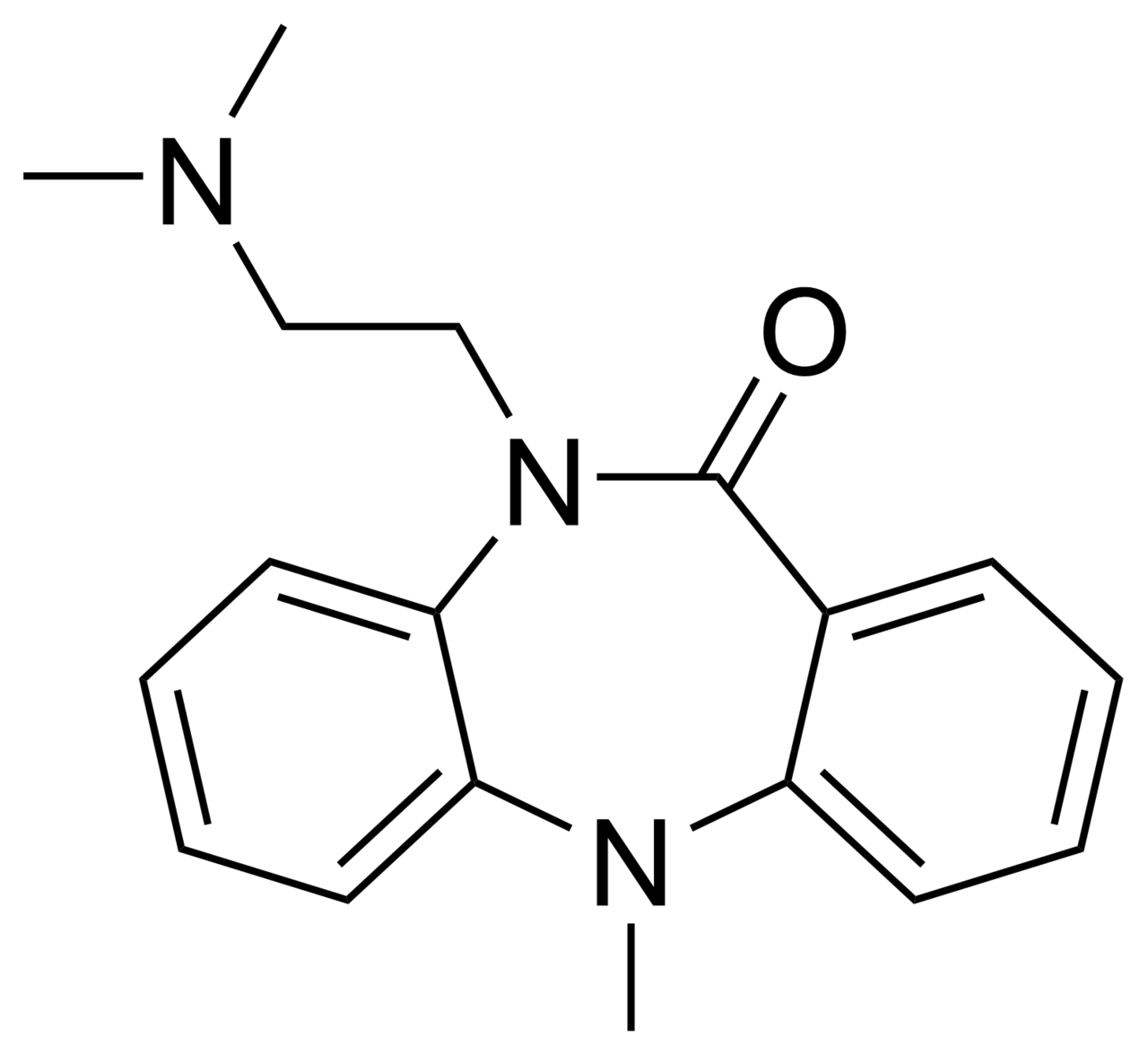
Dibenzepin
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 25% (Oral) |
| Protein binding | 80% |
| Metabolism | Hepatic |
| Elimination half-life | 5 hours |
| Excretion | Urine (80%), Feces (20%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C18H21N3O |
| Molar mass | 295.379 g/mol |
| 3D model (JSmol) | |
| |
| |
| | |
Dibenzepin (Noveril, Anslopax, Deprex, Ecatril, Neodit, Victoril) is a tricyclic antidepressant (TCA) used widely throughout Europe for the treatment of depression.[1][2][3] It has similar efficacy and effects relative to other TCAs like imipramine but with fewer side effects.[4][5][6][7] Dibenzepin acts as a norepinephrine reuptake inhibitor, potent antihistamine, and weak anticholinergic.[4][5][8] It lacks any 5-HT2 antagonistic properties.[9]
Chronic Pain
Like other tricyclic antidepressants, dibenzepin may have potential use in the treatment of chronic neuropathic pain.
Overdose
As tricyclic antidepressants have a relatively narrow therapeutic index, the likelihood of overdose (both accidental and intentional) is fairly high and should be considered carefully by the prescribing physician prior to patient use. Symptoms of overdose are similar to those of other tricyclic antidepressants, with cardiac toxicity (due to inhibition of sodium and calcium channels) generally occurring before the threshold for serotonin syndrome is reached. Due to this risk, tricyclic antidepressants are rarely selected as the first line treatment for depression.
Synthesis
The Ullmann reaction provides the key for preparing the diaryl-amine or -ether starting materials in this series.

Copper catalyzed coupling of methyl N-methylanthranilate () with nitrobromobenzene () leads to the arylaniline (). The ester is then saponified () and the nitro group reduced to the corresponding amine (). That product cyclizes to the lactam () on heating. A strong base preferentially removes the proton on the lactam nitrogen to form an anion. Alkylation with 2-chloroethyldimethylamine then affords dibenzepin (), a compound that shows antidepressant activity.[11]
See also
References
- ↑ Swiss Pharmaceutical Society (2000). Index Nominum 2000: International Drug Directory (Book with CD-ROM). Boca Raton: Medpharm Scientific Publishers. ISBN 3-88763-075-0.
- ↑ Sittig, Marshall (1988). Pharmaceutical manufacturing encyclopedia. Park Ridge, N.J., U.S.A: Noyes Publications. ISBN 0-8155-1144-2.
- ↑ Beresewicz M, Bidzińska E, Koszewska I, Puzyński S (1991). "[Results of using tricyclic antidepressive drugs in the treatment of endogenous depression (comparative analysis of 7 drugs)]". Psychiatria Polska (in Polish). 25 (3–4): 13–8. PMID 1687987.
- ↑ 4.0 4.1 "Novartis (dibenzepin) - Prescribing Information" (PDF).
- ↑ 5.0 5.1 Paloucek, Frank P.; Leikin, Jerrold B. (2007). Poisoning and Toxicology Handbook, Fourth Edition (Poisoning and Toxicology Handbook (Leiken & Paloucek's)). Informa Healthcare. ISBN 1-4200-4479-6.
- ↑ Gowardman M, Brown RA (March 1976). "Dibenzepin and amitriptyline in depressive states: comparative double-blind trial". The New Zealand Medical Journal. 83 (560): 194–7. PMID 6928.
- ↑ Baron DP, Unger HR, Williams HE, Knight RG (April 1976). "A double blind study of the antidepressants dibenzepin (Noveril) and amitriptyline". The New Zealand Medical Journal. 83 (562): 273–4. PMID 8749.
- ↑ Rehavi M, Maayani S, Goldstein L, Assael M, Sokolovsky M (August 1977). "Antimuscarinic properties of antidepressants: dibenzepin (Noveril)". Psychopharmacology. 54 (1): 35–8. doi:10.1007/BF00426538. PMID 20647.
- ↑ Closse A, Jaton AL (July 1984). "Investigation of the influence of lithium upon the down-regulation of serotonin2 receptors in rat frontal cortex induced by long-term treatment with dibenzepin, an antidepressant without appreciable affinity to serotonin2 receptors". Naunyn-Schmiedeberg's Archives of Pharmacology. 326 (4): 291–3. doi:10.1007/bf00501432. PMID 6148707.
- ↑ Hunziker, F.; Lauener, H.; Schmutz, J.; Arzneim. Forsch. 1963, 13, 324.
- ↑ Hunziker, F.; Lauener, H.; Schmutz, J.; Arzneim. Forsch. 1963, 13, 324.
External links
- Pages with script errors
- CS1 maint: Multiple names: authors list
- CS1 maint: Unrecognized language
- Template:drugs.com link with non-standard subpage
- Drugs with non-standard legal status
- Articles with changed EBI identifier
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Infobox drug
- Chemical pages without DrugBank identifier
- Drugboxes which contain changes to verified fields
- Tricyclic antidepressants
- Dibenzodiazepines
- Lactams