Leptin: Difference between revisions
(fixed citation errors and duplicate references) |
(small typo) |
||
| (One intermediate revision by one other user not shown) | |||
| Line 6: | Line 6: | ||
| image = PDB 1ax8 EBI.jpg | | image = PDB 1ax8 EBI.jpg | ||
| width = | | width = | ||
| caption = Structure of the obese protein leptin-E100.<ref name="pmid9144295">{{cite journal | vauthors = Zhang F, Basinski MB, Beals JM, Briggs SL, Churgay LM, Clawson DK, DiMarchi RD, Furman TC, Hale JE, Hsiung HM, Schoner BE, Smith DP, Zhang XY, Wery JP, Schevitz RW | title = Crystal structure of the obese protein leptin-E100 | journal = Nature | volume = 387 | issue = 6629 | pages = | | caption = Structure of the obese protein leptin-E100.<ref name="pmid9144295">{{cite journal | vauthors = Zhang F, Basinski MB, Beals JM, Briggs SL, Churgay LM, Clawson DK, DiMarchi RD, Furman TC, Hale JE, Hsiung HM, Schoner BE, Smith DP, Zhang XY, Wery JP, Schevitz RW | title = Crystal structure of the obese protein leptin-E100 | journal = Nature | volume = 387 | issue = 6629 | pages = 206–09 | date = May 1997 | pmid = 9144295 | doi = 10.1038/387206a0 | bibcode = 1997Natur.387..206Z }}</ref> | ||
| Pfam = PF02024 | | Pfam = PF02024 | ||
| Pfam_clan = CL0053 | | Pfam_clan = CL0053 | ||
| Line 19: | Line 19: | ||
}} | }} | ||
'''Leptin''' (from [[Ancient Greek|Greek]] λεπτός ''leptos'', "thin"), | '''Leptin''' (from [[Ancient Greek|Greek]] λεπτός ''leptos'', "thin"), "the hormone of [[energy expenditure]]",{{efn|Leptin controls the satiety indirectly by saying that we do or not have enough energy on board. Bearing in mind that other hormones such as ghrelin operate in a faster-time scale, it would be misleading to define it as "the satiety hormone".}} is a [[hormone]] predominantly made by [[adipose cells]] that helps to regulate [[Energy homeostasis|energy balance]] by inhibiting [[Hunger (motivational state)|hunger]]. Leptin is opposed by the actions of the hormone [[ghrelin]], the "hunger hormone". Both hormones act on receptors in the [[arcuate nucleus]] of the [[hypothalamus]].<ref name="pmid16932309">{{cite journal | vauthors = Brennan AM, Mantzoros CS | title = Drug Insight: the role of leptin in human physiology and pathophysiology – emerging clinical applications | journal = Nat Clin Pract Endocrinol Metab | volume = 2 | issue = 6 | pages = 318–27 | year = 2006 | pmid = 16932309 | doi = 10.1038/ncpendmet0196 }}</ref> In obesity, a decreased sensitivity to leptin occurs (similar to [[insulin]] resistance in [[type 2 diabetes]]), resulting in an inability to detect satiety despite high energy stores and high levels of leptin.<ref>{{cite journal | vauthors = Pan H, Guo J, Su Z | title = Advances in understanding the interrelations between leptin resistance and obesity | journal = Physiology & Behavior | volume = 130 | pages = 157–69 | date = May 2014 | pmid = 24726399 | doi = 10.1016/j.physbeh.2014.04.003 }}</ref> | ||
Although regulation of fat stores is deemed to be the | Although regulation of fat stores is deemed to be the primary function of leptin, it also plays a role in other physiological processes, as evidenced by its many sites of synthesis other than fat cells, and the many cell types beside hypothalamic cells that have leptin receptors. Many of these additional functions are yet to be defined.<ref name="pmid7624777" /><ref name="pmid7624778" /><ref name="pmid7624776" /><ref name="pmid7584987" /><ref name="pmid7769141">{{cite journal | vauthors = Considine RV, Considine EL, Williams CJ, Nyce MR, Magosin SA, Bauer TL, Rosato EL, Colberg J, Caro JF | title = Evidence against either a premature stop codon or the absence of obese gene mRNA in human obesity | journal = J. Clin. Invest. | volume = 95 | issue = 6 | pages = 2986–88 | date = June 1995 | pmid = 7769141 | pmc = 295988 | doi = 10.1172/JCI118007 }}</ref><ref name="pmid8532024">{{cite journal | vauthors = Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, Ohannesian JP, Marco CC, McKee LJ, Bauer TL | title = Serum immunoreactive-leptin concentrations in normal-weight and obese humans | journal = N. Engl. J. Med. | volume = 334 | issue = 5 | pages = 292–95 | year = 1996 | pmid = 8532024 | doi = 10.1056/NEJM199602013340503 }}</ref> | ||
== Identification of the gene == | == Identification of the encoding gene == | ||
In 1949, a non-obese mouse colony being studied at the [[Jackson Laboratory]] produced a strain of obese offspring, suggesting that a mutation had occurred in a hormone regulating hunger and energy expenditure. Mice homozygous for the so-called ob mutation (ob/ob) ate voraciously and were massively obese.<ref>{{cite journal | vauthors = Dickie MM, Lane PW | journal = Mouse News Lett. | title = Plus letter to Roy Robinson 7/7/70 | year = 1957 | issue = 17 | page = 52}}</ref> In the 1960s, a second mutation causing obesity and a similar phenotype was identified by [[Douglas L. Coleman|Douglas Coleman]], also at the Jackson Laboratory, and was named diabetes (db), as both ob/ob and db/db were obese.<ref name="pmid7906968">{{cite journal | vauthors = Bahary N, Siegel DA, Walsh J, Zhang Y, Leopold L, Leibel R, Proenca R, Friedman JM | title = Microdissection of proximal mouse chromosome 6: identification of RFLPs tightly linked to the ob mutation | journal = Mamm. Genome | volume = 4 | issue = 9 | pages = | In 1949, a non-obese mouse colony being studied at the [[Jackson Laboratory]] produced a strain of obese offspring, suggesting that a mutation had occurred in a hormone regulating hunger and energy expenditure. Mice homozygous for the so-called ob mutation (ob/ob) ate voraciously and were massively obese.<ref>{{cite journal | vauthors = Dickie MM, Lane PW | journal = Mouse News Lett. | title = Plus letter to Roy Robinson 7/7/70 | year = 1957 | issue = 17 | page = 52}}</ref> In the 1960s, a second mutation causing obesity and a similar phenotype was identified by [[Douglas L. Coleman|Douglas Coleman]], also at the Jackson Laboratory, and was named diabetes (db), as both ob/ob and db/db were obese.<ref name="pmid7906968">{{cite journal | vauthors = Bahary N, Siegel DA, Walsh J, Zhang Y, Leopold L, Leibel R, Proenca R, Friedman JM | title = Microdissection of proximal mouse chromosome 6: identification of RFLPs tightly linked to the ob mutation | journal = Mamm. Genome | volume = 4 | issue = 9 | pages = 511–15 | date = September 1993 | pmid = 7906968 | doi = 10.1007/BF00364786 }}</ref><ref name="pmid1686014">{{cite journal | vauthors = Friedman JM, Leibel RL, Siegel DS, Walsh J, Bahary N | title = Molecular mapping of the mouse ob mutation | journal = Genomics | volume = 11 | issue = 4 | pages = 1054–62 | date = December 1991 | pmid = 1686014 | doi = 10.1016/0888-7543(91)90032-A }}</ref><ref name="pmid7984236">{{cite journal | vauthors = Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM | title = Positional cloning of the mouse obese gene and its human homologue | journal = Nature | volume = 372 | issue = 6505 | pages = 425–32 | date = December 1994 | pmid = 7984236 | doi = 10.1038/372425a0 | bibcode = 1994Natur.372..425Z }}</ref> In 1990 [[Rudolph Leibel]] and [[Jeffrey M. Friedman]] reported mapping of the ''db'' gene.<ref name="pmid1973864">{{cite journal | vauthors = Leibel RL, Bahary N, Friedman JM | title = Genetic variation and nutrition in obesity: approaches to the molecular genetics of obesity | journal = World Rev Nutr Diet. | volume = 63 | issue = 1 | pages = 90–101 | date = January 1990 | pmid = 1973864 }}</ref><ref name="pmid1978328">{{cite journal | vauthors = Bahary N, Leibel RL, Joseph L, Friedman JM | title = Molecular mapping of the mouse db mutation | journal = Proc Natl Acad Sci USA | volume = 87 | issue = 21 | pages = 8642–46 | date = November 1990 | pmid = 1978328 | pmc = 55013 | doi = 10.1073/pnas.87.21.8642 | bibcode = 1990PNAS...87.8642B }}</ref><ref name="pmid8357496">{{cite journal | vauthors = Leibel RL, Bahary N, Friedman JM | title = Strategies for the molecular genetic analysis of obesity in humans | journal = Crit Rev Food Sci Nutr | volume = 33 | issue = 4–5 | pages = 351–58 | date = January 1993 | pmid = 8357496 | doi = 10.1080/10408399309527632 }}</ref> | ||
Consistent with Coleman’s and Leibel's hypothesis, several subsequent studies from Leibel's and Friedman’s labs and other groups confirmed that the ob gene encoded a novel hormone that circulated in blood and that could suppress food intake and body weight in ob and wild type mice, but not in db mice.<ref name="pmid7624777">{{cite journal | vauthors = Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D, Lallone RL, Burley SK, Friedman JM | title = Weight-reducing effects of the plasma protein encoded by the obese gene | journal = Science | volume = 269 | issue = 5223 | pages = | Consistent with Coleman’s and Leibel's hypothesis, several subsequent studies from Leibel's and Friedman’s labs and other groups confirmed that the ob gene encoded a novel hormone that circulated in blood and that could suppress food intake and body weight in ob and wild type mice, but not in db mice.<ref name="pmid7624777">{{cite journal | vauthors = Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D, Lallone RL, Burley SK, Friedman JM | title = Weight-reducing effects of the plasma protein encoded by the obese gene | journal = Science | volume = 269 | issue = 5223 | pages = 543–46 | date = July 1995 | pmid = 7624777 | doi = 10.1126/science.7624777 | bibcode = 1995Sci...269..543H }}</ref><ref name="pmid7624778">{{cite journal | vauthors = Campfield LA, Smith FJ, Guisez Y, Devos R, Burn P | title = Recombinant mouse OB protein: evidence for a peripheral signal linking adiposity and central neural networks | journal = Science | volume = 269 | issue = 5223 | pages = 546–49 | date = July 1995 | pmid = 7624778 | doi = 10.1126/science.7624778 | bibcode = 1995Sci...269..546C }}</ref><ref name="pmid7624776">{{cite journal | vauthors = Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T, Collins F | title = Effects of the obese gene product on body weight regulation in ob/ob mice | journal = Science | volume = 269 | issue = 5223 | pages = 540–43 | date = July 1995 | pmid = 7624776 | doi = 10.1126/science.7624776 | bibcode = 1995Sci...269..540P }}</ref><ref name="pmid7584987">{{cite journal | vauthors = Maffei M, Halaas J, Ravussin E, Pratley RE, Lee GH, Zhang Y, Fei H, Kim S, Lallone R, Ranganathan S | title = Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight-reduced subjects | journal = Nat. Med. | volume = 1 | issue = 11 | pages = 1155–61 | date = November 1995 | pmid = 7584987 | doi = 10.1038/nm1195-1155 }}</ref> | ||
In 1994, Friedman's laboratory reported the identification of the gene.<ref name="pmid7984236"/> In 1995, [[Jose F. Caro]]'s laboratory provided evidence that the mutations in the mouse ob gene did not occur in humans. Furthermore, since ob gene expression was increased, not decreased, in human obesity, it suggested resistance to leptin to be a possibility.<ref name="pmid7769141" /> At the suggestion of [[Roger Guillemin]], Friedman named this new hormone "leptin" from the Greek ''lepto'' meaning thin.<ref name="pmid7624777" /><ref name=Neill_2010>{{cite journal | vauthors = Neill US | title = Leaping for leptin: the 2010 Albert Lasker Basic Medical Research Award goes to Douglas Coleman and Jeffrey M. Friedman | journal = Journal of Clinical Investigation | date = 1 October 2010 | volume = 120 | issue = 10 | pages = | In 1994, Friedman's laboratory reported the identification of the gene.<ref name="pmid7984236"/> In 1995, [[Jose F. Caro]]'s laboratory provided evidence that the mutations in the mouse ob gene did not occur in humans. Furthermore, since ob gene expression was increased, not decreased, in human obesity, it suggested resistance to leptin to be a possibility.<ref name="pmid7769141" /> At the suggestion of [[Roger Guillemin]], Friedman named this new hormone "leptin" from the Greek ''lepto'' meaning thin.<ref name="pmid7624777" /><ref name=Neill_2010>{{cite journal | vauthors = Neill US | title = Leaping for leptin: the 2010 Albert Lasker Basic Medical Research Award goes to Douglas Coleman and Jeffrey M. Friedman | journal = Journal of Clinical Investigation | date = 1 October 2010 | volume = 120 | issue = 10 | pages = 3413–18 | doi = 10.1172/JCI45094 | pmc = 2947251 }}</ref> Leptin was the first fat cell-derived hormone ([[adipokine]]) to be discovered.<ref name="pmid22038756">{{cite journal |vauthors=Conde J, Scotece M, Gómez R, López V, Gómez-Reino JJ, Lago F, Gualillo O | title=Adipokines: Biofactors from white adipose tissue. A complex hub among inflammation, metabolism, and immunity | journal= BioFactors | volume=37 | issue=6 | year=2011 | pages=413–20 | doi=10.1002/biof.185 | pmid=22038756}}</ref> | ||
Subsequent studies in 1995 confirmed that the db gene encodes the leptin receptor, and that it is expressed in the [[hypothalamus]], a region of the brain known to regulate the sensation of hunger and body weight.<ref name="pmid8548812">{{cite journal | vauthors = Tartaglia LA, Dembski M, Weng X, Deng N, Culpepper J, Devos R, Richards GJ, Campfield LA, Clark FT, Deeds J, Muir C, Sanker S, Moriarty A, Moore KJ, Smutko JS, Mays GG, Wool EA, Monroe CA, Tepper RI | title = Identification and expression cloning of a leptin receptor, OB-R | journal = Cell | volume = 83 | issue = 7 | pages = 1263–71 | date = December 1995 | pmid = 8548812 | doi = 10.1016/0092-8674(95)90151-5 }}</ref><ref name="pmid8608603">{{cite journal | vauthors = Chen H, Charlat O, Tartaglia LA, Woolf EA, Weng X, Ellis SJ, Lakey ND, Culpepper J, Moore KJ, Breitbart RE, Duyk GM, Tepper RI, Morgenstern JP | title = Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db/db mice | journal = Cell | volume = 84 | issue = 3 | pages = | Subsequent studies in 1995 confirmed that the db gene encodes the [[leptin receptor]], and that it is expressed in the [[hypothalamus]], a region of the brain known to regulate the sensation of hunger and body weight.<ref name="pmid8548812">{{cite journal | vauthors = Tartaglia LA, Dembski M, Weng X, Deng N, Culpepper J, Devos R, Richards GJ, Campfield LA, Clark FT, Deeds J, Muir C, Sanker S, Moriarty A, Moore KJ, Smutko JS, Mays GG, Wool EA, Monroe CA, Tepper RI | title = Identification and expression cloning of a leptin receptor, OB-R | journal = Cell | volume = 83 | issue = 7 | pages = 1263–71 | date = December 1995 | pmid = 8548812 | doi = 10.1016/0092-8674(95)90151-5 }}</ref><ref name="pmid8608603">{{cite journal | vauthors = Chen H, Charlat O, Tartaglia LA, Woolf EA, Weng X, Ellis SJ, Lakey ND, Culpepper J, Moore KJ, Breitbart RE, Duyk GM, Tepper RI, Morgenstern JP | title = Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db/db mice | journal = Cell | volume = 84 | issue = 3 | pages = 491–95 | date = February 1996 | pmid = 8608603 | doi = 10.1016/S0092-8674(00)81294-5 }}</ref><ref name="pmid8628397">{{cite journal | vauthors = Lee GH, Proenca R, Montez JM, Carroll KM, Darvishzadeh JG, Lee JI, Friedman JM | title = Abnormal splicing of the leptin receptor in diabetic mice | journal = Nature | volume = 379 | issue = 6566 | pages = 632–65 | date = February 1996 | pmid = 8628397 | doi = 10.1038/379632a0 | bibcode = 1996Natur.379..632L }}</ref><ref name="pmid8584938">{{cite journal | vauthors = Chua SC, Chung WK, Wu-Peng XS, Zhang Y, Liu SM, Tartaglia L, Leibel RL | title = Phenotypes of mouse diabetes and rat fatty due to mutations in the OB (leptin) receptor | journal = Science | volume = 271 | issue = 5251 | pages = 994–96 | date = February 1996 | pmid = 8584938 | doi = 10.1126/science.271.5251.994 | bibcode = 1996Sci...271..994C }}</ref> | ||
== Recognition of scientific advances == | == Recognition of scientific advances == | ||
Coleman and Friedman have been awarded numerous prizes acknowledging their roles in discovery of leptin, including the [[Gairdner Foundation International Award]] (2005),<ref>{{cite web | author = Bonner J | year = 2005 | title = Jeffrey Friedman, discoverer of leptin, receives Gairdner, Passano awards| url = http://newswire.rockefeller.edu/2005/04/13/jeffrey-friedman-discoverer-of-leptin-receives-gairdner-passano-awards/ | work = Newswire | publisher = The Rockefeller University }}</ref> the [[Shaw Prize]] (2009),<ref>{{cite web | website = News-Medical.net | year = 2009 | title = Jeffrey Friedman receives Shaw Prize for discovery of leptin | url = http://www.news-medical.net/news/20090616/Jeffrey-Friedman-receives-Shaw-Prize-for-discovery-of-leptin.aspx}}</ref> the [[Lasker Award]],<ref name="urlThe Lasker Foundation - 2010 Awards">{{cite web | url = http://www.laskerfoundation.org/awards/2010_b_description.htm | title = The Lasker Foundation – 2010 Awards | year = 2010 | work = | publisher = Lasker Foundation }}</ref> the [[BBVA Foundation Frontiers of Knowledge Award]]<ref name="urlBBVA Foundation Frontiers of Knowledge Awards">{{cite web | url = http://www.fbbva.es/TLFU/tlfu/ing/microsites/premios/fronteras/galardonados/2012/biomedicina.jsp | title = BBVA Foundation Frontiers of Knowledge Awards | year = 2012 | work = | publisher = BBVA Foundation }}</ref> and the [[King Faisal International Prize]],<ref name="urlKFF - KFIP - Winners 2013 - Medicine">{{cite web | url = http://www.kff.com/en01/kfip/1434H2013G/KFIPWinners4MED1434H2013G.html | title = KFF – KFIP – Winners 2013 – Medicine | year = 2013 | work = | publisher = King Faisal Foundation }}</ref> Leibel has not received the same level of recognition from the discovery because he was omitted as a co-author of a scientific paper published by Friedman that reported the discovery of the gene. The various theories surrounding Friedman’s omission of Leibel and others as co-authors of this paper have been presented in a number of publications, including [[Ellen Ruppel Shell]]’s 2002 book ''[[The Hungry Gene]]''.<ref name="Shell2002">{{cite book |author = Shell E | title = The Hungry Gene: The Inside Story of the Obesity Industry | publisher = Atlantic Monthly Press | date = January 1, 2002 | chapter =On the Cutting Edge | isbn = 978-1-4223-5243-4 }}{{page needed| date = November 2013}}</ref><ref name="Shell2002a">{{cite book |author = Shell E | title = The Hungry Gene: The Inside Story of the Obesity Industry | publisher = Atlantic Monthly Press | year = 2002 | chapter = Hunger | isbn = 978-1-4223-5243-4}} {{page needed| date = November 2013}}</ref> | Coleman and Friedman have been awarded numerous prizes acknowledging their roles in discovery of leptin, including the [[Gairdner Foundation International Award]] (2005),<ref>{{cite web | author = Bonner J | year = 2005 | title = Jeffrey Friedman, discoverer of leptin, receives Gairdner, Passano awards| url = http://newswire.rockefeller.edu/2005/04/13/jeffrey-friedman-discoverer-of-leptin-receives-gairdner-passano-awards/ | work = Newswire | publisher = The Rockefeller University }}</ref> the [[Shaw Prize]] (2009),<ref>{{cite web | website = News-Medical.net | year = 2009 | title = Jeffrey Friedman receives Shaw Prize for discovery of leptin | url = http://www.news-medical.net/news/20090616/Jeffrey-Friedman-receives-Shaw-Prize-for-discovery-of-leptin.aspx}}</ref> the [[Lasker Award]],<ref name="urlThe Lasker Foundation - 2010 Awards">{{cite web | url = http://www.laskerfoundation.org/awards/2010_b_description.htm | title = The Lasker Foundation – 2010 Awards | year = 2010 | work = | publisher = Lasker Foundation }}</ref> the [[BBVA Foundation Frontiers of Knowledge Award]]<ref name="urlBBVA Foundation Frontiers of Knowledge Awards">{{cite web | url = http://www.fbbva.es/TLFU/tlfu/ing/microsites/premios/fronteras/galardonados/2012/biomedicina.jsp | title = BBVA Foundation Frontiers of Knowledge Awards | year = 2012 | work = | publisher = BBVA Foundation }}</ref> and the [[King Faisal International Prize]],<ref name="urlKFF - KFIP - Winners 2013 - Medicine">{{cite web | url = http://www.kff.com/en01/kfip/1434H2013G/KFIPWinners4MED1434H2013G.html | title = KFF – KFIP – Winners 2013 – Medicine | year = 2013 | work = | publisher = King Faisal Foundation }}</ref> Leibel has not received the same level of recognition from the discovery because he was omitted as a co-author of a scientific paper published by Friedman that reported the discovery of the gene. The various theories surrounding Friedman’s omission of Leibel and others as co-authors of this paper have been presented in a number of publications, including [[Ellen Ruppel Shell]]’s 2002 book ''[[The Hungry Gene]]''.<ref name="Shell2002">{{cite book |author = Shell E | title = The Hungry Gene: The Inside Story of the Obesity Industry | publisher = Atlantic Monthly Press | date = January 1, 2002 | chapter =On the Cutting Edge | isbn = 978-1-4223-5243-4 }}{{page needed| date = November 2013}}</ref><ref name="Shell2002a">{{cite book |author = Shell E | title = The Hungry Gene: The Inside Story of the Obesity Industry | publisher = Atlantic Monthly Press | year = 2002 | chapter = Hunger | isbn = 978-1-4223-5243-4}} {{page needed| date = November 2013}}</ref> | ||
The discovery of leptin also is documented in a series of books including ''Fat: Fighting the Obesity Epidemic'' by Robert Pool,<ref>{{Cite book | author = Pool R | title = Fat: fighting the obesity epidemic | year = 2001 | publisher = Oxford University Press | location = New York | isbn = 978-0-19-511853-7 | pages = }} {{page needed| date = November 2013}}</ref> ''[[The Hungry Gene]]'' by Ellen Ruppel Shell, and ''Rethinking Thin: The New Science of Weight Loss and the Myths and Realities of Dieting'' by [[Gina Kolata]].<ref>{{cite book | author = Kolata GB | title = Rethinking thin: the new science of weight | The discovery of leptin also is documented in a series of books including ''Fat: Fighting the Obesity Epidemic'' by Robert Pool,<ref>{{Cite book | author = Pool R | title = Fat: fighting the obesity epidemic | year = 2001 | publisher = Oxford University Press | location = New York | isbn = 978-0-19-511853-7 | pages = }} {{page needed| date = November 2013}}</ref> ''[[The Hungry Gene]]'' by Ellen Ruppel Shell, and ''Rethinking Thin: The New Science of Weight Loss and the Myths and Realities of Dieting'' by [[Gina Kolata]].<ref>{{cite book | author = Kolata GB | title = Rethinking thin: the new science of weight loss – and the myths and realities of dietin | year = 2007 | publisher = Farrar | location = New York | isbn = 978-0-374-10398-9 | pages = }} {{page needed| date = November 2013}}</ref><ref>{{cite book |vauthors=Castracane VD, Henson MC | chapter = The Obese (ob/ob) Mouse and the Discovery of Leptin | chapterurl = https://books.google.com/books?id=nQNSJ6XvL6AC&pg=PA1 | pages = 1–9 | doi = 10.1007/978-0-387-31416-7_1 | year = 2006 |veditors=Castracane VD, Henson MC | title = Leptin | series = Endocrine Updates | volume = 25 | isbn = 978-0-387-31415-0 }}</ref> ''Fat: Fighting the Obesity Epidemic'' and ''Rethinking Thin: The New Science of Weight Loss and the Myths and Realities of Dieting'' review the work in the Friedman laboratory that led to the cloning of the ob gene, while The ''Hungry Gene'' draws attention to the contributions of Leibel.{{citation needed|date=August 2016}} | ||
== Location of gene and structure of hormone == | == Location of gene and structure of hormone == | ||
| Line 41: | Line 41: | ||
==Mutations == | ==Mutations == | ||
A human mutant leptin was first described in 1997,<ref name="pmid9202122">{{cite journal | vauthors = Montague CT, Farooqi IS, Whitehead JP, Soos MA, Rau H, Wareham NJ, Sewter CP, Digby JE, Mohammed SN, Hurst JA, Cheetham CH, Earley AR, Barnett AH, Prins JB, O'Rahilly S | title = Congenital leptin deficiency is associated with severe early-onset obesity in humans | journal = Nature | volume = 387 | issue = 6636 | pages = | A human mutant leptin was first described in 1997,<ref name="pmid9202122">{{cite journal | vauthors = Montague CT, Farooqi IS, Whitehead JP, Soos MA, Rau H, Wareham NJ, Sewter CP, Digby JE, Mohammed SN, Hurst JA, Cheetham CH, Earley AR, Barnett AH, Prins JB, O'Rahilly S | title = Congenital leptin deficiency is associated with severe early-onset obesity in humans | journal = Nature | volume = 387 | issue = 6636 | pages = 903–08 | year = 1997 | pmid = 9202122 | doi = 10.1038/43185 }}</ref> and subsequently six additional mutations were described. All of those affected were from Eastern countries; and all had variants of leptin not detected by the standard immunoreactive technique, so leptin levels were low or undetectable. The most recently described eighth mutation reported in January 2015, in a child with Turkish parents, is unique in that it ''is'' detected by the standard immunoreactive technique, where leptin levels are elevated; but the leptin does not turn on the leptin receptor, hence the patient has functional leptin deficiency.<ref name="Wabitsch_2015">{{cite journal | vauthors = Wabitsch M, Funcke JB, Lennerz B, Kuhnle-Krahl U, Lahr G, Debatin KM, Vatter P, Gierschik P, Moepps B, Fischer-Posovszky P | title = Biologically Inactive Leptin and Early-Onset Extreme Obesity | journal = N. Engl. J. Med. | volume = 372 | issue = 1 | pages = 48–54 | pmid = 25551525 | doi = 10.1056/NEJMoa1406653 | date=Jan 2015}}</ref> These eight mutations all cause extreme obesity in infancy, with [[hyperphagia]].<ref name="Wabitsch_2015"/> | ||
===Nonsense=== | ===Nonsense=== | ||
| Line 52: | Line 52: | ||
A Human Genome Equivalent (HuGE) review in 2004 looked at studies of the connection between genetic mutations affecting leptin regulation and obesity. They reviewed a common polymorphism in the leptin gene (A19G; frequency 0.46), three mutations in the [[leptin receptor]] gene (Q223R, K109R and K656N) and two mutations in the ''[[PPARG]]'' gene (P12A and C161T). They found no association between any of the polymorphisms and obesity.<ref name="pmid15972940">{{cite journal | vauthors = Paracchini V, Pedotti P, Taioli E | title = Genetics of leptin and obesity: a HuGE review | journal = Am. J. Epidemiol. | volume = 162 | issue = 2 | pages = 101–14 | year = 2005 | pmid = 15972940 | doi = 10.1093/aje/kwi174 }}</ref> | A Human Genome Equivalent (HuGE) review in 2004 looked at studies of the connection between genetic mutations affecting leptin regulation and obesity. They reviewed a common polymorphism in the leptin gene (A19G; frequency 0.46), three mutations in the [[leptin receptor]] gene (Q223R, K109R and K656N) and two mutations in the ''[[PPARG]]'' gene (P12A and C161T). They found no association between any of the polymorphisms and obesity.<ref name="pmid15972940">{{cite journal | vauthors = Paracchini V, Pedotti P, Taioli E | title = Genetics of leptin and obesity: a HuGE review | journal = Am. J. Epidemiol. | volume = 162 | issue = 2 | pages = 101–14 | year = 2005 | pmid = 15972940 | doi = 10.1093/aje/kwi174 }}</ref> | ||
A 2006 study found a link between the common LEP- | A 2006 study found a link between the common LEP-2548 G/A genotype and morbid obesity in [[Taiwanese aborigines]],<ref name="pmid16571841">{{cite journal | vauthors = Wang TN, Huang MC, Chang WT, Ko AM, Tsai EM, Liu CS, Lee CH, Ko YC | title = G-2548A polymorphism of the leptin gene is correlated with extreme obesity in Taiwanese aborigines | journal = Obesity (Silver Spring) | volume = 14 | issue = 2 | pages = 183–87 | doi=10.1038/oby.2006.23 | pmid=16571841 | date=February 2006}}</ref><ref name="pmid24564125">{{cite journal | vauthors = Zhang L, Lu M, Yuan L, Lai W, Wang Y | title = [Association of leptin gene-2548 G/A polymorphism with obesity: a meta-analysis] | language = Chinese | journal = Wei Sheng Yan Jiu | volume = 43 | issue = 1 | pages = 128–32 | year = 2014 | pmid = 24564125 | doi = }}</ref> but a 2014 meta-analysis did not,<ref name="pmid24564125">{{cite journal | vauthors = Zhang L, Lu M, Yuan L, Lai W, Wang Y | title = [Association of leptin gene-2548 G/A polymorphism with obesity: a meta-analysis] | language = Chinese | journal = Wei Sheng Yan Jiu | volume = 43 | issue = 1 | pages = 128–32 | year = 2014 | pmid = 24564125 | doi = }}</ref> however, this polymorphism has been associated with weight gain in patients taking antipsychotics.<ref name="pmid15864111">{{cite journal | title=Polymorphisms of the 5-HT2C receptor and leptin genes are associated with antipsychotic drug-induced weight gain in Caucasian subjects with a first-episode psychosis. |vauthors=Templeman LA, Reynolds GP, Arranz B, San L | journal=Pharmacogenet Genomics. |date=April 2005 | volume=15 | issue=4 | pages=195–200 | PMID=15864111 | doi=10.1097/01213011-200504000-00002}}</ref><ref name="pmid17804136">{{cite journal | vauthors = Kang SG, Lee HJ, Park YM, Choi JE, Han C, Kim YK, Kim SH, Lee MS, Joe SH, Jung IK, Kim L | title = Possible association between the -2548A/G polymorphism of the leptin gene and olanzapine-induced weight gain | journal = Prog. Neuropsychopharmacol. Biol. Psychiatry | volume = 32 | issue = 1 | pages = 160–63 | year = 2008 | pmid = 17804136 | doi = 10.1016/j.pnpbp.2007.08.002 }}</ref><ref name="pmid21937795">{{cite journal | vauthors = Wu R, Zhao J, Shao P, Ou J, Chang M | title = Genetic predictors of antipsychotic-induced weight gain: a case-matched multi-gene study | journal = Zhong Nan Da Xue Xue Bao Yi Xue Ban | volume = 36 | issue = 8 | pages = 720–73 | year = 2011 | pmid = 21937795 | doi = 10.3969/j.issn.1672-7347.2011.08.003 }}</ref> | ||
The LEP-2548 G/A polymorphism has been linked with an increased risk of prostate cancer,<ref name="pmid15042602">{{cite journal | vauthors = Ribeiro R, Vasconcelos A, Costa S, Pinto D, Morais A, Oliveira J, Lobo F, Lopes C, Medeiros R | title = Overexpressing leptin genetic polymorphism ( | The LEP-2548 G/A polymorphism has been linked with an increased risk of prostate cancer,<ref name="pmid15042602">{{cite journal | vauthors = Ribeiro R, Vasconcelos A, Costa S, Pinto D, Morais A, Oliveira J, Lobo F, Lopes C, Medeiros R | title = Overexpressing leptin genetic polymorphism (−2548 G/A) is associated with susceptibility to prostate cancer and risk of advanced disease | journal = Prostate | volume = 59 | issue = 3 | pages = 268–74 | year = 2004 | pmid = 15042602 | doi = 10.1002/pros.20004 }}</ref> gestational diabetes,<ref name="pmid18850205">{{cite journal | vauthors = Vaskú JA, Vaskú A, Dostálová Z, Bienert P | title = Association of leptin genetic polymorphism -2548 G/A with gestational diabetes mellitus | journal = Genes Nutr | volume = 1 | issue = 2 | pages = 117–23 | year = 2006 | pmid = 18850205 | pmc = 3454683 | doi = 10.1007/BF02829953 }}</ref> and osteoporosis.<ref name="pmid23460508">{{cite journal | vauthors = Ye XL, Lu CF | title = Association of polymorphisms in the leptin and leptin receptor genes with inflammatory mediators in patients with osteoporosis. | journal = Endocrine. | volume = 44 | issue = 2 | pages = 481–88 | date = Oct 2013 | pmid = 23460508 | doi = 10.1007/s12020-013-9899-9}}</ref> | ||
Other rare polymorphisms have been found but their association with obesity are not consistent.<ref name="pmid15972940"/> | Other rare polymorphisms have been found but their association with obesity are not consistent.<ref name="pmid15972940"/> | ||
| Line 62: | Line 62: | ||
== Sites of synthesis == | == Sites of synthesis == | ||
Leptin is produced primarily in the adipocytes of [[white adipose tissue]]. It also is produced by [[brown adipose tissue]], [[placenta]] (syncytiotrophoblasts), [[ovaries]], [[skeletal muscle]], [[stomach]] (the lower part of the [[fundic glands]]), [[mammary gland|mammary]] [[epithelial cell]]s, [[bone marrow]],<ref name=Margetic>{{cite journal | vauthors = Margetic S, Gazzola C, Pegg GG, Hill RA | title = Leptin: a review of its peripheral actions and interactions | journal = Int. J. Obes. Relat. Metab. Disord. | volume = 26 | issue = 11 | pages = | Leptin is produced primarily in the adipocytes of [[white adipose tissue]]. It also is produced by [[brown adipose tissue]], [[placenta]] (syncytiotrophoblasts), [[ovaries]], [[skeletal muscle]], [[stomach]] (the lower part of the [[fundic glands]]), [[mammary gland|mammary]] [[epithelial cell]]s, [[bone marrow]],<ref name=Margetic>{{cite journal | vauthors = Margetic S, Gazzola C, Pegg GG, Hill RA | title = Leptin: a review of its peripheral actions and interactions | journal = Int. J. Obes. Relat. Metab. Disord. | volume = 26 | issue = 11 | pages = 1407–33 | year = 2002 | pmid = 12439643 | doi = 10.1038/sj.ijo.0802142 }}</ref>[[gastric chief cell]]s and [[P/D1 cell]]s.<ref name="pmid9723619">{{cite journal | vauthors = Bado A, Levasseur S, Attoub S, Kermorgant S, Laigneau JP, Bortoluzzi MN, Moizo L, Lehy T, Guerre-Millo M, Le Marchand-Brustel Y, Lewin MJ | title = The stomach is a source of leptin | journal = Nature | volume = 394 | issue = 6695 | pages = 790–93 | date = August 1998 | pmid = 9723619 | doi = 10.1038/29547 | bibcode = 1998Natur.394..790B }}</ref> | ||
== Blood levels== | == Blood levels== | ||
| Line 68: | Line 68: | ||
===Physiologic variation=== | ===Physiologic variation=== | ||
Leptin levels vary exponentially, not linearly, with fat mass.<ref name="Lönnqvist_1995">{{cite journal | vauthors = Lönnqvist F, Arner P, Nordfors L, Schalling M | title = Overexpression of the obese (ob) gene in adipose tissue of human obese subjects | journal = Nat. Med. | volume = 1 | issue = 9 | pages = | Leptin levels vary exponentially, not linearly, with fat mass.<ref name="Lönnqvist_1995">{{cite journal | vauthors = Lönnqvist F, Arner P, Nordfors L, Schalling M | title = Overexpression of the obese (ob) gene in adipose tissue of human obese subjects | journal = Nat. Med. | volume = 1 | issue = 9 | pages = 950–53 | year = 1995 | pmid = 7585223 | doi = 10.1038/nm0995-950 }}</ref><ref name = "Madej_1998">{{cite journal | vauthors = Madej T | title = Considerations in the use of lipid-based drug products | journal = J Intraven Nurs | volume = 21 | issue = 6 | page = 326 | year = 1998 | pmid = 10392096 | doi = }}</ref> Leptin levels in blood are higher between midnight and early morning, perhaps suppressing appetite during the night.<ref name="pmid8636448">{{cite journal | vauthors = Sinha MK, Ohannesian JP, Heiman ML, Kriauciunas A, Stephens TW, Magosin S, Marco C, Caro JF | title = Nocturnal rise of leptin in lean, obese, and non-insulin-dependent diabetes mellitus subjects | journal = J. Clin. Invest. | volume = 97 | issue = 5 | pages = 1344–47 | date = March 1996 | pmid = 8636448 | pmc = 507189 | doi = 10.1172/JCI118551 }}</ref> The diurnal rhythm of blood leptin levels may be modified by meal-timing.<ref name="pmid9312190">{{cite journal | vauthors = Schoeller DA, Cella LK, Sinha MK, Caro JF | title = Entrainment of the diurnal rhythm of plasma leptin to meal timing | journal = J. Clin. Invest. | volume = 100 | issue = 7 | pages = 1882–87 | date = October 1997 | pmid = 9312190 | pmc = 508375 | doi = 10.1172/JCI119717 }}</ref> | ||
=== In specific conditions === | === In specific conditions === | ||
In humans, many instances are seen where leptin dissociates from the strict role of communicating nutritional status between body and brain and no longer correlates with body fat levels: | In humans, many instances are seen where leptin dissociates from the strict role of communicating nutritional status between body and brain and no longer correlates with body fat levels: | ||
{{columns-list| | {{columns-list|colwidth=30em| | ||
*Leptin plays a critical role in the adaptive response to starvation.<ref name="pmid8717038">{{cite journal | vauthors = Ahima RS, Prabakaran D, Mantzoros C, Qu D, Lowell B, Maratos-Flier E, Flier JS | title = Role of leptin in the neuroendocrine response to fasting | journal = Nature | volume = 382 | issue = 6588 | pages = | *Leptin plays a critical role in the adaptive response to starvation.<ref name="pmid8717038">{{cite journal | vauthors = Ahima RS, Prabakaran D, Mantzoros C, Qu D, Lowell B, Maratos-Flier E, Flier JS | title = Role of leptin in the neuroendocrine response to fasting | journal = Nature | volume = 382 | issue = 6588 | pages = 250–52 | date = July 1996 | pmid = 8717038 | doi = 10.1038/382250a0 | bibcode = 1996Natur.382..250A }}</ref><ref name="pmid19190071">{{cite journal | vauthors = Friedman JM | title = Leptin at 14 y of age: an ongoing story | journal = Am. J. Clin. Nutr. | volume = 89 | issue = 3 | pages = 973S–79S | date = March 2009 | pmid = 19190071 | pmc = 2667654 | doi = 10.3945/ajcn.2008.26788B }}</ref> | ||
* Leptin level is decreased after short-term [[fasting]] (24–72 hours), even when changes in fat mass are not observed.<ref name="pmid12727933">{{cite journal | vauthors = Chan JL, Heist K, DePaoli AM, Veldhuis JD, Mantzoros CS | title = The role of falling leptin levels in the neuroendocrine and metabolic adaptation to short-term starvation in healthy men | journal = J. Clin. Invest. | volume = 111 | issue = 9 | pages = | * Leptin level is decreased after short-term [[fasting]] (24–72 hours), even when changes in fat mass are not observed.<ref name="pmid12727933">{{cite journal | vauthors = Chan JL, Heist K, DePaoli AM, Veldhuis JD, Mantzoros CS | title = The role of falling leptin levels in the neuroendocrine and metabolic adaptation to short-term starvation in healthy men | journal = J. Clin. Invest. | volume = 111 | issue = 9 | pages = 1409–21 | date = May 2003 | pmid = 12727933 | pmc = 154448 | doi = 10.1172/JCI17490 }}</ref><ref name="pmid8866554">{{cite journal | vauthors = Kolaczynski JW, Considine RV, Ohannesian J, Marco C, Opentanova I, Nyce MR, Myint M, Caro JF | title = Responses of leptin to short-term fasting and refeeding in humans: a link with ketogenesis but not ketones themselves | journal = Diabetes | volume = 45 | issue = 11 | pages = 1511–15 | date = November 1996 | pmid = 8866554 | doi = 10.2337/diab.45.11.1511 }}</ref><ref name="pmid8923877">{{cite journal | vauthors = Kolaczynski JW, Ohannesian JP, Considine RV, Marco CC, Caro JF | title = Response of leptin to short-term and prolonged overfeeding in humans | journal = J. Clin. Endocrinol. Metab. | volume = 81 | issue = 11 | pages = 4162–65 | date = November 1996 | pmid = 8923877 | doi = 10.1210/JCEM.81.11.8923877 }}</ref> | ||
* Serum level of leptin is reduced by [[sleep deprivation]].<ref name="pmid24732925">{{cite journal | vauthors = Copinschi G, Leproult R, Spiegel K | title = The important role of sleep in metabolism | journal = Front Horm Res | volume = 42 | issue = | pages = 59–72 | year = 2014 | pmid = 24732925 | doi = 10.1159/000358858 | url = }}</ref><ref name="pmid17442599">{{cite journal | vauthors = Knutson KL, Spiegel K, Penev P, Van Cauter E | title = The metabolic consequences of sleep deprivation | journal = Sleep Med Rev | volume = 11 | issue = 3 | pages = | * Serum level of leptin is reduced by [[sleep deprivation]].<ref name="pmid24732925">{{cite journal | vauthors = Copinschi G, Leproult R, Spiegel K | title = The important role of sleep in metabolism | journal = Front Horm Res | volume = 42 | issue = | pages = 59–72 | year = 2014 | pmid = 24732925 | doi = 10.1159/000358858 | url = }}</ref><ref name="pmid17442599">{{cite journal | vauthors = Knutson KL, Spiegel K, Penev P, Van Cauter E | title = The metabolic consequences of sleep deprivation | journal = Sleep Med Rev | volume = 11 | issue = 3 | pages = 163–78 | date = June 2007 | pmid = 17442599 | pmc = 1991337 | doi = 10.1016/j.smrv.2007.01.002 }}</ref> | ||
*Leptin levels are paradoxically increased in [[obesity]].<ref name="pmid8866547">{{cite journal | vauthors = Caro JF, Sinha MK, Kolaczynski JW, Zhang PL, Considine RV | title = Leptin: the tale of an obesity gene | journal = Diabetes | volume = 45 | issue = 11 | pages = 1455–62 | date = November 1996 | pmid = 8866547 | doi = 10.2337/diab.45.11.1455 }}</ref> | *Leptin levels are paradoxically increased in [[obesity]].<ref name="pmid8866547">{{cite journal | vauthors = Caro JF, Sinha MK, Kolaczynski JW, Zhang PL, Considine RV | title = Leptin: the tale of an obesity gene | journal = Diabetes | volume = 45 | issue = 11 | pages = 1455–62 | date = November 1996 | pmid = 8866547 | doi = 10.2337/diab.45.11.1455 }}</ref> | ||
}} | }} | ||
* Leptin level is increased by [[emotional stress]].<ref name="pmid17062814">{{cite journal | vauthors = Otsuka R, Yatsuya H, Tamakoshi K, Matsushita K, Wada K, Toyoshima H | title = psychological stress and serum leptin concentrations in Japanese men | journal = Obesity (Silver Spring) | volume = 14 | issue = 10 | pages = | * Leptin level is increased by [[emotional stress]].<ref name="pmid17062814">{{cite journal | vauthors = Otsuka R, Yatsuya H, Tamakoshi K, Matsushita K, Wada K, Toyoshima H | title = psychological stress and serum leptin concentrations in Japanese men | journal = Obesity (Silver Spring) | volume = 14 | issue = 10 | pages = 1832–38 | date = October 2006 | pmid = 17062814 | doi = 10.1038/oby.2006.211 }}</ref> | ||
* Leptin level is chronically reduced by [[physical exercise]] [[training]].<ref name="pmid20432196">{{cite journal | vauthors = de Salles BF, Simão R, Fleck SJ, Dias I, Kraemer-Aguiar LG, Bouskela E | title = Effects of resistance training on cytokines | journal = Int J Sports Med | volume = 31 | issue = 7 | pages = | * Leptin level is chronically reduced by [[physical exercise]] [[training]].<ref name="pmid20432196">{{cite journal | vauthors = de Salles BF, Simão R, Fleck SJ, Dias I, Kraemer-Aguiar LG, Bouskela E | title = Effects of resistance training on cytokines | journal = Int J Sports Med | volume = 31 | issue = 7 | pages = 441–50 | date = July 2010 | pmid = 20432196 | doi = 10.1055/s-0030-1251994 }}</ref><ref name="pmid8944684">{{cite journal | vauthors = Hickey MS, Considine RV, Israel RG, Mahar TL, McCammon MR, Tyndall GL, Houmard JA, Caro JF | title = Leptin is related to body fat content in male distance runners | journal = Am. J. Physiol. | volume = 271 | issue = 5 Pt 1 | pages = E938–40 | date = November 1996 | pmid = 8944684 | doi = }}</ref><ref name="pmid9142875">{{cite journal | vauthors = Hickey MS, Houmard JA, Considine RV, Tyndall GL, Midgette JB, Gavigan KE, Weidner ML, McCammon MR, Israel RG, Caro JF | title = Gender-dependent effects of exercise training on serum leptin levels in humans | journal = Am. J. Physiol. | volume = 272 | issue = 4 Pt 1 | pages = E562–66 | date = April 1997 | pmid = 9142875 | doi = }}</ref> | ||
* Leptin level is decreased by increases in testosterone levels and increased by increases in estrogen levels.<ref name="pmid10845097">{{cite journal | vauthors = Ahima RS, Flier JS | title = Leptin | journal = Annu. Rev. Physiol. | volume = 62 | issue = 1 | pages = | * Leptin level is decreased by increases in testosterone levels and increased by increases in estrogen levels.<ref name="pmid10845097">{{cite journal | vauthors = Ahima RS, Flier JS | title = Leptin | journal = Annu. Rev. Physiol. | volume = 62 | issue = 1 | pages = 413–37 | year = 2000 | pmid = 10845097 | doi = 10.1146/annurev.physiol.62.1.413 }}</ref> | ||
* Leptin level is increased by [[insulin]].<ref name="pmid8621027">{{cite journal | vauthors = Kolaczynski JW, Nyce MR, Considine RV, Boden G, Nolan JJ, Henry R, Mudaliar SR, Olefsky J, Caro JF | title = Acute and chronic effects of insulin on leptin production in humans: Studies in vivo and in vitro | journal = Diabetes | volume = 45 | issue = 5 | pages = 699–701 | date = May 1996 | pmid = 8621027 | doi = 10.2337/diabetes.45.5.699 }}</ref> | * Leptin level is increased by [[insulin]].<ref name="pmid8621027">{{cite journal | vauthors = Kolaczynski JW, Nyce MR, Considine RV, Boden G, Nolan JJ, Henry R, Mudaliar SR, Olefsky J, Caro JF | title = Acute and chronic effects of insulin on leptin production in humans: Studies in vivo and in vitro | journal = Diabetes | volume = 45 | issue = 5 | pages = 699–701 | date = May 1996 | pmid = 8621027 | doi = 10.2337/diabetes.45.5.699 }}</ref> | ||
* Leptin release is increased by [[dexamethasone]].<ref name="pmid9136082">{{cite journal | vauthors = Considine RV, Nyce MR, Kolaczynski JW, Zhang PL, Ohannesian JP, Moore JH, Fox JW, Caro JF | title = Dexamethasone stimulates leptin release from human adipocytes: unexpected inhibition by insulin | journal = J. Cell. Biochem. | volume = 65 | issue = 2 | pages = | * Leptin release is increased by [[dexamethasone]].<ref name="pmid9136082">{{cite journal | vauthors = Considine RV, Nyce MR, Kolaczynski JW, Zhang PL, Ohannesian JP, Moore JH, Fox JW, Caro JF | title = Dexamethasone stimulates leptin release from human adipocytes: unexpected inhibition by insulin | journal = J. Cell. Biochem. | volume = 65 | issue = 2 | pages = 254–58 | date = May 1997 | pmid = 9136082 | doi = 10.1002/(SICI)1097-4644(199705)65:2<254::AID-JCB10>3.0.CO;2-I }}</ref> | ||
* In obese patients with [[obstructive sleep apnea]], leptin level is increased, but decreased after the administration of [[continuous positive airway pressure]].<ref name="pmid21358603">{{cite journal | vauthors = Zirlik S, Hauck T, Fuchs FS, Neurath MF, Konturek PC, Harsch IA | title = Leptin, Obestatin and Apelin levels in patients with obstructive sleep apnoea syndrome | journal = Med. Sci. Monit. | volume = 17 | issue = 3 | pages = CR159–64 | date = February 2011 | pmid = 21358603 | pmc = 3524733 | doi = 10.12659/MSM.881450 }}</ref><ref name="pmid12952256">{{cite journal | vauthors = Harsch IA, Konturek PC, Koebnick C, Kuehnlein PP, Fuchs FS, Pour Schahin S, Wiest GH, Hahn EG, Lohmann T, Ficker JH | title = Leptin and ghrelin levels in patients with obstructive sleep apnoea: effect of CPAP treatment | journal = Eur. Respir. J. | volume = 22 | issue = 2 | pages = | * In obese patients with [[obstructive sleep apnea]], leptin level is increased, but decreased after the administration of [[continuous positive airway pressure]].<ref name="pmid21358603">{{cite journal | vauthors = Zirlik S, Hauck T, Fuchs FS, Neurath MF, Konturek PC, Harsch IA | title = Leptin, Obestatin and Apelin levels in patients with obstructive sleep apnoea syndrome | journal = Med. Sci. Monit. | volume = 17 | issue = 3 | pages = CR159–64 | date = February 2011 | pmid = 21358603 | pmc = 3524733 | doi = 10.12659/MSM.881450 }}</ref><ref name="pmid12952256">{{cite journal | vauthors = Harsch IA, Konturek PC, Koebnick C, Kuehnlein PP, Fuchs FS, Pour Schahin S, Wiest GH, Hahn EG, Lohmann T, Ficker JH | title = Leptin and ghrelin levels in patients with obstructive sleep apnoea: effect of CPAP treatment | journal = Eur. Respir. J. | volume = 22 | issue = 2 | pages = 251–57 | date = August 2003 | pmid = 12952256 | doi = 10.1183/09031936.03.00010103 }}</ref> In non-obese individuals, however, restful sleep (i.e., 8–12 hours of unbroken sleep) can increase leptin to normal levels. | ||
===Mutant leptins=== | ===Mutant leptins=== | ||
| Line 90: | Line 90: | ||
== {{anchor|Effects}}Effects == | == {{anchor|Effects}}Effects == | ||
{{main|Leptin receptor|Energy expenditure}} | {{main|Leptin receptor|Energy expenditure}} | ||
[[File:Fatmouse.jpg|thumb|alt=Two white mice both with similar sized ears, black eyes, and pink noses: The body of the mouse on the left, however, is about three times the width of the normal-sized mouse on the right.|A comparison of a mouse unable to produce leptin, resulting in [[obesity]] constant [[hunger]] and [[lethargy]] (left), and an active normal weight mouse (right)]] | [[File:Fatmouse.jpg|thumb|alt=Two white mice both with similar sized ears, black eyes, and pink noses: The body of the mouse on the left, however, is about three times the width of the normal-sized mouse on the right.|A comparison of a mouse unable to produce leptin, resulting in [[obesity]], constant [[hunger]], and [[lethargy]] (left), and an active normal weight mouse (right)]] | ||
Predominantly, the "energy expenditure hormone" leptin is made by [[adipose cells]], thus it is labeled ''fat cell-specific''. In the context of its [[Cause and effect|effects]], it is important to recognize that the short [[Adjective|describing words]] ''direct'', ''central'', and ''primary'' are not used interchangeably. In regard to the hormone leptin, central vs peripheral refers to the [[hypothalamus|hypothalamic]] portion of the brain vs non-hypothalamic ''location of action'' of leptin; direct vs indirect refers to whether there is no intermediary, or there is an intermediary in the ''mode of action'' of leptin; and primary vs secondary is an arbitrary description of a particular ''function'' of leptin.<ref name="pmid10215564">{{cite journal | vauthors = Mantzoros CS | title = The role of leptin in human obesity and disease: a review of current evidence | journal = Ann. Intern. Med. | volume = 130 | issue = 8 | pages = 671–80 | year = 1999 | pmid = 10215564 | doi = 10.7326/0003-4819-130-8-199904200-00014 }}</ref> | Predominantly, the "energy expenditure hormone" leptin is made by [[adipose cells]], thus it is labeled ''fat cell-specific''. In the context of its [[Cause and effect|effects]], it is important to recognize that the short [[Adjective|describing words]] ''direct'', ''central'', and ''primary'' are not used interchangeably. In regard to the hormone leptin, central vs peripheral refers to the [[hypothalamus|hypothalamic]] portion of the brain vs non-hypothalamic ''location of action'' of leptin; direct vs indirect refers to whether there is no intermediary, or there is an intermediary in the ''mode of action'' of leptin; and primary vs secondary is an arbitrary description of a particular ''function'' of leptin.<ref name="pmid10215564">{{cite journal | vauthors = Mantzoros CS | title = The role of leptin in human obesity and disease: a review of current evidence | journal = Ann. Intern. Med. | volume = 130 | issue = 8 | pages = 671–80 | year = 1999 | pmid = 10215564 | doi = 10.7326/0003-4819-130-8-199904200-00014 }}</ref> | ||
| Line 99: | Line 99: | ||
;Function: The ''primary'' function of the hormone leptin is the regulation of [[adipose tissue]] mass through central hypothalamus mediated effects on [[hunger]], [[food energy]] use, [[physical exercise]] and [[Energy homeostasis|energy balance]]. Outside the brain, in the periphery of the body, leptin's ''secondary'' functions are: modulation of energy expenditure, modulation between fetal and maternal metabolism, and that of a permissive factor in puberty, activator of immune cells, activator of beta islet cells, and growth factor. | ;Function: The ''primary'' function of the hormone leptin is the regulation of [[adipose tissue]] mass through central hypothalamus mediated effects on [[hunger]], [[food energy]] use, [[physical exercise]] and [[Energy homeostasis|energy balance]]. Outside the brain, in the periphery of the body, leptin's ''secondary'' functions are: modulation of energy expenditure, modulation between fetal and maternal metabolism, and that of a permissive factor in puberty, activator of immune cells, activator of beta islet cells, and growth factor. | ||
=== Central | === Central nervous system === | ||
In vertebrates, the [[nervous system]] consists of two main parts, the [[central nervous system]] (CNS) and the [[peripheral nervous system]] (PNS). The primary effect of | In vertebrates, the [[nervous system]] consists of two main parts, the [[central nervous system]] (CNS) and the [[peripheral nervous system]] (PNS). The primary effect of leptins is in the [[hypothalamus]], a part of the central nervous system. Leptin receptors are [[Gene expression|expressed]] not only in the hypothalamus but also in other brain regions, particularly in the [[hippocampus]]. Thus some leptin receptors in the brain are classified as ''central'' (hypothalamic) and some as ''peripheral'' (non-hypothalamic). | ||
As scientifically known so far, the general effects of leptin in the central nervous system are: | As scientifically known so far, the general effects of leptin in the central nervous system are: | ||
*Deficiency of leptin has been shown to alter brain proteins and neuronal functions of obese mice which can be restored by leptin injection.<ref name="pmid16293343">{{cite journal | vauthors = Farr SA, Banks WA, Morley JE | title = Effects of leptin on memory processing | journal = Peptides | volume = 27 | issue = 6 | pages = | *Deficiency of leptin has been shown to alter brain proteins and neuronal functions of obese mice which can be restored by leptin injection.<ref name="pmid16293343">{{cite journal | vauthors = Farr SA, Banks WA, Morley JE | title = Effects of leptin on memory processing | journal = Peptides | volume = 27 | issue = 6 | pages = 1420–25 | date = June 2006 | pmid = 16293343 | doi = 10.1016/j.peptides.2005.10.006 }}</ref> | ||
*In humans, low circulating plasma leptin has been associated with cognitive changes associated with anorexia,<ref name="pmid9169091">{{cite journal | vauthors = Casanueva FF, Dieguez C, Popovic V, Peino R, Considine RV, Caro JF | title = Serum immunoreactive leptin concentrations in patients with anorexia nervosa before and after partial weight recovery | journal = Biochem. Mol. Med. | volume = 60 | issue = 2 | pages = 116–20 | date = April 1997 | pmid = 9169091 | doi = 10.1006/bmme.1996.2564 }}</ref> depression, and Alzheimer’s Disease .<ref name="pmid20009056">{{cite journal | vauthors = Lieb W, Beiser AS, Vasan RS, Tan ZS, Au R, Harris TB, Roubenoff R, Auerbach S, DeCarli C, Wolf PA, Seshadri S | title = Association of plasma leptin levels with incident Alzheimer disease and MRI measures of brain aging | journal = JAMA | volume = 302 | issue = 23 | pages = 2565–72 | date = December 2009 | pmid = 20009056 | pmc = 2838501 | doi = 10.1001/jama.2009.1836 }}</ref> | *In humans, low circulating plasma leptin has been associated with cognitive changes associated with anorexia,<ref name="pmid9169091">{{cite journal | vauthors = Casanueva FF, Dieguez C, Popovic V, Peino R, Considine RV, Caro JF | title = Serum immunoreactive leptin concentrations in patients with anorexia nervosa before and after partial weight recovery | journal = Biochem. Mol. Med. | volume = 60 | issue = 2 | pages = 116–20 | date = April 1997 | pmid = 9169091 | doi = 10.1006/bmme.1996.2564 }}</ref> depression, and Alzheimer’s Disease .<ref name="pmid20009056">{{cite journal | vauthors = Lieb W, Beiser AS, Vasan RS, Tan ZS, Au R, Harris TB, Roubenoff R, Auerbach S, DeCarli C, Wolf PA, Seshadri S | title = Association of plasma leptin levels with incident Alzheimer disease and MRI measures of brain aging | journal = JAMA | volume = 302 | issue = 23 | pages = 2565–72 | date = December 2009 | pmid = 20009056 | pmc = 2838501 | doi = 10.1001/jama.2009.1836 }}</ref> | ||
*Studies in transgenic mouse models of Alzheimer's disease have shown that chronic administration of leptin can ameliorate brain pathology and improve cognitive performance,<ref name="pmid20308782">{{cite journal | vauthors = Greco SJ, Bryan KJ, Sarkar S, Zhu X, Smith MA, Ashford JW, Johnston JM, Tezapsidis N, Casadesus G | title = Leptin reduces pathology and improves memory in a transgenic mouse model of Alzheimer's disease | journal = J. Alzheimers Dis. | volume = 19 | issue = 4 | pages = 1155–67 | year = 2010 | pmid = 20308782 | pmc = 2862270 | doi = 10.3233/JAD-2010-1308 }}</ref> by reducing b-amyloid and hyperphosphorylated Tau,<ref name="pmid22921154">{{cite journal | vauthors = Doherty GH, Beccano-Kelly D, Yan SD, Gunn-Moore FJ, Harvey J | title = Leptin prevents hippocampal synaptic disruption and neuronal cell death induced by amyloid β | journal = Neurobiol. Aging | volume = 34 | issue = 1 | pages = 226–37 | date = January 2013 | pmid = 22921154 | doi = 10.1016/j.neurobiolaging.2012.08.003 }}</ref><ref name="pmid19166821">{{cite journal | vauthors = Greco SJ, Sarkar S, Johnston JM, Tezapsidis N | title = Leptin regulates tau phosphorylation and amyloid through AMPK in neuronal cells | journal = Biochem. Biophys. Res. Commun. | volume = 380 | issue = 1 | pages = 98–104 | date = February 2009 | pmid = 19166821 | pmc = 2657956 | doi = 10.1016/j.bbrc.2009.01.041 }}</ref> two hallmarks of Alzheimer's pathology. | *Studies in transgenic mouse models of Alzheimer's disease have shown that chronic administration of leptin can ameliorate brain pathology and improve cognitive performance,<ref name="pmid20308782">{{cite journal | vauthors = Greco SJ, Bryan KJ, Sarkar S, Zhu X, Smith MA, Ashford JW, Johnston JM, Tezapsidis N, Casadesus G | title = Leptin reduces pathology and improves memory in a transgenic mouse model of Alzheimer's disease | journal = J. Alzheimers Dis. | volume = 19 | issue = 4 | pages = 1155–67 | year = 2010 | pmid = 20308782 | pmc = 2862270 | doi = 10.3233/JAD-2010-1308 }}</ref> by reducing b-amyloid and hyperphosphorylated Tau,<ref name="pmid22921154">{{cite journal | vauthors = Doherty GH, Beccano-Kelly D, Yan SD, Gunn-Moore FJ, Harvey J | title = Leptin prevents hippocampal synaptic disruption and neuronal cell death induced by amyloid β | journal = Neurobiol. Aging | volume = 34 | issue = 1 | pages = 226–37 | date = January 2013 | pmid = 22921154 | doi = 10.1016/j.neurobiolaging.2012.08.003 }}</ref><ref name="pmid19166821">{{cite journal | vauthors = Greco SJ, Sarkar S, Johnston JM, Tezapsidis N | title = Leptin regulates tau phosphorylation and amyloid through AMPK in neuronal cells | journal = Biochem. Biophys. Res. Commun. | volume = 380 | issue = 1 | pages = 98–104 | date = February 2009 | pmid = 19166821 | pmc = 2657956 | doi = 10.1016/j.bbrc.2009.01.041 }}</ref> two hallmarks of Alzheimer's pathology. | ||
Generally, leptin is thought to enter the brain at the [[choroid plexus]], where the intense expression of a form of leptin receptor molecule could act as a transport mechanism.<ref name="pmid8645274">{{cite journal | vauthors = Lynn RB, Cao GY, Considine RV, Hyde TM, Caro JF | title = Autoradiographic localization of leptin binding in the choroid plexus of ob/ob and db/db mice | journal = Biochem. Biophys. Res. Commun. | volume = 219 | issue = 3 | pages = | Generally, leptin is thought to enter the brain at the [[choroid plexus]], where the intense expression of a form of leptin receptor molecule could act as a transport mechanism.<ref name="pmid8645274">{{cite journal | vauthors = Lynn RB, Cao GY, Considine RV, Hyde TM, Caro JF | title = Autoradiographic localization of leptin binding in the choroid plexus of ob/ob and db/db mice | journal = Biochem. Biophys. Res. Commun. | volume = 219 | issue = 3 | pages = 884–89 | date = February 1996 | pmid = 8645274 | doi = 10.1006/bbrc.1996.0328 }}</ref> | ||
Increased levels of [[melatonin]] causes a downregulation of leptin,<ref name="pmid15311999">{{cite journal | vauthors = Kus I, Sarsilmaz M, Colakoglu N, Kukne A, Ozen OA, Yilmaz B, Kelestimur H | title = Pinealectomy increases and exogenous melatonin decreases leptin production in rat anterior pituitary cells: an immunohistochemical study | journal = Physiol Res | volume = 53 | issue = 4 | pages = | Increased levels of [[melatonin]] causes a downregulation of leptin,<ref name="pmid15311999">{{cite journal | vauthors = Kus I, Sarsilmaz M, Colakoglu N, Kukne A, Ozen OA, Yilmaz B, Kelestimur H | title = Pinealectomy increases and exogenous melatonin decreases leptin production in rat anterior pituitary cells: an immunohistochemical study | journal = Physiol Res | volume = 53 | issue = 4 | pages = 403–08 | year = 2004 | pmid = 15311999 | doi = }}</ref> however, melatonin also appears to increase leptin levels in the presence of [[insulin]], therefore causing a decrease in appetite during sleeping.<ref name="pmid15572654">{{cite journal | vauthors = Alonso-Vale MI, Andreotti S, Peres SB, Anhê GF, das Neves Borges-Silva C, Neto JC, Lima FB | title = Melatonin enhances leptin expression by rat adipocytes in the presence of insulin | journal = Am. J. Physiol. Endocrinol. Metab. | volume = 288 | issue = 4 | pages = E805–12 | date = April 2005 | pmid = 15572654 | doi = 10.1152/ajpendo.00478.2004 }}</ref> Partial sleep deprivation has also been associated with decreased leptin levels.<ref>{{cite journal | vauthors = Copinschi G | title = Metabolic and endocrine effects of sleep deprivation | journal = Essential psychopharmacology | volume = 6 | issue = 6 | pages = 341–47 | year = 2005 | pmid = 16459757 }}</ref> | ||
Mice with type 1 diabetes treated with leptin or leptin plus insulin, compared to insulin alone had better metabolic profiles: blood sugar did not fluctuate so much; cholesterol levels decreased; less body fat formed.<ref name="pmid20194735">{{cite journal | vauthors = Wang MY, Chen L, Clark GO, Lee Y, Stevens RD, Ilkayeva OR, Wenner BR, Bain JR, Charron MJ, Newgard CB, Unger RH | title = Leptin therapy in insulin-deficient type I diabetes | journal = Proc. Natl. Acad. Sci. | Mice with type 1 diabetes treated with leptin or leptin plus insulin, compared to insulin alone had better metabolic profiles: blood sugar did not fluctuate so much; cholesterol levels decreased; less body fat formed.<ref name="pmid20194735">{{cite journal | vauthors = Wang MY, Chen L, Clark GO, Lee Y, Stevens RD, Ilkayeva OR, Wenner BR, Bain JR, Charron MJ, Newgard CB, Unger RH | title = Leptin therapy in insulin-deficient type I diabetes | journal = Proc. Natl. Acad. Sci. USA | volume = 107 | issue = 11 | pages = 4813–19 | date = March 2010 | pmid = 20194735 | pmc = 2841945 | doi = 10.1073/pnas.0909422107 | laysummary = http://www.medicinenet.com/script/main/art.asp?articlekey=113886 | laysource = medicinenet.com | bibcode = 2010PNAS..107.4813W }}</ref> | ||
====Hypothalamus==== | ====Hypothalamus==== | ||
| Line 118: | Line 118: | ||
**counteracting the effects of [[neuropeptide Y]], a potent hunger promoter secreted by cells in the gut and in the hypothalamus | **counteracting the effects of [[neuropeptide Y]], a potent hunger promoter secreted by cells in the gut and in the hypothalamus | ||
**counteracting the effects of [[anandamide]], another potent hunger promoter that binds to the same receptors as [[THC]] | **counteracting the effects of [[anandamide]], another potent hunger promoter that binds to the same receptors as [[THC]] | ||
*In the medial hypothalamus, leptin stimulates satiety<ref>{{cite journal | vauthors = Fekete C, Légrádi G, Mihály E, Huang QH, Tatro JB, Rand WM, Emerson CH, Lechan RM | title = alpha-Melanocyte-stimulating hormone is contained in nerve terminals innervating thyrotropin-releasing hormone-synthesizing neurons in the hypothalamic paraventricular nucleus and prevents fasting-induced suppression of prothyrotropin-releasing hormone gene expression | journal = The Journal of Neuroscience | volume = 20 | issue = 4 | pages = | *In the medial hypothalamus, leptin stimulates satiety<ref>{{cite journal | vauthors = Fekete C, Légrádi G, Mihály E, Huang QH, Tatro JB, Rand WM, Emerson CH, Lechan RM | title = alpha-Melanocyte-stimulating hormone is contained in nerve terminals innervating thyrotropin-releasing hormone-synthesizing neurons in the hypothalamic paraventricular nucleus and prevents fasting-induced suppression of prothyrotropin-releasing hormone gene expression | journal = The Journal of Neuroscience | volume = 20 | issue = 4 | pages = 1550–58 | date = Feb 2000 | pmid = 10662844 | url = http://www.jneurosci.org/content/20/4/1550 }}</ref> by | ||
**promoting the synthesis of [[α-MSH]], a hunger suppressant | **promoting the synthesis of [[α-MSH]], a hunger suppressant | ||
Thus, a lesion in the lateral hypothalamus causes anorexia (due to a lack of hunger signals) and a lesion in the medial hypothalamus causes excessive hunger (due to a lack of satiety signals).<ref name="Elmquist_1999"/> | Thus, a lesion in the lateral hypothalamus causes anorexia (due to a lack of hunger signals) and a lesion in the medial hypothalamus causes excessive hunger (due to a lack of satiety signals).<ref name="Elmquist_1999"/> | ||
This appetite inhibition is long-term, in contrast to the rapid inhibition of hunger by [[cholecystokinin]] (CCK) and the slower suppression of hunger between meals mediated by [[PYY3-36]]. The absence of leptin (or its receptor) leads to uncontrolled hunger and resulting obesity. Fasting or following a very-low-calorie diet lowers leptin levels.<ref>{{cite journal | vauthors = Dubuc GR, Phinney SD, Stern JS, Havel PJ | title = Changes of serum leptin and endocrine and metabolic parameters after 7 days of energy restriction in men and women | journal = Metab. Clin. Exp. | volume = 47 | issue = 4 | pages = 429–34 | year = 1998 | pmid = 9550541 | doi = 10.1016/S0026-0495(98)90055-5 }}</ref><ref>{{cite journal | vauthors = Pratley RE, Nicolson M, Bogardus C, Ravussin E | title = Plasma leptin responses to fasting in Pima Indians | journal = Am. J. Physiol. | volume = 273 | issue = 3 Pt 1 | pages = | This appetite inhibition is long-term, in contrast to the rapid inhibition of hunger by [[cholecystokinin]] (CCK) and the slower suppression of hunger between meals mediated by [[PYY3-36]]. The absence of leptin (or its receptor) leads to uncontrolled hunger and resulting obesity. Fasting or following a very-low-calorie diet lowers leptin levels.<ref>{{cite journal | vauthors = Dubuc GR, Phinney SD, Stern JS, Havel PJ | title = Changes of serum leptin and endocrine and metabolic parameters after 7 days of energy restriction in men and women | journal = Metab. Clin. Exp. | volume = 47 | issue = 4 | pages = 429–34 | year = 1998 | pmid = 9550541 | doi = 10.1016/S0026-0495(98)90055-5 }}</ref><ref>{{cite journal | vauthors = Pratley RE, Nicolson M, Bogardus C, Ravussin E | title = Plasma leptin responses to fasting in Pima Indians | journal = Am. J. Physiol. | volume = 273 | issue = 3 Pt 1 | pages = E644–49 | year = 1997 | pmid = 9316457 }}</ref><ref>{{cite journal | vauthors = Weigle DS, Duell PB, Connor WE, Steiner RA, Soules MR, Kuijper JL | title = Effect of fasting, refeeding, and dietary fat restriction on plasma leptin levels | journal = J. Clin. Endocrinol. Metab. | volume = 82 | issue = 2 | pages = 561–65 | date = February 1997 | pmid = 9024254 | doi = 10.1210/jc.82.2.561 }}</ref><ref name="Nov 26 2013">{{cite journal | vauthors = Wadden TA, Considine RV, Foster GD, Anderson DA, Sarwer DB, Caro JS | title = Short- and long-term changes in serum leptin dieting obese women: effects of caloric restriction and weight loss | journal = J. Clin. Endocrinol. Metab. | volume = 83 | issue = 1 | pages = 214–18 | date = January 1998 | pmid = 9435444 | doi = 10.1210/jcem.83.1.4494 }}</ref> | ||
Leptin levels change more when food intake decreases than when it increases.<ref>{{cite journal | vauthors = Chin-Chance C, Polonsky KS, Schoeller DA | title = Twenty-four-hour leptin levels respond to cumulative short-term energy imbalance and predict subsequent intake | journal = J. Clin. Endocrinol. Metab. | volume = 85 | issue = 8 | pages = | Leptin levels change more when food intake decreases than when it increases.<ref>{{cite journal | vauthors = Chin-Chance C, Polonsky KS, Schoeller DA | title = Twenty-four-hour leptin levels respond to cumulative short-term energy imbalance and predict subsequent intake | journal = J. Clin. Endocrinol. Metab. | volume = 85 | issue = 8 | pages = 2685–91 | year = 2000 | pmid = 10946866 | doi = 10.1210/jc.85.8.2685 }}</ref> The dynamics of leptin due to an acute change in energy balance may be related to appetite and eventually, to food intake rather than fat stores.<ref>{{cite journal | vauthors = Keim NL, Stern JS, Havel PJ | title = Relation between circulating leptin concentrations and appetite during a prolonged, moderate energy deficit in women | journal = Am. J. Clin. Nutr. | volume = 68 | issue = 4 | pages = 794–801 | year = 1998 | pmid = 9771856 }}</ref><ref>{{cite journal | vauthors = Mars M, de Graaf C, de Groot CP, van Rossum CT, Kok FJ | title = Fasting leptin and appetite responses induced by a 4-day 65%-energy-restricted diet | journal = International journal of obesity (Lond) | volume = 30 | issue = 1 | pages = 122–28 | year = 2006 | pmid = 16158086 | doi = 10.1038/sj.ijo.0803070 }}</ref> | ||
* It controls food intake and energy expenditure by acting on receptors in the mediobasal [[hypothalamus]].<ref name="pmid19176744">{{cite journal | vauthors = Williams KW, Scott MM, Elmquist JK | title = From observation to experimentation: leptin action in the mediobasal hypothalamus | journal = Am. J. Clin. Nutr. | volume = 89 | issue = 3 | pages = | * It controls food intake and energy expenditure by acting on receptors in the mediobasal [[hypothalamus]].<ref name="pmid19176744">{{cite journal | vauthors = Williams KW, Scott MM, Elmquist JK | title = From observation to experimentation: leptin action in the mediobasal hypothalamus | journal = Am. J. Clin. Nutr. | volume = 89 | issue = 3 | pages = 985S–90S | date = March 2009 | pmid = 19176744 | pmc = 2667659 | doi = 10.3945/ajcn.2008.26788D }}</ref> | ||
Leptin binds to [[neuropeptide Y]] (NPY) neurons in the [[arcuate nucleus]] in such a way as to decrease the activity of these neurons. Leptin signals to the hypothalamus which produces a feeling of satiety. Moreover, leptin signals may make it easier for people to resist the temptation of foods high in calories.<ref name="pmid17986612">{{cite journal | vauthors = Baicy K, London ED, Monterosso J, Wong ML, Delibasi T, Sharma A, Licinio J | title = Leptin replacement alters brain response to food cues in genetically leptin-deficient adults | journal = Proc. Natl. Acad. Sci. | Leptin binds to [[neuropeptide Y]] (NPY) neurons in the [[arcuate nucleus]] in such a way as to decrease the activity of these neurons. Leptin signals to the hypothalamus which produces a feeling of satiety. Moreover, leptin signals may make it easier for people to resist the temptation of foods high in calories.<ref name="pmid17986612">{{cite journal | vauthors = Baicy K, London ED, Monterosso J, Wong ML, Delibasi T, Sharma A, Licinio J | title = Leptin replacement alters brain response to food cues in genetically leptin-deficient adults | journal = Proc. Natl. Acad. Sci. USA | volume = 104 | issue = 46 | pages = 18276–79 | date = November 2007 | pmid = 17986612 | pmc = 2084333 | doi = 10.1073/pnas.0706481104 | laysummary = http://www.webmd.com/brain/news/20071029/hormone-leptin-tweaks-hungry-brain | laysource = WebMD | bibcode = 2007PNAS..10418276B }}</ref> | ||
Leptin receptor activation inhibits | Leptin receptor activation inhibits neuropeptide Y and [[agouti-related peptide]] (AgRP), and activates [[α-melanocyte-stimulating hormone]] (α-MSH). The NPY neurons are a key element in the regulation of hunger; small doses of NPY injected into the brains of experimental animals stimulates feeding, while selective destruction of the NPY neurons in mice causes them to become anorexic. Conversely, α-MSH is an important mediator of satiety, and differences in the gene for the α-MSH receptor are linked to obesity in humans. | ||
Leptin interacts with six types of receptors (Ob- | Leptin interacts with six types of receptors (Ob-Ra–Ob-Rf, or LepRa-LepRf), which in turn are encoded by a single gene, [[leptin receptor|LEPR]].<ref name="pmid8772180">{{cite journal | vauthors = Wang MY, Zhou YT, Newgard CB, Unger RH | title = A novel leptin receptor isoform in rat | journal = FEBS Lett. | volume = 392 | issue = 2 | pages = 87–90 | date = August 1996 | pmid = 8772180 | doi = 10.1016/0014-5793(96)00790-9 }}</ref> Ob-Rb is the only receptor isoform that can signal [[intracellular]]ly via the [[JAK-STAT signaling pathway|Jak-Stat]] and [[MAPK]] [[signal transduction pathways]],<ref name="pmid16964413">{{cite journal | vauthors = Malendowicz W, Rucinski M, Macchi C, Spinazzi R, Ziolkowska A, Nussdorfer GG, Kwias Z | title = Leptin and leptin receptors in the prostate and seminal vesicles of the adult rat | journal = Int. J. Mol. Med. | volume = 18 | issue = 4 | pages = 615–18 | date = October 2006 | pmid = 16964413 | doi = 10.3892/ijmm.18.4.615 | url = http://www.spandidos-publications.com/ijmm/article.jsp?article_id=ijmm_18_4_615 }}</ref> and is present in [[hypothalamus|hypothalamic nuclei]].<ref>{{cite web|url=http://www.neuromics.com/ittrium/visit?path=A1x66x1y1x9fx1y1x246x1y1x372x1x82y1x35d4x1x7f|title=LepRb antibody (commercial site)}}</ref> | ||
Once leptin has bound to the Ob-Rb receptor, it activates the stat3, which is phosphorylated and travels to the nucleus to effect changes in gene expression, one of the main effects being the down-regulation of the expression of [[endocannabinoids]], responsible for increasing hunger.<ref name="pmid18563385">{{cite journal | vauthors = Di Marzo V | title = The endocannabinoid system in obesity and type 2 diabetes | journal = Diabetologia | volume = 51 | issue = 8 | pages = 1356–67 | year = 2008 | pmid = 18563385 | doi = 10.1007/s00125-008-1048-2 }}</ref> In response to leptin, receptor neurons have been shown to remodel themselves, changing the number and types of synapses that fire onto them. | Once leptin has bound to the Ob-Rb receptor, it activates the stat3, which is phosphorylated and travels to the nucleus to effect changes in gene expression, one of the main effects being the down-regulation of the expression of [[endocannabinoids]], responsible for increasing hunger.<ref name="pmid18563385">{{cite journal | vauthors = Di Marzo V | title = The endocannabinoid system in obesity and type 2 diabetes | journal = Diabetologia | volume = 51 | issue = 8 | pages = 1356–67 | year = 2008 | pmid = 18563385 | doi = 10.1007/s00125-008-1048-2 }}</ref> In response to leptin, receptor neurons have been shown to remodel themselves, changing the number and types of synapses that fire onto them. | ||
=== Circulatory system === | === Circulatory system === | ||
The role of leptin/leptin receptors in modulation of [[T cell]] activity in the immune system was shown in experimentation with mice. It modulates the immune response to atherosclerosis, of which obesity is a predisposing factor.<ref name=Taleb>{{cite journal | vauthors = Taleb S, Herbin O, Ait-Oufella H, Verreth W, Gourdy P, Barateau V, Merval R, Esposito B, Clément K, Holvoet P, Tedgui A, Mallat Z | title = Defective leptin/leptin receptor signaling improves regulatory T cell immune response and protects mice from atherosclerosis | journal = Arterioscler Thromb Vasc Biol | The role of leptin/leptin receptors in modulation of [[T cell]] activity in the immune system was shown in experimentation with mice. It modulates the immune response to atherosclerosis, of which obesity is a predisposing factor.<ref name=Taleb>{{cite journal | vauthors = Taleb S, Herbin O, Ait-Oufella H, Verreth W, Gourdy P, Barateau V, Merval R, Esposito B, Clément K, Holvoet P, Tedgui A, Mallat Z | title = Defective leptin/leptin receptor signaling improves regulatory T cell immune response and protects mice from atherosclerosis | journal = Arterioscler Thromb Vasc Biol | volume = 27 | issue = 12 | pages = 2691–98 | year = 2007 | pmid = 17690315 | doi = 10.1161/ATVBAHA.107.149567 }}</ref> | ||
Exogenous leptin can promote [[angiogenesis]] by increasing [[vascular endothelial growth factor]] levels. | Exogenous leptin can promote [[angiogenesis]] by increasing [[vascular endothelial growth factor]] levels. | ||
Hyperleptinemia produced by infusion or adenoviral gene transfer decreases blood pressure in rats.<ref name=Zhang>{{cite journal | vauthors = Zhang W, Telemaque S, Augustyniak RA, Anderson P, Thomas GD, An J, Wang Z, Newgard CB, Victor RG | title = Adenovirus-mediated leptin expression normalises hypertension associated with diet-induced obesity | journal = J Neuroendocrinol. | volume = 22 | issue = 3 | pages = | Hyperleptinemia produced by infusion or adenoviral gene transfer decreases blood pressure in rats.<ref name=Zhang>{{cite journal | vauthors = Zhang W, Telemaque S, Augustyniak RA, Anderson P, Thomas GD, An J, Wang Z, Newgard CB, Victor RG | title = Adenovirus-mediated leptin expression normalises hypertension associated with diet-induced obesity | journal = J Neuroendocrinol. | volume = 22 | issue = 3 | pages = 175–80 | year = 2010 | pmid = 20059648 | doi = 10.1111/j.1365-2826.2010.01953.x }}</ref><ref name=Knight>{{cite journal | vauthors = Knight WD, Seth R, Boron J, Overton JM | title = Short-term physiological hyperleptinemia decreases arterial blood pressure | journal = Regul Pept. | volume = 154 | issue = 1–3 | pages = 60–68 | year = 2009 | pmid = 19323984 | doi = 10.1016/j.regpep.2009.02.001 }}</ref> | ||
Leptin microinjections into the nucleus of the solitary tract (NTS) have been shown to elicit sympathoexcitatory responses, and potentiate the cardiovascular responses to activation of the chemoreflex.<ref>{{cite journal | vauthors = Ciriello J, Moreau JM | title = Systemic administration of leptin potentiates the response of neurons in the nucleus of the solitary tract to chemoreceptor activation in the rat | journal = Journal of Neuroscience | volume = 229 | pages = 88–99 | date = November 2012 | pmid = 23159310 | doi = 10.1016/j.neuroscience.2012.10.065 }}</ref> | Leptin microinjections into the nucleus of the solitary tract (NTS) have been shown to elicit sympathoexcitatory responses, and potentiate the cardiovascular responses to activation of the chemoreflex.<ref>{{cite journal | vauthors = Ciriello J, Moreau JM | title = Systemic administration of leptin potentiates the response of neurons in the nucleus of the solitary tract to chemoreceptor activation in the rat | journal = Journal of Neuroscience | volume = 229 | pages = 88–99 | date = November 2012 | pmid = 23159310 | doi = 10.1016/j.neuroscience.2012.10.065 }}</ref> | ||
=== Fetal lung === | === Fetal lung === | ||
In [[fetal]] lung, leptin is induced in the alveolar interstitial fibroblasts ("lipofibroblasts") by the action of [[PTHrP]] secreted by formative alveolar epithelium (endoderm) under moderate stretch. The leptin from the mesenchyme, in turn, acts back on the epithelium at the leptin receptor carried in the alveolar type II pneumocytes and induces surfactant expression, which is one of the main functions of these type II pneumocytes.<ref name="pmid16940239">{{cite journal | vauthors = Torday JS, Rehan VK | title = Up-regulation of fetal rat lung parathyroid hormone-related protein gene regulatory network down-regulates the Sonic Hedgehog/Wnt/beta-catenin gene regulatory network | journal = Pediatr. Res. | volume = 60 | issue = 4 | pages = | In [[fetal]] lung, leptin is induced in the alveolar interstitial fibroblasts ("lipofibroblasts") by the action of [[PTHrP]] secreted by formative alveolar epithelium (endoderm) under moderate stretch. The leptin from the mesenchyme, in turn, acts back on the epithelium at the leptin receptor carried in the alveolar type II pneumocytes and induces surfactant expression, which is one of the main functions of these type II pneumocytes.<ref name="pmid16940239">{{cite journal | vauthors = Torday JS, Rehan VK | title = Up-regulation of fetal rat lung parathyroid hormone-related protein gene regulatory network down-regulates the Sonic Hedgehog/Wnt/beta-catenin gene regulatory network | journal = Pediatr. Res. | volume = 60 | issue = 4 | pages = 382–88 | date = October 2006 | pmid = 16940239 | doi = 10.1203/01.pdr.0000238326.42590.03 }}</ref> | ||
=== Reproductive system === | === Reproductive system === | ||
==== Ovulatory cycle ==== | ==== Ovulatory cycle ==== | ||
In mice, and to a lesser extent in humans, leptin is required for male and female [[fertility]]. Ovulatory cycles in females are linked to energy balance (positive or negative depending on whether a female is losing or gaining weight) and energy flux (how much energy is consumed and expended) much more than energy status (fat levels). When energy balance is highly negative (meaning the woman is starving) or energy flux is very high (meaning the woman is exercising at extreme levels, but still consuming enough calories), the ovarian cycle stops and females stop menstruating. Only if a female has an extremely low body fat percentage does energy status affect menstruation. Leptin levels outside an ideal range may have a negative effect on egg quality and outcome during ''in vitro'' fertilization.<ref name="pmid15798029">{{cite journal | vauthors = Anifandis G, Koutselini E, Louridas K, Liakopoulos V, Leivaditis K, Mantzavinos T, Sioutopoulou D, Vamvakopoulos N | title = Estradiol and leptin as conditional prognostic IVF markers | journal = Reproduction | volume = 129 | issue = 4 | pages = | In mice, and to a lesser extent in humans, leptin is required for male and female [[fertility]]. Ovulatory cycles in females are linked to energy balance (positive or negative depending on whether a female is losing or gaining weight) and energy flux (how much energy is consumed and expended) much more than energy status (fat levels). When energy balance is highly negative (meaning the woman is starving) or energy flux is very high (meaning the woman is exercising at extreme levels, but still consuming enough calories), the ovarian cycle stops and females stop menstruating. Only if a female has an extremely low body fat percentage does energy status affect menstruation. Leptin levels outside an ideal range may have a negative effect on egg quality and outcome during ''in vitro'' fertilization.<ref name="pmid15798029">{{cite journal | vauthors = Anifandis G, Koutselini E, Louridas K, Liakopoulos V, Leivaditis K, Mantzavinos T, Sioutopoulou D, Vamvakopoulos N | title = Estradiol and leptin as conditional prognostic IVF markers | journal = Reproduction | volume = 129 | issue = 4 | pages = 531–34 | date = April 2005 | pmid = 15798029 | doi = 10.1530/rep.1.00567 }}</ref> Leptin is involved in reproduction by stimulating [[gonadotropin-releasing hormone]] from the [[hypothalamus]].<ref name="pmid24173881">{{cite journal | vauthors = Comninos AN, Jayasena CN, Dhillo WS | title = The relationship between gut and adipose hormones, and reproduction | journal = Hum. Reprod. Update | volume = 20 | issue = 2 | pages = 153–74 | year = 2014 | pmid = 24173881 | doi = 10.1093/humupd/dmt033 }}</ref> | ||
==== Pregnancy ==== | ==== Pregnancy ==== | ||
The placenta produces leptin.<ref>{{cite journal | vauthors = Zhao J, Townsend KL, Schulz LC, Kunz TH, Li C, Widmaier EP | title = Leptin receptor expression increases in placenta, but not hypothalamus, during gestation in Mus musculus and Myotis lucifugus | journal = Placenta | volume = 25 | issue = 8–9 | pages = | The placenta produces leptin.<ref>{{cite journal | vauthors = Zhao J, Townsend KL, Schulz LC, Kunz TH, Li C, Widmaier EP | title = Leptin receptor expression increases in placenta, but not hypothalamus, during gestation in Mus musculus and Myotis lucifugus | journal = Placenta | volume = 25 | issue = 8–9 | pages = 712–22 | year = 2004 | pmid = 15450389 | doi = 10.1016/j.placenta.2004.01.017 }}</ref> Leptin levels rise during pregnancy and fall after childbirth. Leptin is also expressed in fetal membranes and the uterine tissue. Uterine contractions are inhibited by leptin.<ref>{{cite journal | vauthors = Moynihan AT, Hehir MP, Glavey SV, Smith TJ, Morrison JJ | title = Inhibitory effect of leptin on human uterine contractility ''in vitro'' | journal = Am. J. Obstet. Gynecol. | volume = 195 | issue = 2 | pages = 504–09 | year = 2006 | pmid = 16647683 | doi = 10.1016/j.ajog.2006.01.106 }}</ref> Leptin plays a role in [[hyperemesis gravidarum]] (severe [[morning sickness]] of pregnancy),<ref>{{cite journal | vauthors = Aka N, Atalay S, Sayharman S, Kiliç D, Köse G, Küçüközkan T | title = Leptin and leptin receptor levels in pregnant women with hyperemesis gravidarum | journal = The Australian & New Zealand journal of obstetrics & gynaecology | volume = 46 | issue = 4 | pages = 274–77 | year = 2006 | pmid = 16866785 | doi = 10.1111/j.1479-828X.2006.00590.x }}</ref> in [[polycystic ovary syndrome]]<ref>{{cite journal | vauthors = Cervero A, Domínguez F, Horcajadas JA, Quiñonero A, Pellicer A, Simón C | title = The role of the leptin in reproduction | journal = Current Opinion in Obstetrics and Gynecology | volume = 18 | issue = 3 | pages = 297–303 | year = 2006 | pmid = 16735830 | doi = 10.1097/01.gco.0000193004.35287.89 }}</ref><!-- to check: literature is controversial --> and hypothalamic leptin is implicated in bone growth in mice.<ref>{{cite journal | vauthors = Iwaniec UT, Boghossian S, Lapke PD, Turner RT, Kalra SP | title = Central leptin gene therapy corrects skeletal abnormalities in leptin-deficient ob/ob mice | journal = Peptides | volume = 28 | issue = 5 | pages = 1012–19 | year = 2007 | pmid = 17346852 | pmc = 1986832 | doi = 10.1016/j.peptides.2007.02.001 }}</ref> | ||
==== Lactation ==== | ==== Lactation ==== | ||
Immunoreactive leptin has been found in human breast milk; and leptin from mother's milk has been found in the blood of suckling infant animals.<ref name="pmid9398752">{{cite journal | vauthors = Casabiell X, Piñeiro V, Tomé MA, Peinó R, Diéguez C, Casanueva FF | title = Presence of leptin in colostrum and/or breast milk from lactating mothers: a potential role in the regulation of neonatal food intake | journal = J. Clin. Endocrinol. Metab. | volume = 82 | issue = 12 | pages = | Immunoreactive leptin has been found in human breast milk; and leptin from mother's milk has been found in the blood of suckling infant animals.<ref name="pmid9398752">{{cite journal | vauthors = Casabiell X, Piñeiro V, Tomé MA, Peinó R, Diéguez C, Casanueva FF | title = Presence of leptin in colostrum and/or breast milk from lactating mothers: a potential role in the regulation of neonatal food intake | journal = J. Clin. Endocrinol. Metab. | volume = 82 | issue = 12 | pages = 4270–73 | year = 1997 | pmid = 9398752 | doi = 10.1210/jcem.82.12.4590 }}</ref> | ||
==== Puberty ==== | ==== Puberty ==== | ||
| Line 160: | Line 160: | ||
=== Bone === | === Bone === | ||
Leptin's ability to regulate bone mass was first recognized in 2000.<ref name="pmid10660043">{{cite journal | vauthors = Ducy P, Amling M, Takeda S, Priemel M, Schilling AF, Beil FT, Shen J, Vinson C, Rueger JM, Karsenty G | title = Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass | journal = Cell | volume = 100 | issue = 2 | pages = 197–207 | date = January 2000 | pmid = 10660043 | doi = 10.1016/S0092-8674(00)81558-5 }}</ref> Leptin can affect [[bone metabolism]] via direct signalling from the brain. Leptin decreases [[cancellous bone]], but increases [[cortical bone]]. This "cortical-cancellous dichotomy" may represent a mechanism for enlarging bone size, and thus bone resistance, to cope with increased body weight.<ref name="pmid17924050">{{cite journal | vauthors = Hamrick MW, Ferrari SL | title = Leptin and the sympathetic connection of fat to bone | journal = Osteoporos Int | volume = 19 | issue = 7 | pages = | Leptin's ability to regulate bone mass was first recognized in 2000.<ref name="pmid10660043">{{cite journal | vauthors = Ducy P, Amling M, Takeda S, Priemel M, Schilling AF, Beil FT, Shen J, Vinson C, Rueger JM, Karsenty G | title = Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass | journal = Cell | volume = 100 | issue = 2 | pages = 197–207 | date = January 2000 | pmid = 10660043 | doi = 10.1016/S0092-8674(00)81558-5 }}</ref> Leptin can affect [[bone metabolism]] via direct signalling from the brain. Leptin decreases [[cancellous bone]], but increases [[cortical bone]]. This "cortical-cancellous dichotomy" may represent a mechanism for enlarging bone size, and thus bone resistance, to cope with increased body weight.<ref name="pmid17924050">{{cite journal | vauthors = Hamrick MW, Ferrari SL | title = Leptin and the sympathetic connection of fat to bone | journal = Osteoporos Int | volume = 19 | issue = 7 | pages = 905–12 | date = July 2008 | pmid = 17924050 | doi = 10.1007/s00198-007-0487-9 }}</ref> | ||
Bone metabolism can be regulated by central sympathetic outflow, since sympathetic pathways innervate bone tissue.<ref name="Allison_2006">{{cite journal | vauthors = Allison SJ, Herzog H | title = NPY and bone | journal = EXS | volume = | issue = 95 | pages = 171–82 | year = 2006 | pmid = 16383006 | doi = }}</ref> A number of brain-signalling molecules ([[neuropeptides]] and [[neurotransmitters]]) have been found in bone, including [[adrenaline]], [[noradrenaline]], [[serotonin]], [[calcitonin gene-related peptide]], [[vasoactive intestinal peptide]] and [[neuropeptide Y]].<ref name="Allison_2006"/><ref name="pmid12577304">{{cite journal | vauthors = Gordeladze JO, Reseland JE | title = A unified model for the action of leptin on bone turnover | journal = J. Cell. Biochem. | volume = 88 | issue = 4 | pages = | Bone metabolism can be regulated by central sympathetic outflow, since sympathetic pathways innervate bone tissue.<ref name="Allison_2006">{{cite journal | vauthors = Allison SJ, Herzog H | title = NPY and bone | journal = EXS | volume = | issue = 95 | pages = 171–82 | year = 2006 | pmid = 16383006 | doi = }}</ref> A number of brain-signalling molecules ([[neuropeptides]] and [[neurotransmitters]]) have been found in bone, including [[adrenaline]], [[noradrenaline]], [[serotonin]], [[calcitonin gene-related peptide]], [[vasoactive intestinal peptide]] and [[neuropeptide Y]].<ref name="Allison_2006"/><ref name="pmid12577304">{{cite journal | vauthors = Gordeladze JO, Reseland JE | title = A unified model for the action of leptin on bone turnover | journal = J. Cell. Biochem. | volume = 88 | issue = 4 | pages = 706–12 | date = March 2003 | pmid = 12577304 | doi = 10.1002/jcb.10385 }}</ref> Leptin binds to its receptors in the hypothalamus, where it acts through the [[sympathetic nervous system]] to regulate bone metabolism.<ref name="pmid12419242">{{cite journal | vauthors = Takeda S, Elefteriou F, Levasseur R, Liu X, Zhao L, Parker KL, Armstrong D, Ducy P, Karsenty G | title = Leptin regulates bone formation via the sympathetic nervous system | journal = Cell | volume = 111 | issue = 3 | pages = 305–17 | date = November 2002 | pmid = 12419242 | doi = 10.1016/S0092-8674(02)01049-8 }}</ref> Leptin may also act directly on bone metabolism via a balance between energy intake and the IGF-I pathway.<ref name="pmid17924050"/><ref name="pmid17431002">{{cite journal | vauthors = Martin A, David V, Malaval L, Lafage-Proust MH, Vico L, Thomas T | title = Opposite effects of leptin on bone metabolism: a dose-dependent balance related to energy intake and insulin-like growth factor-I pathway | journal = Endocrinology | volume = 148 | issue = 7 | pages = 3419–25 | year = 2007 | pmid = 17431002 | doi = 10.1210/en.2006-1541 }}</ref> There is a potential for treatment of diseases of bone formation - such as impaired fracture healing - with leptin.<ref name="pmid24343796">{{cite journal | vauthors = Rőszer T, Józsa T, Kiss-Tóth ED, De Clerck N, Balogh L | title = Leptin receptor deficient diabetic (db/db) mice are compromised in postnatal bone regeneration. | journal = Cell and Tissue Research | volume = 356 | issue = 1 | pages = 195–206 | date = April 2014 | pmid = 24343796 | doi = 10.1007/s00441-013-1768-6 }}</ref> | ||
=== Immune system === | === Immune system === | ||
Factors that acutely affect leptin levels are also factors that influence other markers of inflammation, e.g., testosterone, sleep, emotional stress, caloric restriction, and body fat levels. While it is well-established that leptin is involved in the regulation of the [[Inflammation|inflammatory]] response,<ref name="pmid9732873">{{cite journal | vauthors = Lord GM, Matarese G, Howard JK, Baker RJ, Bloom SR, Lechler RI | title = Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression | journal = Nature | volume = 394 | issue = 6696 | pages = 897–901 | date = August 1998 | pmid = 9732873 | doi = 10.1038/29795 }}</ref><ref name="Fantuzzi_2000">{{cite journal | vauthors = Fantuzzi G, Faggioni R | title = Leptin in the regulation of immunity, inflammation, and hematopoiesis | journal = J. Leukoc. Biol. | volume = 68 | issue = 4 | pages = 437–46 | date = October 2000 | pmid = 11037963 | doi = }}</ref><ref name="Caldefie-Chezet 2001">{{cite journal | vauthors = Caldefie-Chezet F, Poulin A, Tridon A, Sion B, Vasson MP | title = Leptin: a potential regulator of polymorphonuclear neutrophil bactericidal action? | journal = J. Leukoc. Biol. | volume = 69 | issue = 3 | pages = | Factors that acutely affect leptin levels are also factors that influence other markers of inflammation, e.g., testosterone, sleep, emotional stress, caloric restriction, and body fat levels. While it is well-established that leptin is involved in the regulation of the [[Inflammation|inflammatory]] response,<ref name="pmid9732873">{{cite journal | vauthors = Lord GM, Matarese G, Howard JK, Baker RJ, Bloom SR, Lechler RI | title = Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression | journal = Nature | volume = 394 | issue = 6696 | pages = 897–901 | date = August 1998 | pmid = 9732873 | doi = 10.1038/29795 | bibcode = 1998Natur.394..897L }}</ref><ref name="Fantuzzi_2000">{{cite journal | vauthors = Fantuzzi G, Faggioni R | title = Leptin in the regulation of immunity, inflammation, and hematopoiesis | journal = J. Leukoc. Biol. | volume = 68 | issue = 4 | pages = 437–46 | date = October 2000 | pmid = 11037963 | doi = }}</ref><ref name="Caldefie-Chezet 2001">{{cite journal | vauthors = Caldefie-Chezet F, Poulin A, Tridon A, Sion B, Vasson MP | title = Leptin: a potential regulator of polymorphonuclear neutrophil bactericidal action? | journal = J. Leukoc. Biol. | volume = 69 | issue = 3 | pages = 414–18 | date = March 2001 | pmid = 11261788 | doi = }}</ref> it has been further theorized that leptin's role as an inflammatory marker is to respond specifically to adipose-derived inflammatory [[cytokines]]. | ||
In terms of both structure and function, leptin resembles [[Interleukin 6|IL-6]] and is a member of the cytokine [[Protein family|superfamily]].<ref name="pmid9144295"/><ref name="Fantuzzi_2000"/><ref name="Madej_1995">{{cite journal | vauthors = Madej T, Boguski MS, Bryant SH | title = Threading analysis suggests that the obese gene product may be a helical cytokine | journal = FEBS Lett. | volume = 373 | issue = 1 | pages = 13–18 | date = October 1995 | pmid = 7589424 | doi = 10.1016/0014-5793(95)00977-H }}</ref> Circulating leptin seems to affect the [[Hypothalamic–pituitary–adrenal axis|HPA axis]], suggesting a role for leptin in stress response.<ref name="pmid9275075">{{cite journal | vauthors = Heiman ML, Ahima RS, Craft LS, Schoner B, Stephens TW, Flier JS | title = Leptin inhibition of the hypothalamic-pituitary-adrenal axis in response to stress | journal = Endocrinology | volume = 138 | issue = 9 | pages = | In terms of both structure and function, leptin resembles [[Interleukin 6|IL-6]] and is a member of the cytokine [[Protein family|superfamily]].<ref name="pmid9144295"/><ref name="Fantuzzi_2000"/><ref name="Madej_1995">{{cite journal | vauthors = Madej T, Boguski MS, Bryant SH | title = Threading analysis suggests that the obese gene product may be a helical cytokine | journal = FEBS Lett. | volume = 373 | issue = 1 | pages = 13–18 | date = October 1995 | pmid = 7589424 | doi = 10.1016/0014-5793(95)00977-H }}</ref> Circulating leptin seems to affect the [[Hypothalamic–pituitary–adrenal axis|HPA axis]], suggesting a role for leptin in stress response.<ref name="pmid9275075">{{cite journal | vauthors = Heiman ML, Ahima RS, Craft LS, Schoner B, Stephens TW, Flier JS | title = Leptin inhibition of the hypothalamic-pituitary-adrenal axis in response to stress | journal = Endocrinology | volume = 138 | issue = 9 | pages = 3859–63 | date = September 1997 | pmid = 9275075 | doi = 10.1210/en.138.9.3859 }}</ref> Elevated leptin concentrations are associated with elevated white blood cell counts in both men and women.<ref name="pmid15724240">{{cite journal | vauthors = Mabuchi T, Yatsuya H, Tamakoshi K, Otsuka R, Nagasawa N, Zhang H, Murata C, Wada K, Ishikawa M, Hori Y, Kondo T, Hashimoto S, Toyoshima H | title = Association between serum leptin concentration and white blood cell count in middle-aged Japanese men and women | journal = Diabetes Metab. Res. Rev. | volume = 21 | issue = 5 | pages = 441–47 | year = 2005 | pmid = 15724240 | doi = 10.1002/dmrr.540 }}</ref> | ||
Similar to what is observed in chronic inflammation, chronically elevated leptin levels are associated with obesity, overeating, and inflammation-related diseases, including [[hypertension]], [[metabolic syndrome]], and [[cardiovascular disease]]. While leptin is associated with body fat mass, however, the size of individual fat cells, and the act of overeating, it is interesting that it is not affected by exercise (for comparison, [[Inflammation#Post-inflammatory muscle growth and repair|IL-6 is released in response to muscular contractions]]). Thus, it is speculated that leptin responds specifically to adipose-derived inflammation.<ref name="pmid7585224">{{cite journal | vauthors = Hamilton BS, Paglia D, Kwan AY, Deitel M | title = Increased obese mRNA expression in omental fat cells from massively obese humans | journal = Nat. Med. | volume = 1 | issue = 9 | pages = | Similar to what is observed in chronic inflammation, chronically elevated leptin levels are associated with obesity, overeating, and inflammation-related diseases, including [[hypertension]], [[metabolic syndrome]], and [[cardiovascular disease]]. While leptin is associated with body fat mass, however, the size of individual fat cells, and the act of overeating, it is interesting that it is not affected by exercise (for comparison, [[Inflammation#Post-inflammatory muscle growth and repair|IL-6 is released in response to muscular contractions]]). Thus, it is speculated that leptin responds specifically to adipose-derived inflammation.<ref name="pmid7585224">{{cite journal | vauthors = Hamilton BS, Paglia D, Kwan AY, Deitel M | title = Increased obese mRNA expression in omental fat cells from massively obese humans | journal = Nat. Med. | volume = 1 | issue = 9 | pages = 953–56 | date = September 1995 | pmid = 7585224 | doi = 10.1038/nm0995-953 }}</ref> Leptin is a pro-angiogenic, pro-inflammatory and mitogenic factor, the actions of which are reinforced through crosstalk with IL-1 family cytokines in cancer.<ref name="pmid19111549">{{cite journal | vauthors = Perrier S, Caldefie-Chézet F, Vasson MP | title = IL-1 family in breast cancer: potential interplay with leptin and other adipocytokines | journal = FEBS Lett. | volume = 583 | issue = 2 | pages = 259–65 | date = January 2009 | pmid = 19111549 | doi = 10.1016/j.febslet.2008.12.030 }}</ref> | ||
Taken as such, increases in leptin levels (in response to caloric intake) function as an acute pro-inflammatory response mechanism to prevent excessive cellular stress induced by overeating. When high caloric intake overtaxes the ability of fat cells to [[hypertrophy|grow larger]] or [[hyperplasia|increase in number]] in step with caloric intake, the ensuing stress response leads to inflammation at the cellular level and ectopic fat storage, i.e., the unhealthy storage of body fat within internal organs, arteries, and/or muscle. The insulin increase in response to the caloric load provokes a dose-dependent rise in leptin, an effect potentiated by high cortisol levels.<ref name="pmid8826983">{{cite journal | vauthors = Wabitsch M, Jensen PB, Blum WF, Christoffersen CT, Englaro P, Heinze E, Rascher W, Teller W, Tornqvist H, Hauner H | title = Insulin and cortisol promote leptin production in cultured human fat cells | journal = Diabetes | volume = 45 | issue = 10 | pages = | Taken as such, increases in leptin levels (in response to caloric intake) function as an acute pro-inflammatory response mechanism to prevent excessive cellular stress induced by overeating. When high caloric intake overtaxes the ability of fat cells to [[hypertrophy|grow larger]] or [[hyperplasia|increase in number]] in step with caloric intake, the ensuing stress response leads to inflammation at the cellular level and ectopic fat storage, i.e., the unhealthy storage of body fat within internal organs, arteries, and/or muscle. The insulin increase in response to the caloric load provokes a dose-dependent rise in leptin, an effect potentiated by high cortisol levels.<ref name="pmid8826983">{{cite journal | vauthors = Wabitsch M, Jensen PB, Blum WF, Christoffersen CT, Englaro P, Heinze E, Rascher W, Teller W, Tornqvist H, Hauner H | title = Insulin and cortisol promote leptin production in cultured human fat cells | journal = Diabetes | volume = 45 | issue = 10 | pages = 1435–38 | date = October 1996 | pmid = 8826983 | doi = 10.2337/diabetes.45.10.1435 }}</ref> (This insulin-leptin relationship is notably similar to insulin's effect on the increase of IL-6 gene expression and secretion from [[adipocytes|preadipocytes]] in a time- and dose-dependent manner.)<ref name="pmid18617614">{{cite journal | vauthors = LaPensee CR, Hugo ER, Ben-Jonathan N | title = Insulin stimulates interleukin-6 expression and release in LS14 human adipocytes through multiple signaling pathways | journal = Endocrinology | volume = 149 | issue = 11 | pages = 5415–22 | date = November 2008 | pmid = 18617614 | pmc = 2584585 | doi = 10.1210/en.2008-0549 }}</ref> Furthermore, plasma leptin concentrations have been observed to gradually increase when [[acipimox]] is administered to prevent [[lipolysis]], concurrent hypocaloric dieting and weight loss notwithstanding.<ref name="pmid11022182">{{cite journal | vauthors = Worm D, Vinten J, Vaag A, Henriksen JE, Beck-Nielsen H | title = The nicotinic acid analogue acipimox increases plasma leptin and decreases free fatty acids in type 2 diabetic patients | journal = Eur. J. Endocrinol. | volume = 143 | issue = 3 | pages = 389–95 | date = September 2000 | pmid = 11022182 | doi = 10.1530/eje.0.1430389 }}</ref> Such findings appear to demonstrate high caloric loads in excess of storage rate capacities of fat cells lead to stress responses that induce an increase in leptin, which then operates as an adipose-derived inflammation stopgap signaling for the cessation of food intake so as to prevent adipose-derived inflammation from reaching elevated levels. This response may then protect against the harmful process of ectopic fat storage, which perhaps explains the connection between chronically elevated leptin levels and ectopic fat storage in obese individuals.<ref name="pmid8866547"/> | ||
== Role in obesity and weight loss == | == Role in obesity and weight loss == | ||
===Obesity=== | ===Obesity=== | ||
[[File:Energy Balance.png|thumbnail|300 px|Leptin and Ghrelin on the metabolism control]] | [[File:Energy Balance.png|thumbnail|300 px|Leptin and Ghrelin on the metabolism control]] | ||
Although leptin reduces appetite as a circulating signal, obese individuals generally exhibit a higher circulating concentration of leptin than normal weight individuals due to their higher [[Body fat percentage|percentage body fat]].<ref name="pmid8532024" /> These people show resistance to leptin, similar to [[Insulin resistance|resistance of insulin]] in [[type 2 diabetes]], with the elevated levels failing to control hunger and modulate their weight. A number of explanations have been proposed to explain this. An important contributor to leptin resistance is changes to leptin receptor signalling, particularly in the [[arcuate nucleus]], however, deficiency of, or major changes to, the leptin receptor itself are not thought to be a major cause. Other explanations suggested include changes to the way leptin crosses the [[blood brain barrier]] (BBB) or alterations occurring during development.<ref name="pmid17937601">{{cite journal | vauthors = Myers MG, Cowley MA, Münzberg H | title = Mechanisms of leptin action and leptin resistance | journal = Annu. Rev. Physiol. | volume = 70 | issue = 1 | pages = | Although leptin reduces appetite as a circulating signal, obese individuals generally exhibit a higher circulating concentration of leptin than normal weight individuals due to their higher [[Body fat percentage|percentage body fat]].<ref name="pmid8532024" /> These people show resistance to leptin, similar to [[Insulin resistance|resistance of insulin]] in [[type 2 diabetes]], with the elevated levels failing to control hunger and modulate their weight. A number of explanations have been proposed to explain this. An important contributor to leptin resistance is changes to leptin receptor signalling, particularly in the [[arcuate nucleus]], however, deficiency of, or major changes to, the leptin receptor itself are not thought to be a major cause. Other explanations suggested include changes to the way leptin crosses the [[blood brain barrier]] (BBB) or alterations occurring during development.<ref name="pmid17937601">{{cite journal | vauthors = Myers MG, Cowley MA, Münzberg H | title = Mechanisms of leptin action and leptin resistance | journal = Annu. Rev. Physiol. | volume = 70 | issue = 1 | pages = 537–56 | year = 2008 | pmid = 17937601 | doi = 10.1146/annurev.physiol.70.113006.100707 }}</ref> | ||
Studies on leptin [[cerebrospinal fluid]] (CSF) levels provide evidence for the reduction in leptin crossing the BBB and reaching obesity-relevant targets, such as the hypothalamus, in obese people.<ref name="pmid24039946">{{cite journal | vauthors = Veyrat-Durebex C, Poher AL, Caillon A, Somm E, Vallet P, Charnay Y, Rohner-Jeanrenaud F | title = Improved leptin sensitivity as a potential candidate responsible for the spontaneous food restriction of the Lou/C rat | journal = PLoS ONE | volume = 8 | issue = 9 | pages = e73452 | year = 2013 | pmid = 24039946 | pmc = 3765307 | doi = 10.1371/journal.pone.0073452 }}</ref> In humans it has been observed that the ratio of leptin in the CSF compared to the blood is lower in obese people than in people of a normal weight.<ref name="pmid8684156">{{cite journal | vauthors = Caro JF, Kolaczynski JW, Nyce MR, Ohannesian JP, Opentanova I, Goldman WH, Lynn RB, Zhang PL, Sinha MK, Considine RV | title = Decreased cerebrospinal-fluid/serum leptin ratio in obesity: a possible mechanism for leptin resistance | journal = Lancet | volume = 348 | issue = 9021 | pages = 159–61 | date = 20 July 1996 | pmid = 8684156 | doi = 10.1016/S0140-6736(96)03173-X }}</ref> The reason for this may be high levels of [[triglycerides]] affecting the transport of leptin across the BBB or due to the leptin transporter becoming saturated.<ref name="pmid24039946"/> Although deficits in the transfer of leptin from the plasma to the CSF is seen in obese people, they are still found to have 30% more leptin in their CSF than lean individuals.<ref name="pmid8684156"/> These higher CSF levels fail to prevent their obesity. Since the amount and quality of leptin receptors in the hypothalamus appears to be normal in the majority of obese humans (as judged from leptin-mRNA studies),<ref name="pmid8666155">{{cite journal | vauthors = Considine RV, Considine EL, Williams CJ, Hyde TM, Caro JF | title = The hypothalamic leptin receptor in humans: identification of incidental sequence polymorphisms and absence of the db/db mouse and fa/fa rat mutations | journal = Diabetes | volume = 45 | issue = 7 | pages = | Studies on leptin [[cerebrospinal fluid]] (CSF) levels provide evidence for the reduction in leptin crossing the BBB and reaching obesity-relevant targets, such as the hypothalamus, in obese people.<ref name="pmid24039946">{{cite journal | vauthors = Veyrat-Durebex C, Poher AL, Caillon A, Somm E, Vallet P, Charnay Y, Rohner-Jeanrenaud F | title = Improved leptin sensitivity as a potential candidate responsible for the spontaneous food restriction of the Lou/C rat | journal = PLoS ONE | volume = 8 | issue = 9 | pages = e73452 | year = 2013 | pmid = 24039946 | pmc = 3765307 | doi = 10.1371/journal.pone.0073452 | bibcode = 2013PLoSO...873452V }}</ref> In humans it has been observed that the ratio of leptin in the CSF compared to the blood is lower in obese people than in people of a normal weight.<ref name="pmid8684156">{{cite journal | vauthors = Caro JF, Kolaczynski JW, Nyce MR, Ohannesian JP, Opentanova I, Goldman WH, Lynn RB, Zhang PL, Sinha MK, Considine RV | title = Decreased cerebrospinal-fluid/serum leptin ratio in obesity: a possible mechanism for leptin resistance | journal = Lancet | volume = 348 | issue = 9021 | pages = 159–61 | date = 20 July 1996 | pmid = 8684156 | doi = 10.1016/S0140-6736(96)03173-X }}</ref> The reason for this may be high levels of [[triglycerides]] affecting the transport of leptin across the BBB or due to the leptin transporter becoming saturated.<ref name="pmid24039946"/> Although deficits in the transfer of leptin from the plasma to the CSF is seen in obese people, they are still found to have 30% more leptin in their CSF than lean individuals.<ref name="pmid8684156"/> These higher CSF levels fail to prevent their obesity. Since the amount and quality of leptin receptors in the hypothalamus appears to be normal in the majority of obese humans (as judged from leptin-mRNA studies),<ref name="pmid8666155">{{cite journal | vauthors = Considine RV, Considine EL, Williams CJ, Hyde TM, Caro JF | title = The hypothalamic leptin receptor in humans: identification of incidental sequence polymorphisms and absence of the db/db mouse and fa/fa rat mutations | journal = Diabetes | volume = 45 | issue = 7 | pages = 992–94 | year = 1996 | pmid = 8666155 | doi = 10.2337/diabetes.45.7.992 }}</ref> it is likely that the leptin resistance in these individuals is due to a post leptin-receptor deficit, similar to the post-insulin receptor defect seen in type 2 diabetes.<ref name="pmid9451823">{{cite journal | vauthors = Considine RV, Caro JF | title = Leptin and the regulation of body weight | journal = Int. J. Biochem. Cell Biol. | volume = 29 | issue = 11 | pages = 1255–72 | date = November 1997 | pmid = 9451823 | doi = 10.1016/S1357-2725(97)00050-2 }}</ref> | ||
When leptin binds with the leptin receptor, it activates a number of pathways. Leptin resistance may be caused by defects in one or more part of this process, particularly the [[Janus kinase 1|JAK]]/[[STAT protein|STAT]] pathway. Mice with a mutation in the leptin receptor gene that prevents the activation of [[STAT3]] are obese and exhibit hyperphagia. The [[Phosphoinositide 3-kinase|PI3K]] pathway may also be involved in leptin resistance, as has been demonstrated in mice by artificial blocking of PI3K signalling. The PI3K pathway also is activated by the insulin receptor and is therefore an important area where leptin and insulin act together as part of energy homeostasis. The insulin-pI3K pathway can cause [[POMC]] neurons to become insensitive to leptin through [[Hyperpolarization (biology)|hyperpolarization]].<ref name="pmid19644451"/> | When leptin binds with the leptin receptor, it activates a number of pathways. Leptin resistance may be caused by defects in one or more part of this process, particularly the [[Janus kinase 1|JAK]]/[[STAT protein|STAT]] pathway. Mice with a mutation in the leptin receptor gene that prevents the activation of [[STAT3]] are obese and exhibit hyperphagia. The [[Phosphoinositide 3-kinase|PI3K]] pathway may also be involved in leptin resistance, as has been demonstrated in mice by artificial blocking of PI3K signalling. The PI3K pathway also is activated by the insulin receptor and is therefore an important area where leptin and insulin act together as part of energy homeostasis. The insulin-pI3K pathway can cause [[POMC]] neurons to become insensitive to leptin through [[Hyperpolarization (biology)|hyperpolarization]].<ref name="pmid19644451"/> | ||
The consumption of a high [[fructose]] diet from birth has been associated with a reduction in leptin levels and reduced expression of leptin receptor mRNA in rats. Long-term consumption of fructose in rats has been shown to increase levels of triglycerides and trigger leptin and insulin resistance,<ref name="pmid18703413">{{cite journal | vauthors = Shapiro A, Mu W, Roncal C, Cheng KY, Johnson RJ, Scarpace PJ | title = Fructose-induced leptin resistance exacerbates weight gain in response to subsequent high-fat feeding | journal = Am. J. Physiol. Regul. Integr. Comp. Physiol. | volume = 295 | issue = 5 | pages = | The consumption of a high [[fructose]] diet from birth has been associated with a reduction in leptin levels and reduced expression of leptin receptor mRNA in rats. Long-term consumption of fructose in rats has been shown to increase levels of triglycerides and trigger leptin and insulin resistance,<ref name="pmid18703413">{{cite journal | vauthors = Shapiro A, Mu W, Roncal C, Cheng KY, Johnson RJ, Scarpace PJ | title = Fructose-induced leptin resistance exacerbates weight gain in response to subsequent high-fat feeding | journal = Am. J. Physiol. Regul. Integr. Comp. Physiol. | volume = 295 | issue = 5 | pages = R1370–75 | date = November 2008 | pmid = 18703413 | pmc = 2584858 | doi = 10.1152/ajpregu.00195.2008 | url = }}</ref><ref name="pmid18784330">{{cite journal | vauthors = Vasselli JR | title = Fructose-induced leptin resistance: discovery of an unsuspected form of the phenomenon and its significance. Focus on "Fructose-induced leptin resistance exacerbates weight gain in response to subsequent high-fat feeding," by Shapiro et al | journal = Am. J. Physiol. Regul. Integr. Comp. Physiol. | volume = 295 | issue = 5 | pages = R1365–69 | date = November 2008 | pmid = 18784330 | doi = 10.1152/ajpregu.90674.2008 }}</ref> however, another study found that leptin resistance only developed in the presence of both high fructose and high fat levels in the diet. A third study found that high fructose levels reversed leptin resistance in rats given a high fat diet. The contradictory results mean that it is uncertain whether leptin resistance is caused by high levels of carbohydrates or fats, or if an increase of both, is needed.<ref name="pmid22496363">{{cite journal | vauthors = Harris RB, Apolzan JW | title = Changes in glucose tolerance and leptin responsiveness of rats offered a choice of lard, sucrose, and chow. | journal = Am J Physiol Regul Integr Comp Physiol | volume = 302 | issue = 11 | pages = R1327–39 | date = Jun 2012 | pmid = 22496363 | pmc = 3378343 | doi = 10.1152/ajpregu.00477.2011 }}</ref> | ||
Leptin is known to interact with [[amylin]], a hormone involved in gastric emptying and creating a feeling of fullness. When both leptin and amylin were given to obese, leptin-resistant rats, sustained weight loss was seen. Due to its apparent ability to reverse leptin resistance, amylin has been suggested as possible therapy for obesity.<ref name="pmid18458326">{{cite journal | vauthors = Roth JD, Roland BL, Cole RL, Trevaskis JL, Weyer C, Koda JE, Anderson CM, Parkes DG, Baron AD | title = Leptin responsiveness restored by amylin agonism in diet-induced obesity: evidence from nonclinical and clinical studies | journal = Proc. Natl. Acad. Sci. | Leptin is known to interact with [[amylin]], a hormone involved in gastric emptying and creating a feeling of fullness. When both leptin and amylin were given to obese, leptin-resistant rats, sustained weight loss was seen. Due to its apparent ability to reverse leptin resistance, amylin has been suggested as possible therapy for obesity.<ref name="pmid18458326">{{cite journal | vauthors = Roth JD, Roland BL, Cole RL, Trevaskis JL, Weyer C, Koda JE, Anderson CM, Parkes DG, Baron AD | title = Leptin responsiveness restored by amylin agonism in diet-induced obesity: evidence from nonclinical and clinical studies | journal = Proc. Natl. Acad. Sci. USA | volume = 105 | issue = 20 | pages = 7257–62 | date = May 2008 | pmid = 18458326 | pmc = 2438237 | doi = 10.1073/pnas.0706473105 | bibcode = 2008PNAS..105.7257R }}</ref> | ||
It has been suggested that the main role of leptin is to act as a starvation signal when levels are low, to help maintain fat stores for survival during times of starvation, rather than a satiety signal to prevent overeating. Leptin levels signal when an animal has enough stored energy to spend it in pursuits besides acquiring food.<ref name="pmid19644451">{{cite journal | vauthors = Oswal A, Yeo G | title = Leptin and the control of body weight: a review of its diverse central targets, signaling mechanisms, and role in the pathogenesis of obesity | journal = Obesity (Silver Spring) | volume = 18 | issue = 2 | pages = | It has been suggested that the main role of leptin is to act as a starvation signal when levels are low, to help maintain fat stores for survival during times of starvation, rather than a satiety signal to prevent overeating. Leptin levels signal when an animal has enough stored energy to spend it in pursuits besides acquiring food.<ref name="pmid19644451">{{cite journal | vauthors = Oswal A, Yeo G | title = Leptin and the control of body weight: a review of its diverse central targets, signaling mechanisms, and role in the pathogenesis of obesity | journal = Obesity (Silver Spring) | volume = 18 | issue = 2 | pages = 221–29 | date = February 2010 | pmid = 19644451 | doi = 10.1038/oby.2009.228 }}</ref><ref name="pmid16781741">{{cite journal | vauthors = Banks WA, Farr SA, Morley JE | title = The effects of high fat diets on the blood-brain barrier transport of leptin: failure or adaptation? | journal = Physiol. Behav. | volume = 88 | issue = 3 | pages = 244–48 | date = June 2006 | pmid = 16781741 | doi = 10.1016/j.physbeh.2006.05.037 }}</ref> This would mean that leptin resistance in obese people is a normal part of mammalian physiology and possibly, could confer a survival advantage.<ref name="pmid17937601"/> Leptin resistance (in combination with insulin resistance and weight gain) is seen in rats after they are given unlimited access to palatable, energy-dense foods.<ref name="pmid11723062">{{cite journal | vauthors = Wang J, Obici S, Morgan K, Barzilai N, Feng Z, Rossetti L | title = Overfeeding rapidly induces leptin and insulin resistance | journal = Diabetes | volume = 50 | issue = 12 | pages = 2786–91 | date = December 2001 | pmid = 11723062 | doi = 10.2337/diabetes.50.12.2786 }}</ref> This effect is reversed when the animals are put back on a low-energy diet.<ref name="pmid17339026">{{cite journal | vauthors = Enriori PJ, Evans AE, Sinnayah P, Jobst EE, Tonelli-Lemos L, Billes SK, Glavas MM, Grayson BE, Perello M, Nillni EA, Grove KL, Cowley MA | title = Diet-induced obesity causes severe but reversible leptin resistance in arcuate melanocortin neurons | journal = Cell Metab. | volume = 5 | issue = 3 | pages = 181–94 | date = March 2007 | pmid = 17339026 | doi = 10.1016/j.cmet.2007.02.004 }}</ref> This also may have an evolutionary advantage: allowing energy to be stored efficiently when food is plentiful would be advantageous in populations where food frequently may be scarce.<ref name="pmid12970158">{{cite journal | vauthors = Obici S, Rossetti L | title = Minireview: nutrient sensing and the regulation of insulin action and energy balance | journal = Endocrinology | volume = 144 | issue = 12 | pages = 5172–78 | date = December 2003 | pmid = 12970158 | doi = 10.1210/en.2003-0999 }}</ref> | ||
=== Response to weight loss === | === Response to weight loss === | ||
Dieters who lose weight, particularly those with an overabundance of fat cells, experience a drop in levels of circulating leptin. This drop causes reversible decreases in thyroid activity, sympathetic tone, and energy expenditure in skeletal muscle, and increases in muscle efficiency and parasympathetic tone. Many of these changes are reversed by peripheral administration ( intravenously into the veins of the arms, hands, legs, or feet ) of recombinant leptin to restore pre-diet levels.<ref name="Ahima_2008">{{cite journal | vauthors = Ahima RS | title = Revisiting leptin's role in obesity and weight loss | journal = J. Clin. Invest. | volume = 118 | issue = 7 | pages = 2380–83 | date = July 2008 | pmid = 18568083 | pmc = 2430504 | doi = 10.1172/JCI36284 }}</ref> | |||
Dieters who lose weight, particularly those with an overabundance of fat cells, experience a drop in levels of circulating leptin. This drop causes reversible decreases in thyroid activity, sympathetic tone, and energy expenditure in skeletal muscle, and increases in muscle efficiency and parasympathetic tone. | |||
A decline in levels of circulating leptin also changes brain activity in areas involved in the regulatory, emotional, and cognitive control of appetite that are reversed by administration of leptin.<ref name="Ahima_2008"/> | A decline in levels of circulating leptin also changes brain activity in areas involved in the regulatory, emotional, and cognitive control of appetite that are reversed by administration of leptin.<ref name="Ahima_2008"/> | ||
| Line 199: | Line 198: | ||
Osteoarthritis and obesity are closely linked. Obesity is one of the most important preventable factors for the development of osteoarthritis. | Osteoarthritis and obesity are closely linked. Obesity is one of the most important preventable factors for the development of osteoarthritis. | ||
Originally, the relationship between osteoarthritis and obesity was considered to be exclusively biomechanically based, according to which the excess weight caused the joint to become worn down more quickly. However, today we recognise that there is also a metabolic component which explains why obesity is a risk factor for osteoarthritis, not only for weight-bearing joints (for example, the knees), but also for joints that do not bear weight (for example, the hands).<ref name="pmid19487215">{{cite journal | vauthors = Yusuf E, Nelissen RG, Ioan-Facsinay A, Stojanovic-Susulic V, DeGroot J, van Osch G, Middeldorp S, Huizinga TW, Kloppenburg M | title = Association between weight or body mass index and hand osteoarthritis: a systematic review | journal = Annals of the Rheumatic Diseases | volume = 69 | issue = 4 | pages = | Originally, the relationship between osteoarthritis and obesity was considered to be exclusively biomechanically based, according to which the excess weight caused the joint to become worn down more quickly. However, today we recognise that there is also a metabolic component which explains why obesity is a risk factor for osteoarthritis, not only for weight-bearing joints (for example, the knees), but also for joints that do not bear weight (for example, the hands).<ref name="pmid19487215">{{cite journal | vauthors = Yusuf E, Nelissen RG, Ioan-Facsinay A, Stojanovic-Susulic V, DeGroot J, van Osch G, Middeldorp S, Huizinga TW, Kloppenburg M | title = Association between weight or body mass index and hand osteoarthritis: a systematic review | journal = Annals of the Rheumatic Diseases | volume = 69 | issue = 4 | pages = 761–65 | year = 2010 | pmid = 19487215 | doi = 10.1136/ard.2008.106930 }}</ref> Consequently, it has been shown that decreasing body fat lessens osteoarthritis to a greater extent than weight loss per se.<ref name="pmid20485173">{{cite journal | vauthors = Sowers MR, Karvonen-Gutierrez CA | title = The evolving role of obesity in knee osteoarthritis | journal = Current Opinion in Rheumatology | volume = 22 | issue = 5 | pages = 533–37 | year = 2010 | pmid = 20485173 | pmc = 3291123 | doi = 10.1097/BOR.0b013e32833b4682 }}</ref> This metabolic component related with the release of systemic factors, of a pro-inflammatory nature, by the adipose tissues, which frequently are critically associated with the development of osteoarthritis.<ref name="pmid11297982">{{cite journal | vauthors = Aspden RM, Scheven BA, Hutchison JD | title = Osteoarthritis as a systemic disorder including stromal cell differentiation and lipid metabolism | journal = Lancet | volume = 357 | issue = 9262 | pages = 1118–20 | year = 2001 | pmid = 11297982 | doi = 10.1016/S0140-6736(00)04264-1 }}</ref><ref name="pmid17038451">{{cite journal | vauthors = Pottie P, Presle N, Terlain B, Netter P, Mainard D, Berenbaum F | title = Obesity and osteoarthritis: more complex than predicted! | journal = Annals of the Rheumatic Diseases | volume = 65 | issue = 11 | pages = 1403–05 | year = 2006 | pmid = 17038451 | pmc = 1798356 | doi = 10.1136/ard.2006.061994 }}</ref><ref name="pmid18836239">{{cite journal | vauthors = Griffin TM, Guilak F | title = Why is obesity associated with osteoarthritis? Insights from mouse models of obesity | journal = Biorheology | volume = 45 | issue = 3-4 | pages = 387–98 | year = 2008 | pmid = 18836239 | pmc = 2748656 | doi = 10.3233/BIR-2008-0485 }}</ref><ref name="pmid19473582">{{cite journal | vauthors = Masuko K, Murata M, Suematsu N, Okamoto K, Yudoh K, Nakamura H, Kato T | title = A metabolic aspect of osteoarthritis: lipid as a possible contributor to the pathogenesis of cartilage degradation | journal = Clinical and Experimental Rheumatology | volume = 27 | issue = 2 | pages = 347–53 | year = 2009 | pmid = 19473582 | doi = }}</ref><ref name="pmid20480243">{{cite journal | vauthors = Hu PF, Bao JP, Wu LD | title = The emerging role of adipokines in osteoarthritis: a narrative review | journal = Molecular Biology Reports | volume = 38 | issue = 2 | pages = 873–78 | year = 2011 | pmid = 20480243 | doi = 10.1007/s11033-010-0179-y }}</ref> | ||
Thus, the deregulated production of adipokines and inflammatory mediators, hyperlipidaemia, and the increase of systemic oxidative stress are conditions frequently associated with obesity which can favour joint degeneration. Furthermore, many regulation factors have been implicated in the development, maintenance and function, both of adipose tissues, as well as of the cartilage and other joint tissues. Alterations in these factors can be the additional link between obesity and osteoarthritis. | Thus, the deregulated production of adipokines and inflammatory mediators, hyperlipidaemia, and the increase of systemic oxidative stress are conditions frequently associated with obesity which can favour joint degeneration. Furthermore, many regulation factors have been implicated in the development, maintenance and function, both of adipose tissues, as well as of the cartilage and other joint tissues. Alterations in these factors can be the additional link between obesity and osteoarthritis. | ||
| Line 206: | Line 205: | ||
Adipocytes interact with other cells through producing and secreting a variety of signalling molecules, including the cell signalling proteins known as adipokines. Certain adipokines can be considered as hormones, as they regulate the functions of organs at a distance, and several of them have been specifically involved in the physiopathology of joint diseases. In particular, there is one, leptin, which has been the focus of attention for research in recent years. | Adipocytes interact with other cells through producing and secreting a variety of signalling molecules, including the cell signalling proteins known as adipokines. Certain adipokines can be considered as hormones, as they regulate the functions of organs at a distance, and several of them have been specifically involved in the physiopathology of joint diseases. In particular, there is one, leptin, which has been the focus of attention for research in recent years. | ||
The circulating leptin levels are positively correlated with the Body Mass Index (BMI), more specifically with fatty mass, and obese individuals have higher leptin levels in their | The circulating leptin levels are positively correlated with the Body Mass Index (BMI), more specifically with fatty mass, and obese individuals have higher leptin levels in their blood circulation, compared with non-obese individuals.<ref name="pmid8532024" /> In obese individuals, the increased circulating leptin levels induce unwanted responses, that is, reduced food intake or losing body weight does not occur as there is a resistance to leptin (ref 9). In addition to the function of regulating energy homeostasis, leptin carries out a role in other physiological functions such as neuroendocrine communication, reproduction, angiogenesis and bone formation. More recently, leptin has been recognised as a cytokine factor as well as with pleiotropic actions also in the immune response and inflammation.<ref name="pmid22935803">{{cite journal | vauthors = Coppari R, Bjørbæk C | title = Leptin revisited: its mechanism of action and potential for treating diabetes | journal = Nature Reviews. Drug Discovery | volume = 11 | issue = 9 | pages = 692–708 | year = 2012 | pmid = 22935803 | pmc = 4019022 | doi = 10.1038/nrd3757 }}</ref><ref name="pmid17560812">{{cite journal | vauthors = Gualillo O | title = Further evidence for leptin involvement in cartilage homeostases | journal = Osteoarthritis and Cartilage | volume = 15 | issue = 8 | pages = 857–60 | year = 2007 | pmid = 17560812 | doi = 10.1016/j.joca.2007.04.015 }}</ref><ref name="pmid21252989">{{cite journal | vauthors = Ouchi N, Parker JL, Lugus JJ, Walsh K | title = Adipokines in inflammation and metabolic disease | journal = Nature Reviews. Immunology | volume = 11 | issue = 2 | pages = 85–97 | year = 2011 | pmid = 21252989 | pmc = 3518031 | doi = 10.1038/nri2921 }}</ref><ref name="pmid23906693">{{cite journal | vauthors = Scotece M, Conde J, Vuolteenaho K, Koskinen A, López V, Gómez-Reino J, Lago F, Moilanen E, Gualillo O | title = Adipokines as drug targets in joint and bone disease | journal = Drug Discovery Today | volume = 19 | issue = 3 | pages = 241–58 | year = 2014 | pmid = 23906693 | doi = 10.1016/j.drudis.2013.07.012 }}</ref> For example, leptin can be found in the synovial fluid in correlation with the body mass index, and the leptin receptors are expressed in the cartilage, where leptin mediates and modulates many inflammatory responses that can damage cartilage and other joint tissues. Leptin has thus emerged as a candidate to link obesity and osteoarthritis and serves as an apparent objective as a nutritional treatment for osteoarthritis. | ||
As in the plasma, the leptin levels in the synovial fluid are positively correlated with BMI.<ref name="Dumond_2003">{{cite journal | vauthors = Dumond H, Presle N, Terlain B, Mainard D, Loeuille D, Netter P, Pottie P | title = Evidence for a key role of leptin in osteoarthritis | journal = Arthritis and Rheumatism | volume = 48 | issue = 11 | pages = 3118–29 | year = 2003 | pmid = 14613274 | doi = 10.1002/art.11303 }}</ref><ref name="Simopoulou_2007">{{cite journal | vauthors = Simopoulou T, Malizos KN, Iliopoulos D, Stefanou N, Papatheodorou L, Ioannou M, Tsezou A | title = Differential expression of leptin and leptin's receptor isoform (Ob-Rb) mRNA between advanced and minimally affected osteoarthritic cartilage; effect on cartilage metabolism | journal = Osteoarthritis and Cartilage | volume = 15 | issue = 8 | pages = 872–83 | year = 2007 | pmid = 17350295 | doi = 10.1016/j.joca.2007.01.018 }}</ref><ref name="pmid22689314">{{cite journal | vauthors = Vuolteenaho K, Koskinen A, Moilanen T, Moilanen E | title = Leptin levels are increased and its negative regulators, SOCS-3 and sOb-R are decreased in obese patients with osteoarthritis: a link between obesity and osteoarthritis | journal = Annals of the Rheumatic Diseases | volume = 71 | issue = 11 | pages = | As in the plasma, the leptin levels in the synovial fluid are positively correlated with BMI.<ref name="Dumond_2003">{{cite journal | vauthors = Dumond H, Presle N, Terlain B, Mainard D, Loeuille D, Netter P, Pottie P | title = Evidence for a key role of leptin in osteoarthritis | journal = Arthritis and Rheumatism | volume = 48 | issue = 11 | pages = 3118–29 | year = 2003 | pmid = 14613274 | doi = 10.1002/art.11303 }}</ref><ref name="Simopoulou_2007">{{cite journal | vauthors = Simopoulou T, Malizos KN, Iliopoulos D, Stefanou N, Papatheodorou L, Ioannou M, Tsezou A | title = Differential expression of leptin and leptin's receptor isoform (Ob-Rb) mRNA between advanced and minimally affected osteoarthritic cartilage; effect on cartilage metabolism | journal = Osteoarthritis and Cartilage | volume = 15 | issue = 8 | pages = 872–83 | year = 2007 | pmid = 17350295 | doi = 10.1016/j.joca.2007.01.018 }}</ref><ref name="pmid22689314">{{cite journal | vauthors = Vuolteenaho K, Koskinen A, Moilanen T, Moilanen E | title = Leptin levels are increased and its negative regulators, SOCS-3 and sOb-R are decreased in obese patients with osteoarthritis: a link between obesity and osteoarthritis | journal = Annals of the Rheumatic Diseases | volume = 71 | issue = 11 | pages = 1912–13 | year = 2012 | pmid = 22689314 | doi = 10.1136/annrheumdis-2011-201242 }}</ref><ref name="pmid19780190">{{cite journal | vauthors = Gandhi R, Takahashi M, Syed K, Davey JR, Mahomed NN | title = Relationship between body habitus and joint leptin levels in a knee osteoarthritis population | journal = Journal of Orthopaedic Research : Official Publication of the Orthopaedic Research Society | volume = 28 | issue = 3 | pages = 329–33 | year = 2010 | pmid = 19780190 | doi = 10.1002/jor.21000 }}</ref> The leptin of the synovial fluid is synthesised at least partially in the joint and may originate in part in the circulation. Leptin has been shown to be produced by chondrocytes, as well as by other tissues in the joints, including the synovial tissue, osteophytes, the meniscus and bone.<ref name="Dumond_2003" /><ref name="Simopoulou_2007" /><ref name="pmid16527497">{{cite journal | vauthors = Presle N, Pottie P, Dumond H, Guillaume C, Lapicque F, Pallu S, Mainard D, Netter P, Terlain B | title = Differential distribution of adipokines between serum and synovial fluid in patients with osteoarthritis. Contribution of joint tissues to their articular production | journal = Osteoarthritis and Cartilage | volume = 14 | issue = 7 | pages = 690–95 | year = 2006 | pmid = 16527497 | doi = 10.1016/j.joca.2006.01.009 }}</ref><ref name="pmid15447688">{{cite journal | vauthors = Morroni M, De Matteis R, Palumbo C, Ferretti M, Villa I, Rubinacci A, Cinti S, Marotti G | title = In vivo leptin expression in cartilage and bone cells of growing rats and adult humans | journal = Journal of Anatomy | volume = 205 | issue = 4 | pages = 291–96 | year = 2004 | pmid = 15447688 | pmc = 1571344 | doi = 10.1111/j.0021-8782.2004.00333.x }}</ref><ref name="pmid18565249">{{cite journal | vauthors = Järvinen K, Vuolteenaho K, Nieminen R, Moilanen T, Knowles RG, Moilanen E | title = Selective iNOS inhibitor 1400W enhances anti-catabolic IL-10 and reduces destructive MMP-10 in OA cartilage. Survey of the effects of 1400W on inflammatory mediators produced by OA cartilage as detected by protein antibody array | journal = Clinical and Experimental Rheumatology | volume = 26 | issue = 2 | pages = 275–82 | year = 2008 | pmid = 18565249 | doi = }}</ref><ref name="Distel_2009">{{cite journal | vauthors = Distel E, Cadoudal T, Durant S, Poignard A, Chevalier X, Benelli C | title = The infrapatellar fat pad in knee osteoarthritis: an important source of interleukin-6 and its soluble receptor | journal = Arthritis and Rheumatism | volume = 60 | issue = 11 | pages = 3374–77 | year = 2009 | pmid = 19877065 | doi = 10.1002/art.24881 }}</ref> An infrapatellar fat pad located extrasynovially within the knee joint is also adjacent to the synovial membrane and cartilage, and has recently been highly appreciated as an important source of leptin, as well as other adipokines and mediators which contribute to the pathogenesis of osteoarthritis <ref name="Distel_2009" /><ref name="pmid20417297">{{cite journal | vauthors = Clockaerts S, Bastiaansen-Jenniskens YM, Runhaar J, Van Osch GJ, Van Offel JF, Verhaar JA, De Clerck LS, Somville J | title = The infrapatellar fat pad should be considered as an active osteoarthritic joint tissue: a narrative review | journal = Osteoarthritis and Cartilage | volume = 18 | issue = 7 | pages = 876–82 | year = 2010 | pmid = 20417297 | doi = 10.1016/j.joca.2010.03.014 }}</ref><ref name="pmid21242232">{{cite journal | vauthors = Klein-Wieringa IR, Kloppenburg M, Bastiaansen-Jenniskens YM, Yusuf E, Kwekkeboom JC, El-Bannoudi H, Nelissen RG, Zuurmond A, Stojanovic-Susulic V, Van Osch GJ, Toes RE, Ioan-Facsinay A | title = The infrapatellar fat pad of patients with osteoarthritis has an inflammatory phenotype | journal = Annals of the Rheumatic Diseases | volume = 70 | issue = 5 | pages = 851–57 | year = 2011 | pmid = 21242232 | doi = 10.1136/ard.2010.140046 }}</ref><ref name="pmid22072016">{{cite journal | vauthors = Hui W, Litherland GJ, Elias MS, Kitson GI, Cawston TE, Rowan AD, Young DA | title = Leptin produced by joint white adipose tissue induces cartilage degradation via upregulation and activation of matrix metalloproteinases | journal = Annals of the Rheumatic Diseases | volume = 71 | issue = 3 | pages = 455–62 | year = 2012 | pmid = 22072016 | doi = 10.1136/annrheumdis-2011-200372 }}</ref> | ||
The risk of suffering osteoarthritis can be decreased with weight loss. This reduction of risk is related in part with the decrease of the load on the joint, but also in the decrease of fatty mass, the central adipose tissue and the low-level inflammation associated with obesity and systemic factors. | The risk of suffering osteoarthritis can be decreased with weight loss. This reduction of risk is related in part with the decrease of the load on the joint, but also in the decrease of fatty mass, the central adipose tissue and the low-level inflammation associated with obesity and systemic factors. | ||
This growing evidence points to leptin as a cartilage degradation factor in the pathogenesis of osteoarthritis, and as a potential biomarker in the progression of the disease, which suggests that leptin, as well as regulation and signalling mechanisms, can be a new and promising target in the treatment of osteoarthritis, especially in obese patients. | This growing evidence points to leptin as a cartilage degradation factor in the pathogenesis of osteoarthritis, and as a potential biomarker in the progression of the disease, which suggests that leptin, as well as regulation and signalling mechanisms, can be a new and promising target in the treatment of osteoarthritis, especially in obese patients. | ||
| Line 222: | Line 221: | ||
=== Leptin === | === Leptin === | ||
Leptin was approved in the United States in 2014 for use in congenital leptin deficiency and generalized [[lipodystrophy]].<ref name="pmid24714458">{{cite journal | vauthors = Sinha G | title = Leptin therapy gains FDA approval | journal = Nat. Biotechnol. | volume = 32 | issue = 4 | pages = | Leptin was approved in the United States in 2014 for use in congenital leptin deficiency and generalized [[lipodystrophy]].<ref name="pmid24714458">{{cite journal | vauthors = Sinha G | title = Leptin therapy gains FDA approval | journal = Nat. Biotechnol. | volume = 32 | issue = 4 | pages = 300–02 | year = 2014 | pmid = 24714458 | doi = 10.1038/nbt0414-300b }}</ref> | ||
=== Analog metreleptin=== | === Analog metreleptin=== | ||
{{main|Metreleptin}} | {{main|Metreleptin}} | ||
An analog of human leptin [[metreleptin]] (trade | An analog of human leptin [[metreleptin]] (trade names Mylept, Mylepta) was first approved in Japan in 2013, and in the United States in February 2014 and in Europe in 2018. In the US it is indicated as a treatment for complications of leptin deficiency, and for the diabetes and [[hypertriglyceridemia]] associated with congenital or acquired generalized [[lipodystrophy]].<ref>{{cite journal | vauthors = Chou K, Perry CM | title = Metreleptin: first global approval | journal = Drugs | volume = 73 | issue = 9 | pages = 989–97 | year = 2013 | pmid = 23740412 | doi = 10.1007/s40265-013-0074-7 }}</ref><ref name="fda">{{cite web | url=http://www.fda.gov/newsevents/newsroom/pressannouncements/ucm387060.htm | title=FDA approves Myalept to treat rare metabolic disease | publisher=FDA | date=25 February 2014 | accessdate=30 April 2014}}</ref> In Europe based on [[European Medicines Agency|EMA]], metreleptin should be used in addition to diet to treat lipodystrophy, where patients have loss of fatty tissue under the skin and build-up of fat elsewhere in the body such as in the liver and muscles. The medicine is used in: adults and children above the age of 2 years with [[Generalized lipodystrophy|generalised lipodystrophy]] ([[Berardinelli-Seip syndrome]] and [[Acquired generalized lipodystrophy|Lawrence syndrome]]) and in adults and children above the age of 12 years with [[partial lipodystrophy]] (including [[Barraquer–Simons syndrome|Barraquer-Simons syndrome]]), when standard treatments have failed.<ref>{{Cite web|url=https://www.ema.europa.eu/en/medicines/human/EPAR/myalepta|title=Myalepta {{!}} European Medicines Agency|website=www.ema.europa.eu|access-date=2019-01-09}}</ref> | ||
== | == History == | ||
{{ | The leptine was discovered by Jeffrey Freidman in 1994 after several decades of research conducted by others institutions since 1950 on obese mouse models <ref>{{Cite web|url=http://centennial.rucares.org/index.php?page=Discovery_Leptin|title=The Rockefeller University » Hospital Centennial|website=centennial.rucares.org|language=en|access-date=2018-10-11}}</ref> | ||
== See also == | == See also == | ||
* [[NAPEs]] | * [[NAPEs]] | ||
* [[Teleost leptins]] | * [[Teleost leptins]] | ||
==Notes== | |||
{{notelist}} | |||
== References == | == References == | ||
{{reflist | {{reflist}} | ||
== External links == | == External links == | ||
*[http://neuroendo.org.uk/index.php/content/view/8/11/ Leptin: Your brain, appetite and obesity by the British Society of Neuroendocrinology] | *[https://web.archive.org/web/20060627212342/http://neuroendo.org.uk/index.php/content/view/8/11/ Leptin: Your brain, appetite and obesity by the British Society of Neuroendocrinology] | ||
*[http://www.vivo.colostate.edu/hbooks/pathphys/endocrine/bodyweight/leptin.html Leptin by Colorado State University] | *[http://www.vivo.colostate.edu/hbooks/pathphys/endocrine/bodyweight/leptin.html Leptin by Colorado State University] – nice illustrations, but last updated 1998 | ||
*[http://www.3dchem.com/molecules.asp?ID=154 Leptin at 3Dchem.com], description and structure diagrams | *[http://www.3dchem.com/molecules.asp?ID=154 Leptin at 3Dchem.com], description and structure diagrams | ||
Latest revision as of 08:28, 11 January 2019
| VALUE_ERROR (nil) | |||||||
|---|---|---|---|---|---|---|---|
| Identifiers | |||||||
| Aliases | |||||||
| External IDs | GeneCards: [1] | ||||||
| Orthologs | |||||||
| Species | Human | Mouse | |||||
| Entrez |
|
| |||||
| Ensembl |
|
| |||||
| UniProt |
|
| |||||
| RefSeq (mRNA) |
|
| |||||
| RefSeq (protein) |
|
| |||||
| Location (UCSC) | n/a | n/a | |||||
| PubMed search | n/a | n/a | |||||
| Wikidata | |||||||
| |||||||
| Leptin | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| File:PDB 1ax8 EBI.jpg Structure of the obese protein leptin-E100.[1] | |||||||||
| Identifiers | |||||||||
| Symbol | Leptin | ||||||||
| Pfam | PF02024 | ||||||||
| Pfam clan | CL0053 | ||||||||
| InterPro | IPR000065 | ||||||||
| SCOP | 1ax8 | ||||||||
| SUPERFAMILY | 1ax8 | ||||||||
| |||||||||
Leptin (from Greek λεπτός leptos, "thin"), "the hormone of energy expenditure",[lower-alpha 1] is a hormone predominantly made by adipose cells that helps to regulate energy balance by inhibiting hunger. Leptin is opposed by the actions of the hormone ghrelin, the "hunger hormone". Both hormones act on receptors in the arcuate nucleus of the hypothalamus.[2] In obesity, a decreased sensitivity to leptin occurs (similar to insulin resistance in type 2 diabetes), resulting in an inability to detect satiety despite high energy stores and high levels of leptin.[3]
Although regulation of fat stores is deemed to be the primary function of leptin, it also plays a role in other physiological processes, as evidenced by its many sites of synthesis other than fat cells, and the many cell types beside hypothalamic cells that have leptin receptors. Many of these additional functions are yet to be defined.[4][5][6][7][8][9]
Identification of the encoding gene
In 1949, a non-obese mouse colony being studied at the Jackson Laboratory produced a strain of obese offspring, suggesting that a mutation had occurred in a hormone regulating hunger and energy expenditure. Mice homozygous for the so-called ob mutation (ob/ob) ate voraciously and were massively obese.[10] In the 1960s, a second mutation causing obesity and a similar phenotype was identified by Douglas Coleman, also at the Jackson Laboratory, and was named diabetes (db), as both ob/ob and db/db were obese.[11][12][13] In 1990 Rudolph Leibel and Jeffrey M. Friedman reported mapping of the db gene.[14][15][16]
Consistent with Coleman’s and Leibel's hypothesis, several subsequent studies from Leibel's and Friedman’s labs and other groups confirmed that the ob gene encoded a novel hormone that circulated in blood and that could suppress food intake and body weight in ob and wild type mice, but not in db mice.[4][5][6][7]
In 1994, Friedman's laboratory reported the identification of the gene.[13] In 1995, Jose F. Caro's laboratory provided evidence that the mutations in the mouse ob gene did not occur in humans. Furthermore, since ob gene expression was increased, not decreased, in human obesity, it suggested resistance to leptin to be a possibility.[8] At the suggestion of Roger Guillemin, Friedman named this new hormone "leptin" from the Greek lepto meaning thin.[4][17] Leptin was the first fat cell-derived hormone (adipokine) to be discovered.[18]
Subsequent studies in 1995 confirmed that the db gene encodes the leptin receptor, and that it is expressed in the hypothalamus, a region of the brain known to regulate the sensation of hunger and body weight.[19][20][21][22]
Recognition of scientific advances
Coleman and Friedman have been awarded numerous prizes acknowledging their roles in discovery of leptin, including the Gairdner Foundation International Award (2005),[23] the Shaw Prize (2009),[24] the Lasker Award,[25] the BBVA Foundation Frontiers of Knowledge Award[26] and the King Faisal International Prize,[27] Leibel has not received the same level of recognition from the discovery because he was omitted as a co-author of a scientific paper published by Friedman that reported the discovery of the gene. The various theories surrounding Friedman’s omission of Leibel and others as co-authors of this paper have been presented in a number of publications, including Ellen Ruppel Shell’s 2002 book The Hungry Gene.[28][29]
The discovery of leptin also is documented in a series of books including Fat: Fighting the Obesity Epidemic by Robert Pool,[30] The Hungry Gene by Ellen Ruppel Shell, and Rethinking Thin: The New Science of Weight Loss and the Myths and Realities of Dieting by Gina Kolata.[31][32] Fat: Fighting the Obesity Epidemic and Rethinking Thin: The New Science of Weight Loss and the Myths and Realities of Dieting review the work in the Friedman laboratory that led to the cloning of the ob gene, while The Hungry Gene draws attention to the contributions of Leibel.[citation needed]
Location of gene and structure of hormone
The Ob(Lep) gene (Ob for obese, Lep for leptin) is located on chromosome 7 in humans.[33] Human leptin is a 16-kDa protein of 167 amino acids.
Mutations
A human mutant leptin was first described in 1997,[34] and subsequently six additional mutations were described. All of those affected were from Eastern countries; and all had variants of leptin not detected by the standard immunoreactive technique, so leptin levels were low or undetectable. The most recently described eighth mutation reported in January 2015, in a child with Turkish parents, is unique in that it is detected by the standard immunoreactive technique, where leptin levels are elevated; but the leptin does not turn on the leptin receptor, hence the patient has functional leptin deficiency.[35] These eight mutations all cause extreme obesity in infancy, with hyperphagia.[35]
Nonsense
A nonsense mutation in the leptin gene that results in a stop codon and lack of leptin production was first observed in mice in 1950. In the mouse gene, arginine-105 is encoded by CGA and only requires one nucleotide change to create the stop codon TGA. The corresponding amino acid in humans is encoded by the sequence CGG and would require two nucleotides to be changed to produce a stop codon, which is much less likely to happen.[8]
Frameshift
A recessive frameshift mutation resulting in a reduction of leptin has been observed in two consanguineous children with juvenile obesity.
Polymorphisms
A Human Genome Equivalent (HuGE) review in 2004 looked at studies of the connection between genetic mutations affecting leptin regulation and obesity. They reviewed a common polymorphism in the leptin gene (A19G; frequency 0.46), three mutations in the leptin receptor gene (Q223R, K109R and K656N) and two mutations in the PPARG gene (P12A and C161T). They found no association between any of the polymorphisms and obesity.[36]
A 2006 study found a link between the common LEP-2548 G/A genotype and morbid obesity in Taiwanese aborigines,[37][38] but a 2014 meta-analysis did not,[38] however, this polymorphism has been associated with weight gain in patients taking antipsychotics.[39][40][41]
The LEP-2548 G/A polymorphism has been linked with an increased risk of prostate cancer,[42] gestational diabetes,[43] and osteoporosis.[44]
Other rare polymorphisms have been found but their association with obesity are not consistent.[36]
Transversion
A single case of a homozygous transversion mutation of the gene encoding for leptin was reported in January 2015.[35] It leads to functional leptin deficiency with high leptin levels in circulation. The transversion of (c.298G → T) changed aspartic acid to tyrosine at position 100 (p.D100Y). The mutant leptin could neither bind to nor activate the leptin receptor in vitro, nor in leptin-deficient mice in vivo. It was found in a two-year-old boy with extreme obesity with recurrent ear and pulmonary infections. Treatment with metreleptin led to "rapid change in eating behavior, a reduction in daily energy intake, and substantial weight loss".[35]
Sites of synthesis
Leptin is produced primarily in the adipocytes of white adipose tissue. It also is produced by brown adipose tissue, placenta (syncytiotrophoblasts), ovaries, skeletal muscle, stomach (the lower part of the fundic glands), mammary epithelial cells, bone marrow,[45]gastric chief cells and P/D1 cells.[46]
Blood levels
Leptin circulates in blood in free form and bound to proteins.[47]
Physiologic variation
Leptin levels vary exponentially, not linearly, with fat mass.[48][49] Leptin levels in blood are higher between midnight and early morning, perhaps suppressing appetite during the night.[50] The diurnal rhythm of blood leptin levels may be modified by meal-timing.[51]
In specific conditions
In humans, many instances are seen where leptin dissociates from the strict role of communicating nutritional status between body and brain and no longer correlates with body fat levels:
- Leptin plays a critical role in the adaptive response to starvation.[52][53]
- Leptin level is decreased after short-term fasting (24–72 hours), even when changes in fat mass are not observed.[54][55][56]
- Serum level of leptin is reduced by sleep deprivation.[57][58]
- Leptin levels are paradoxically increased in obesity.[59]
Mutant leptins
All known leptin mutations except one are associated with low to undetectable immunoreactive leptin blood levels. The exception is a mutant leptin reported in January 2015 which is not functional, but is detected with standard immunoreactive methods. It was found in a massively obese 2-1/2-year-old boy who had high levels of circulating leptin which had no effect on leptin receptors, so he was functionally leptin-deficient.[35]
Effects
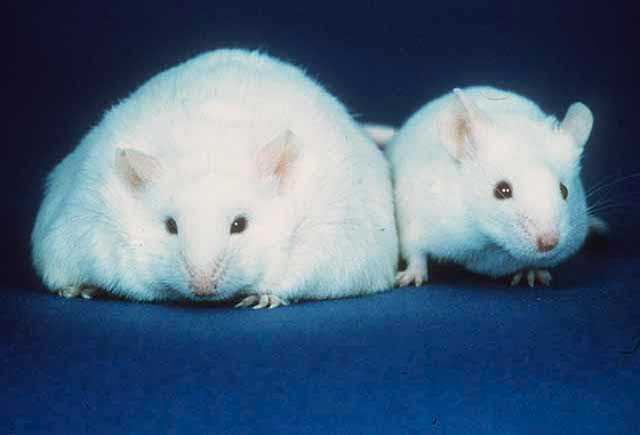
Predominantly, the "energy expenditure hormone" leptin is made by adipose cells, thus it is labeled fat cell-specific. In the context of its effects, it is important to recognize that the short describing words direct, central, and primary are not used interchangeably. In regard to the hormone leptin, central vs peripheral refers to the hypothalamic portion of the brain vs non-hypothalamic location of action of leptin; direct vs indirect refers to whether there is no intermediary, or there is an intermediary in the mode of action of leptin; and primary vs secondary is an arbitrary description of a particular function of leptin.[69]
- Location of action
- Leptin acts directly on leptin receptors in the cell membrane of different types of cells in the human body in particular, and in vertebrates in general. The leptin receptor is found on a wide range of cell types. It is a single-transmembrane-domain type I cytokine receptor,[70] a special class of cytokine receptors. Further, leptin interacts with other hormones and energy regulators, indirectly mediating the effects of: insulin, glucagon, insulin-like growth factor, growth hormone, glucocorticoids, cytokines, and metabolites.[45]
- Mode of action
- The central location of action (effect) of the fat cell-specific hormone leptin is the hypothalamus, a part of the brain, which is a part of the central nervous system. Non-hypothalamic targets of leptin are referred to as peripheral targets. There is a different relative importance of central and peripheral leptin interactions under different physiologic states, and variations between species.[45]
- Function
- The primary function of the hormone leptin is the regulation of adipose tissue mass through central hypothalamus mediated effects on hunger, food energy use, physical exercise and energy balance. Outside the brain, in the periphery of the body, leptin's secondary functions are: modulation of energy expenditure, modulation between fetal and maternal metabolism, and that of a permissive factor in puberty, activator of immune cells, activator of beta islet cells, and growth factor.
Central nervous system
In vertebrates, the nervous system consists of two main parts, the central nervous system (CNS) and the peripheral nervous system (PNS). The primary effect of leptins is in the hypothalamus, a part of the central nervous system. Leptin receptors are expressed not only in the hypothalamus but also in other brain regions, particularly in the hippocampus. Thus some leptin receptors in the brain are classified as central (hypothalamic) and some as peripheral (non-hypothalamic).
As scientifically known so far, the general effects of leptin in the central nervous system are:
- Deficiency of leptin has been shown to alter brain proteins and neuronal functions of obese mice which can be restored by leptin injection.[71]
- In humans, low circulating plasma leptin has been associated with cognitive changes associated with anorexia,[72] depression, and Alzheimer’s Disease .[73]
- Studies in transgenic mouse models of Alzheimer's disease have shown that chronic administration of leptin can ameliorate brain pathology and improve cognitive performance,[74] by reducing b-amyloid and hyperphosphorylated Tau,[75][76] two hallmarks of Alzheimer's pathology.
Generally, leptin is thought to enter the brain at the choroid plexus, where the intense expression of a form of leptin receptor molecule could act as a transport mechanism.[77]
Increased levels of melatonin causes a downregulation of leptin,[78] however, melatonin also appears to increase leptin levels in the presence of insulin, therefore causing a decrease in appetite during sleeping.[79] Partial sleep deprivation has also been associated with decreased leptin levels.[80]
Mice with type 1 diabetes treated with leptin or leptin plus insulin, compared to insulin alone had better metabolic profiles: blood sugar did not fluctuate so much; cholesterol levels decreased; less body fat formed.[81]
Hypothalamus
Leptin acts on receptors in the lateral hypothalamus to inhibit hunger and the medial hypothalamus to stimulate satiety.[82]
- In the lateral hypothalamus, leptin inhibits hunger[83] by
- counteracting the effects of neuropeptide Y, a potent hunger promoter secreted by cells in the gut and in the hypothalamus
- counteracting the effects of anandamide, another potent hunger promoter that binds to the same receptors as THC
- In the medial hypothalamus, leptin stimulates satiety[84] by
- promoting the synthesis of α-MSH, a hunger suppressant
Thus, a lesion in the lateral hypothalamus causes anorexia (due to a lack of hunger signals) and a lesion in the medial hypothalamus causes excessive hunger (due to a lack of satiety signals).[82] This appetite inhibition is long-term, in contrast to the rapid inhibition of hunger by cholecystokinin (CCK) and the slower suppression of hunger between meals mediated by PYY3-36. The absence of leptin (or its receptor) leads to uncontrolled hunger and resulting obesity. Fasting or following a very-low-calorie diet lowers leptin levels.[85][86][87][88] Leptin levels change more when food intake decreases than when it increases.[89] The dynamics of leptin due to an acute change in energy balance may be related to appetite and eventually, to food intake rather than fat stores.[90][91]
- It controls food intake and energy expenditure by acting on receptors in the mediobasal hypothalamus.[92]
Leptin binds to neuropeptide Y (NPY) neurons in the arcuate nucleus in such a way as to decrease the activity of these neurons. Leptin signals to the hypothalamus which produces a feeling of satiety. Moreover, leptin signals may make it easier for people to resist the temptation of foods high in calories.[93]
Leptin receptor activation inhibits neuropeptide Y and agouti-related peptide (AgRP), and activates α-melanocyte-stimulating hormone (α-MSH). The NPY neurons are a key element in the regulation of hunger; small doses of NPY injected into the brains of experimental animals stimulates feeding, while selective destruction of the NPY neurons in mice causes them to become anorexic. Conversely, α-MSH is an important mediator of satiety, and differences in the gene for the α-MSH receptor are linked to obesity in humans.
Leptin interacts with six types of receptors (Ob-Ra–Ob-Rf, or LepRa-LepRf), which in turn are encoded by a single gene, LEPR.[94] Ob-Rb is the only receptor isoform that can signal intracellularly via the Jak-Stat and MAPK signal transduction pathways,[95] and is present in hypothalamic nuclei.[96]
Once leptin has bound to the Ob-Rb receptor, it activates the stat3, which is phosphorylated and travels to the nucleus to effect changes in gene expression, one of the main effects being the down-regulation of the expression of endocannabinoids, responsible for increasing hunger.[97] In response to leptin, receptor neurons have been shown to remodel themselves, changing the number and types of synapses that fire onto them.
Circulatory system
The role of leptin/leptin receptors in modulation of T cell activity in the immune system was shown in experimentation with mice. It modulates the immune response to atherosclerosis, of which obesity is a predisposing factor.[98]
Exogenous leptin can promote angiogenesis by increasing vascular endothelial growth factor levels.
Hyperleptinemia produced by infusion or adenoviral gene transfer decreases blood pressure in rats.[99][100]
Leptin microinjections into the nucleus of the solitary tract (NTS) have been shown to elicit sympathoexcitatory responses, and potentiate the cardiovascular responses to activation of the chemoreflex.[101]
Fetal lung
In fetal lung, leptin is induced in the alveolar interstitial fibroblasts ("lipofibroblasts") by the action of PTHrP secreted by formative alveolar epithelium (endoderm) under moderate stretch. The leptin from the mesenchyme, in turn, acts back on the epithelium at the leptin receptor carried in the alveolar type II pneumocytes and induces surfactant expression, which is one of the main functions of these type II pneumocytes.[102]
Reproductive system
Ovulatory cycle
In mice, and to a lesser extent in humans, leptin is required for male and female fertility. Ovulatory cycles in females are linked to energy balance (positive or negative depending on whether a female is losing or gaining weight) and energy flux (how much energy is consumed and expended) much more than energy status (fat levels). When energy balance is highly negative (meaning the woman is starving) or energy flux is very high (meaning the woman is exercising at extreme levels, but still consuming enough calories), the ovarian cycle stops and females stop menstruating. Only if a female has an extremely low body fat percentage does energy status affect menstruation. Leptin levels outside an ideal range may have a negative effect on egg quality and outcome during in vitro fertilization.[103] Leptin is involved in reproduction by stimulating gonadotropin-releasing hormone from the hypothalamus.[104]
Pregnancy
The placenta produces leptin.[105] Leptin levels rise during pregnancy and fall after childbirth. Leptin is also expressed in fetal membranes and the uterine tissue. Uterine contractions are inhibited by leptin.[106] Leptin plays a role in hyperemesis gravidarum (severe morning sickness of pregnancy),[107] in polycystic ovary syndrome[108] and hypothalamic leptin is implicated in bone growth in mice.[109]
Lactation
Immunoreactive leptin has been found in human breast milk; and leptin from mother's milk has been found in the blood of suckling infant animals.[110]
Puberty
Leptin along with kisspeptin controls the onset of puberty.[111] High levels of leptin, as usually observed in obese females, can trigger neuroendocrine cascade resulting in early menarche.[112] This may eventually lead to shorter stature as oestrogen secretion starts during menarche and causes early closure of epiphyses.
Bone
Leptin's ability to regulate bone mass was first recognized in 2000.[113] Leptin can affect bone metabolism via direct signalling from the brain. Leptin decreases cancellous bone, but increases cortical bone. This "cortical-cancellous dichotomy" may represent a mechanism for enlarging bone size, and thus bone resistance, to cope with increased body weight.[114]
Bone metabolism can be regulated by central sympathetic outflow, since sympathetic pathways innervate bone tissue.[115] A number of brain-signalling molecules (neuropeptides and neurotransmitters) have been found in bone, including adrenaline, noradrenaline, serotonin, calcitonin gene-related peptide, vasoactive intestinal peptide and neuropeptide Y.[115][116] Leptin binds to its receptors in the hypothalamus, where it acts through the sympathetic nervous system to regulate bone metabolism.[117] Leptin may also act directly on bone metabolism via a balance between energy intake and the IGF-I pathway.[114][118] There is a potential for treatment of diseases of bone formation - such as impaired fracture healing - with leptin.[119]
Immune system
Factors that acutely affect leptin levels are also factors that influence other markers of inflammation, e.g., testosterone, sleep, emotional stress, caloric restriction, and body fat levels. While it is well-established that leptin is involved in the regulation of the inflammatory response,[120][121][122] it has been further theorized that leptin's role as an inflammatory marker is to respond specifically to adipose-derived inflammatory cytokines.
In terms of both structure and function, leptin resembles IL-6 and is a member of the cytokine superfamily.[1][121][123] Circulating leptin seems to affect the HPA axis, suggesting a role for leptin in stress response.[124] Elevated leptin concentrations are associated with elevated white blood cell counts in both men and women.[125]
Similar to what is observed in chronic inflammation, chronically elevated leptin levels are associated with obesity, overeating, and inflammation-related diseases, including hypertension, metabolic syndrome, and cardiovascular disease. While leptin is associated with body fat mass, however, the size of individual fat cells, and the act of overeating, it is interesting that it is not affected by exercise (for comparison, IL-6 is released in response to muscular contractions). Thus, it is speculated that leptin responds specifically to adipose-derived inflammation.[126] Leptin is a pro-angiogenic, pro-inflammatory and mitogenic factor, the actions of which are reinforced through crosstalk with IL-1 family cytokines in cancer.[127]
Taken as such, increases in leptin levels (in response to caloric intake) function as an acute pro-inflammatory response mechanism to prevent excessive cellular stress induced by overeating. When high caloric intake overtaxes the ability of fat cells to grow larger or increase in number in step with caloric intake, the ensuing stress response leads to inflammation at the cellular level and ectopic fat storage, i.e., the unhealthy storage of body fat within internal organs, arteries, and/or muscle. The insulin increase in response to the caloric load provokes a dose-dependent rise in leptin, an effect potentiated by high cortisol levels.[128] (This insulin-leptin relationship is notably similar to insulin's effect on the increase of IL-6 gene expression and secretion from preadipocytes in a time- and dose-dependent manner.)[129] Furthermore, plasma leptin concentrations have been observed to gradually increase when acipimox is administered to prevent lipolysis, concurrent hypocaloric dieting and weight loss notwithstanding.[130] Such findings appear to demonstrate high caloric loads in excess of storage rate capacities of fat cells lead to stress responses that induce an increase in leptin, which then operates as an adipose-derived inflammation stopgap signaling for the cessation of food intake so as to prevent adipose-derived inflammation from reaching elevated levels. This response may then protect against the harmful process of ectopic fat storage, which perhaps explains the connection between chronically elevated leptin levels and ectopic fat storage in obese individuals.[59]
Role in obesity and weight loss
Obesity
Although leptin reduces appetite as a circulating signal, obese individuals generally exhibit a higher circulating concentration of leptin than normal weight individuals due to their higher percentage body fat.[9] These people show resistance to leptin, similar to resistance of insulin in type 2 diabetes, with the elevated levels failing to control hunger and modulate their weight. A number of explanations have been proposed to explain this. An important contributor to leptin resistance is changes to leptin receptor signalling, particularly in the arcuate nucleus, however, deficiency of, or major changes to, the leptin receptor itself are not thought to be a major cause. Other explanations suggested include changes to the way leptin crosses the blood brain barrier (BBB) or alterations occurring during development.[131]
Studies on leptin cerebrospinal fluid (CSF) levels provide evidence for the reduction in leptin crossing the BBB and reaching obesity-relevant targets, such as the hypothalamus, in obese people.[132] In humans it has been observed that the ratio of leptin in the CSF compared to the blood is lower in obese people than in people of a normal weight.[133] The reason for this may be high levels of triglycerides affecting the transport of leptin across the BBB or due to the leptin transporter becoming saturated.[132] Although deficits in the transfer of leptin from the plasma to the CSF is seen in obese people, they are still found to have 30% more leptin in their CSF than lean individuals.[133] These higher CSF levels fail to prevent their obesity. Since the amount and quality of leptin receptors in the hypothalamus appears to be normal in the majority of obese humans (as judged from leptin-mRNA studies),[134] it is likely that the leptin resistance in these individuals is due to a post leptin-receptor deficit, similar to the post-insulin receptor defect seen in type 2 diabetes.[135]
When leptin binds with the leptin receptor, it activates a number of pathways. Leptin resistance may be caused by defects in one or more part of this process, particularly the JAK/STAT pathway. Mice with a mutation in the leptin receptor gene that prevents the activation of STAT3 are obese and exhibit hyperphagia. The PI3K pathway may also be involved in leptin resistance, as has been demonstrated in mice by artificial blocking of PI3K signalling. The PI3K pathway also is activated by the insulin receptor and is therefore an important area where leptin and insulin act together as part of energy homeostasis. The insulin-pI3K pathway can cause POMC neurons to become insensitive to leptin through hyperpolarization.[136]
The consumption of a high fructose diet from birth has been associated with a reduction in leptin levels and reduced expression of leptin receptor mRNA in rats. Long-term consumption of fructose in rats has been shown to increase levels of triglycerides and trigger leptin and insulin resistance,[137][138] however, another study found that leptin resistance only developed in the presence of both high fructose and high fat levels in the diet. A third study found that high fructose levels reversed leptin resistance in rats given a high fat diet. The contradictory results mean that it is uncertain whether leptin resistance is caused by high levels of carbohydrates or fats, or if an increase of both, is needed.[139]
Leptin is known to interact with amylin, a hormone involved in gastric emptying and creating a feeling of fullness. When both leptin and amylin were given to obese, leptin-resistant rats, sustained weight loss was seen. Due to its apparent ability to reverse leptin resistance, amylin has been suggested as possible therapy for obesity.[140]
It has been suggested that the main role of leptin is to act as a starvation signal when levels are low, to help maintain fat stores for survival during times of starvation, rather than a satiety signal to prevent overeating. Leptin levels signal when an animal has enough stored energy to spend it in pursuits besides acquiring food.[136][141] This would mean that leptin resistance in obese people is a normal part of mammalian physiology and possibly, could confer a survival advantage.[131] Leptin resistance (in combination with insulin resistance and weight gain) is seen in rats after they are given unlimited access to palatable, energy-dense foods.[142] This effect is reversed when the animals are put back on a low-energy diet.[143] This also may have an evolutionary advantage: allowing energy to be stored efficiently when food is plentiful would be advantageous in populations where food frequently may be scarce.[144]
Response to weight loss
Dieters who lose weight, particularly those with an overabundance of fat cells, experience a drop in levels of circulating leptin. This drop causes reversible decreases in thyroid activity, sympathetic tone, and energy expenditure in skeletal muscle, and increases in muscle efficiency and parasympathetic tone. Many of these changes are reversed by peripheral administration ( intravenously into the veins of the arms, hands, legs, or feet ) of recombinant leptin to restore pre-diet levels.[145]
A decline in levels of circulating leptin also changes brain activity in areas involved in the regulatory, emotional, and cognitive control of appetite that are reversed by administration of leptin.[145]
Role in joint problems and obesity
Obesity and osteoarthritis
Osteoarthritis and obesity are closely linked. Obesity is one of the most important preventable factors for the development of osteoarthritis.
Originally, the relationship between osteoarthritis and obesity was considered to be exclusively biomechanically based, according to which the excess weight caused the joint to become worn down more quickly. However, today we recognise that there is also a metabolic component which explains why obesity is a risk factor for osteoarthritis, not only for weight-bearing joints (for example, the knees), but also for joints that do not bear weight (for example, the hands).[146] Consequently, it has been shown that decreasing body fat lessens osteoarthritis to a greater extent than weight loss per se.[147] This metabolic component related with the release of systemic factors, of a pro-inflammatory nature, by the adipose tissues, which frequently are critically associated with the development of osteoarthritis.[148][149][150][151][152]
Thus, the deregulated production of adipokines and inflammatory mediators, hyperlipidaemia, and the increase of systemic oxidative stress are conditions frequently associated with obesity which can favour joint degeneration. Furthermore, many regulation factors have been implicated in the development, maintenance and function, both of adipose tissues, as well as of the cartilage and other joint tissues. Alterations in these factors can be the additional link between obesity and osteoarthritis.
Leptin and osteoarthritis
Adipocytes interact with other cells through producing and secreting a variety of signalling molecules, including the cell signalling proteins known as adipokines. Certain adipokines can be considered as hormones, as they regulate the functions of organs at a distance, and several of them have been specifically involved in the physiopathology of joint diseases. In particular, there is one, leptin, which has been the focus of attention for research in recent years.
The circulating leptin levels are positively correlated with the Body Mass Index (BMI), more specifically with fatty mass, and obese individuals have higher leptin levels in their blood circulation, compared with non-obese individuals.[9] In obese individuals, the increased circulating leptin levels induce unwanted responses, that is, reduced food intake or losing body weight does not occur as there is a resistance to leptin (ref 9). In addition to the function of regulating energy homeostasis, leptin carries out a role in other physiological functions such as neuroendocrine communication, reproduction, angiogenesis and bone formation. More recently, leptin has been recognised as a cytokine factor as well as with pleiotropic actions also in the immune response and inflammation.[153][154][155][156] For example, leptin can be found in the synovial fluid in correlation with the body mass index, and the leptin receptors are expressed in the cartilage, where leptin mediates and modulates many inflammatory responses that can damage cartilage and other joint tissues. Leptin has thus emerged as a candidate to link obesity and osteoarthritis and serves as an apparent objective as a nutritional treatment for osteoarthritis.
As in the plasma, the leptin levels in the synovial fluid are positively correlated with BMI.[157][158][159][160] The leptin of the synovial fluid is synthesised at least partially in the joint and may originate in part in the circulation. Leptin has been shown to be produced by chondrocytes, as well as by other tissues in the joints, including the synovial tissue, osteophytes, the meniscus and bone.[157][158][161][162][163][164] An infrapatellar fat pad located extrasynovially within the knee joint is also adjacent to the synovial membrane and cartilage, and has recently been highly appreciated as an important source of leptin, as well as other adipokines and mediators which contribute to the pathogenesis of osteoarthritis [164][165][166][167]
The risk of suffering osteoarthritis can be decreased with weight loss. This reduction of risk is related in part with the decrease of the load on the joint, but also in the decrease of fatty mass, the central adipose tissue and the low-level inflammation associated with obesity and systemic factors.
This growing evidence points to leptin as a cartilage degradation factor in the pathogenesis of osteoarthritis, and as a potential biomarker in the progression of the disease, which suggests that leptin, as well as regulation and signalling mechanisms, can be a new and promising target in the treatment of osteoarthritis, especially in obese patients.
Obese individuals are predisposed to developing osteoarthritis, not only due to the excess mechanical load, but also due to the excess expression of soluble factors, that is, leptin and pro-inflammatory cytokines, which contribute to joint inflammation and cartilage destruction. As such, obese individuals are in an altered state, due to a metabolic insufficiency, which requires specific nutritional treatment capable of normalising the leptin production and reducing the systematic low-level inflammation, in order to reduce the harmful impact of these systematic mediators on the joint health.
There are nutritional supplements and pharmacological agents capable of directing these factors and improving both conditions.
Therapeutic use
Leptin
Leptin was approved in the United States in 2014 for use in congenital leptin deficiency and generalized lipodystrophy.[168]
Analog metreleptin
An analog of human leptin metreleptin (trade names Mylept, Mylepta) was first approved in Japan in 2013, and in the United States in February 2014 and in Europe in 2018. In the US it is indicated as a treatment for complications of leptin deficiency, and for the diabetes and hypertriglyceridemia associated with congenital or acquired generalized lipodystrophy.[169][170] In Europe based on EMA, metreleptin should be used in addition to diet to treat lipodystrophy, where patients have loss of fatty tissue under the skin and build-up of fat elsewhere in the body such as in the liver and muscles. The medicine is used in: adults and children above the age of 2 years with generalised lipodystrophy (Berardinelli-Seip syndrome and Lawrence syndrome) and in adults and children above the age of 12 years with partial lipodystrophy (including Barraquer-Simons syndrome), when standard treatments have failed.[171]
History
The leptine was discovered by Jeffrey Freidman in 1994 after several decades of research conducted by others institutions since 1950 on obese mouse models [172]
See also
Notes
- ↑ Leptin controls the satiety indirectly by saying that we do or not have enough energy on board. Bearing in mind that other hormones such as ghrelin operate in a faster-time scale, it would be misleading to define it as "the satiety hormone".
References
- ↑ 1.0 1.1 Zhang F, Basinski MB, Beals JM, Briggs SL, Churgay LM, Clawson DK, DiMarchi RD, Furman TC, Hale JE, Hsiung HM, Schoner BE, Smith DP, Zhang XY, Wery JP, Schevitz RW (May 1997). "Crystal structure of the obese protein leptin-E100". Nature. 387 (6629): 206–09. Bibcode:1997Natur.387..206Z. doi:10.1038/387206a0. PMID 9144295.
- ↑ Brennan AM, Mantzoros CS (2006). "Drug Insight: the role of leptin in human physiology and pathophysiology – emerging clinical applications". Nat Clin Pract Endocrinol Metab. 2 (6): 318–27. doi:10.1038/ncpendmet0196. PMID 16932309.
- ↑ Pan H, Guo J, Su Z (May 2014). "Advances in understanding the interrelations between leptin resistance and obesity". Physiology & Behavior. 130: 157–69. doi:10.1016/j.physbeh.2014.04.003. PMID 24726399.
- ↑ 4.0 4.1 4.2 Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D, Lallone RL, Burley SK, Friedman JM (July 1995). "Weight-reducing effects of the plasma protein encoded by the obese gene". Science. 269 (5223): 543–46. Bibcode:1995Sci...269..543H. doi:10.1126/science.7624777. PMID 7624777.
- ↑ 5.0 5.1 Campfield LA, Smith FJ, Guisez Y, Devos R, Burn P (July 1995). "Recombinant mouse OB protein: evidence for a peripheral signal linking adiposity and central neural networks". Science. 269 (5223): 546–49. Bibcode:1995Sci...269..546C. doi:10.1126/science.7624778. PMID 7624778.
- ↑ 6.0 6.1 Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T, Collins F (July 1995). "Effects of the obese gene product on body weight regulation in ob/ob mice". Science. 269 (5223): 540–43. Bibcode:1995Sci...269..540P. doi:10.1126/science.7624776. PMID 7624776.
- ↑ 7.0 7.1 Maffei M, Halaas J, Ravussin E, Pratley RE, Lee GH, Zhang Y, Fei H, Kim S, Lallone R, Ranganathan S (November 1995). "Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight-reduced subjects". Nat. Med. 1 (11): 1155–61. doi:10.1038/nm1195-1155. PMID 7584987.
- ↑ 8.0 8.1 8.2 Considine RV, Considine EL, Williams CJ, Nyce MR, Magosin SA, Bauer TL, Rosato EL, Colberg J, Caro JF (June 1995). "Evidence against either a premature stop codon or the absence of obese gene mRNA in human obesity". J. Clin. Invest. 95 (6): 2986–88. doi:10.1172/JCI118007. PMC 295988. PMID 7769141.
- ↑ 9.0 9.1 9.2 Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, Ohannesian JP, Marco CC, McKee LJ, Bauer TL (1996). "Serum immunoreactive-leptin concentrations in normal-weight and obese humans". N. Engl. J. Med. 334 (5): 292–95. doi:10.1056/NEJM199602013340503. PMID 8532024.
- ↑ Dickie MM, Lane PW (1957). "Plus letter to Roy Robinson 7/7/70". Mouse News Lett. (17): 52.
- ↑ Bahary N, Siegel DA, Walsh J, Zhang Y, Leopold L, Leibel R, Proenca R, Friedman JM (September 1993). "Microdissection of proximal mouse chromosome 6: identification of RFLPs tightly linked to the ob mutation". Mamm. Genome. 4 (9): 511–15. doi:10.1007/BF00364786. PMID 7906968.
- ↑ Friedman JM, Leibel RL, Siegel DS, Walsh J, Bahary N (December 1991). "Molecular mapping of the mouse ob mutation". Genomics. 11 (4): 1054–62. doi:10.1016/0888-7543(91)90032-A. PMID 1686014.
- ↑ 13.0 13.1 Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM (December 1994). "Positional cloning of the mouse obese gene and its human homologue". Nature. 372 (6505): 425–32. Bibcode:1994Natur.372..425Z. doi:10.1038/372425a0. PMID 7984236.
- ↑ Leibel RL, Bahary N, Friedman JM (January 1990). "Genetic variation and nutrition in obesity: approaches to the molecular genetics of obesity". World Rev Nutr Diet. 63 (1): 90–101. PMID 1973864.
- ↑ Bahary N, Leibel RL, Joseph L, Friedman JM (November 1990). "Molecular mapping of the mouse db mutation". Proc Natl Acad Sci USA. 87 (21): 8642–46. Bibcode:1990PNAS...87.8642B. doi:10.1073/pnas.87.21.8642. PMC 55013. PMID 1978328.
- ↑ Leibel RL, Bahary N, Friedman JM (January 1993). "Strategies for the molecular genetic analysis of obesity in humans". Crit Rev Food Sci Nutr. 33 (4–5): 351–58. doi:10.1080/10408399309527632. PMID 8357496.
- ↑ Neill US (1 October 2010). "Leaping for leptin: the 2010 Albert Lasker Basic Medical Research Award goes to Douglas Coleman and Jeffrey M. Friedman". Journal of Clinical Investigation. 120 (10): 3413–18. doi:10.1172/JCI45094. PMC 2947251.
- ↑ Conde J, Scotece M, Gómez R, López V, Gómez-Reino JJ, Lago F, Gualillo O (2011). "Adipokines: Biofactors from white adipose tissue. A complex hub among inflammation, metabolism, and immunity". BioFactors. 37 (6): 413–20. doi:10.1002/biof.185. PMID 22038756.
- ↑ Tartaglia LA, Dembski M, Weng X, Deng N, Culpepper J, Devos R, Richards GJ, Campfield LA, Clark FT, Deeds J, Muir C, Sanker S, Moriarty A, Moore KJ, Smutko JS, Mays GG, Wool EA, Monroe CA, Tepper RI (December 1995). "Identification and expression cloning of a leptin receptor, OB-R". Cell. 83 (7): 1263–71. doi:10.1016/0092-8674(95)90151-5. PMID 8548812.
- ↑ Chen H, Charlat O, Tartaglia LA, Woolf EA, Weng X, Ellis SJ, Lakey ND, Culpepper J, Moore KJ, Breitbart RE, Duyk GM, Tepper RI, Morgenstern JP (February 1996). "Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db/db mice". Cell. 84 (3): 491–95. doi:10.1016/S0092-8674(00)81294-5. PMID 8608603.
- ↑ Lee GH, Proenca R, Montez JM, Carroll KM, Darvishzadeh JG, Lee JI, Friedman JM (February 1996). "Abnormal splicing of the leptin receptor in diabetic mice". Nature. 379 (6566): 632–65. Bibcode:1996Natur.379..632L. doi:10.1038/379632a0. PMID 8628397.
- ↑ Chua SC, Chung WK, Wu-Peng XS, Zhang Y, Liu SM, Tartaglia L, Leibel RL (February 1996). "Phenotypes of mouse diabetes and rat fatty due to mutations in the OB (leptin) receptor". Science. 271 (5251): 994–96. Bibcode:1996Sci...271..994C. doi:10.1126/science.271.5251.994. PMID 8584938.
- ↑ Bonner J (2005). "Jeffrey Friedman, discoverer of leptin, receives Gairdner, Passano awards". Newswire. The Rockefeller University.
- ↑ "Jeffrey Friedman receives Shaw Prize for discovery of leptin". News-Medical.net. 2009.
- ↑ "The Lasker Foundation – 2010 Awards". Lasker Foundation. 2010.
- ↑ "BBVA Foundation Frontiers of Knowledge Awards". BBVA Foundation. 2012.
- ↑ "KFF – KFIP – Winners 2013 – Medicine". King Faisal Foundation. 2013.
- ↑ Shell E (January 1, 2002). "On the Cutting Edge". The Hungry Gene: The Inside Story of the Obesity Industry. Atlantic Monthly Press. ISBN 978-1-4223-5243-4.[page needed]
- ↑ Shell E (2002). "Hunger". The Hungry Gene: The Inside Story of the Obesity Industry. Atlantic Monthly Press. ISBN 978-1-4223-5243-4.[page needed]
- ↑ Pool R (2001). Fat: fighting the obesity epidemic. New York: Oxford University Press. ISBN 978-0-19-511853-7.[page needed]
- ↑ Kolata GB (2007). Rethinking thin: the new science of weight loss – and the myths and realities of dietin. New York: Farrar. ISBN 978-0-374-10398-9.[page needed]
- ↑ Castracane VD, Henson MC (2006). "The Obese (ob/ob) Mouse and the Discovery of Leptin". In Castracane VD, Henson MC. Leptin. Endocrine Updates. 25. pp. 1–9. doi:10.1007/978-0-387-31416-7_1. ISBN 978-0-387-31415-0.
- ↑ Green ED, Maffei M, Braden VV, Proenca R, DeSilva U, Zhang Y, Chua SC, Leibel RL, Weissenbach J, Friedman JM (August 1995). "The human obese (OB) gene: RNA expression pattern and mapping on the physical, cytogenetic, and genetic maps of chromosome 7". Genome Res. 5 (1): 5–12. doi:10.1101/gr.5.1.5. PMID 8717050.
- ↑ Montague CT, Farooqi IS, Whitehead JP, Soos MA, Rau H, Wareham NJ, Sewter CP, Digby JE, Mohammed SN, Hurst JA, Cheetham CH, Earley AR, Barnett AH, Prins JB, O'Rahilly S (1997). "Congenital leptin deficiency is associated with severe early-onset obesity in humans". Nature. 387 (6636): 903–08. doi:10.1038/43185. PMID 9202122.
- ↑ 35.0 35.1 35.2 35.3 35.4 Wabitsch M, Funcke JB, Lennerz B, Kuhnle-Krahl U, Lahr G, Debatin KM, Vatter P, Gierschik P, Moepps B, Fischer-Posovszky P (Jan 2015). "Biologically Inactive Leptin and Early-Onset Extreme Obesity". N. Engl. J. Med. 372 (1): 48–54. doi:10.1056/NEJMoa1406653. PMID 25551525.
- ↑ 36.0 36.1 Paracchini V, Pedotti P, Taioli E (2005). "Genetics of leptin and obesity: a HuGE review". Am. J. Epidemiol. 162 (2): 101–14. doi:10.1093/aje/kwi174. PMID 15972940.
- ↑ Wang TN, Huang MC, Chang WT, Ko AM, Tsai EM, Liu CS, Lee CH, Ko YC (February 2006). "G-2548A polymorphism of the leptin gene is correlated with extreme obesity in Taiwanese aborigines". Obesity (Silver Spring). 14 (2): 183–87. doi:10.1038/oby.2006.23. PMID 16571841.
- ↑ 38.0 38.1 Zhang L, Lu M, Yuan L, Lai W, Wang Y (2014). "[Association of leptin gene-2548 G/A polymorphism with obesity: a meta-analysis]". Wei Sheng Yan Jiu (in Chinese). 43 (1): 128–32. PMID 24564125.
- ↑ Templeman LA, Reynolds GP, Arranz B, San L (April 2005). "Polymorphisms of the 5-HT2C receptor and leptin genes are associated with antipsychotic drug-induced weight gain in Caucasian subjects with a first-episode psychosis". Pharmacogenet Genomics. 15 (4): 195–200. doi:10.1097/01213011-200504000-00002. PMID 15864111.
- ↑ Kang SG, Lee HJ, Park YM, Choi JE, Han C, Kim YK, Kim SH, Lee MS, Joe SH, Jung IK, Kim L (2008). "Possible association between the -2548A/G polymorphism of the leptin gene and olanzapine-induced weight gain". Prog. Neuropsychopharmacol. Biol. Psychiatry. 32 (1): 160–63. doi:10.1016/j.pnpbp.2007.08.002. PMID 17804136.
- ↑ Wu R, Zhao J, Shao P, Ou J, Chang M (2011). "Genetic predictors of antipsychotic-induced weight gain: a case-matched multi-gene study". Zhong Nan Da Xue Xue Bao Yi Xue Ban. 36 (8): 720–73. doi:10.3969/j.issn.1672-7347.2011.08.003. PMID 21937795.
- ↑ Ribeiro R, Vasconcelos A, Costa S, Pinto D, Morais A, Oliveira J, Lobo F, Lopes C, Medeiros R (2004). "Overexpressing leptin genetic polymorphism (−2548 G/A) is associated with susceptibility to prostate cancer and risk of advanced disease". Prostate. 59 (3): 268–74. doi:10.1002/pros.20004. PMID 15042602.
- ↑ Vaskú JA, Vaskú A, Dostálová Z, Bienert P (2006). "Association of leptin genetic polymorphism -2548 G/A with gestational diabetes mellitus". Genes Nutr. 1 (2): 117–23. doi:10.1007/BF02829953. PMC 3454683. PMID 18850205.
- ↑ Ye XL, Lu CF (Oct 2013). "Association of polymorphisms in the leptin and leptin receptor genes with inflammatory mediators in patients with osteoporosis". Endocrine. 44 (2): 481–88. doi:10.1007/s12020-013-9899-9. PMID 23460508.
- ↑ 45.0 45.1 45.2 Margetic S, Gazzola C, Pegg GG, Hill RA (2002). "Leptin: a review of its peripheral actions and interactions". Int. J. Obes. Relat. Metab. Disord. 26 (11): 1407–33. doi:10.1038/sj.ijo.0802142. PMID 12439643.
- ↑ Bado A, Levasseur S, Attoub S, Kermorgant S, Laigneau JP, Bortoluzzi MN, Moizo L, Lehy T, Guerre-Millo M, Le Marchand-Brustel Y, Lewin MJ (August 1998). "The stomach is a source of leptin". Nature. 394 (6695): 790–93. Bibcode:1998Natur.394..790B. doi:10.1038/29547. PMID 9723619.
- ↑ Sinha MK, Opentanova I, Ohannesian JP, Kolaczynski JW, Heiman ML, Hale J, Becker GW, Bowsher RR, Stephens TW, Caro JF (September 1996). "Evidence of free and bound leptin in human circulation. Studies in lean and obese subjects and during short-term fasting". J. Clin. Invest. 98 (6): 1277–82. doi:10.1172/JCI118913. PMC 507552. PMID 8823291.
- ↑ Lönnqvist F, Arner P, Nordfors L, Schalling M (1995). "Overexpression of the obese (ob) gene in adipose tissue of human obese subjects". Nat. Med. 1 (9): 950–53. doi:10.1038/nm0995-950. PMID 7585223.
- ↑ Madej T (1998). "Considerations in the use of lipid-based drug products". J Intraven Nurs. 21 (6): 326. PMID 10392096.
- ↑ Sinha MK, Ohannesian JP, Heiman ML, Kriauciunas A, Stephens TW, Magosin S, Marco C, Caro JF (March 1996). "Nocturnal rise of leptin in lean, obese, and non-insulin-dependent diabetes mellitus subjects". J. Clin. Invest. 97 (5): 1344–47. doi:10.1172/JCI118551. PMC 507189. PMID 8636448.
- ↑ Schoeller DA, Cella LK, Sinha MK, Caro JF (October 1997). "Entrainment of the diurnal rhythm of plasma leptin to meal timing". J. Clin. Invest. 100 (7): 1882–87. doi:10.1172/JCI119717. PMC 508375. PMID 9312190.
- ↑ Ahima RS, Prabakaran D, Mantzoros C, Qu D, Lowell B, Maratos-Flier E, Flier JS (July 1996). "Role of leptin in the neuroendocrine response to fasting". Nature. 382 (6588): 250–52. Bibcode:1996Natur.382..250A. doi:10.1038/382250a0. PMID 8717038.
- ↑ Friedman JM (March 2009). "Leptin at 14 y of age: an ongoing story". Am. J. Clin. Nutr. 89 (3): 973S–79S. doi:10.3945/ajcn.2008.26788B. PMC 2667654. PMID 19190071.
- ↑ Chan JL, Heist K, DePaoli AM, Veldhuis JD, Mantzoros CS (May 2003). "The role of falling leptin levels in the neuroendocrine and metabolic adaptation to short-term starvation in healthy men". J. Clin. Invest. 111 (9): 1409–21. doi:10.1172/JCI17490. PMC 154448. PMID 12727933.
- ↑ Kolaczynski JW, Considine RV, Ohannesian J, Marco C, Opentanova I, Nyce MR, Myint M, Caro JF (November 1996). "Responses of leptin to short-term fasting and refeeding in humans: a link with ketogenesis but not ketones themselves". Diabetes. 45 (11): 1511–15. doi:10.2337/diab.45.11.1511. PMID 8866554.
- ↑ Kolaczynski JW, Ohannesian JP, Considine RV, Marco CC, Caro JF (November 1996). "Response of leptin to short-term and prolonged overfeeding in humans". J. Clin. Endocrinol. Metab. 81 (11): 4162–65. doi:10.1210/JCEM.81.11.8923877. PMID 8923877.
- ↑ Copinschi G, Leproult R, Spiegel K (2014). "The important role of sleep in metabolism". Front Horm Res. 42: 59–72. doi:10.1159/000358858. PMID 24732925.
- ↑ Knutson KL, Spiegel K, Penev P, Van Cauter E (June 2007). "The metabolic consequences of sleep deprivation". Sleep Med Rev. 11 (3): 163–78. doi:10.1016/j.smrv.2007.01.002. PMC 1991337. PMID 17442599.
- ↑ 59.0 59.1 Caro JF, Sinha MK, Kolaczynski JW, Zhang PL, Considine RV (November 1996). "Leptin: the tale of an obesity gene". Diabetes. 45 (11): 1455–62. doi:10.2337/diab.45.11.1455. PMID 8866547.
- ↑ Otsuka R, Yatsuya H, Tamakoshi K, Matsushita K, Wada K, Toyoshima H (October 2006). "psychological stress and serum leptin concentrations in Japanese men". Obesity (Silver Spring). 14 (10): 1832–38. doi:10.1038/oby.2006.211. PMID 17062814.
- ↑ de Salles BF, Simão R, Fleck SJ, Dias I, Kraemer-Aguiar LG, Bouskela E (July 2010). "Effects of resistance training on cytokines". Int J Sports Med. 31 (7): 441–50. doi:10.1055/s-0030-1251994. PMID 20432196.
- ↑ Hickey MS, Considine RV, Israel RG, Mahar TL, McCammon MR, Tyndall GL, Houmard JA, Caro JF (November 1996). "Leptin is related to body fat content in male distance runners". Am. J. Physiol. 271 (5 Pt 1): E938–40. PMID 8944684.
- ↑ Hickey MS, Houmard JA, Considine RV, Tyndall GL, Midgette JB, Gavigan KE, Weidner ML, McCammon MR, Israel RG, Caro JF (April 1997). "Gender-dependent effects of exercise training on serum leptin levels in humans". Am. J. Physiol. 272 (4 Pt 1): E562–66. PMID 9142875.
- ↑ Ahima RS, Flier JS (2000). "Leptin". Annu. Rev. Physiol. 62 (1): 413–37. doi:10.1146/annurev.physiol.62.1.413. PMID 10845097.
- ↑ Kolaczynski JW, Nyce MR, Considine RV, Boden G, Nolan JJ, Henry R, Mudaliar SR, Olefsky J, Caro JF (May 1996). "Acute and chronic effects of insulin on leptin production in humans: Studies in vivo and in vitro". Diabetes. 45 (5): 699–701. doi:10.2337/diabetes.45.5.699. PMID 8621027.
- ↑ Considine RV, Nyce MR, Kolaczynski JW, Zhang PL, Ohannesian JP, Moore JH, Fox JW, Caro JF (May 1997). "Dexamethasone stimulates leptin release from human adipocytes: unexpected inhibition by insulin". J. Cell. Biochem. 65 (2): 254–58. doi:10.1002/(SICI)1097-4644(199705)65:2<254::AID-JCB10>3.0.CO;2-I. PMID 9136082.
- ↑ Zirlik S, Hauck T, Fuchs FS, Neurath MF, Konturek PC, Harsch IA (February 2011). "Leptin, Obestatin and Apelin levels in patients with obstructive sleep apnoea syndrome". Med. Sci. Monit. 17 (3): CR159–64. doi:10.12659/MSM.881450. PMC 3524733. PMID 21358603.
- ↑ Harsch IA, Konturek PC, Koebnick C, Kuehnlein PP, Fuchs FS, Pour Schahin S, Wiest GH, Hahn EG, Lohmann T, Ficker JH (August 2003). "Leptin and ghrelin levels in patients with obstructive sleep apnoea: effect of CPAP treatment". Eur. Respir. J. 22 (2): 251–57. doi:10.1183/09031936.03.00010103. PMID 12952256.
- ↑ Mantzoros CS (1999). "The role of leptin in human obesity and disease: a review of current evidence". Ann. Intern. Med. 130 (8): 671–80. doi:10.7326/0003-4819-130-8-199904200-00014. PMID 10215564.
- ↑ Cirillo D, Rachiglio AM, la Montagna R, Giordano A, Normanno N (2008). "Leptin signaling in breast cancer: an overview". Journal of Cellular Biochemistry. 105 (4): 956–64. doi:10.1002/jcb.21911. PMID 18821585.
- ↑ Farr SA, Banks WA, Morley JE (June 2006). "Effects of leptin on memory processing". Peptides. 27 (6): 1420–25. doi:10.1016/j.peptides.2005.10.006. PMID 16293343.
- ↑ Casanueva FF, Dieguez C, Popovic V, Peino R, Considine RV, Caro JF (April 1997). "Serum immunoreactive leptin concentrations in patients with anorexia nervosa before and after partial weight recovery". Biochem. Mol. Med. 60 (2): 116–20. doi:10.1006/bmme.1996.2564. PMID 9169091.
- ↑ Lieb W, Beiser AS, Vasan RS, Tan ZS, Au R, Harris TB, Roubenoff R, Auerbach S, DeCarli C, Wolf PA, Seshadri S (December 2009). "Association of plasma leptin levels with incident Alzheimer disease and MRI measures of brain aging". JAMA. 302 (23): 2565–72. doi:10.1001/jama.2009.1836. PMC 2838501. PMID 20009056.
- ↑ Greco SJ, Bryan KJ, Sarkar S, Zhu X, Smith MA, Ashford JW, Johnston JM, Tezapsidis N, Casadesus G (2010). "Leptin reduces pathology and improves memory in a transgenic mouse model of Alzheimer's disease". J. Alzheimers Dis. 19 (4): 1155–67. doi:10.3233/JAD-2010-1308. PMC 2862270. PMID 20308782.
- ↑ Doherty GH, Beccano-Kelly D, Yan SD, Gunn-Moore FJ, Harvey J (January 2013). "Leptin prevents hippocampal synaptic disruption and neuronal cell death induced by amyloid β". Neurobiol. Aging. 34 (1): 226–37. doi:10.1016/j.neurobiolaging.2012.08.003. PMID 22921154.
- ↑ Greco SJ, Sarkar S, Johnston JM, Tezapsidis N (February 2009). "Leptin regulates tau phosphorylation and amyloid through AMPK in neuronal cells". Biochem. Biophys. Res. Commun. 380 (1): 98–104. doi:10.1016/j.bbrc.2009.01.041. PMC 2657956. PMID 19166821.
- ↑ Lynn RB, Cao GY, Considine RV, Hyde TM, Caro JF (February 1996). "Autoradiographic localization of leptin binding in the choroid plexus of ob/ob and db/db mice". Biochem. Biophys. Res. Commun. 219 (3): 884–89. doi:10.1006/bbrc.1996.0328. PMID 8645274.
- ↑ Kus I, Sarsilmaz M, Colakoglu N, Kukne A, Ozen OA, Yilmaz B, Kelestimur H (2004). "Pinealectomy increases and exogenous melatonin decreases leptin production in rat anterior pituitary cells: an immunohistochemical study". Physiol Res. 53 (4): 403–08. PMID 15311999.
- ↑ Alonso-Vale MI, Andreotti S, Peres SB, Anhê GF, das Neves Borges-Silva C, Neto JC, Lima FB (April 2005). "Melatonin enhances leptin expression by rat adipocytes in the presence of insulin". Am. J. Physiol. Endocrinol. Metab. 288 (4): E805–12. doi:10.1152/ajpendo.00478.2004. PMID 15572654.
- ↑ Copinschi G (2005). "Metabolic and endocrine effects of sleep deprivation". Essential psychopharmacology. 6 (6): 341–47. PMID 16459757.
- ↑ Wang MY, Chen L, Clark GO, Lee Y, Stevens RD, Ilkayeva OR, Wenner BR, Bain JR, Charron MJ, Newgard CB, Unger RH (March 2010). "Leptin therapy in insulin-deficient type I diabetes". Proc. Natl. Acad. Sci. USA. 107 (11): 4813–19. Bibcode:2010PNAS..107.4813W. doi:10.1073/pnas.0909422107. PMC 2841945. PMID 20194735. Lay summary – medicinenet.com.
- ↑ 82.0 82.1 Elmquist JK, Elias CF, Saper CB (Feb 1999). "From lesions to leptin: hypothalamic control of food intake and body weight". Neuron. 22 (2): 221–32. doi:10.1016/S0896-6273(00)81084-3. PMID 10069329.
- ↑ Elias CF, Aschkenasi C, Lee C, Kelly J, Ahima RS, Bjorbaek C, Flier JS, Saper CB, Elmquist JK (Aug 1999). "Leptin differentially regulates NPY and POMC neurons projecting to the lateral hypothalamic area". Neuron. 23 (4): 775–86. doi:10.1016/S0896-6273(01)80035-0. PMID 10482243.
- ↑ Fekete C, Légrádi G, Mihály E, Huang QH, Tatro JB, Rand WM, Emerson CH, Lechan RM (Feb 2000). "alpha-Melanocyte-stimulating hormone is contained in nerve terminals innervating thyrotropin-releasing hormone-synthesizing neurons in the hypothalamic paraventricular nucleus and prevents fasting-induced suppression of prothyrotropin-releasing hormone gene expression". The Journal of Neuroscience. 20 (4): 1550–58. PMID 10662844.
- ↑ Dubuc GR, Phinney SD, Stern JS, Havel PJ (1998). "Changes of serum leptin and endocrine and metabolic parameters after 7 days of energy restriction in men and women". Metab. Clin. Exp. 47 (4): 429–34. doi:10.1016/S0026-0495(98)90055-5. PMID 9550541.
- ↑ Pratley RE, Nicolson M, Bogardus C, Ravussin E (1997). "Plasma leptin responses to fasting in Pima Indians". Am. J. Physiol. 273 (3 Pt 1): E644–49. PMID 9316457.
- ↑ Weigle DS, Duell PB, Connor WE, Steiner RA, Soules MR, Kuijper JL (February 1997). "Effect of fasting, refeeding, and dietary fat restriction on plasma leptin levels". J. Clin. Endocrinol. Metab. 82 (2): 561–65. doi:10.1210/jc.82.2.561. PMID 9024254.
- ↑ Wadden TA, Considine RV, Foster GD, Anderson DA, Sarwer DB, Caro JS (January 1998). "Short- and long-term changes in serum leptin dieting obese women: effects of caloric restriction and weight loss". J. Clin. Endocrinol. Metab. 83 (1): 214–18. doi:10.1210/jcem.83.1.4494. PMID 9435444.
- ↑ Chin-Chance C, Polonsky KS, Schoeller DA (2000). "Twenty-four-hour leptin levels respond to cumulative short-term energy imbalance and predict subsequent intake". J. Clin. Endocrinol. Metab. 85 (8): 2685–91. doi:10.1210/jc.85.8.2685. PMID 10946866.
- ↑ Keim NL, Stern JS, Havel PJ (1998). "Relation between circulating leptin concentrations and appetite during a prolonged, moderate energy deficit in women". Am. J. Clin. Nutr. 68 (4): 794–801. PMID 9771856.
- ↑ Mars M, de Graaf C, de Groot CP, van Rossum CT, Kok FJ (2006). "Fasting leptin and appetite responses induced by a 4-day 65%-energy-restricted diet". International journal of obesity (Lond). 30 (1): 122–28. doi:10.1038/sj.ijo.0803070. PMID 16158086.
- ↑ Williams KW, Scott MM, Elmquist JK (March 2009). "From observation to experimentation: leptin action in the mediobasal hypothalamus". Am. J. Clin. Nutr. 89 (3): 985S–90S. doi:10.3945/ajcn.2008.26788D. PMC 2667659. PMID 19176744.
- ↑ Baicy K, London ED, Monterosso J, Wong ML, Delibasi T, Sharma A, Licinio J (November 2007). "Leptin replacement alters brain response to food cues in genetically leptin-deficient adults". Proc. Natl. Acad. Sci. USA. 104 (46): 18276–79. Bibcode:2007PNAS..10418276B. doi:10.1073/pnas.0706481104. PMC 2084333. PMID 17986612. Lay summary – WebMD.
- ↑ Wang MY, Zhou YT, Newgard CB, Unger RH (August 1996). "A novel leptin receptor isoform in rat". FEBS Lett. 392 (2): 87–90. doi:10.1016/0014-5793(96)00790-9. PMID 8772180.
- ↑ Malendowicz W, Rucinski M, Macchi C, Spinazzi R, Ziolkowska A, Nussdorfer GG, Kwias Z (October 2006). "Leptin and leptin receptors in the prostate and seminal vesicles of the adult rat". Int. J. Mol. Med. 18 (4): 615–18. doi:10.3892/ijmm.18.4.615. PMID 16964413.
- ↑ "LepRb antibody (commercial site)".
- ↑ Di Marzo V (2008). "The endocannabinoid system in obesity and type 2 diabetes". Diabetologia. 51 (8): 1356–67. doi:10.1007/s00125-008-1048-2. PMID 18563385.
- ↑ Taleb S, Herbin O, Ait-Oufella H, Verreth W, Gourdy P, Barateau V, Merval R, Esposito B, Clément K, Holvoet P, Tedgui A, Mallat Z (2007). "Defective leptin/leptin receptor signaling improves regulatory T cell immune response and protects mice from atherosclerosis". Arterioscler Thromb Vasc Biol. 27 (12): 2691–98. doi:10.1161/ATVBAHA.107.149567. PMID 17690315.
- ↑ Zhang W, Telemaque S, Augustyniak RA, Anderson P, Thomas GD, An J, Wang Z, Newgard CB, Victor RG (2010). "Adenovirus-mediated leptin expression normalises hypertension associated with diet-induced obesity". J Neuroendocrinol. 22 (3): 175–80. doi:10.1111/j.1365-2826.2010.01953.x. PMID 20059648.
- ↑ Knight WD, Seth R, Boron J, Overton JM (2009). "Short-term physiological hyperleptinemia decreases arterial blood pressure". Regul Pept. 154 (1–3): 60–68. doi:10.1016/j.regpep.2009.02.001. PMID 19323984.
- ↑ Ciriello J, Moreau JM (November 2012). "Systemic administration of leptin potentiates the response of neurons in the nucleus of the solitary tract to chemoreceptor activation in the rat". Journal of Neuroscience. 229: 88–99. doi:10.1016/j.neuroscience.2012.10.065. PMID 23159310.
- ↑ Torday JS, Rehan VK (October 2006). "Up-regulation of fetal rat lung parathyroid hormone-related protein gene regulatory network down-regulates the Sonic Hedgehog/Wnt/beta-catenin gene regulatory network". Pediatr. Res. 60 (4): 382–88. doi:10.1203/01.pdr.0000238326.42590.03. PMID 16940239.
- ↑ Anifandis G, Koutselini E, Louridas K, Liakopoulos V, Leivaditis K, Mantzavinos T, Sioutopoulou D, Vamvakopoulos N (April 2005). "Estradiol and leptin as conditional prognostic IVF markers". Reproduction. 129 (4): 531–34. doi:10.1530/rep.1.00567. PMID 15798029.
- ↑ Comninos AN, Jayasena CN, Dhillo WS (2014). "The relationship between gut and adipose hormones, and reproduction". Hum. Reprod. Update. 20 (2): 153–74. doi:10.1093/humupd/dmt033. PMID 24173881.
- ↑ Zhao J, Townsend KL, Schulz LC, Kunz TH, Li C, Widmaier EP (2004). "Leptin receptor expression increases in placenta, but not hypothalamus, during gestation in Mus musculus and Myotis lucifugus". Placenta. 25 (8–9): 712–22. doi:10.1016/j.placenta.2004.01.017. PMID 15450389.
- ↑ Moynihan AT, Hehir MP, Glavey SV, Smith TJ, Morrison JJ (2006). "Inhibitory effect of leptin on human uterine contractility in vitro". Am. J. Obstet. Gynecol. 195 (2): 504–09. doi:10.1016/j.ajog.2006.01.106. PMID 16647683.
- ↑ Aka N, Atalay S, Sayharman S, Kiliç D, Köse G, Küçüközkan T (2006). "Leptin and leptin receptor levels in pregnant women with hyperemesis gravidarum". The Australian & New Zealand journal of obstetrics & gynaecology. 46 (4): 274–77. doi:10.1111/j.1479-828X.2006.00590.x. PMID 16866785.
- ↑ Cervero A, Domínguez F, Horcajadas JA, Quiñonero A, Pellicer A, Simón C (2006). "The role of the leptin in reproduction". Current Opinion in Obstetrics and Gynecology. 18 (3): 297–303. doi:10.1097/01.gco.0000193004.35287.89. PMID 16735830.
- ↑ Iwaniec UT, Boghossian S, Lapke PD, Turner RT, Kalra SP (2007). "Central leptin gene therapy corrects skeletal abnormalities in leptin-deficient ob/ob mice". Peptides. 28 (5): 1012–19. doi:10.1016/j.peptides.2007.02.001. PMC 1986832. PMID 17346852.
- ↑ Casabiell X, Piñeiro V, Tomé MA, Peinó R, Diéguez C, Casanueva FF (1997). "Presence of leptin in colostrum and/or breast milk from lactating mothers: a potential role in the regulation of neonatal food intake". J. Clin. Endocrinol. Metab. 82 (12): 4270–73. doi:10.1210/jcem.82.12.4590. PMID 9398752.
- ↑ Sanchez-Garrido MA, Tena-Sempere M (2013). "Metabolic control of puberty: roles of leptin and kisspeptins". Horm Behav. 64 (2): 187–94. doi:10.1016/j.yhbeh.2013.01.014. PMID 23998663.
- ↑ Matkovic V, Ilich JZ, Skugor M, Badenhop NE, Goel P, Clairmont A, Klisovic D, Nahhas RW, Landoll JD (October 1997). "Leptin is inversely related to age at menarche in human females". J. Clin. Endocrinol. Metab. 82 (10): 3239–45. doi:10.1210/jc.82.10.3239. PMID 9329346.
- ↑ Ducy P, Amling M, Takeda S, Priemel M, Schilling AF, Beil FT, Shen J, Vinson C, Rueger JM, Karsenty G (January 2000). "Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass". Cell. 100 (2): 197–207. doi:10.1016/S0092-8674(00)81558-5. PMID 10660043.
- ↑ 114.0 114.1 Hamrick MW, Ferrari SL (July 2008). "Leptin and the sympathetic connection of fat to bone". Osteoporos Int. 19 (7): 905–12. doi:10.1007/s00198-007-0487-9. PMID 17924050.
- ↑ 115.0 115.1 Allison SJ, Herzog H (2006). "NPY and bone". EXS (95): 171–82. PMID 16383006.
- ↑ Gordeladze JO, Reseland JE (March 2003). "A unified model for the action of leptin on bone turnover". J. Cell. Biochem. 88 (4): 706–12. doi:10.1002/jcb.10385. PMID 12577304.
- ↑ Takeda S, Elefteriou F, Levasseur R, Liu X, Zhao L, Parker KL, Armstrong D, Ducy P, Karsenty G (November 2002). "Leptin regulates bone formation via the sympathetic nervous system". Cell. 111 (3): 305–17. doi:10.1016/S0092-8674(02)01049-8. PMID 12419242.
- ↑ Martin A, David V, Malaval L, Lafage-Proust MH, Vico L, Thomas T (2007). "Opposite effects of leptin on bone metabolism: a dose-dependent balance related to energy intake and insulin-like growth factor-I pathway". Endocrinology. 148 (7): 3419–25. doi:10.1210/en.2006-1541. PMID 17431002.
- ↑ Rőszer T, Józsa T, Kiss-Tóth ED, De Clerck N, Balogh L (April 2014). "Leptin receptor deficient diabetic (db/db) mice are compromised in postnatal bone regeneration". Cell and Tissue Research. 356 (1): 195–206. doi:10.1007/s00441-013-1768-6. PMID 24343796.
- ↑ Lord GM, Matarese G, Howard JK, Baker RJ, Bloom SR, Lechler RI (August 1998). "Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression". Nature. 394 (6696): 897–901. Bibcode:1998Natur.394..897L. doi:10.1038/29795. PMID 9732873.
- ↑ 121.0 121.1 Fantuzzi G, Faggioni R (October 2000). "Leptin in the regulation of immunity, inflammation, and hematopoiesis". J. Leukoc. Biol. 68 (4): 437–46. PMID 11037963.
- ↑ Caldefie-Chezet F, Poulin A, Tridon A, Sion B, Vasson MP (March 2001). "Leptin: a potential regulator of polymorphonuclear neutrophil bactericidal action?". J. Leukoc. Biol. 69 (3): 414–18. PMID 11261788.
- ↑ Madej T, Boguski MS, Bryant SH (October 1995). "Threading analysis suggests that the obese gene product may be a helical cytokine". FEBS Lett. 373 (1): 13–18. doi:10.1016/0014-5793(95)00977-H. PMID 7589424.
- ↑ Heiman ML, Ahima RS, Craft LS, Schoner B, Stephens TW, Flier JS (September 1997). "Leptin inhibition of the hypothalamic-pituitary-adrenal axis in response to stress". Endocrinology. 138 (9): 3859–63. doi:10.1210/en.138.9.3859. PMID 9275075.
- ↑ Mabuchi T, Yatsuya H, Tamakoshi K, Otsuka R, Nagasawa N, Zhang H, Murata C, Wada K, Ishikawa M, Hori Y, Kondo T, Hashimoto S, Toyoshima H (2005). "Association between serum leptin concentration and white blood cell count in middle-aged Japanese men and women". Diabetes Metab. Res. Rev. 21 (5): 441–47. doi:10.1002/dmrr.540. PMID 15724240.
- ↑ Hamilton BS, Paglia D, Kwan AY, Deitel M (September 1995). "Increased obese mRNA expression in omental fat cells from massively obese humans". Nat. Med. 1 (9): 953–56. doi:10.1038/nm0995-953. PMID 7585224.
- ↑ Perrier S, Caldefie-Chézet F, Vasson MP (January 2009). "IL-1 family in breast cancer: potential interplay with leptin and other adipocytokines". FEBS Lett. 583 (2): 259–65. doi:10.1016/j.febslet.2008.12.030. PMID 19111549.
- ↑ Wabitsch M, Jensen PB, Blum WF, Christoffersen CT, Englaro P, Heinze E, Rascher W, Teller W, Tornqvist H, Hauner H (October 1996). "Insulin and cortisol promote leptin production in cultured human fat cells". Diabetes. 45 (10): 1435–38. doi:10.2337/diabetes.45.10.1435. PMID 8826983.
- ↑ LaPensee CR, Hugo ER, Ben-Jonathan N (November 2008). "Insulin stimulates interleukin-6 expression and release in LS14 human adipocytes through multiple signaling pathways". Endocrinology. 149 (11): 5415–22. doi:10.1210/en.2008-0549. PMC 2584585. PMID 18617614.
- ↑ Worm D, Vinten J, Vaag A, Henriksen JE, Beck-Nielsen H (September 2000). "The nicotinic acid analogue acipimox increases plasma leptin and decreases free fatty acids in type 2 diabetic patients". Eur. J. Endocrinol. 143 (3): 389–95. doi:10.1530/eje.0.1430389. PMID 11022182.
- ↑ 131.0 131.1 Myers MG, Cowley MA, Münzberg H (2008). "Mechanisms of leptin action and leptin resistance". Annu. Rev. Physiol. 70 (1): 537–56. doi:10.1146/annurev.physiol.70.113006.100707. PMID 17937601.
- ↑ 132.0 132.1 Veyrat-Durebex C, Poher AL, Caillon A, Somm E, Vallet P, Charnay Y, Rohner-Jeanrenaud F (2013). "Improved leptin sensitivity as a potential candidate responsible for the spontaneous food restriction of the Lou/C rat". PLoS ONE. 8 (9): e73452. Bibcode:2013PLoSO...873452V. doi:10.1371/journal.pone.0073452. PMC 3765307. PMID 24039946.
- ↑ 133.0 133.1 Caro JF, Kolaczynski JW, Nyce MR, Ohannesian JP, Opentanova I, Goldman WH, Lynn RB, Zhang PL, Sinha MK, Considine RV (20 July 1996). "Decreased cerebrospinal-fluid/serum leptin ratio in obesity: a possible mechanism for leptin resistance". Lancet. 348 (9021): 159–61. doi:10.1016/S0140-6736(96)03173-X. PMID 8684156.
- ↑ Considine RV, Considine EL, Williams CJ, Hyde TM, Caro JF (1996). "The hypothalamic leptin receptor in humans: identification of incidental sequence polymorphisms and absence of the db/db mouse and fa/fa rat mutations". Diabetes. 45 (7): 992–94. doi:10.2337/diabetes.45.7.992. PMID 8666155.
- ↑ Considine RV, Caro JF (November 1997). "Leptin and the regulation of body weight". Int. J. Biochem. Cell Biol. 29 (11): 1255–72. doi:10.1016/S1357-2725(97)00050-2. PMID 9451823.
- ↑ 136.0 136.1 Oswal A, Yeo G (February 2010). "Leptin and the control of body weight: a review of its diverse central targets, signaling mechanisms, and role in the pathogenesis of obesity". Obesity (Silver Spring). 18 (2): 221–29. doi:10.1038/oby.2009.228. PMID 19644451.
- ↑ Shapiro A, Mu W, Roncal C, Cheng KY, Johnson RJ, Scarpace PJ (November 2008). "Fructose-induced leptin resistance exacerbates weight gain in response to subsequent high-fat feeding". Am. J. Physiol. Regul. Integr. Comp. Physiol. 295 (5): R1370–75. doi:10.1152/ajpregu.00195.2008. PMC 2584858. PMID 18703413.
- ↑ Vasselli JR (November 2008). "Fructose-induced leptin resistance: discovery of an unsuspected form of the phenomenon and its significance. Focus on "Fructose-induced leptin resistance exacerbates weight gain in response to subsequent high-fat feeding," by Shapiro et al". Am. J. Physiol. Regul. Integr. Comp. Physiol. 295 (5): R1365–69. doi:10.1152/ajpregu.90674.2008. PMID 18784330.
- ↑ Harris RB, Apolzan JW (Jun 2012). "Changes in glucose tolerance and leptin responsiveness of rats offered a choice of lard, sucrose, and chow". Am J Physiol Regul Integr Comp Physiol. 302 (11): R1327–39. doi:10.1152/ajpregu.00477.2011. PMC 3378343. PMID 22496363.
- ↑ Roth JD, Roland BL, Cole RL, Trevaskis JL, Weyer C, Koda JE, Anderson CM, Parkes DG, Baron AD (May 2008). "Leptin responsiveness restored by amylin agonism in diet-induced obesity: evidence from nonclinical and clinical studies". Proc. Natl. Acad. Sci. USA. 105 (20): 7257–62. Bibcode:2008PNAS..105.7257R. doi:10.1073/pnas.0706473105. PMC 2438237. PMID 18458326.
- ↑ Banks WA, Farr SA, Morley JE (June 2006). "The effects of high fat diets on the blood-brain barrier transport of leptin: failure or adaptation?". Physiol. Behav. 88 (3): 244–48. doi:10.1016/j.physbeh.2006.05.037. PMID 16781741.
- ↑ Wang J, Obici S, Morgan K, Barzilai N, Feng Z, Rossetti L (December 2001). "Overfeeding rapidly induces leptin and insulin resistance". Diabetes. 50 (12): 2786–91. doi:10.2337/diabetes.50.12.2786. PMID 11723062.
- ↑ Enriori PJ, Evans AE, Sinnayah P, Jobst EE, Tonelli-Lemos L, Billes SK, Glavas MM, Grayson BE, Perello M, Nillni EA, Grove KL, Cowley MA (March 2007). "Diet-induced obesity causes severe but reversible leptin resistance in arcuate melanocortin neurons". Cell Metab. 5 (3): 181–94. doi:10.1016/j.cmet.2007.02.004. PMID 17339026.
- ↑ Obici S, Rossetti L (December 2003). "Minireview: nutrient sensing and the regulation of insulin action and energy balance". Endocrinology. 144 (12): 5172–78. doi:10.1210/en.2003-0999. PMID 12970158.
- ↑ 145.0 145.1 Ahima RS (July 2008). "Revisiting leptin's role in obesity and weight loss". J. Clin. Invest. 118 (7): 2380–83. doi:10.1172/JCI36284. PMC 2430504. PMID 18568083.
- ↑ Yusuf E, Nelissen RG, Ioan-Facsinay A, Stojanovic-Susulic V, DeGroot J, van Osch G, Middeldorp S, Huizinga TW, Kloppenburg M (2010). "Association between weight or body mass index and hand osteoarthritis: a systematic review". Annals of the Rheumatic Diseases. 69 (4): 761–65. doi:10.1136/ard.2008.106930. PMID 19487215.
- ↑ Sowers MR, Karvonen-Gutierrez CA (2010). "The evolving role of obesity in knee osteoarthritis". Current Opinion in Rheumatology. 22 (5): 533–37. doi:10.1097/BOR.0b013e32833b4682. PMC 3291123. PMID 20485173.
- ↑ Aspden RM, Scheven BA, Hutchison JD (2001). "Osteoarthritis as a systemic disorder including stromal cell differentiation and lipid metabolism". Lancet. 357 (9262): 1118–20. doi:10.1016/S0140-6736(00)04264-1. PMID 11297982.
- ↑ Pottie P, Presle N, Terlain B, Netter P, Mainard D, Berenbaum F (2006). "Obesity and osteoarthritis: more complex than predicted!". Annals of the Rheumatic Diseases. 65 (11): 1403–05. doi:10.1136/ard.2006.061994. PMC 1798356. PMID 17038451.
- ↑ Griffin TM, Guilak F (2008). "Why is obesity associated with osteoarthritis? Insights from mouse models of obesity". Biorheology. 45 (3–4): 387–98. doi:10.3233/BIR-2008-0485. PMC 2748656. PMID 18836239.
- ↑ Masuko K, Murata M, Suematsu N, Okamoto K, Yudoh K, Nakamura H, Kato T (2009). "A metabolic aspect of osteoarthritis: lipid as a possible contributor to the pathogenesis of cartilage degradation". Clinical and Experimental Rheumatology. 27 (2): 347–53. PMID 19473582.
- ↑ Hu PF, Bao JP, Wu LD (2011). "The emerging role of adipokines in osteoarthritis: a narrative review". Molecular Biology Reports. 38 (2): 873–78. doi:10.1007/s11033-010-0179-y. PMID 20480243.
- ↑ Coppari R, Bjørbæk C (2012). "Leptin revisited: its mechanism of action and potential for treating diabetes". Nature Reviews. Drug Discovery. 11 (9): 692–708. doi:10.1038/nrd3757. PMC 4019022. PMID 22935803.
- ↑ Gualillo O (2007). "Further evidence for leptin involvement in cartilage homeostases". Osteoarthritis and Cartilage. 15 (8): 857–60. doi:10.1016/j.joca.2007.04.015. PMID 17560812.
- ↑ Ouchi N, Parker JL, Lugus JJ, Walsh K (2011). "Adipokines in inflammation and metabolic disease". Nature Reviews. Immunology. 11 (2): 85–97. doi:10.1038/nri2921. PMC 3518031. PMID 21252989.
- ↑ Scotece M, Conde J, Vuolteenaho K, Koskinen A, López V, Gómez-Reino J, Lago F, Moilanen E, Gualillo O (2014). "Adipokines as drug targets in joint and bone disease". Drug Discovery Today. 19 (3): 241–58. doi:10.1016/j.drudis.2013.07.012. PMID 23906693.
- ↑ 157.0 157.1 Dumond H, Presle N, Terlain B, Mainard D, Loeuille D, Netter P, Pottie P (2003). "Evidence for a key role of leptin in osteoarthritis". Arthritis and Rheumatism. 48 (11): 3118–29. doi:10.1002/art.11303. PMID 14613274.
- ↑ 158.0 158.1 Simopoulou T, Malizos KN, Iliopoulos D, Stefanou N, Papatheodorou L, Ioannou M, Tsezou A (2007). "Differential expression of leptin and leptin's receptor isoform (Ob-Rb) mRNA between advanced and minimally affected osteoarthritic cartilage; effect on cartilage metabolism". Osteoarthritis and Cartilage. 15 (8): 872–83. doi:10.1016/j.joca.2007.01.018. PMID 17350295.
- ↑ Vuolteenaho K, Koskinen A, Moilanen T, Moilanen E (2012). "Leptin levels are increased and its negative regulators, SOCS-3 and sOb-R are decreased in obese patients with osteoarthritis: a link between obesity and osteoarthritis". Annals of the Rheumatic Diseases. 71 (11): 1912–13. doi:10.1136/annrheumdis-2011-201242. PMID 22689314.
- ↑ Gandhi R, Takahashi M, Syed K, Davey JR, Mahomed NN (2010). "Relationship between body habitus and joint leptin levels in a knee osteoarthritis population". Journal of Orthopaedic Research : Official Publication of the Orthopaedic Research Society. 28 (3): 329–33. doi:10.1002/jor.21000. PMID 19780190.
- ↑ Presle N, Pottie P, Dumond H, Guillaume C, Lapicque F, Pallu S, Mainard D, Netter P, Terlain B (2006). "Differential distribution of adipokines between serum and synovial fluid in patients with osteoarthritis. Contribution of joint tissues to their articular production". Osteoarthritis and Cartilage. 14 (7): 690–95. doi:10.1016/j.joca.2006.01.009. PMID 16527497.
- ↑ Morroni M, De Matteis R, Palumbo C, Ferretti M, Villa I, Rubinacci A, Cinti S, Marotti G (2004). "In vivo leptin expression in cartilage and bone cells of growing rats and adult humans". Journal of Anatomy. 205 (4): 291–96. doi:10.1111/j.0021-8782.2004.00333.x. PMC 1571344. PMID 15447688.
- ↑ Järvinen K, Vuolteenaho K, Nieminen R, Moilanen T, Knowles RG, Moilanen E (2008). "Selective iNOS inhibitor 1400W enhances anti-catabolic IL-10 and reduces destructive MMP-10 in OA cartilage. Survey of the effects of 1400W on inflammatory mediators produced by OA cartilage as detected by protein antibody array". Clinical and Experimental Rheumatology. 26 (2): 275–82. PMID 18565249.
- ↑ 164.0 164.1 Distel E, Cadoudal T, Durant S, Poignard A, Chevalier X, Benelli C (2009). "The infrapatellar fat pad in knee osteoarthritis: an important source of interleukin-6 and its soluble receptor". Arthritis and Rheumatism. 60 (11): 3374–77. doi:10.1002/art.24881. PMID 19877065.
- ↑ Clockaerts S, Bastiaansen-Jenniskens YM, Runhaar J, Van Osch GJ, Van Offel JF, Verhaar JA, De Clerck LS, Somville J (2010). "The infrapatellar fat pad should be considered as an active osteoarthritic joint tissue: a narrative review". Osteoarthritis and Cartilage. 18 (7): 876–82. doi:10.1016/j.joca.2010.03.014. PMID 20417297.
- ↑ Klein-Wieringa IR, Kloppenburg M, Bastiaansen-Jenniskens YM, Yusuf E, Kwekkeboom JC, El-Bannoudi H, Nelissen RG, Zuurmond A, Stojanovic-Susulic V, Van Osch GJ, Toes RE, Ioan-Facsinay A (2011). "The infrapatellar fat pad of patients with osteoarthritis has an inflammatory phenotype". Annals of the Rheumatic Diseases. 70 (5): 851–57. doi:10.1136/ard.2010.140046. PMID 21242232.
- ↑ Hui W, Litherland GJ, Elias MS, Kitson GI, Cawston TE, Rowan AD, Young DA (2012). "Leptin produced by joint white adipose tissue induces cartilage degradation via upregulation and activation of matrix metalloproteinases". Annals of the Rheumatic Diseases. 71 (3): 455–62. doi:10.1136/annrheumdis-2011-200372. PMID 22072016.
- ↑ Sinha G (2014). "Leptin therapy gains FDA approval". Nat. Biotechnol. 32 (4): 300–02. doi:10.1038/nbt0414-300b. PMID 24714458.
- ↑ Chou K, Perry CM (2013). "Metreleptin: first global approval". Drugs. 73 (9): 989–97. doi:10.1007/s40265-013-0074-7. PMID 23740412.
- ↑ "FDA approves Myalept to treat rare metabolic disease". FDA. 25 February 2014. Retrieved 30 April 2014.
- ↑ "Myalepta | European Medicines Agency". www.ema.europa.eu. Retrieved 2019-01-09.
- ↑ "The Rockefeller University » Hospital Centennial". centennial.rucares.org. Retrieved 2018-10-11.
External links
- Leptin: Your brain, appetite and obesity by the British Society of Neuroendocrinology
- Leptin by Colorado State University – nice illustrations, but last updated 1998
- Leptin at 3Dchem.com, description and structure diagrams
| Wikimedia Commons has media related to Leptin. |
- Wikipedia articles needing page number citations from November 2013
- Articles with invalid date parameter in template
- CS1 maint: Unrecognized language
- Pages with script errors
- Genes on human chromosome
- Pages with broken file links
- All articles with unsourced statements
- Articles with unsourced statements from August 2016
- Commons category link is locally defined
- Commons category link is on Wikidata using P373
- Leptin receptor agonists
- Peptide hormones
- Mutated genes
- Obesity