Tapentadol
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Vignesh Ponnusamy, M.B.B.S. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Tapentadol is an opioid analgesic that is FDA approved for the {{{indicationType}}} of moderate to severe acute pain in adults. Common adverse reactions include nausea, dizziness, vomiting and somnolence.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Moderate to Severe Acute Pain
- The dose is 50 mg, 75 mg, or 100 mg every 4 to 6 hours depending upon pain intensity.
- On the first day of dosing, the second dose may be administered as soon as one hour after the first dose, if adequate pain relief is not attained with the first dose. Subsequent dosing is 50 mg, 75 mg, or 100 mg every 4 to 6 hours and should be adjusted to maintain adequate analgesia with acceptable tolerability.
- Daily doses greater than 700 mg on the first day of therapy and 600 mg on subsequent days have not been studied and are not recommended.
- NUCYNTA® may be given with or without food.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Tapentadol in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Tapentadol in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Tapentadol in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Tapentadol in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Tapentadol in pediatric patients.
Contraindications
- Patients with significant respiratory depression.
- Patients with acute or severe bronchial asthma or hypercarbia in an unmonitored setting or in the absence of resuscitative equipment.
- Patients with known or suspected paralytic ileus.
- Patients with hypersensitivity (e.g. anaphylaxis, angioedema) to tapentadol or to any other ingredients of the product.
- Patients who are receiving monoamine oxidase (MAO) inhibitors or who have taken them within the last 14 days due to potential additive effects on norepinephrine levels which may result in adverse cardiovascular events.
Warnings
Precautions
- Abuse Potential
- NUCYNTA® contains tapentadol, an opioid agonist and a Schedule II controlled substance. Tapentadol can be abused in a manner similar to other opioid agonists legal or illicit. Opioid agonists are sought by drug abusers and people with addiction disorders and are subject to criminal diversion. Consider these risks when prescribing or dispensing NUCYNTA® in situations where there is concern about increased risks of misuse, abuse, or diversion. Concerns about abuse, addiction, and diversion should not, however, prevent the proper management of pain.
- Assess each patient's risk for opioid abuse or addiction prior to prescribing NUCYNTA®. The risk for opioid abuse is increased in patients with a personal or family history of substance abuse (including drug or alcohol abuse or addiction) or mental illness (e.g., major depression). Patients at increased risk may still be appropriately treated with opioids; however these patients will require intensive monitoring for signs of misuse, abuse, or addiction. Routinely monitor all patients receiving opioids for signs of misuse, abuse, and addiction because these drugs carry a risk for addiction even under appropriate medical use.
- Misuse or abuse of NUCYNTA® by crushing, chewing, snorting or injecting will pose a significant risk that could result in overdose and death.
- Contact local state professional licensing board or state controlled substances authority for information on how to prevent and detect abuse or diversion of this product.
- Life Threatening Respiratory Depression
- Respiratory depression is the chief hazard of opioid agonists, including NUCYNTA®. Respiratory depression, if not immediately recognized and treated, may lead to respiratory arrest and death. Respiratory depression from opioids is manifested by a reduced urge to breathe and a decreased rate of respiration, often associated with a "sighing" pattern of breathing (deep breaths separated by abnormally long pauses). Carbon dioxide (CO2) retention from opioid-induced respiratory depression can exacerbate the sedating effects of opioids. Management of respiratory depression may include close observation, supportive measures, and use of opioid antagonists, depending on the patient's clinical status.
- Instruct patients against use by individuals other than the patient for whom NUCYNTA® was prescribed and to keep NUCYNTA® out of the reach of children, as such inappropriate use may result in fatal respiratory depression.
- Patients with conditions accompanied by hypoxia, hypercarbia or decreased respiratory reserve such as: asthma, chronic obstructive pulmonary disease or cor pulmonale, central nervous system (CNS) depression, or coma may be at increased risk for increased airway resistance and decreased respiratory drive to the point of apnea even with usual therapeutic doses of NUCYNTA®. Consider the use of alternative non-mu-opioid agonist analgesics and use NUCYNTA® only under careful medical supervision at the lowest effective dose in such patients. If respiratory depression occurs, treat the patient for mu-opioid agonist-induced respiratory depression. To reduce the risk of respiratory depression, proper dosing of NUCYNTA® is essential.
- Accidental Exposure
- Accidental ingestion of NUCYNTA®, especially in children, can result in a fatal overdose of tapentadol.
- Interactions with Alcohol, Other Opioids, and Drugs of Abuse
- Due to its mu-opioid agonist activity, NUCYNTA® may be expected to have additive effects when used in conjunction with alcohol, other opioids, or illicit drugs that cause central nervous system depression, respiratory depression, hypotension, and profound sedation, coma or death. Instruct patients not to consume alcoholic beverages or use prescription or non-prescription products containing alcohol, other opioids, or drugs of abuse while on NUCYNTA® therapy.
- Elderly, Cachectic, and Debilitated Patients
- Respiratory depression is more likely to occur in elderly, cachectic, or debilitated patients as they may have altered pharmacokinetics or altered clearance compared to younger, healthier patients. Therefore, closely monitor such patients, particularly when NUCYNTA® is given concomitantly with other drugs that depress respiration.
- Use in Patients with Chronic Pulmonary Disease
- Monitor for respiratory depression those patients with significant chronic obstructive pulmonary disease or cor pulmonale, and patients having a substantially decreased respiratory reserve, hypoxia, hypercarbia, or pre-existing respiratory depression, as in these patients, even usual therapeutic doses of NUCYNTA® may decrease respiratory drive to the point of apnea. Consider the use of alternative non-opioid analgesics in these patients if possible.
- Interactions with CNS Depressants and Illicit Drugs
- Hypotension, and profound sedation, coma or respiratory depression may result if NUCYNTA® is used concomitantly with other CNS depressants (e.g., sedatives, anxiolytics, hypnotics, neuroleptics, muscle relaxants, other opioids and illicit drugs). When considering the use of NUCYNTA® in a patient taking a CNS depressant, assess the duration of use of the CNS depressant and the patient's response, including the degree of tolerance that has developed to CNS depression. Additionally, consider the patient's use, if any, of alcohol and/or illicit drugs that can cause CNS depression. If NUCYNTA® therapy is to be initiated in a patient taking a CNS depressant, start with a lower NUCYNTA® dose than usual and monitor patients for signs of sedation and respiratory depression and consider using a lower dose of the concomitant CNS depressant.
- Hypotensive Effect
- NUCYNTA® may cause severe hypotension. There is an increased risk in patients whose ability to maintain blood pressure has already been compromised by a reduced blood volume or concurrent administration of certain CNS depressant drugs (e.g., phenothiazines or general anesthetics). Monitor these patients for signs of hypotension after the dose of NUCYNTA®. In patients with circulatory shock, NUCYNTA® may cause vasodilation that can further reduce cardiac output and blood pressure. Avoid the use of NUCYNTA® in patients with circulatory shock.
- Use in Patients with Head Injury or Increased Intracranial Pressure
- Monitor patients taking NUCYNTA® who may be susceptible to the intracranial effects of CO2 retention (e.g., those with evidence of increased intracranial pressure or brain tumors) for signs of sedation and respiratory depression. NUCYNTA® may reduce respiratory drive, and the resultant CO2 retention can further increase intracranial pressure. Opioids may also obscure the clinical course in a patient with a head injury.
- Avoid the use of NUCYNTA® in patients with impaired consciousness or coma.
- Seizures
- NUCYNTA® has not been evaluated in patients with a predisposition to a seizure disorder, and such patients were excluded from clinical studies. The active ingredient tapentadol in NUCYNTA® may aggravate convulsions in patients with convulsive disorders, and may induce or aggravate seizures in some clinical settings. Monitor patients with a history of seizure disorders for worsened seizure control during NUCYNTA® therapy.
- Serotonin Syndrome Risk
- Cases of life-threatening serotonin syndrome have been reported with the concurrent use of tapentadol and serotonergic drugs. Serotonergic drugs comprise Selective Serotonin Reuptake Inhibitors (SSRIs), Serotonin and Norepinephrine Reuptake Inhibitors (SNRIs), tricyclic antidepressants (TCAs), triptans, drugs that affect the serotonergic neurotransmitter system (e.g. mirtazapine, trazodone, and tramadol), and drugs that impair metabolism of serotonin (including MAOIs). This may occur within the recommended dose. Serotonin syndrome may include mental-status changes (e.g., agitation, hallucinations, coma), autonomic instability (e.g., tachycardia, labile blood pressure, hyperthermia), neuromuscular aberrations (e.g., hyperreflexia, incoordination) and/or gastrointestinal symptoms (e.g., nausea, vomiting, diarrhea) and can be fatal [see Drug Interactions (7.4)].
- Use in Patients with Gastrointestinal Conditions
- NUCYNTA® is contraindicated in patients with GI obstruction, including paralytic ileus. The tapentadol in NUCYNTA® may cause spasm of the sphincter of Oddi. Monitor patients with biliary tract disease, including acute pancreatitis, for worsening symptoms.
- Withdrawal
- Withdrawal symptoms may occur if NUCYNTA® is discontinued abruptly. These symptoms may include: anxiety, sweating, insomnia, rigors, pain, nausea, tremors, diarrhea, upper respiratory symptoms, piloerection, and rarely, hallucinations. Withdrawal symptoms may be reduced by tapering NUCYNTA®.
- Driving and Operating Heavy Machinery
- NUCYNTA® may impair the mental or physical abilities needed to perform potentially hazardous activities such as driving a car or operating machinery. Warn patients not to drive or operate dangerous machinery unless they are tolerant to the effects of NUCYNTA® and know how they will react to the medication.
- Hepatic Impairment
- A study with NUCYNTA® in subjects with hepatic impairment showed higher serum concentrations of tapentadol than in those with normal hepatic function. Avoid use of NUCYNTA® in patients with severe hepatic impairment. Reduce the dose of NUCYNTA® in patients with moderate hepatic impairment. Closely monitor patients with moderate hepatic impairment for respiratory and central nervous system depression when receiving NUCYNTA®.
- Renal Impairment
- Use of NUCYNTA® in patients with severe renal impairment is not recommended due to accumulation of a metabolite formed by glucuronidation of tapentadol. The clinical relevance of the elevated metabolite is not known.
Adverse Reactions
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
- Based on data from nine Phase 2/3 studies that administered multiple doses (seven placebo- and/or active-controlled, one noncontrolled and one Phase 3 active-controlled safety study) the most common adverse reactions (reported by ≥10% in any NUCYNTA® dose group) were: nausea, dizziness, vomiting and somnolence.
- The most common reasons for discontinuation due to adverse reactions in the studies described above (reported by ≥1% in any NUCYNTA® dose group) were dizziness (2.6% vs. 0.5%), nausea (2.3% vs. 0.6%), vomiting (1.4% vs. 0.2%), somnolence (1.3% vs. 0.2%) and headache (0.9% vs. 0.2%) for NUCYNTA®- and placebo-treated patients, respectively.
- Seventy-six percent of NUCYNTA®-treated patients from the nine studies experienced adverse events.
- NUCYNTA® was studied in multiple-dose, active- or placebo-controlled studies, or noncontrolled studies (n = 2178), in single-dose studies (n = 870), in open-label study extension (n = 483) and in Phase 1 studies (n = 597). Of these, 2034 patients were treated with doses of 50 mg to 100 mg of NUCYNTA® dosed every 4 to 6 hours.
- The data described below reflect exposure to NUCYNTA® in 3161 patients, including 449 exposed for 45 days. NUCYNTA® was studied primarily in placebo- and active-controlled studies (n = 2266, and n = 2944, respectively). The population was 18 to 85 years old (mean age 46 yea), 68% were female, 75% white and 67% were postoperative. Most patients received NUCYNTA® doses of 50 mg, 75 mg, or 100 mg every 4 to 6 hours.
- The following adverse drug reactions occurred in less than 1% of NUCYNTA®-treated patients in the pooled safety data from nine Phase 2/3 clinical studies:
Cardiac disorders
Heart rate increased, heart rate decreased
Eye disorders
Visual disturbance
Gastrointestinal disorders
Abdominal discomfort, impaired gastric emptying
General disorders and administration site conditions
Irritability, edema, drug withdrawal syndrome, feeling drunk
Immune system disorders
Hypersensitivity
Investigations
Gamma-glutamyltransferase increased, alanine aminotransferase increased, aspartate aminotransferase increased
Musculoskeletal and connective tissue disorders
Involuntary muscle contractions, sensation of heaviness
Nervous system disorders
Hypoesthesia, paresthesia, disturbance in attention, sedation, dysarthria, depressed level of consciousness, memory impairment, ataxia, presyncope, syncope, coordination abnormal, seizure
Psychiatric disorders
Euphoric mood, disorientation, restlessness, agitation, nervousness, thinking abnormal
Renal and urinary disorders
Urinary hesitation, pollakiuria
Respiratory, thoracic and mediastinal disorders
Oxygen saturation decreased, cough, dyspnea, respiratory depression
Skin and subcutaneous tissue disorders
Urticaria
Vascular disorders
Blood pressure decreased
- In the pooled safety data, the overall incidence of adverse reactions increased with increased dose of NUCYNTA®, as did the percentage of patients with adverse reactions of nausea, dizziness, vomiting, somnolence, and pruritus.
Postmarketing Experience
- The following additional adverse reactions have been identified during post-approval use of NUCYNTA®. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to estimate their frequency reliably.
Gastrointestinal disorders
Diarrhea
Nervous system disorders
Headache
Psychiatric disorders
Hallucination, suicidal ideation, panic attack
Cardiac disorders
Palpitations
- Anaphylaxis, angioedema, and anaphylactic shock have been reported very rarely with ingredients contained in NUCYNTA®. Advise patients how to recognize such reactions and when to seek medical attention.
Drug Interactions
- Alcohol, Other Opioids, and Drugs of Abuse
- Due to its mu-opioid agonist activity, NUCYNTA® may be expected to have additive effects when used in conjunction with alcohol, other opioids, or illicit drugs that cause central nervous system depression, respiratory depression, hypotension, and profound sedation, coma or death. Instruct patients not to consume alcoholic beverages or use prescription or non-prescription products containing alcohol, other opioids, or drugs of abuse while on NUCYNTA® therapy [see Warnings and Precautions (5.4)].
- Monoamine Oxidase Inhibitors
- NUCYNTA® is contraindicated in patients who are receiving monoamine oxidase (MAO) inhibitors or who have taken them within the last 14 days due to potential additive effects on norepinephrine levels which may result in adverse cardiovascular events [see Contraindications (4)].
- CNS Depressants
- Concurrent use of NUCYNTA® and other central nervous system (CNS) depressants including sedatives or hypnotics, general anesthetics, phenothiazines, tranquilizers, and alcohol can increase the risk of respiratory depression, hypotension, profound sedation or coma. Monitor patients receiving CNS depressants and NUCYNTA® for signs of respiratory depression and hypotension. When such combined therapy is contemplated, start NUCYNTA® at ⅓ to ½ of the usual dosage and consider using a lower dose of the concomitant CNS depressant [see Warnings and Precautions (5.7)].
- Serotonergic Drugs
- There have been post-marketing reports of serotonin syndrome with the concomitant use of tapentadol and serotonergic drugs (e.g., SSRIs and SNRIs). Caution is advised when NUCYNTA® is co-administered with other drugs that may affect serotonergic neurotransmitter systems such as SSRIs, SNRIs, MAOIs, and triptans. If concomitant treatment of NUCYNTA® with a drug affecting the serotonergic neurotransmitter system is clinically warranted, careful observation of the patient is advised [see Warning and Precautions (5.11)].
- Mixed Agonist/Antagonist Opioid Analgesics
- The concomitant use of NUCYNTA® with mixed agonist/antagonists (e.g., butorphanol, nalbuphine, and pentazocine) and partial agonists (e.g., buprenorphine) may precipitate withdrawal symptoms. Avoid the use of agonist/antagonists and partial agonists with NUCYNTA®.
- Anticholinergics
- The use of NUCYNTA® with anticholinergic products may increase the risk of urinary retention and/or severe constipation, which may lead to paralytic ileus.
Use in Specific Populations
Pregnancy
- Pregnancy Category C
- There are no adequate and well-controlled studies of NUCYNTA® in pregnant women. NUCYNTA® should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
- Tapentadol HCl was evaluated for teratogenic effects in pregnant rats and rabbits following intravenous and subcutaneous exposure during the period of embryofetal organogenesis. When tapentadol was administered twice daily by the subcutaneous route in rats at dose levels of 10, 20, or 40 mg/kg/day [producing up to 1 times the plasma exposure at the maximum recommended human dose (MRHD) of 700 mg/day based on an area under the time-curve (AUC) comparison], no teratogenic effects were observed. Evidence of embryofetal toxicity included transient delays in skeletal maturation (i.e. reduced ossification) at the 40 mg/kg/day dose which was associated with significant maternal toxicity. Administration of tapentadol HCl in rabbits at doses of 4, 10, or 24 mg/kg/day by subcutaneous injection [producing 0.2, 0.6, and 1.85 times the plasma exposure at the MRHD based on an AUC comparison] revealed embryofetal toxicity at doses ≥10 mg/kg/day. Findings included reduced fetal viability, skeletal delays and other variations. In addition, there were multiple malformations including gastroschisis/thoracogastroschisis, amelia/phocomelia, and cleft palate at doses ≥10 mg/kg/day and above, and ablepharia, encephalopathy, and spina bifida at the high dose of 24 mg/kg/day. Embryofetal toxicity, including malformations, may be secondary to the significant maternal toxicity observed in the study.
- In a study of pre- and postnatal development in rats, oral administration of tapentadol at doses of 20, 50, 150, or 300 mg/kg/day to pregnant and lactating rats during the late gestation and early postnatal period [resulting in up to 1.7 times the plasma exposure at the MRHD on an AUC basis] did not influence physical or reflex development, the outcome of neurobehavioral tests or reproductive parameters. Treatment-related developmental delay was observed, including incomplete ossification, and significant reductions in pup body weights and body weight gains at doses associated with maternal toxicity (150 mg/kg/day and above). At maternal tapentadol doses ≥150 mg/kg/day, a dose-related increase in pup mortality was observed through postnatal Day 4.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Tapentadol in women who are pregnant.
Labor and Delivery
- NUCYNTA® is not for use in women during and immediately prior to labor. Occasionally, opioid analgesics may prolong labor by temporarily reducing the strength, duration, and frequency of uterine contractions. However, these effects are not consistent and may be offset by an increased rate of cervical dilatation which tends to shorten labor.
- Opioids cross the placenta and may produce respiratory depression and psychophysiologic effects in neonates. Closely observe neonates whose mothers received opioid analgesics during labor for signs of respiratory depression. An opioid antagonist, such as naloxone, should be available for reversal of opioid-induced respiratory depression in the neonate in such situations.
Nursing Mothers
- There is insufficient/limited information on the excretion of tapentadol in human or animal breast milk. Physicochemical and available pharmacodynamic/toxicological data on tapentadol point to excretion in breast milk and risk to the breastfeeding child cannot be excluded.
- Because of the potential for adverse reactions in nursing infants from NUCYNTA®, a decision should be made whether to discontinue nursing or discontinue the drug, taking into account the importance of the drug to the mother.
- Withdrawal symptoms can occur in breast-feeding infants when maternal administration of NUCYNTA® is stopped.
Pediatric Use
- The safety and effectiveness of NUCYNTA® in pediatric patients less than 18 years of age have not been established.
Geriatic Use
- Of the total number of patients in Phase 2/3 double-blind, multiple-dose clinical studies of NUCYNTA®, 19% were 65 and over, while 5% were 75 and over. No overall differences in effectiveness were observed between these patients and younger patients. The rate of constipation was higher in subjects greater than or equal to 65 years than those less than 65 years (12% vs. 7%).
- In general, recommended dosing for elderly patients with normal renal and hepatic function is the same as for younger adult patients with normal renal and hepatic function. Because elderly patients are more likely to have decreased renal and hepatic function, consideration should be given to starting elderly patients with the lower range of recommended doses.
Gender
There is no FDA guidance on the use of Tapentadol with respect to specific gender populations.
Race
There is no FDA guidance on the use of Tapentadol with respect to specific racial populations.
Renal Impairment
- The safety and effectiveness of NUCYNTA® has not been established in patients with severe renal impairment (CLCR <30 mL/min). Use of NUCYNTA® in patients with severe renal impairment is not recommended due to accumulation of a metabolite formed by glucuronidation of tapentadol. The clinical relevance of the elevated metabolite is not known.
Hepatic Impairment
- Administration of tapentadol resulted in higher exposures and serum levels of tapentadol in subjects with impaired hepatic function compared to subjects with normal hepatic function. The dose of NUCYNTA® should be reduced in patients with moderate hepatic impairment (Child-Pugh Score 7 to 9).
- Use of NUCYNTA® is not recommended in patients with severe hepatic impairment (Child-Pugh Score 10 to 15).
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Tapentadol in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Tapentadol in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
Monitoring
There is limited information regarding Monitoring of Tapentadol in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Tapentadol in the drug label.
Overdosage
Acute Overdose
Signs and Symptoms
- Acute overdosage with opioids can be manifested by respiratory depression, somnolence progressing to stupor or coma, skeletal muscle flaccidity, cold and clammy skin, constricted pupils, and sometimes pulmonary edema, bradycardia, hypotension and death. Marked mydriasis rather than miosis may be seen due to severe hypoxia in overdose situations.
Management
- In case of overdose, priorities are the re-establishment of a patent and protected airway and institution of assisted or controlled ventilation if needed. Employ other supportive measures (including oxygen, vasopressors) in the management of circulatory shock and pulmonary edema as indicated. Cardiac arrest or arrhythmias will require advanced life support techniques.
- The opioid antagonists, naloxone or nalmefene, are specific antidotes to respiratory depression resulting from opioid overdose. Opioid antagonists should not be administered in the absence of clinically significant respiratory or circulatory depression secondary to tapentadol overdose. Such agents should be administered cautiously to patients who are known, or suspected to be, physically dependent on NUCYNTA®. In such cases, an abrupt or complete reversal of opioid effects may precipitate an acute withdrawal syndrome.
- Because the duration of reversal would be expected to be less than the duration of action of tapentadol in NUCYNTA®, carefully monitor the patient until spontaneous respiration is reliably re-established. If the response to opioid antagonists is suboptimal or not sustained, additional antagonist should be given as directed in the product's prescribing information.
- In an individual physically dependent on opioids, administration of an opioid receptor antagonist may precipitate an acute withdrawal. The severity of the withdrawal produced will depend on the degree of physical dependence and the dose of the antagonist administered. If a decision is made to treat serious respiratory depression in the physically dependent patient, administration of the antagonist should be begun with care and by titration with smaller than usual doses of the antagonist.
Chronic Overdose
There is limited information regarding Chronic Overdose of Tapentadol in the drug label.
Pharmacology
Mechanism of Action
- Tapentadol is a centrally-acting synthetic analgesic. The exact mechanism of action is unknown. Although the clinical relevance is unclear, preclinical studies have shown that tapentadol is a mu-opioid receptor (MOR) agonist and a norepinephrine reuptake inhibitor (NRI). Analgesia in animal models is derived from both of these properties.
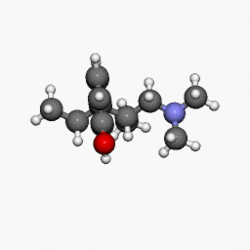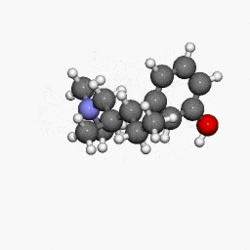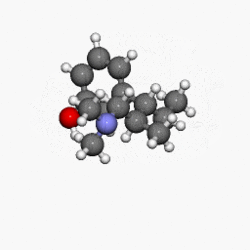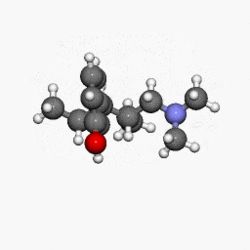
Structure
- NUCYNTA® (tapentadol) is a mu-opioid receptor agonist, supplied in immediate-release film-coated tablets for oral administration, containing 58.24, 87.36 and 116.48 mg of tapentadol hydrochloride in each tablet strength, corresponding to 50, 75, and 100 mg of tapentadol free-base, respectively. The chemical name is 3-[(1R,2R)-3-(dimethylamino)-1-ethyl-2-methylpropyl]phenol monohydrochloride. The structural formula is:
- The molecular weight of tapentadol HCl is 257.80, and the molecular formula is C14H23NO•HCl. The n-octanol:water partition coefficient log P value is 2.87. The pKa values are 9.34 and 10.45.
- In addition to the active ingredient tapentadol HCl, NUCYNTA® tablets also contain the following inactive ingredients: croscarmellose sodium, lactose monohydrate, magnesium stearate, microcrystalline cellulose, povidone. The film coatings for all tablet strengths contain polyvinyl alcohol, titanium dioxide, polyethylene glycol, talc, and the colorant FD&C Yellow #6 aluminum lake; the film coatings for the 50 mg and 75 mg tablets also contain the additional colorant D&C Yellow #10 aluminum lake.
Pharmacodynamics
- Tapentadol is 18 times less potent than morphine in binding to the human mu-opioid receptor and is 2–3 times less potent in producing analgesia in animal models. Tapentadol has been shown to inhibit norepinephrine reuptake in the brains of rats resulting in increased norepinephrine concentrations. In preclinical models, the analgesic activity due to the mu-opioid receptor agonist activity of tapentadol can be antagonized by selective mu-opioid antagonists (e.g., naloxone), whereas the norepinephrine reuptake inhibition is sensitive to norepinephrine modulators. Tapentadol exerts its analgesic effects without a pharmacologically active metabolite.
- Concentration-Efficacy Relationships
- The minimum effective plasma concentration of tapentadol for analgesia varies widely among patients, especially among patients who have been previously treated with agonist opioids.
- Concentration-Adverse Experience Relationships
- There is a general relationship between increasing opioid plasma concentration and increasing frequency of adverse experiences such as nausea, vomiting, CNS effects, and respiratory depression.
- Effects on the Cardiovascular System
- There was no effect of therapeutic and supratherapeutic doses of tapentadol on the QT interval. In a randomized, double-blind, placebo- and positive-controlled crossover study, healthy subjects were administered five consecutive doses of NUCYNTA® 100 mg every 6 hours, NUCYNTA® 150 mg every 6 hours, placebo and a single oral dose of moxifloxacin. Similarly, NUCYNTA® had no relevant effect on other ECG parameters (heart rate, PR interval, QRS duration, T-wave or U-wave morphology).
- Tapentadol produces peripheral vasodilation which may result in orthostatic hypotension.
- Effects on the Central Nervous System (CNS)
- The principal therapeutic action of tapentadol is analgesia. Tapentadol causes respiratory depression, in part by a direct effect on the brainstem respiratory centers. The respiratory depression involves a reduction in the responsiveness of the brain stem respiratory centers to both increases in carbon dioxide tension and electrical stimulation. Tapentadol depresses the cough reflex by direct effect on the cough center in the medulla.
- Tapentadol causes miosis, even in total darkness. Pinpoint pupils are a sign of opioid overdose but are not pathognomonic (e.g., pontine lesions of hemorrhagic or ischemic origin may produce similar findings). Marked mydriasis rather than miosis may be seen with hypoxia in overdose situations [see Overdosage (10)]. Other effects of tapentadol include anxiolysis, euphoria, and feeling of relaxation, drowsiness and changes in mood.
- Effects on the Gastrointestinal Tract and on Other Smooth Muscle
- Gastric, biliary and pancreatic secretions are decreased by tapentadol. Tapentadol causes a reduction in motility and is associated with an increase in tone in the antrum of the stomach and duodenum. Digestion of food in the small intestine is delayed and propulsive contractions are decreased. Propulsive peristaltic waves in the colon are decreased, while tone is increased to the point of spasm. The end result is constipation. Tapentadol can cause a marked increase in biliary tract pressure as a result of spasm of the sphincter of Oddi, and transient elevations in serum amylase. Tapentadol may also cause spasm of the sphincter of the urinary bladder.
- Effects on the Endocrine System
- Opioid agonists have been shown to have a variety of effects on the secretion of hormones. Opioids inhibit the secretion of ACTH, cortisol, and luteinizing hormone (LH) in humans. They also stimulate prolactin, growth hormone (GH) secretion, and pancreatic secretion of insulin and glucagon.
- Effects on the Immune System
- Opioids have been shown to have a variety of effects on components of the immune system in in vitro and animal models. The clinical significance of these findings is unknown.
- CNS Depressant/Alcohol Interaction
- Additive pharmacodynamic effects may be expected when NUCYNTA® is used in conjunction with alcohol, other opioids, or illicit drugs that cause central nervous system depression.
Pharmacokinetics
- Absorption
- The mean absolute bioavailability after single-dose administration (fasting) of NUCYNTA® is approximately 32% due to extensive first-pass metabolism. Maximum serum concentrations of tapentadol are typically observed at around 1.25 hours after dosing.
- Dose-proportional increases in the Cmax and AUC values of tapentadol have been observed over the 50 to 150 mg dose range.
- A multiple (every 6 hour) dose study with doses ranging from 75 to 175 mg tapentadol showed a mean accumulation factor of 1.6 for the parent drug and 1.8 for the major metabolite tapentadol-O-glucuronide, which are primarily determined by the dosing interval and apparent half-life of tapentadol and its metabolite.
- Food Effect
- The AUC and Cmax increased by 25% and 16%, respectively, when NUCYNTA® was administered after a high-fat, high-calorie breakfast. NUCYNTA® may be given with or without food.
- Distribution
- Tapentadol is widely distributed throughout the body. Following intravenous administration, the volume of distribution (Vz) for tapentadol is 540 +/- 98 L. The plasma protein binding is low and amounts to approximately 20%.
- Metabolism and Elimination
- In humans, about 97% of the parent compound is metabolized. Tapentadol is mainly metabolized via Phase 2 pathways, and only a small amount is metabolized by Phase 1 oxidative pathways. The major pathway of tapentadol metabolism is conjugation with glucuronic acid to produce glucuronides. After oral administration approximately 70% (55% O-glucuronide and 15% sulfate of tapentadol) of the dose is excreted in urine in the conjugated form. A total of 3% of drug was excreted in urine as unchanged drug. Tapentadol is additionally metabolized to N-desmethyl tapentadol (13%) by CYP2C9 and CYP2C19 and to hydroxy tapentadol (2%) by CYP2D6, which are further metabolized by conjugation. Therefore, drug metabolism mediated by cytochrome P450 system is of less importance than phase 2 conjugation.
- None of the metabolites contribute to the analgesic activity.
- Tapentadol and its metabolites are excreted almost exclusively (99%) via the kidneys. The terminal half-life is on average 4 hours after oral administration. The total clearance is 1530 +/- 177 mL/min.
- Special Populations
- Geriatric Patients
- The mean exposure (AUC) to tapentadol was similar in elderly subjects compared to young adults, with a 16% lower mean Cmax observed in the elderly subject group compared to young adult subjects.
- Renal Impairment
- AUC and Cmax of tapentadol were comparable in subjects with varying degrees of renal function (from normal to severely impaired). In contrast, increasing exposure (AUC) to tapentadol-O-glucuronide was observed with increasing degree of renal impairment. In subjects with mild (CLCR = 50 to <80 mL/min), moderate (CLCR = 30 to <50 mL/min), and severe (CLCR = <30 mL/min) renal impairment, the AUC of tapentadol-O-glucuronide was 1.5-, 2.5-, and 5.5-fold higher compared with normal renal function, respectively.
- Hepatic Impairment
- Administration of NUCYNTA® resulted in higher exposures and serum levels to tapentadol in subjects with impaired hepatic function compared to subjects with normal hepatic function. The ratio of tapentadol pharmacokinetic parameters for the mild hepatic impairment group (Child-Pugh Score 5 to 6) and moderate hepatic impairment group (Child-Pugh Score 7 to 9) in comparison to the normal hepatic function group were 1.7 and 4.2, respectively, for AUC; 1.4 and 2.5, respectively, for Cmax; and 1.2 and 1.4, respectively, for t1/2. The rate of formation of tapentadol-O-glucuronide was lower in subjects with increased liver impairment.
- Pharmacokinetic Drug Interactions
- Tapentadol is mainly metabolized by Phase 2 glucuronidation, a high capacity/low affinity system; therefore, clinically relevant interactions caused by Phase 2 metabolism are unlikely to occur. Naproxen and probenecid increased the AUC of tapentadol by 17% and 57%, respectively. These changes are not considered clinically relevant and no change in dose is required.
- No changes in the pharmacokinetic parameters of tapentadol were observed when acetaminophen and acetylsalicylic acid were given concomitantly.
- In vitro studies did not reveal any potential of tapentadol to either inhibit or induce cytochrome P450 enzymes. Furthermore, a minor amount of NUCYNTA® is metabolized via the oxidative pathway. Thus, clinically relevant interactions mediated by the cytochrome P450 system are unlikely to occur.
- The pharmacokinetics of tapentadol were not affected when gastric pH or gastrointestinal motility were increased by omeprazole and metoclopramide, respectively.
- Plasma protein binding of tapentadol is low (approximately 20%). Therefore, the likelihood of pharmacokinetic drug-drug interactions by displacement from the protein binding site is low.
Nonclinical Toxicology
- Carcinogenesis
- Tapentadol was administered to rats (diet) and mice (oral gavage) for two years.
- In mice, tapentadol HCl was administered by oral gavage at dosages of 50, 100 and 200 mg/kg/day for 2 years (up to 0.2 times the plasma exposure at the maximum recommended human dose [MRHD] on an area under the time-curve [AUC] basis). No increase in tumor incidence was observed at any dose level.
- In rats, tapentadol HCl was administered in diet at dosages of 10, 50, 125 and 250 mg/kg/day for two years (up to 0.2 times in the male rats and 0.6 times in the female rats the MRHD on an AUC basis). No increase in tumor incidence was observed at any dose level.
- Mutagenesis
- Tapentadol did not induce gene mutations in bacteria, but was clastogenic with metabolic activation in a chromosomal aberration test in V79 cells. The test was repeated and was negative in the presence and absence of metabolic activation. The one positive result for tapentadol was not confirmed in vivo in rats, using the two endpoints of chromosomal aberration and unscheduled DNA synthesis, when tested up to the maximum tolerated dose.
- Impairment of Fertility
- Tapentadol HCl was administered intravenously to male or female rats at dosages of 3, 6, or 12 mg/kg/day (representing exposures of up to approximately 0.4 times the exposure at the MRHD on an AUC basis, based on extrapolation from toxicokinetic analyses in a separate 4-week intravenous study in rats). Tapentadol did not alter fertility at any dose level. Maternal toxicity and adverse effects on embryonic development, including decreased number of implantations, decreased numbers of live conceptuses, and increased pre- and post-implantation losses occurred at dosages ≥6 mg/kg/day.
Clinical Studies
- The efficacy and safety of NUCYNTA® in the treatment of moderate to severe acute pain has been established in two randomized, double-blind, placebo- and active-controlled studies of moderate to severe pain from first metatarsal bunionectomy and end-stage degenerative joint disease.
Orthopedic Surgery – Bunionectomy
- A randomized, double-blind, parallel-group, active- and placebo-controlled, multiple-dose study demonstrated the efficacy of 50 mg, 75 mg, and 100 mg NUCYNTA® given every 4 to 6 hours for 72 hours in patients aged 18 to 80 years experiencing moderate to severe pain following unilateral, first metatarsal bunionectomy surgery. Patients who qualified for the study with a baseline pain score of ≥4 on an 11-point rating scale ranging from 0 to 10 were randomized to 1 of 5 treatments. Patients were allowed to take a second dose of study medication as soon as 1 hour after the first dose on study Day 1, with subsequent dosing every 4 to 6 hours. If rescue analgesics were required, the patients were discontinued for lack of efficacy. Efficacy was evaluated by comparing the sum of pain intensity difference over the first 48 hours (SPID48) versus placebo. NUCYNTA® at each dose provided a greater reduction in pain compared to placebo based on SPID48 values.
- For various degrees of improvement from baseline to the 48-hour endpoint, Figure 1 shows the fraction of patients achieving that level of improvement. The figures are cumulative, such that every patient that achieves a 50% reduction in pain from baseline is included in every level of improvement below 50%. Patients who did not complete the 48-hour observation period in the study were assigned 0% improvement.
- The proportions of patients who showed reduction in pain intensity at 48 hours of 30% or greater, or 50% or greater were significantly higher in patients treated with NUCYNTA® at each dose versus placebo.
End-Stage Degenerative Joint Disease
- A randomized, double-blind, parallel-group, active- and placebo-controlled, multiple-dose study evaluated the efficacy and safety of 50 mg and 75 mg NUCYNTA® given every 4 to 6 hours during waking hours for 10 days in patients aged 18 to 80 years, experiencing moderate to severe pain from end stage degenerative joint disease of the hip or knee, defined as a 3-day mean pain score of ≥5 on an 11-point pain intensity scale, ranging from 0 to 10. Pain scores were assessed twice daily and assessed the pain the patient had experienced over the previous 12 hours. Patients were allowed to continue non-opioid analgesic therapy for which they had been on a stable regimen before screening throughout the study. Eighty-three percent (83%) of patients in the tapentadol treatment groups and the placebo group took such analgesia during the study. The 75 mg treatment group was dosed at 50 mg for the first day of the study, followed by 75 mg for the remaining nine days. Patients requiring rescue analgesics other than study medication were discontinued for lack of efficacy. Efficacy was evaluated by comparing the sum of pain intensity difference (SPID) versus placebo over the first five days of treatment. NUCYNTA® 50 mg and 75 mg provided improvement in pain compared with placebo based on the 5-Day SPID.
- For various degrees of improvement from baseline to the Day 5 endpoint, Figure 2 shows the fraction of patients achieving that level of improvement. The figures are cumulative, such that every patient that achieves a 50% reduction in pain from baseline is included in every level of improvement below 50%. Patients who did not complete the 5-day observation period in the study were assigned 0% improvement.
- The proportions of patients who showed reduction in pain intensity at 5 days of 30% or greater, or 50% or greater were significantly higher in patients treated with NUCYNTA® at each dose versus placebo.
How Supplied
- NUCYNTA® Tablets are available in the following strengths and packages. All tablets are round and biconvex-shaped.
- 50 mg tablets are yellow and debossed with "O-M" on one side and "50" on the other side, and are available in bottles of 100 (NDC 50458-820-04) and hospital unit dose blister packs of 10 (NDC 50458-820-02).
- 75 mg tablets are yellow-orange and debossed with "O-M" on one side and "75" on the other side, and are available in bottles of 100 (NDC 50458-830-04) and hospital unit dose blister packs of 10 (NDC 50458-830-02).
- 100 mg tablets are orange and debossed with "O-M" on one side and "100" on the other side, and are available in bottles of 100 (NDC 50458-840-04) and hospital unit dose blister packs of 10 (NDC 50458-840-02).
- Storage and Handling
- Store up to 25°C (77°F); excursions permitted to 15° – 30°C (59° – 86°F) [see USP Controlled Room Temperature]. Protect from moisture.
- Keep NUCYNTA® in a secure place out of reach of children.
- NUCYNTA® tablets that are no longer needed should be destroyed by flushing down the toilet.
Storage
There is limited information regarding Tapentadol Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Tapentadol |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Tapentadol |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Instruct patients to take NUCYNTA® only as prescribed.
- Abuse Potential
- Inform patients that NUCYNTA® contains tapentadol, a Schedule II controlled substance that is subject to abuse. Instruct patients not to share NUCYNTA® with others and to take steps to protect NUCYNTA® from theft or misuse.
- Life-threatening Respiratory Depression
- Discuss the risk of respiratory depression with patients, explaining that the risk is greatest when starting NUCYNTA® or when the dose is increased. Advise patients how to recognize respiratory depression and to seek medical attention if they are experiencing breathing difficulties.
- Accidental Exposure
- Instruct patients to take steps to store NUCYNTA® securely. Accidental exposure, especially in children, may results in serious harm or death. Advise patients to dispose of unused NUCYNTA® by flushing the tablets down the toilet.
- Important Administration Instructions
- Instruct patients how to properly take NUCYNTA®, including the following:
- Using NUCYNTA® exactly as prescribed to reduce the risk of life-threatening adverse reactions (e.g., respiratory depression).
- Not discontinuing NUCYNTA® without first discussing the need for a tapering regimen with the prescriber.
- Risks from Concomitant Use of Alcohol and other CNS Depressants
- Inform patients that the concomitant use of alcohol with NUCYNTA® can increase the risk of life-threatening respiratory depression. Instruct patients not to consume alcoholic beverages, as well as prescription and over-the-counter drug products that contain alcohol, during treatment with NUCYNTA®.
- Inform patients that potentially serious additive effects may occur if NUCYNTA® is used with other CNS depressants, and not to use such drugs unless supervised by a health care provider.
- Concurrent use of MAOI
- Inform patients not to take NUCYNTA® while using any drugs that inhibit monoamine oxidase. Patients should not start any new medications while taking NUCYNTA®.
- Seizures
- Inform patients that NUCYNTA® could cause seizures if they are at risk for seizures or have epilepsy. Patients should be advised to stop taking NUCYNTA® if they have a seizure while taking NUCYNTA® and call their healthcare provider right away.
- Serotonin Syndrome
- Inform patients that NUCYNTA® could cause a rare but potentially life-threatening condition resulting from concomitant administration of serotonergic drugs (including Serotonin Reuptake Inhibitors, Serotonin and Norepinephrine Reuptake Inhibitors and tricyclic antidepressants). Warn patients of the symptoms of serotonin syndrome and to seek medical attention right away if symptoms develop.
- Instruct patients to inform their physicians if they are taking or plan to take additional medications, including CNS Depressants, MAO inhibitors, mixed agonists/antagonist opioid analgesics, anticholinergics, SSRIs, SNRIs, or tricyclic antidepressants.
- Hypotension
- Inform patients that NUCYNTA® may cause orthostatic hypotension and syncope. Instruct patients how to recognize symptoms of low blood pressure and how to reduce the risk of serious consequences should hypotension occur (e.g., sit or lie down, carefully rise from a sitting or lying position).
- Driving or Operating Heavy Machinery
- Inform patients that NUCYNTA® may impair the ability to perform potentially hazardous activities such as driving a car or operating heavy machinery. Advise patients not to perform such tasks until they know how they will react to the medication.
- Constipation
- Advise patients of the potential for severe constipation, including management instructions and when to seek medical attention.
- Anaphylaxis
- Inform patients that anaphylaxis has been reported with ingredients contained in NUCYNTA®. Advise patients how to recognize such a reaction and when to seek medical attention.
- Pregnancy
- Advise female patients that NUCYNTA® can cause fetal harm and to inform the prescriber if they are pregnant or plan to become pregnant.
Precautions with Alcohol
- Alcohol-Tapentadol interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- NUCYNTA®[3]
Look-Alike Drug Names
There is limited information regarding Tapentadol Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Terlinden, R.; Ossig, J.; Fliegert, F.; Gohler, K. (2006). "Pharmacokinetics, Excretion and Metabolism of Tapentadol HCl, a Novel Centrally Acting Analgesic in Healthy Subjects". Program and Abstracts of the 25th Annual Scientific Meeting of the American Pain Society; May 3–6, 2006 San Antonio, Texas. Poster 689.
- ↑ 2.0 2.1 2.2 2.3 Brayfield, A, ed. (14 November 2011). "Tapentadol". Martindale: The Complete Drug Reference. Pharmaceutical Press. Retrieved 2 April 2014.
- ↑ "NUCYNTA- tapentadol hydrochloride tablet, film coated".
{{#subobject:
|Page Name=Tapentadol |Pill Name=No image.jpg |Drug Name= |Pill Ingred=|+sep=; |Pill Imprint= |Pill Dosage= |Pill Color=|+sep=; |Pill Shape= |Pill Size (mm)= |Pill Scoring= |Pill Image= |Drug Author= |NDC=
}}
{{#subobject:
|Label Page=Tapentadol |Label Name=Tapentadol05.png
}}
{{#subobject:
|Label Page=Tapentadol |Label Name=Tapentadol06.png
}}
{{#subobject:
|Label Page=Tapentadol |Label Name=Tapentadol07.png
}}
{{#subobject:
|Label Page=Tapentadol |Label Name=Tapentadol08.png
}}