Oxycodone
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Chetan Lokhande, M.B.B.S [2]
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Oxycodone is an analgesic opioid that is FDA approved for the {{{indicationType}}} of pain, chronic (severe), in patients requiring a long-term daily around-the-clock opioid analgesic, pain (moderate to severe). Common adverse reactions include dermatologic: pruritus (controlled-release, 13% ; immediate-release, 3% or greater ), sweating (controlled-release, 5% ; immediate-release, less than 3% ), gastrointestinal: abdominal pain (up to 5% ), constipation (controlled-release, 23% ; immediate-release, 3% or greater ), nausea (controlled-release, 23% ; immediate-release, 3% or greater ), vomiting (controlled-release, 12% ; immediate-release, 3% or greater ), xerostomia (controlled-release, 6% ; immediate-release, less than 3% ), neurologic: Asthenia (controlled-release, 6% ; immediate-release, 3% or greater ), dizziness (controlled-release, 13% ; immediate-release, 3% or greater ), headache (controlled-release, 7% ; immediate-release, 3% or greater ), somnolence (controlled-release, 23% ; immediate-release, 3% or greater ).
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- Reserve use of single doses greater than 40 mg, total daily doses greater than 80 mg , oral solution concentration of 100 mg per 5 mL (20 mg per mL), and controlled-release 60 mg or 80 mg tablets, for opioid-tolerant patients only (oral solution) to avoid dosing errors include total dose in mg and mL when prescribing, administering, and dispensing
- Do not discontinue abruptly in physically dependent patient; taper gradually to prevent withdrawal.
- Pain, chronic (Severe), in patients requiring a long-term daily around-the-clock opioid analgesic: individualize dose; initial dose selection must take into account patient's prior analgesic treatment experience and risk factors for addiction, abuse, and misuse; due to substantial inter-patient variability in relative potency of different opioid products, when converting it is recommended to underestimate a patient's 24-hour oral oxycodone requirements and provide rescue mediation as needed.
- Pain, chronic (Severe), in patients requiring a long-term daily around-the-clock opioid analgesic: (as first opioid and for patients who are not opioid-tolerant) initial, 10 mg or every 12 hours; titrate by 25% to 50% of the current dose every 1 to 2 days based on analgesic requirement and tolerance.
- Pain, chronic (Severe), in patients requiring a long-term daily around-the-clock opioid analgesic: (conversion from other oral oxycodone formulations) initial, one-half of total daily oxycodone requirement orally every 12 hours; titrate by 25% to 50% of the current dose every 1 to 2 days based on analgesic requirement and tolerance.
- Pain, chronic (Severe), in patients requiring a long-term daily around-the-clock opioid analgesic: (conversion from other opioids) initial, 10 mg orally every 12 hours; titrate by 25% to 50% of the current dose every 1 to 2 days based on analgesic requirement and tolerance.
- Pain, chronic (Severe), in patients requiring a long-term daily around-the-clock opioid analgesic: (conversion from transdermal fentanyl) 18 hours following patch removal initiate 10 mg orally every 12 hours for every 25 mcg/hr of transdermal fentanyl; titrate by 25% to 50% of the current dose every 1 to 2 days based on analgesic requirement and tolerance.
- Pain (Moderate to Severe): (immediate-release capsules, tablets, or oral solution 5 mg/5 mL) opioid-naive patients; initial, 5 to 15 mg orally every 4 to 6 hours as needed for pain; titrate based on pain severity and patient response.
- Pain (Moderate to Severe): (oral solution 100 mg/5 mL) reserve for opioid-tolerant patients who have been titrated to a stable analgesic regimen with lower doses of oxycodone and can benefit from use of a smaller volume of oral solution; always use the enclosed oral syringe to measure dose.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information about Off-Label Guideline-Supported Use of Oxycodone in adult patients.
Non–Guideline-Supported Use
There is limited information about Off-Label Non–Guideline-Supported Use of Oxycodone in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
safety and efficacy not established in pediatric patients
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information about Off-Label Guideline-Supported Use of Oxycodone in pediatric patients.
Non–Guideline-Supported Use
There is limited information about Off-Label Non–Guideline-Supported Use of Oxycodone in pediatric patients.
Contraindications
- Oxycodone hydrochloride tablets are contraindicated in patients with known hypersensitivity to oxycodone, or in any situation where opioids are contraindicated. This includes patients with significant respiratory depression (in unmonitored settings or the absence of resuscitative equipment) and patients with acute or severe bronchial asthma or hypercarbia. Oxycodone hydrochloride tablets are contraindicated in any patient who has or is suspected of having paralytic ileus.
Warnings
Respiratory Depression:
- Respiratory depression is the chief hazard from all opioid agonist preparations. Respiratory depression occurs most frequently in elderly or debilitated patients, usually following large initial doses in non-tolerant patients, or when opioids are given in conjunction with other agents that depress respiration.
- Oxycodone hydrochloride tablets should be used with extreme caution in patients with significant chronic obstructive pulmonary disease or cor pulmonale, and in patients having substantially decreased respiratory reserve, hypoxia, hypercapnia, or pre-existing respiratory depression. In such patients, even usual therapeutic doses of oxycodone hydrochloride tablets may decrease respiratory drive to the point of apnea. In these patients alternative non-opioid analgesics should be considered, and opioids should be employed only under careful medical supervision at the lowest effective dose.
Hypotensive Effect:
- Oxycodone hydrochloride tablets, like all opioid analgesics, may cause severe hypotension in an individual whose ability to maintain blood pressure has been compromised by a depleted blood volume, or after concurrent administration with drugs such as phenothiazines or other agents which compromise vasomotor tone. Oxycodone hydrochloride tablets may produce orthostatic hypotension in ambulatory patients. Oxycodone hydrochloride tablets, like all opioid analgesics, should be administered with caution to patients in circulatory shock, since vasodilatation produced by the drug may further reduce cardiac output and blood pressure.
Head Injury and Increased Intracranial Pressure:
- The respiratory depressant effects of narcotics and their capacity to elevate cerebrospinal fluid pressure may be markedly exaggerated in the presence of head injury, other intracranial lesions or a pre-existing increase in intracranial pressure. Furthermore, narcotics produce adverse reactions which may obscure the clinical course of patients with head injuries.
Adverse Reactions
Clinical Trials Experience
- Oxycodone hydrochloride tablets have been evaluated in open label clinical trials in patients with cancer and nonmalignant pain. Oxycodone hydrochloride tablets are associated with adverse experiences similar to those seen with other opioids.
- Serious adverse reactions that may be associated with oxycodone hydrochloride tablets therapy in clinical use are those observed with other opioid analgesics and include: respiratory depression, respiratory arrest, circulatory depression, cardiac arrest, hypotension, and/or shock (see overdosage, warnings).
- The less severe adverse events seen on initiation of therapy with oxycodone hydrochloride tablets are also typical opioid side effects. These events are dose dependent, and their frequency depends on the clinical setting, the patient's level of opioid tolerance, and host factors specific to the individual. They should be expected and managed as a part of opioid analgesia. The most frequent of these include nausea, constipation, vomiting, headache, and pruritus.
- In many cases the frequency of adverse events during initiation of opioid therapy may be minimized by careful individualization of starting dosage, slow titration and the avoidance of large rapid swings in plasma concentration of the opioid. Many of these adverse events will abate as therapy is continued and some degree of tolerance is developed, but others may be expected to remain throughout therapy.
- In all patients for whom dosing information was available (n=191) from the open-label and double-blind studies involving oxycodone hydrochloride tablets, the following adverse events were recorded in oxycodone hydrochloride tablets treated patients with an incidence ≥ 3%. In descending order of frequency they were: nausea, constipation, vomiting, headache, pruritus, insomnia, dizziness, asthenia, and somnolence.
- The following adverse experiences occurred in less than 3% of patients involved in clinical trials with oxycodone:
Body as a Whole
- Abdominal pain, accidental injury, allergic reaction, back pain, chills and fever, fever, flu syndrome, infection, neck pain, pain, photosensitivity reaction, and sepsis.
Cardiovascular
- Deep thrombophlebitis, heart failure, hemorrhage, hypotension, migraine, palpitation, and tachycardia.
Digestive
Hemic and Lymphatic
- Anemia and leukopenia.
Metabolic and Nutritional
Musculoskeletal
- Arthralgia, arthritis, bone pain, myalgia and pathological fracture.
Nervous
- Agitation, anxiety, confusion, dry mouth, hypertonia, hypesthesia, nervousness, neuralgia, personality disorder, tremor, and vasodilation.
Respiratory
- Bronchitis, cough increased, dyspnea, epistaxis, laryngismus, lung disorder, pharyngitis, rhinitis, and sinusitis.
Skin and Appendages
- Herpes simplex, rash, sweating, and urticaria.
Special Senses
Urogenital
Drug Abuse and Dependence
Controlled Substance:
- Oxycodone Hydrochloride Tablets contain Oxycodone, a mu-agonist opioid if the morphine type and is a Schedule II controlled substance.
- Oxycodone hydrochloride, like other opioids used in analgesia, can be abused and is subject to criminal diversion.
Abuse
- Drug addiction is characterized by compulsive use, use for non-medical purposes, and continued use despite harm or risk of harm. Drug addiction is a treatable disease, utilizing a multi-disciplinary approach, but relapse is common.
- “Drug-seeking” behavior is very common in addicts and drug abusers. Drug-seeking tactics include emergency calls or visits near the end of office hours, refusal to undergo appropriate examination, testing or referral, repeated “loss” of prescriptions, tampering with prescriptions and reluctance to provide prior medical records or contact information for other treating physician(s). “Doctor shopping” to obtain additional prescriptions is common among drug abusers and people suffering from untreated addiction.
- Abuse and addiction are separate and distinct from physical dependence and tolerance. Physicians should be aware that addiction may not be accompanied by concurrent tolerance and symptoms of physical dependence. In addition, abuse of opioids can occur in the absence of true addiction and is characterized by misuse for nonmedical purposes, often in combination with other psychoactive substances. Careful record-keeping of prescribing information, including quantity, frequency, and renewal requests is strongly advised.
- Oxycodone hydrochloride is intended for oral use only. Abuse of oxycodone hydrochloride tablets poses a risk of overdose and death. The risk is increased with concurrent abuse of alcohol and other substances. Parenteral drug abuse is commonly associated with transmission of infectious diseases such as hepatitis and HIV.
- Proper assessment of the patient, proper prescribing practices, periodic re-evaluation of therapy, and proper dispening and storage are appropriate measures that help to limit abuse of opioid drugs.
- Infants born to mothers physically dependent on opioids will also be physically dependent and may exhibit respiratory difficulties and withdrawal symptoms.
Dependence
- Tolerance is the need for increasing doses of opioids to maintain a defined effect such as analgesia (in the absence of disease progression or other external factors). Physical dependence is manifested by withdrawal symptoms after abrupt discontinuation of a drug or upon administration of an antagonist. Physical dependence and tolerance are not unusual during chronic opioid therapy.
- The opioid abstinence or withdrawal syndrome is characterized by some or all of the following: restlessness, lacrimation, rhinorrhea, yawning, perspiration, chills, myalgia, and mydriasis. Other symptoms also may develop, including irritability, anxiety, backache, joint pain, weakness, abdominal cramps, insomnia, nausea, anorexia, vomiting, diarrhea, or increased blood pressure, respiratory rate, or heart rate. In general, opioids should not be abruptly discontinued.
Postmarketing Experience
There is limited information regarding Oxycodone Postmarketing Experience in the drug label.
Drug Interactions
- Oxycodone is metabolized in part to oxymorphone via the cytochrome p450 isoenzyme CYP2D6. While this pathway may be blocked by a variety of drugs (e.g., certain cardiovascular drugs and antidepressants), such blockade has not yet been shown to be of clinical significance with this agent. However, clinicians should be aware of this possible interaction.
- Neuromuscular Blocking Agents: Oxycodone, as well as other opioid analgesics, may enhance the neuromuscular blocking action of skeletal muscle relaxants and produce an increased degree of respiratory depression.
CNS Depressants
- Patients receiving narcotic analgesics, general anesthetics, phenothiazines, other tranquilizers, sedative-hypnotics or other CNS depressants (including alcohol) concomitantly with oxycodone hydrochloride tablets may exhibit an additive CNS depression. Interactive effects resulting in respiratory depression, hypotension, profound sedation, or coma may result if these drugs are taken in combination with the usual dosage of oxycodone hydrochloride tablets. When such combined therapy is contemplated, the dose of one or both agents should be reduced.
Mixed Agonist/Antagonist Opioid Analgesics
- Agonist/antagonist analgesics (i.e., pentazocine, nalbuphine, butorphanol and buprenorphine) should be administered with caution to patients who have received or are receiving a course of therapy with a pure opioid agonist analgesic such as oxycodone hydrochloride tablets. In this situation, mixed agonist/antagonist analgesics may reduce the analgesic effect of oxycodone hydrochloride tablets and/or may precipitate withdrawal symptoms in these patients.
Monoamine Oxidase Inhibitors (MAOIs)
- MAOIs have been reported to intensify the effects of at least one opioid drug causing anxiety, confusion and significant depression of respiration or coma. The use of oxycodone hydrochloride tablets is not recommended for patients taking MAOIs or within 14 days of stopping such treatment.
Use in Specific Populations
Pregnancy
Teratogenic Effects: Category B
- Reproduction studies in Sprague-Dawley rats and New Zealand rabbits revealed that when oxycodone was administered orally at doses up to 16 mg/kg (approximately 2 times the daily oral dose of 90 mg for adults on a mg/m2 basis) and 25 mg/kg (approximately 5 times the daily oral dose of 90 mg on a mg/m2 basis), respectively was not teratogenic or embryo-fetal toxic. There are no adequate and well controlled studies of oxycodone in pregnant women. Because animal reproductive studies are not always predictive of human responses, oxycodone hydrochloride tablets should be used during pregnancy only if potential benefit justifies the potential risk to the fetus.
Nonteratogenic Effects
- Neonates whose mothers have taken oxycodone chronically may exhibit respiratory depression and/or withdrawal symptoms, either at birth and/or in the nursery.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Oxycodone in women who are pregnant.
Labor and Delivery
- Oxycodone hydrochloride tablets are not recommended for use in women during or immediately prior to labor. Occasionally, opioid analgesics may prolong labor through actions which temporarily reduce the strength, duration and frequency of uterine contractions. Neonates, whose mothers received opioid analgesics during labor, should be observed closely for signs of respiratory depression. A specific narcotic antagonist, naloxone, should be available for reversal of narcotic-induced respiratory depression in the neonate.
Nursing Mothers
- Oxycodone has been detected in breast milk. Withdrawal symptoms can occur in breast-feeding infants when maternal administration of an opioid analgesic is stopped. Ordinarily, nursing should not be undertaken while a patient is receiving oxycodone hydrochloride tablets since oxycodone may be excreted in milk.
Pediatric Use
- The safety and efficacy of oxycodone in pediatric patients have not been evaluated.
Geriatic Use
- Population pharmacokinetic studies conducted with oxycodone hydrochloride tablets indicated that the plasma concentrations of oxycodone did not appear to be increased in patients over the age of 65.
Gender
- Population pharmacokinetic analyses performed in the clinical study support the lack of gender effect on the pharmacokinetics of oxycodone from oxycodone hydrochloride tablets.
Race
- Population pharmacokinetic analyses support the lack of race effect on oxycodone pharmacokinetics after administration of oxycodone hydrochloride tablets, but these data should be interpreted conservatively, since the majority of patients enrolled into the studies were Caucasians (94%).
Renal Impairment
- In a clinical trial supporting the development of oxycodone hydrochloride tablets, too few patients with decreased renal function were evaluated to study these potential differences. In previous studies, patients with renal impairment (defined as a creatinine clearance < 60 mL/min) had concentrations of oxycodone in the plasma that were higher than in subjects with normal renal function. Based on information available on the metabolism and excretion of oxycodone, dose initiation in patients with renal impairment should follow a conservative approach. Dosages should be adjusted according to the clinical situation.
Hepatic Impairment
- In a clinical trial supporting the development of oxycodone hydrochloride tablets, too few patients with decreased hepatic function were evaluated to study these potential differences. However, since oxycodone is extensively metabolized, its clearance may decrease in hepatic failure patients. Dose initiation in patients with hepatic impairment should follow a conservative approach. Dosages should be adjusted according to the clinical situation.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Oxycodone in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Oxycodone in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Oxycodone Administration in the drug label.
Monitoring
There is limited information regarding Oxycodone Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Oxycodone and IV administrations.
Overdosage
Signs and Symptoms
- Acute overdose with oxycodone hydrochloride tablets can be manifested by respiratory depression, somnolence progressing to stupor or coma, skeletal muscle flaccidity, cold and clammy skin, constricted pupils, bradycardia, hypotension, and death.
Treatment
- To treat oxycodone hydrochloride tablets overdose, primary attention should be given to the re-establishment of a patent airway and institution of assisted or controlled ventilation. Supportive measures (including oxygen and vasopressors) should be employed in the management of circulatory shock and pulmonary edema accompanying overdose as indicated. Cardiac arrest or arrhythmias may require cardiac massage or defibrillation.
- The narcotic antagonists, naloxone or nalmefene, are specific antidotes for opioid overdose. Opioid antagonists should not be administered in the absence of clinically significant respiratory or circulatory depression secondary to oxycodone hydrochloride tablets overdose. If needed the appropriate dose of naloxone hydrochloride or nalmefene should be administered simultaneously with efforts at respiratory resuscitation (see package insert for each drug for the details). Since the duration of action of oxycodone may exceed that of the antagonist, the patient should be kept under continued surveillance and repeated doses of the antagonist should be administered as needed to maintain adequate respiration. Gastric emptying may be useful in removing unabsorbed drug.
- Opioid antagonists should be administered cautiously to persons who are suspected to be physically dependent on any opioid agonist, including Oxycodone. (see Opioid-Tolerant Individuals.)
- Opioid-Tolerant Individuals:In an individual physically dependent on opioids, administration of a usual dose of antagonist will precipitate an acute withdrawal. The severity of the withdrawal syndrome produced will depend on the degree of physical dependence and the dose of the antagonist administered. Use of an opioid antagonist should be reserved for cases where such treatment is clearly needed. If it is necessary to treat serious respiratory depression in the physically dependent patient, administration of the antagonist should be begun with care and by titration with smaller than usual doses.
Pharmacology
Mechanism of Action
- The analgesic ingredient, oxycodone, is a semi-synthetic narcotic with multiple actions qualitatively similar to those of morphine; the most prominent of these involves the central nervous system and organs composed of smooth muscle.
- Oxycodone, as the hydrochloride salt, is a pure agonist opioid whose principal therapeutic action is analgesia and has been in clinical use since 1917. Like all pure opioid agonists, there is no ceiling effect to analgesia, such as is seen with partial agonists or non-opioid analgesics. Based upon a single-dose, relative-potency study conducted in humans with cancer pain, 10 to 15 mg of oxycodone given intramuscularly produced an analgesic effect similar to 10 mg of morphine given intramuscularly. Both drugs have a 3 to 4 hour duration of action. Oxycodone retains approximately one half of its analgesic activity when administered orally
Effects on Central Nervous System
- The precise mechanism of the analgesic action is unknown. However, specific CNS opioid receptors for endogenous compounds with opioid-like activity have been identified throughout the brain and spinal cord and play a role in the analgesic effects of this drug. A significant feature of opioid-induced analgesia is that it occurs without loss of consciousness. The relief of pain by morphine-like opioids is relatively selective, in that other sensory modalities, (e.g., touch, vibrations, vision, hearing, etc.) are not obtunded.
- Oxycodone produces respiratory depression by direct action on brain stem respiratory centers. The respiratory depression involves both a reduction in the responsiveness of the brain stem respiratory centers to increases in carbon dioxide tension and to electrical stimulation.
- Oxycodone depresses the cough reflex by direct effect on the cough center in the medulla. Antitussive effects may occur with doses lower than those usually required for analgesia. Oxycodone causes miosis, even in total darkness. Pinpoint pupils are a sign of opioid overdose but are not pathognomonic (e.g., pontine lesions of hemorrhagic or ischemic origins may produce similar findings). Marked mydriasis rather than miosis may be seen due to hypoxia in overdose situations.
Effects on Gastrointestinal Tract and Other Smooth Muscle
- Oxycodone, like other opioid analgesics, produces some degree of nausea and vomiting which is caused by direct stimulation of the chemoreceptor trigger zone (CTZ) located in the medulla. The frequency and severity of emesis gradually diminishes with time.
- Oxycodone may cause a decrease in the secretion of hydrochloric acid in the stomach that reduces motility while increasing the tone of the antrum, stomach, and duodenum. Digestion of food in the small intestine is delayed and propulsive contractions are decreased. Propulsive peristaltic waves in the colon are decreased, while tone may be increased to the point of spasm resulting in constipation. Other opioid-induced effects may include a reduction in biliary and pancreatic secretions, spasm of sphincter of Oddi, and transient elevations in serum amylase.
Effects on Cardiovascular System
- Oxycodone, in therapeutic doses, produces peripheral vasodilatation (arteriolar and venous), decreased peripheral resistance, and inhibits baroreceptor reflexes. Manifestations of histamine release and/or peripheral vasodilatation may include pruritus, flushing, red eyes, sweating, and/or orthostatic hypotension.
- Caution should be used in hypovolemic patients, such as those suffering acute myocardial infarction, because oxycodone may cause or further aggravate their hypotension. Caution should also be used in patients with cor pulmonale who have received therapeutic doses of opioids.
Structure
- Oxycodone hydrochloride tablets USP is an opioid analgesic.
- Each tablet for oral administration contains 5 mg, 10 mg, 15 mg, 20 mg or 30 mg of oxycodone hydrochloride USP.
- Oxycodone hydrochloride is a white, odorless crystalline powder derived from the opium alkaloid, thebaine. Oxycodone hydrochloride dissolves in water (1 g in 6 to 7 mL) and is considered slightly soluble in alcohol (octanol water partition coefficient is 0.7).
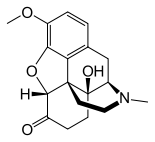
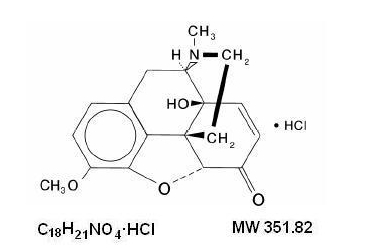
- Chemically, oxycodone hydrochloride is 4, 5α-epoxy-14-hydroxy-3-methoxy-17-methylmorphinan-6-one hydrochloride and has the following structural formula:
- The tablets contain the following inactive ingredients: corn starch; lactose monohydrate; microcrystalline cellulose; silicon dioxide; sodium starch glycolate; and stearic acid. The 10 mg tablet also contains D&C Red No. 27 aluminum lake. The 15 mg tablet also contains D&C Yellow No. 10 aluminum lake and FD&C Blue No. 2 aluminum lake. The 20 mg tablet also contains FD&C Blue No. 2 aluminum lake; FD&C Red No. 40 aluminum lake; and FD&C Yellow No. 6 aluminum lake. The 30 mg tablet also contains FD&C Blue No. 2 aluminum lake.
- The 5 mg, 10 mg, 15 mg, 20 mg, and 30 mg tablets contain the equivalent of 4.5 mg, 9 mg, 13.5 mg, 18 mg, and 27 mg, respectively, of oxycodone free base.
Pharmacodynamics
- The relationship between the plasma level of oxycodone and the analgesic response will depend on the patient's age, state of health, medical condition and extent of previous opioid treatment.
- The minimum effective plasma concentration of oxycodone to achieve analgesia will vary widely among patients, especially among patients who have been previously treated with potent agonist opioids. Thus, patients need to be treated with individualized titration of dosage to the desired effect. The minimum effective analgesic concentration of oxycodone for any individual patient may increase with repeated dosing due to an increase in pain and/or development of tolerance.
Pharmacokinetics
- The activity of oxycodone hydrochloride tablets is primarily due to the parent drug oxycodone. Oxycodone hydrochloride tablets are designed to provide immediate release of oxycodone.
Absorption
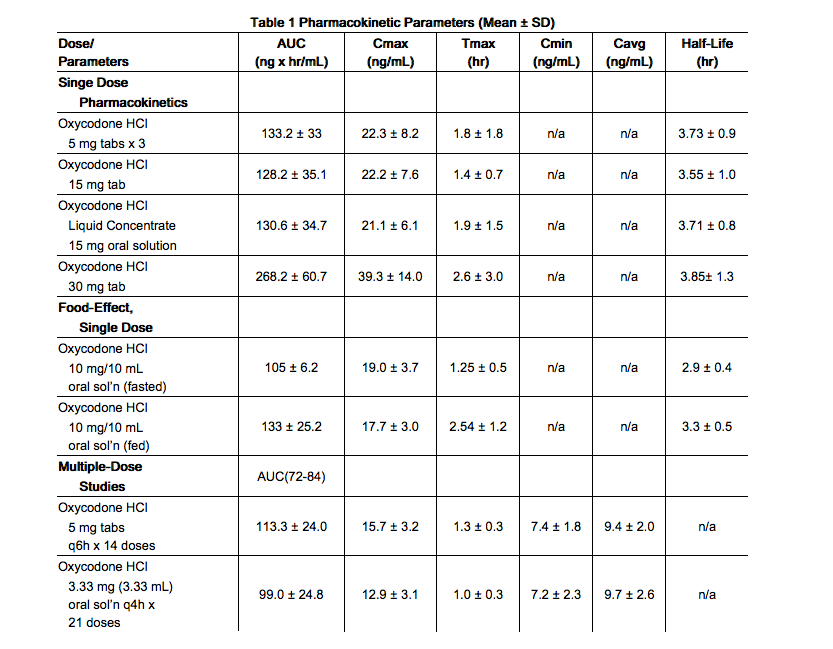
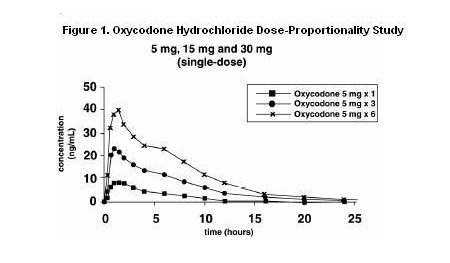
- About 60% to 87% of an oral dose of oxycodone reaches the systemic circulation in comparison to a parenteral dose. This high oral bioavailability (compared to other oral opioids) is due to lower pre-systemic and/or first-pass metabolism of oxycodone. The relative oral bioavailability of oxycodone hydrochloride 15 mg and 30 mg tablets, compared to the 5 mg oxycodone hydrochloride tablets, is 96% and 101% respectively. Oxycodone hydrochloride 15 mg tablets and 30 mg tablets are bioequivalent to the 5 mg oxycodone hydrochloride tablets (see Table 1 for pharmacokinetic parameters). Dose proportionality of oxycodone has been established using the oxycodone hydrochloride 5 mg tablets at doses of 5 mg, 15 mg (three 5 mg tablets) and 30 mg (six 5 mg tablets) based on extent of absorption (AUC) (see Figure 1). It takes approximately 18 to 24 hours to reach steady-state plasma concentrations of oxycodone with oxycodone hydrochloride tablets.
Food Effect
- A single-dose food effect study was conducted in normal volunteers using the 5 mg/5 mL solution. The concurrent intake of a high fat meal was shown to enhance the extent (27% increase in AUC), but not the rate of oxycodone absorption from the oral solution. (see Table 1). In addition, food caused a delay in Tmax (1.25 to 2.54 hour). Similar effects of food are expected with the 15 mg and 30 mg tablets.
Distribution
- Following intravenous administration, the volume of distribution (Vss) for oxycodone was 2.6 L/kg. Plasma protein binding of oxycodone at 37°C and a pH of 7.4 was about 45%. Oxycodone has been found in breast milk. (See PRECAUTIONS-Nursing Mothers.)
Metabolism
- Oxycodone hydrochloride is extensively metabolized to noroxycodone, oxymorphone, and their glucuronides. The major circulating metabolite is noroxycodone with an AUC ratio of 0.6 relative to that of oxycodone. Oxymorphone is present in the plasma only in low concentrations. The analgesic activity profile of other metabolites is not known at present.
- The formation of oxymorphone, but not noroxycodone, is mediated by CYP2D6 and as such its formation can, in theory, be affected by other drugs. (See Precautions-Drug Interactions.)
Elimination
- Oxycodone and its metabolites are excreted primarily via the kidney. The amounts measured in the urine have been reported as follows: free oxycodone up to 19%; conjugated oxycodone up to 50%; free oxymorphone 0%; conjugated oxymorphone ≤ 14%; both free and conjugated noroxycodone have been found in the urine but not quantified. The total plasma clearance was 0.8 L/min for adults. Apparent elimination half-life of oxycodone following the administration of oxycodone hydrochloride tablets was 3.5 to 4 hours.
Special Populations
Geriatric
- Population pharmacokinetic studies conducted with oxycodone hydrochloride tablets indicated that the plasma concentrations of oxycodone did not appear to be increased in patients over the age of 65.
Gender
- Population pharmacokinetic analyses performed in the clinical study support the lack of gender effect on the pharmacokinetics of oxycodone from oxycodone hydrochloride tablets.
Race
- Population pharmacokinetic analyses support the lack of race effect on oxycodone pharmacokinetics after administration of oxycodone hydrochloride tablets, but these data should be interpreted conservatively, since the majority of patients enrolled into the studies were Caucasians (94%).
Renal Insufficiency
- In a clinical trial supporting the development of oxycodone hydrochloride tablets, too few patients with decreased renal function were evaluated to study these potential differences. In previous studies, patients with renal impairment (defined as a creatinine clearance < 60 mL/min) had concentrations of oxycodone in the plasma that were higher than in subjects with normal renal function. Based on information available on the metabolism and excretion of oxycodone, dose initiation in patients with renal impairment should follow a conservative approach. Dosages should be adjusted according to the clinical situation.
Hepatic Failure
- In a clinical trial supporting the development of oxycodone hydrochloride tablets, too few patients with decreased hepatic function were evaluated to study these potential differences. However, since oxycodone is extensively metabolized, its clearance may decrease in hepatic failure patients. Dose initiation in patients with hepatic impairment should follow a conservative approach. Dosages should be adjusted according to the clinical situation.
Nonclinical Toxicology
There is limited information regarding Oxycodone Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Oxycodone Clinical Studies in the drug label.
How Supplied
- Oxycodone hydrochloride tablets, USP are available as follows:
- Oxycodone hydrochloride tablets, USP 5 mg are supplied as white round biconvex tablets debossed “K” on left and “18” on right of the bisect on one side and plain on the other side.
- NDC 10702-018-01 Bottles of 100 tablets
- NDC 10702-018-50 Bottles of 500 tablets
- Oxycodone hydrochloride tablets, USP 10 mg are supplied as pink round biconvex tablets debossed “K” on left and “56” on right of the bisect on one side and plain on the other side.
- NDC 10702-056-01 Bottles of 100 tablets
- NDC 10702-056-50 Bottles of 500 tablets
- Oxycodone hydrochloride tablets, USP 15 mg are supplied as green round biconvex tablets debossed “K” on left and “8” on right of the bisect on one side and plain on the other side.
- NDC 10702-008-01 Bottles of 100 tablets
- NDC 10702-008-50 Bottles of 500 tablets
- Oxycodone hydrochloride tablets, USP 20 mg are supplied as gray round biconvex tablets debossed “K” on left and “57” on right of the bisect on one side and plain on the other side.
- NDC 10702-057-01 Bottles of 100 tablets
- NDC 10702-057-50 Bottles of 500 tablets
- Oxycodone hydrochloride tablets, USP 30 mg are supplied as blue round biconvex tablets debossed “K” on left and “9” on right of the bisect on one side and plain on the other side.
- NDC 10702-009-01 Bottles of 100 tablets
- NDC 10702-009-50 Bottles of 500 tablets
- DEA Order Form Required
Storage
- Dispense in a tight, light-resistant and child-resistant container as defined in the USP. Protect from moisture.
- Store at 20° to 25°C (68° to 77°F) with excursions permitted between 15° to 30°C (59° to 86°F) [See USP Controlled Room Temperature].
- Manufactured by:
KVK-TECH, INC. 110 Terry Dr. Suite 200 Newtown, PA 18940-1850 Item ID # 6102/03 10/13 Manufacturer’s Code: 10702
Images
Drug Images
{{#ask: Page Name::Oxycodone |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Oxycodone |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Oxycodone Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Oxycodone interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Oxycodone Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Oxycodone Look-Alike Drug Names in the drug label.
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Label Page=Oxycodone |Label Name=Oxycodone label.png
}}
Synonyms / Brand Names: Dihydrohydroxycondeinone, Dihydrohydroxycodeinone, Dihydrone, Oxycodone Hydrochloride, Oxicodona, Oxycodone Hcl, Oxycodonum, oxycodone, PTI-821, Combunox, Dihydroxycodeinone, Dinarkon, Diphydrone, Endocet, Endodan, Endone, Eubine, Eucodal, Eucodalum, Eukodal, Eutagen, Oxanest, Oxicon, Oxicone, Oxikon, Oxycodeinone, Oxycodon, Oxycon, Oxycontin, Pancodine, Percobarb, Roxicodone, Supendol, Tecodin, Tekodin, Thecodine, Thekodin, OxyIR, OxyNorm, Percolone, OxyFAST, Supeudol, Remoxy Street Names/Slangs: Blue, Hillbilly Heroin, Kicker, OC, OX, Oxy, Oxycotton, Poor man’s heroin