™ should be used with caution when taking medications such as tranquilizers, hypnotics or other opiate containing analgesics.
- Instruct female patients to inform the prescriber if they are pregnant, think they might become pregnant, or are trying to become pregnant.
- ConZip™ is to be taken once-a-day and at approximately the same time every day. Also, exceeding these recommendations and the maximum recommended daily dose can result in respiratory depression, seizures or death.
- Elderly patients, especially those over 75 years of age, and other patients who have renal or hepatic impairments may need to be cautioned about reduced dosages.
- Not to abruptly withdraw or discontinue tramadol therapy, as clinical experience with tramadol suggests the possible onset of signs and symptoms of withdrawal. These affects may be reduced by tapering tramadol therapy.
- ConZip™ must be kept out of reach of children.
Precautions with Alcohol
- Alcohol-Tramadol interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- TRAMADOL HCL®[1]
Look-Alike Drug Names
There is limited information regarding Tramadol Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Label Page=Tramadol |Label Name=Tramadol06.jpg
}}
{{#subobject:
|Label Page=Tramadol |Label Name=Tramadol07.jpg
}}