Piritramide
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Dipidolor |
| AHFS/Drugs.com | International Drug Names |
| Pregnancy category |
|
| Routes of administration | Oral, IM, IV |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | 95%[1] |
| Metabolism | Liver |
| Elimination half-life | 4-10 hours (acute dosing), 17.4 hours (chronic dosing) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
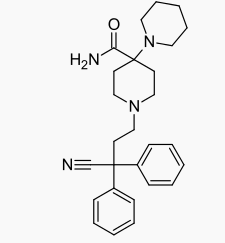
| Formula | C27H34N4O |
| Molar mass | 430.585 g/mol |
| 3D model (JSmol) | |
| |
| |
| | |
|
WikiDoc Resources for Piritramide |
|
Articles |
|---|
|
Most recent articles on Piritramide Most cited articles on Piritramide |
|
Media |
|
Powerpoint slides on Piritramide |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Piritramide at Clinical Trials.gov Clinical Trials on Piritramide at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Piritramide
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Piritramide Discussion groups on Piritramide Patient Handouts on Piritramide Directions to Hospitals Treating Piritramide Risk calculators and risk factors for Piritramide
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Piritramide |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Piritramide (R-3365, trade names Dipidolor, Piridolan, Pirium and others) is a synthetic opioid analgesic (narcotic painkiller) that is marketed in certain European countries including: Austria, Belgium, Czech Republic, Germany and the Netherlands.[2] It comes in free form, is about 0.75x times as potent as morphine and is given parenterally (by injection) for the treatment of severe pain.[2][3] Nausea, vomiting, respiratory depression and constipation are believed to be less frequent with piritramide than with morphine (which is the gold standard opioid against which other opioids are compared and contrasted against) and it produces more rapid-onset analgesia (pain relief) when compared to morphine and pethidine, after intravenous administration the onset of analgesia is as little as 1–2 minutes, which may be related to its great lipophilicity.[4] The analgesic and sedative effects of piritramide are believed to be potentiated with phenothiazines and its emetic (nausea/vomiting-inducing) effects are suppressed.[4] The volume of distribution is 0.7-1 L/kg after a single dose, 4.7-6 L/kg after steady-state concentrations are achieved and up to 11.1 L/kg after prolonged dosing.[4]
History & Regulation
Piritramide was developed and patented in the Netherlands, at Janssen, in 1960. It is part of an eponymous two-member class of opioids in clinical use with the other being bezitramide (Burgodin). The closest chemical and structural relatives of piritramide in clinical use include the diphenoxylate family, fentanyl (both Janssen discoveries) and somewhat more distantly alphaprodine.
Not being in clinical use in the United States, it is a Schedule I Narcotic controlled substance with a DEA ACSCN of 9642 and manufacturing quota of zero.[5] It presumably has abuse potential, and appears on the European black market on occasion and has a handful of street names including "Pierrette" and "P".[6] LEXIS-NEXIS and other database searches do not show mentions of law enforcement contact with this drug in the United States. Piritramide is specifically exempted from the Canadian controlled-substances law, and its relative bezitramide is a Schedule II controlled substance in the US, although not used there as of 2014.
Synthesis
See also
References
- ↑ Jage, J; Laufenberg-Feldmann, R; Heid, F (May 2008). "Medikamente zur postoperativen Schmerztherapie: Bewährtes und Neues". Der Anaesthesist (in German). 57 (5): 491–8. doi:10.1007/s00101-008-1327-9. PMID 18409073. Unknown parameter
|trans_title=ignored (help) - ↑ 2.0 2.1 Brayfield, A, ed. (23 September 2011). "Piritramide". Martindale: The Complete Drug Reference. Pharmaceutical Press. Retrieved 22 April 2014.
- ↑ Kay, B (December 1971). "A clinical investigation of piritramide in the treatment of postoperative pain". British Journal of Anaesthesia. 43 (12): 1167–71. doi:10.1093/bja/43.12.1167. PMID 4945251.
- ↑ 4.0 4.1 4.2 "FACHINFORMATION (Zusammenfassung der Merkmale des Arzneimittels)" (PDF). Janssen. Janssen - Cilag Pharma GmbH. November 2013. Retrieved 9 April 2014. Unknown parameter
|trans_title=ignored (help) - ↑ http://www.deadiversion.usdoj.gov/fed_regs/quotas/2013/fr0620.htm
- ↑ Inside Narcotics, 5th Edition, pp 347 (sidebar) "Piritramide -- what they say - Wien 8/iii/2002"
- Pages with script errors
- Pages with citations using unsupported parameters
- CS1 maint: Unrecognized language
- Template:drugs.com link with non-standard subpage
- Articles with changed CASNo identifier
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Infobox drug
- Chemical pages without DrugBank identifier
- Drugboxes which contain changes to verified fields
- Drugboxes which contain changes to watched fields
- Drug
- Opioids
- Piperidines
- Amides
- Nitriles
- Mu-opioid agonists
- Janssen Pharmaceutica
- Belgian inventions