Salsalate
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Ammu Susheela, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
Boxed Warning
See full prescribing information for complete Boxed Warning.
Cardiovascular Risk
Gastrointestinal Risk
|
Overview
Salsalate is an analgesic that is FDA approved for the treatment of relief of the signs and symptoms of rheumatoid arthritis, osteoarthritis and related rheumatic disorder. There is a Black Box Warning for this drug as shown here. Common adverse reactions include rash, nausea, vertigo, hearing disorder, tinnitus.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- Carefully consider the potential benefits and risks of Salsalate tablets, USP and other treatment options before deciding to use Salsalate tablets, USP. Use the lowest effective dose for the shortest duration consistent with individual patient treatment goals.
- Salsalate is indicated for relief of the signs and symptoms of rheumatoid arthritis, osteoarthritis and related rheumatic disorder.
Carefully consider the potential benefits and risks of Salsalate tablet, USP and other treatment options before deciding to use Salsalate tablet, USP. Use the lowest effective dose for the shortest duration consistent with individual patient treatment goals.
- After observing the response to initial therapy with Salsalate tablet, USP, the dose and frequency should be adjusted to suit an individual patient's needs.
- Salsalate is indicated for relief of the signs and symptoms of rheumatoid arthritis, osteoarthritis and related rheumatic disorder.
- Adults
- The usual dosage is 3000 mg daily, given in divided doses as follows: 1) two doses of two 750 mg tablets; 2) two doses of three 500 mg tablets; or 3) three doses of two 500 mg tablets. Some patients, e.g., the elderly, may require a lower dosage to achieve therapeutic blood concentrations and to avoid the more common side effects such as auditory.
- Alleviation of symptoms is gradual, and full benefit may not be evident for 3 to 4 days, when plasma salicylate levels have achieved steady state. There is no evidence for development of tissue tolerance (tachyphylaxis), but salicylate therapy may induce increased activity of metabolizing liver enzymes, causing a greater rate of salicyluric acid production and excretion, with a resultant increase in dosage requirement for maintenance of therapeutic serum salicylate levels.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Salsalate in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Salsalate in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Dosage recommendations and indications for salsalate use in children have not been established.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Salsalate in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Salsalate in pediatric patients.
Contraindications
- Salsalate tablet, USP is contraindicated in patients with known hypersensitivity to salsalate.
- Salsalate tablet, USP should not be given to patients who have experienced asthma, urticaria, or allergic-type reactions after taking aspirin or other NSAIDs. Severe, rarely fatal, anaphylactic-like reactions to NSAIDs have been reported in such patients.
- Salsalate tablet, USP is contraindicated for the treatment of peri-operative pain in the setting of coronary artery bypass graft (CABG) surgery.
Warnings
|
Boxed Warning
See full prescribing information for complete Boxed Warning.
Cardiovascular Risk
Gastrointestinal Risk
|
- Reye's Syndrome may develop in individuals who have chicken pox, influenza, or flu symptoms. Some studies suggest a possible association between the development of Reye's Syndrome and the use of medicines containing salicylate or aspirin. Salsalate contains a salicylate and therefore is not recommended for use in patients with chicken pox, influenza, or flu symptoms.
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Clinical Trial Experience of Salsalate in the drug label.
Postmarketing Experience
- In two well-controlled clinical trials, the following reversible adverse experiences characteristic of salicylates were most commonly reported with salsalate (n-280 pts; listed in descending order of frequency): tinnitus, nausea, hearing impairment, rash, and vertigo. These common symptoms of salicylates, i.e., tinnitus or reversible hearing impairment, are often used as a guide to therapy.
- Although cause-and-effect relationships have not been established, spontaneous reports over a ten-year period have included the following additional medically significant adverse experiences: abdominal pain, abnormal hepatic function, anaphylactic shock, angioedema, bronchospasm, decreased creatinine clearance, diarrhea, G.I. bleeding, hepatitis, hypotension, nephritis and urticaria.
Drug Interactions
There is limited information regarding Salsalate Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
- Reproductive studies conducted in rats and rabbits have not demonstrated evidence of developmental abnormalities. However, animal reproduction studies are not always predictive of human response. There are no adequate and well-controlled studies in pregnant women.
Nonteratogenic Effects
- Because of the known effects of nonsteroidal anti-inflammatory drugs on the fetal cardiovascular system (closure of ductus arteriosus), use during pregnancy (particularly late pregnancy) should be avoided.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Salsalate in women who are pregnant.
Labor and Delivery
- There exist no adequate and well-controlled studies in pregnant women. Although adverse effects on mother or infant have not been reported with salsalate use during labor, caution is advised when anti-inflammatory dosage is involved. However, other salicylates have been associated with prolonged gestation and labor, maternal and neonatal bleeding sequelae, potentiation of narcotic and barbiturate effects (respiratory or cardiac arrest in the mother), delivery problems and stillbirth.
- In rat studies with NSAIDs, as with other drugs known to inhibit prostaglandin synthesis, an increased incidence of dystocia, delayed parturition, and decreased pup survival occurred. The effects of Salsalate tablet, USP on labor and delivery in pregnant women are unknown.
Nursing Mothers
- It is not known whether salsalate per se is excreted in human milk; salicylic acid, the primary metabolite of salsalate, has been shown to appear in human milk in concentrations approximating the maternal blood level. Thus, the infant of a mother on salsalate therapy might ingest in mother’s milk 30 to 80% as much salicylate per kg body weight as the mother is taking. Accordingly, caution should be exercised when salsalate is administered to a nursing woman.
Pediatric Use
Safety and effectiveness of salsalate use in children have not been established.
Geriatic Use
As with any NSAIDs, caution should be exercised in treating the elderly (65 years and older).
Gender
There is no FDA guidance on the use of Salsalate with respect to specific gender populations.
Race
There is no FDA guidance on the use of Salsalate with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Salsalate in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Salsalate in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Salsalate in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Salsalate in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral.
Monitoring
- Blood pressure (BP) should be monitored closely during the initiation of NSAID treatment and throughout the course of therapy.
- If Salsalate tablet, USP therapy must be initiated, close monitoring of the patient's renal function is advisable
- Patients receiving Salsalate tablet, USP, who may be adversely affected by alterations in platelet function, such as those with coagulation disorders or patients receiving anticoagulants, should be carefully monitored.
IV Compatibility
There is limited information regarding IV Compatibility of Salsalate in the drug label.
Overdosage
Death has followed ingestion of 10 to 30 g of salicylates in adults, but much larger amounts have been ingested without fatal outcome.
Symptoms The usual symptoms of salicylism tinnitus, vertigo, headache, confusion, drowsiness, sweating, hyperventilation, vomiting and diarrhea will occur. More severe intoxication will lead to disruption of electrolyte balance and blood pH, and hyperthermia and dehydration.
Treatment Further absorption of salsalate from the G.I. tract should be prevented by emesis (syrup of ipecac), and, if necessary, by gastric lavage.
Fluid and electrolyte imbalance should be corrected by the administration of appropriate I.V. therapy. Adequate renal function should be maintained. Hemodialysis or peritoneal dialysis may be required in extreme cases.
Pharmacology
| |
Salsalate
| |
| Systematic (IUPAC) name | |
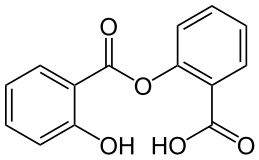
| 2-(2-Hydroxybenzoyl)oxybenzoic acid | |
| Identifiers | |
| CAS number | |
| ATC code | N02 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 258.23 g/mol |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
C(US) |
| Legal status |
[[Prescription drug|Template:Unicode-only]](US) |
| Routes | ? |
Mechanism of Action
- Salsalate is a nonsteroidal anti-inflammatory drug (NSAID). The anti-inflammatory property may be attributed to its ability to inhibit prostaglandin synthesis.
Structure
- Salsalate, is a nonsteroidal anti-inflammatory agent for oral administration. Chemically, salsalate (salicylsalicylic acid or 2-hydroxy-benzoic acid, 2- carboxyphenyl ester) is a dimer of salicylic acid; its structural formula is shown below.
Each tablet, for oral administration contains 500 mg or 750 mg of salsalate, USP. In addition each tablet contains the following inactive ingredients: Microcrystalline Cellulose, Sodium Starch Glycolate, Povidone, and Stearic Acid. Also contains: Hydroxypropyl Methylcellulose, Polydextrose, Titanium Dioxide, Triacetin, FD&C Blue No. 1 Aluminum Lake, FD&C Blue No. 2 Aluminum Lake and Polyethylene Glycol.
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Salsalate in the drug label.
Pharmacokinetics
- Salsalate is insoluble in acid gastric fluids (<0.1 mg/ml at pH 1.0), but readily soluble in the small intestine where it is partially hydrolyzed to two molecules of salicylic acid. A significant portion of the parent compound is absorbed unchanged and undergoes rapid esterase hydrolysis in the body: its half-life is about one hour. About 13% is excreted through the kidneys as a glucuronide conjugate of the parent compound, the remainder as salicylic acid and its metabolites. Thus, the amount of salicylic acid available from salsalate is about 15% less than from aspirin, when the two drugs are administered on a salicylic acid molar equivalent basis (3.6 g salsalate/5 g aspirin).
- Salicylic acid biotransformation is saturated at anti-inflammatory doses of salsalate. Such capacity-limited biotransformation results in an increase in the half-life of salicylic acid from 3.5 to 16 or more hours. Thus, dosing with salsalate twice a day will satisfactorily maintain blood levels within the desired therapeutic range (10 to 30 mg/100 ml) throughout the 12-hour intervals. Therapeutic blood levels continue for up to 16 hours after the last dose. The parent compound does not show capacity-limited biotransformation, nor does it accumulate in the plasma on multiple dosing. Food slows the absorption of all salicylates including salsalate.
- The mode of anti-inflammatory action of salsalate and other nonsteroidal anti-inflammatory drugs is not fully defined. Although salicylic acid (the primary metabolite of salsalate) is a weak inhibitor of prostaglandin synthesis in vitro, salsalate appears to selectively inhibit prostaglandin synthesis in vivo1, providing anti-inflammatory activity equivalent to aspirin2 and indomethacin3. Unlike aspirin, salsalate does not inhibit platelet aggregation4.
- The usefulness of salicylic acid, the active in vivo product of salsalate, in the treatment of arthritic disorders has been established 5,6. In contrast to aspirin, salsalate causes no greater fecal gastrointestinal blood loss than placebo.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Salsalate in the drug label.
Clinical Studies
There is limited information regarding Salsalate Clinical Studies in the drug label.
How Supplied
- Salsalate is insoluble in acid gastric fluids (<0.1 mg/ml at pH 1.0), but readily soluble in the small intestine where it is partially hydrolyzed to two molecules of salicylic acid. A significant portion of the parent compound is absorbed unchanged and undergoes rapid esterase hydrolysis in the body: its half-life is about one hour. About 13% is excreted through the kidneys as a glucuronide conjugate of the parent compound, the remainder as salicylic acid and its metabolites. Thus, the amount of salicylic acid available from salsalate is about 15% less than from aspirin, when the two drugs are administered on a salicylic acid molar equivalent basis (3.6 g salsalate/5 g aspirin).
- Salicylic acid biotransformation is saturated at anti-inflammatory doses of salsalate. Such capacity-limited biotransformation results in an increase in the half-life of salicylic acid from 3.5 to 16 or more hours. Thus, dosing with salsalate twice a day will satisfactorily maintain blood levels within the desired therapeutic range (10 to 30 mg/100 ml) throughout the 12-hour intervals. Therapeutic blood levels continue for up to 16 hours after the last dose. The parent compound does not show capacity-limited biotransformation, nor does it accumulate in the plasma on multiple dosing. Food slows the absorption of all salicylates including salsalate.
- The mode of anti-inflammatory action of salsalate and other nonsteroidal anti-inflammatory drugs is not fully defined. Although salicylic acid (the primary metabolite of salsalate) is a weak inhibitor of prostaglandin synthesis in vitro, salsalate appears to selectively inhibit prostaglandin synthesis in vivo, providing anti-inflammatory activity equivalent to aspirin2 and indomethacin3. Unlike aspirin, salsalate does not inhibit platelet aggregation.
- The usefulness of salicylic acid, the active in vivo product of salsalate, in the treatment of arthritic disorders has been established. In contrast to aspirin, salsalate causes no greater fecal gastrointestinal blood loss than placebo.
Storage
Store at controlled room temperature 15-30°C (59-86°F).
Images
Drug Images
{{#ask: Page Name::Salsalate |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel







{{#ask: Label Page::Salsalate |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Salsalate Patient Counseling Information in the drug label.
Precautions with Alcohol
- Alcohol-Salsalate interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- SALSALATE ®[1]
Look-Alike Drug Names
There is limited information regarding Salsalate Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
