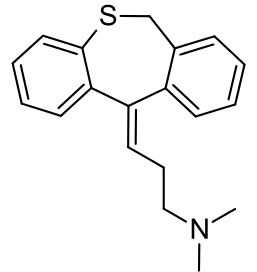
Dosulepin
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Dothep, Prothiaden |
| AHFS/Drugs.com | International Drug Names |
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 30%[2] |
| Protein binding | 84%[1] |
| Metabolism | Hepatic (via N-demethylation to active metabolite northiaden, S-oxidation and glucuronidation)[1] |
| Elimination half-life | 51 hours,[1] 14-45 hours,[3][4][5] 54 hours (elderly)[5][6] |
| Excretion | Urine (56%), faeces (15%)[1] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| UNII | |
| KEGG | |
| ChEMBL | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C19H21NS |
| Molar mass | 295.45 g/mol |
| | |
|
WikiDoc Resources for Dosulepin |
|
Articles |
|---|
|
Most recent articles on Dosulepin |
|
Media |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Dosulepin at Clinical Trials.gov Clinical Trials on Dosulepin at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Dosulepin
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Dosulepin Discussion groups on Dosulepin Directions to Hospitals Treating Dosulepin Risk calculators and risk factors for Dosulepin
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Dosulepin |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Dosulepin (INN, BAN) formerly known as dothiepin (USAN), and marketed under the brand names Prothiaden, Dothep, Thaden, and Dopress, is a tricyclic antidepressant that is used in several European and South Asian countries, as well as Australia, South Africa, and New Zealand. It is not used in the United States.[7]
Medical uses
Dosulepin is used for the treatment of major depressive disorder and neuropathic pain.[2] Dosulepin is only TGA- and MHRA-approved for the treatment of major depressive disorder.[8][9] There is clear evidence of the efficacy of dosulepin in psychogenic facial pain, though the drug may be needed for up to a year.[10]
Adverse effects
Common adverse effects:[1]
- Drowsiness
- Extrapyramidal symptoms
- Tremor
- Confusion
- Disorientation
- Dizziness
- Paresthesias
- Alterations to ECG patterns
- Dry mouth
- Sweating
- Urinary retention
- Hypotension
- Postural hypotension
- Tachycardia
- Palpitations
- Arrhythmias
- Conduction defects
- Increased or decreased libido
- Nausea
- Vomiting
- Constipation
- Blurred vision
Less common adverse effects:[1]
- Disturbed concentration
- Delusions
- Hallucinations
- Anxiety
- Fatigue
- Headaches
- Restlessness
- Excitement
- Insomnia
- Hypomania
- Nightmares
- Peripheral neuropathy
- Ataxia
- Incoordination
- Seizures
- Paralytic ileus
- Hypertension
- Heart block
- Myocardial infarction
- Stroke
- Gynecomastia (swelling of breast tissue in males)
- Testicular swelling
- Impotence
- Epigastric distress
- Abdominal cramps
- Parotid swellings
- Diarrhea
- Stomatitis (swelling of the mouth)
- Black tongue
- Peculiar taste sensations
- Cholestatic jaundice
- Altered liver function
- Hepatitis (swelling of the liver)
- Skin rash
- Urticaria (hives)
- Photosensitisation
- Skin blisters
- Angioneurotic edema
- Weight loss
- Urinary frequency
- Mydriasis
- Weight gain
- Hyponatremia (low blood sodium)
- Movement disorders
- Dyspepsia (indigestion)
- Increased intraocular pressure
- Changes in blood sugar levels
- Thrombocytopenia (an abnormally low number of platelets in the blood. This makes one more susceptible to bleeds)
- Eosinophilia (an abnormally high amount of eosinophils in the blood)
- Agranulocytosis (a dangerously low number of white blood cells in the blood leaving one open to potentially life-threatening infections)
- Galactorrhea (lactation that is unassociated with breastfeeding and lactation)
Contraindications
Contraindications include:[1]
- Epilepsy as it can lower the seizure threshold
- TCAs should not be used concomitantly or within 14 days of treatment with monoamine oxidase inhibitors due to the risk for serotonin syndrome
- Acute recovery phase following myocardial infarction as TCAs may produce conduction defects and arrhythmias
- Liver failure
- Hypersensitivity to dothiepin
Drug interactions
Dosulepin can potentiate the effects of alcohol and at least one death has been attributed to this combination.[1] TCAs potentiate the sedative effects of barbiturates, tranquillisers and CNS depressants.[1] Guanethidine and other adrenergic neurone blocking drugs can have their antihypertensive effects blocked by dosulepin.[1] Sympathomimetics may potentiate the sympathomimetic effects of dosulepin.[1] Due to the anticholinergic and antihistamine effects of dosulepin anticholinergic and antihistamine medications may have their effects potentiated by dosulepin and hence these combinations are advised against.[1] Dosulepin may have its postural hypotensive effects potentiated by diuretics.[1] Anticonvulsants may have their efficacy reduced by dosulepin due to its ability to reduce the seizure threshold.[1]
Overdose
The symptoms and the treatment of an overdose are largely the same as for the other tricyclic antidepressants.[8] Dosulepin may be particularly toxic in overdose compared to other TCAs.[8] The onset of toxic effects is around 4–6 hours after dosulepin is ingested.[1] In order to minimise the risk of overdose it is advised that patients only receive a limited number of tablets at a time so as to limit their risk of overdosing.[1] It is also advised that patients are not prescribed any medications that are known to increase the risk of toxicity in those receiving dosulepin due to the potential for mixed overdoses.[1] The medication should also be kept out of reach of children.[1]
Mechanism of action
Dosulepin is a serotonin-norepinephrine reuptake inhibitor (SNRI) with anticholinergic, antihistamine, and antiadrenergic effects.[11]
| Receptor/Transporter | Ki (nM)[12] |
|---|---|
| SERT | 8.6 |
| NET | 46 |
| DAT | 5,310 |
| 5-HT1A | 4,004 |
| 5-HT2A | 152 |
| M1 | 18 |
| M2 | 109 |
| M3 | 38 |
| M4 | 61 |
| M5 | 92 |
| α1 | 419 |
| α2 | 12 |
| H1 | 4 |
Pharmacokinetics
Dothiepin is readily absorbed from the small intestine and is extensively metabolised on first-pass through the liver into its chief active metabolite, northiaden (desmethyldosulepin).[1] Peak plasma concentrations of between 30.4 ng/mL to 278.8 ng/mL occur within 2–3 hours of oral administration.[1] It is distributed in breast milk and crosses the placenta and blood-brain barrier.[1] It is highly bound to plasma proteins (84%), and has a whole-body elimination half-life of 51 hours.[1]
Chemistry
Dosulepin is also the dibenzothiepine analogue of doxepin, from which it differs by the replacement of the oxygen position with that of sulfur.
The synthesis of this agent is quite similar to that used for its oxygen analogue, doxepin (), though with a reversed functionality. The sequence in the present case starts with the alkylation of thiosalicylic acid () with benzyl chloride () to give the thioether (). The product is then cyclized by means of polyphosphoric acid to give the ketone (). Condensation with the familiar dimethylpropylamine Grignard reagent serves to introduce the side chain (). Dehydration of the tertiary alcohol then affords dothiepin () as a mixture of isomers.[13]
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 1.15 1.16 1.17 1.18 1.19 1.20 1.21 "Dothep Dothiepin hydrochloride" (PDF). TGA eBusiness Services. Alphapharm Pty Limited. 1 November 2013. Retrieved 3 December 2013.
- ↑ 2.0 2.1 Lancaster, SG; Gonzalez, JP (July 1989). "Dothiepin: a review of its pharmcodynamic and pharmacokinetic properties, and therapeutic efficacy in depressive illness". Drugs. 38 (1): 123–147. doi:10.2165/00003495-198938010-00005. PMID 2670509.
- ↑ Rees, JA (1981). "Clinical interpretation of pharmacokinetic data on dothiepin hydrochloride (Dosulepin, Prothiaden)". Journal of International Medical Research. 9 (2): 98–102. PMID 7227628.
- ↑ Maguire, KP; Burrows, GD; Norman, TR; Scoggins, BA (September 1981). "Metabolism and pharmacokinetics of dothiepin" (PDF). British Journal of Clinical Pharmacology. 12 (3): 405–409. doi:10.1111/j.1365-2125.1981.tb01235.x. PMC 1401810. PMID 7295471.
- ↑ 5.0 5.1 Bareggi, SR; Cavallaro, R; Pirola, R; Altamura, AC (1990). "Pharmacokinetics and adverse effects of single doses of dothiepin in young and elderly subjects". Progress in Neuro-Psychopharmacology and Biological Psychiatry. 14 (2): 163–170. doi:10.1016/0278-5846(90)90098-2. PMID 2309034.
- ↑ Ogura, C; Kishimoto, A; Mizukawa, R; Hazama, H; Honma, H; Kawahara, K (1983). "Age differences in effects on blood pressure, flicker fusion frequency, salivation and pharmacokinetics of single oral doses of dothiepin and amitriptyline". European Journal of Clinical Pharmacology. 25 (6): 811–814. doi:10.1007/BF00542525. PMID 6662179.
- ↑ Dosulepin Hydrochloride. Martindale: The Complete Drug Reference. London, UK: Pharmaceutical Press. 5 December 2011. Retrieved 3 December 2013.
- ↑ 8.0 8.1 8.2 Template:Cite isbn
- ↑ Template:Cite isbn
- ↑ C Feinmann, M Harris, and R Cawley (11 February 1984). "Psychogenic facial pain: presentation and treatment". http://www.ncbi.nlm.nih.gov/. The National Center for Biotechnology Information. Retrieved 6 April 2014. External link in
|website=(help) - ↑ Template:Cite isbn
- ↑ Roth, BL; Driscol, J (12 January 2011). "PDSP Ki Database". Psychoactive Drug Screening Program (PDSP). University of North Carolina at Chapel Hill and the United States National Institute of Mental Health. Retrieved 3 December 2013.
- ↑ Zirkle, C. L.; 1971, Template:US Patent.
Template:Antidepressants
Template:Neuropathic pain and fibromyalgia pharmacotherapies
- Pages with script errors
- CS1 maint: Multiple names: authors list
- CS1 errors: external links
- Template:drugs.com link with non-standard subpage
- Drugs with non-standard legal status
- Articles with changed EBI identifier
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without InChI source
- Drugboxes which contain changes to verified fields
- Pages using div col with unknown parameters
- Pages with broken file links
- Tricyclic antidepressants
- Analgesics
- Dibenzothiepines