Levosulpiride
| |
| Names | |
|---|---|
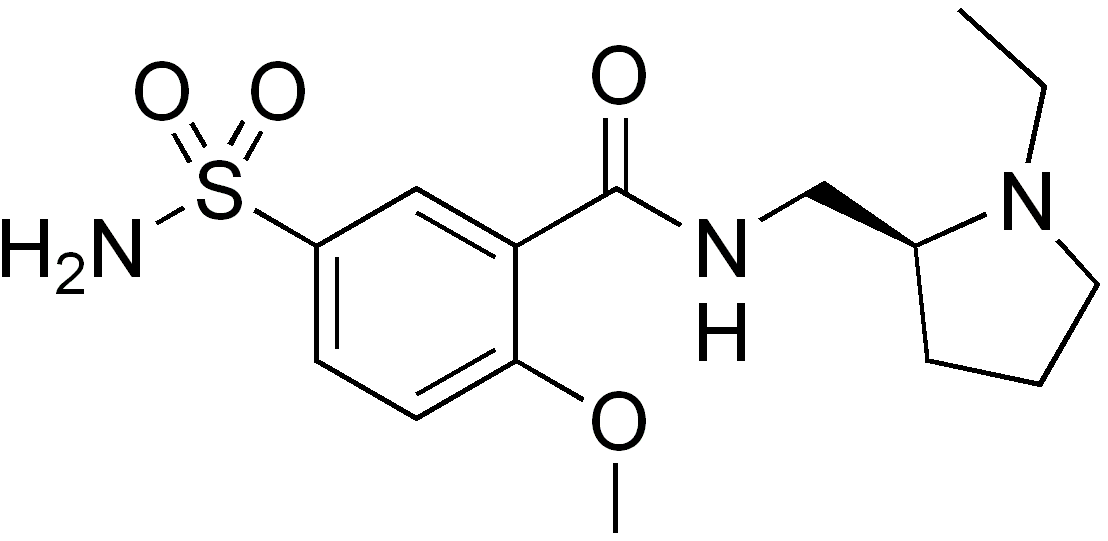
| IUPAC name
N-[[(2S)-1-Ethylpyrrolidin-2-yl]methyl]-2-methoxy-5-sulfamoylbenzamide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | Lua error in Module:Wikidata at line 879: attempt to index field 'wikibase' (a nil value). Lua error in Module:Wikidata at line 879: attempt to index field 'wikibase' (a nil value). |
| KEGG | |
PubChem CID
|
|
| |
| |
| Properties | |
| C15H23N3O4S | |
| Molar mass | 341.43 g·mol−1 |
|
WikiDoc Resources for Levosulpiride |
|
Articles |
|---|
|
Most recent articles on Levosulpiride Most cited articles on Levosulpiride |
|
Media |
|
Powerpoint slides on Levosulpiride |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Levosulpiride at Clinical Trials.gov Trial results on Levosulpiride Clinical Trials on Levosulpiride at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Levosulpiride NICE Guidance on Levosulpiride
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Levosulpiride Discussion groups on Levosulpiride Patient Handouts on Levosulpiride Directions to Hospitals Treating Levosulpiride Risk calculators and risk factors for Levosulpiride
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Levosulpiride |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
verify (what is
?)
Infobox references
Levosulpiride is a substituted benzamide antipsychotic, reported to be a selective antagonist of dopamine D2 receptor activity on both central and peripheral levels. It is an atypical neuroleptic and a prokinetic agent. Levosulpiride is also claimed to have mood elevating properties. Levosulpiride is used in the treatment of psychoses, particularly negative symptoms of schizophrenia, anxiety disorders, dysthymia, vertigo, dyspepsia, irritable bowel syndrome and premature ejaculation.
Chemically, it is the (S)-enantiomer of sulpiride.
Side effect
Side effects include amenorrhoea, gynaecomastia, galactorrhoea, changes in libido, and neuroleptic malignant syndrome.[1] It also effects GI motility may be antagonized by anticholinergic agents, narcotics and analgesics.[citation needed]
Mechanism of action
In contrast to most other neuroleptics which block both dopamine D1 and D2 receptors, sulpiride is more selective and acts primarily as a dopamine D2 antagonist. Sulpiride appears to lack effects on norepinephrine, acetylcholine, serotonin, histamine, or gamma-aminobutyric acid (GABA) receptors. [2]
Pharmacodynamics
Sulpiride is a substituted benzamide derivative and a selective dopamine D2 antagonist with antipsychotic and antidepressant activity. Other benzamide derivatives include metoclopramide, tiapride, and sultopride.[3]
References
- Pages with script errors
- Articles without UNII source
- Chemical articles with unknown parameter in Chembox
- Articles with changed EBI identifier
- Articles with changed ChemSpider identifier
- ECHA InfoCard ID from Wikidata
- Articles with changed InChI identifier
- Articles containing unverified chemical infoboxes
- All articles with unsourced statements
- Articles with unsourced statements from July 2012
- Articles with invalid date parameter in template
- Sulfonamides
- Pyrrolidines
- Phenol ethers