Vitamin K
|
WikiDoc Resources for Vitamin K |
|
Articles |
|---|
|
Most recent articles on Vitamin K |
|
Media |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Vitamin K at Clinical Trials.gov Clinical Trials on Vitamin K at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Vitamin K
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Vitamin K Discussion groups on Vitamin K Directions to Hospitals Treating Vitamin K Risk calculators and risk factors for Vitamin K
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Vitamin K |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief:

Overview
Vitamin K denotes a group of lipophilic, and hydrophobic, vitamins that are needed for the posttranslational modification of certain proteins, mostly required for blood coagulation. Chemically they are 2-methyl-1,4-naphthoquinone derivatives.
Vitamin K2 (menaquinone, menatetrenone) is normally produced by bacteria in the intestines, and dietary deficiency is extremely rare unless the intestines are heavily damaged.
Chemical structure
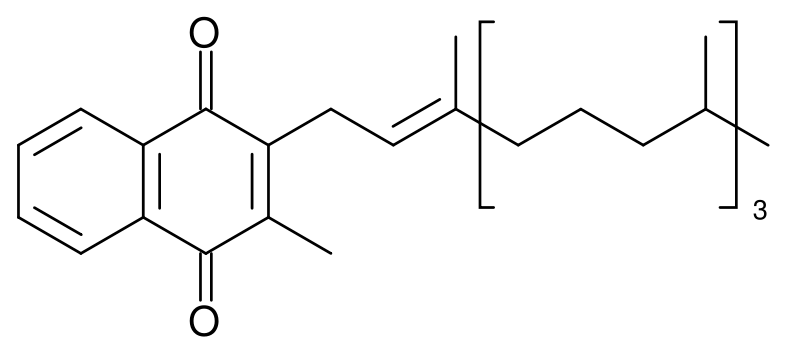
Vitamin K ("Koagulation" in German) is a group name for a number of related compounds, which have in common a methylated naphthoquinone ring structure, and which vary in the aliphatic side chain attached at the 3-position (see figure 1). Phylloquinone (also known as vitamin K1) invariably contains in its side chain four isoprenoid residues, one of which is unsaturated.
Menaquinones have side chains composed of a variable number of unsaturated isoprenoid residues; generally they are designated as MK-n, where n specifies the number of isoprenoids.
It is generally accepted that the naphthoquinone is the functional group, so that the mechanism of action is similar for all K-vitamins. Substantial differences may be expected, however, with respect to intestinal absorption, transport, tissue distribution, and bio-availability. These differences are caused by the different lipophilicity of the various side chains, and by the different food matrices in which they occur.
Physiology
Vitamin K is involved in the carboxylation of certain glutamate residues in proteins to form gamma-carboxyglutamate residues (abbreviated Gla-residues). Gla-residues are usually involved in binding calcium. The Gla-residues are essential for the biological activity of all known Gla-proteins.[1]
At this time 14 human Gla-proteins have been discovered, and they play key roles in the regulation of three physiological processes: blood coagulation[2], bone metabolism[3] and vascular biology[4].
Blood Coagulation
- Vitamin K plays a role in blood coagulation through the activation of blood coagulation and anticoagulation pathways. In fact vitamin K is involved in the gamma carboxylation of glutamate residues of prothrombin (factor II), factors VII, IX, X, protein C, protein S and protein Z.[2]
- Vitamin K is transformed into its active form by the enzyme vitamin K epoxide reductase.
Bone Metabolism
- Vitamin K is involved in bone mineralization through the gamma carboxylation of vitamin K dependent proteins osteocalcin, a protein synthesized by osteoblasts , and matrix gla protein (MGP) which is a protein found in cartilage.[3][5]
- Vitamin K affects calcium balance by regulating the synthesis and excretion of nephrocalcin and interleukin 1 and 6.[6]
- High dose vitamin K2 and low dose vitamin K1 were shown to increase bone density in osteoporosis and decrease fracture rates. In fact, vitamin K's effect on bone is synergistic with co-administration of vitamin D.[5]
Vascular Biology
- Matrix gla protein (MGP) is not only found in cartilage but also in vessels and it plays a role in preventing atherosclerosis and in decreasing age related arterial stiffness.[4][7]
Recommended amounts
The U.S. Dietary Reference Intake (DRI) for an Adequate Intake (AI) for a 25-year old male for Vitamin K is 120 micrograms/day. No Tolerable Upper Intake Level (UL) has been set. The human body stores Vitamin K, so it is not necessary to take Vitamin K daily.
Sources of Vitamin K
Vitamin K is found in leafy green vegetables such as spinach and lettuce; Brassica vegetables such as kale, cabbage, cauliflower, broccoli, and Brussels sprouts; wheat bran; organ meats; cereals; some fruits, such as avocado, kiwifruit and bananas; meats; cow milk and other dairy products; eggs; and other soy products. Two tablespoons of parsley contains 153% of the recommended daily amount of vitamin K.[8]
Phylloquinone (vitamin K1) is the major dietary form of vitamin K. Vitamin K1 is found in chicken egg yolk, butter, cow liver, most cheeses, and products. It is also found in some types of mayonnaise.
Vitamin K deficiency
- Poor dietary intake (malnutrition, starvation, fasting, post-chemotherapy induced anorexia, total parenteral nutrition)
- Malabsorption (celiac disease)
- Antibiotics
Role in disease
Vitamin K-deficiency may occur by disturbed intestinal uptake (such as would occur in a bile duct obstruction), by therapeutic or accidental intake of vitamin K-antagonists or, very rarely, by nutritional vitamin K-deficiency. As a result of the acquired vitamin K-deficiency, Gla-residues are not or incompletely formed and hence the Gla-proteins are inactive. Lack of control of the three processes mentioned above may lead to the following: risk of massive, uncontrolled internal bleeding, cartilage calcification and severe malformation of developing bone, or deposition of insoluble calcium salts in the arterial vessel walls.
Vitamin K indications
Warfarin overdose
For detailed description of role of vitamin K in warfarin overdose click here
Superwarfarin toxicity
- Superwarfarins are long-acting rat poisons that are 100 times more potent than warfarin
- Market name: Brodifacoum, bromadiolone, coumafuryl, difenacoum
- Increased risks of superwarfarin poisoning can be seen in:
- Occupational hazard
- Drug addicts
- Accidental exposure
- Suicide cases
- Presentation
- Diagnosis
- Treatment - High doses of vitamin K for longer durations (may be weeks to months)
Use on newborn babies
In some countries, injections of Vitamin K are routinely given to newborn babies. Vitamin K is used as prophylactic measure to prevent late-onset haemorrhagic disease (HDN). However, HDN is a relatively rare problem, and many parents now choose for their babies not to have such an injection.
Vitamin K: route of administration in patients with warfarin overanticoagulation
In patients with warfarin overanticoagulation, vitamin K administration can decrease the risks of bleeding. Oral and intravenous routes of vitamin K administration are found to be more effective than subcutaneous routes. Also, oral routes are preferred over intravenous routes, due to ease of administration and absence of anaphylaxsis seen with oral routes [9].
Biochemistry
Discovery
In the late 1920s, Danish scientist Henrik Dam investigated the role of cholesterol by feeding chickens a cholesterol-depleted diet.[10] After several weeks, the animals developed hemorrhages and started bleeding. These defects could not be restored by adding purified cholesterol to the diet. It appeared that - together with the cholesterol - a second compound had been extracted from the food, and this compound was called the coagulation vitamin. The new vitamin received the letter K because the initial discoveries were reported in a German journal, in which it was designated as Koagulationsvitamin. Edward Adelbert Doisy (of Saint Louis University) did much of the research that led to the discovery of the structure and chemical nature of Vitamin K.[11] Dam and Doisy shared the 1943 Nobel Prize for medicine for their work on Vitamin K. Several laboratories synthesized the compound in 1939.[12]
For several decades the vitamin K-deficient chick model was the only method of quantitating of vitamin K in various foods: the chicks were made vitamin K-deficient and subsequently fed with known amounts of vitamin K-containing food. The extent to which blood coagulation was restored by the diet was taken as a measure for its vitamin K content.
The first published report of successful treatment with vitamin K of life-threatening hemorrhage in a jaundiced patient with prothrombin deficiency was made in 1938 at the University of Iowa Department of Pathology by Drs. Harry Pratt Smith, Emory Warner, Kenneth Brinkhous, and Walter Seegers.[13]
Function in the cell
The precise function of vitamin K was not discovered until 1974, when three laboratories (Stenflo et al.[14], Nelsestuen et al.[15], and Magnusson et al.[16]) isolated the vitamin K-dependent coagulation factor prothrombin (Factor II) from cows that received a high dose of a vitamin K antagonist, warfarin. It was shown that while warfarin-treated cows had a form of prothrombin that contained 10 glutamate amino acid residues near the amino terminus of this protein, the normal (untreated) cows contained 10 unusual residues which were chemically identified as gamma-carboxyglutamate, or Gla. The extra carboxyl group in Gla made clear that vitamin K plays a role in a carboxylation reaction during which Glu is converted into Gla.
The biochemistry of how Vitamin K is used to convert Glu to Gla has been elucidated over the past thirty years in academic laboratories throughout the world. Within the cell, Vitamin K undergoes electron reduction to a reduced form of Vitamin K (called Vitamin K hydroquinone) by the enzyme Vitamin K epoxide reductase (or VKOR).[17] Another enzyme then oxidizes Vitamin K hydroquinone to allow carboxylation of Glu to Gla; this enzyme is called the gamma-glutamyl carboxylase[18][19] or the Vitamin K-dependent carboxylase. The carboxylation reaction will only proceed if the carboxylase enzyme is able to oxidize Vitamin K hydroquinone to vitamin K epoxide at the same time; the carboxylation and epoxidation reactions are said to be coupled reactions. Vitamin K epoxide is then re-converted to Vitamin K by the Vitamin K epoxide reductase. These two enzymes comprise the so-called Vitamin K cycle.[20] One of the reasons why Vitamin K is rarely deficient in a human diet is because Vitamin K is continually recycled in our cells.
Warfarin and other coumadin drugs block the action of the Vitamin K epoxide reductase.[21] This results in decreased concentrations of Vitamin K and Vitamin K hydroquinone in the tissues, such that the carboxylation reaction catalyzed by the glutamyl carboxylase is inefficient. This results in the production of clotting factors with a greatly diminished or a complete absence of Gla. Without Gla on the amino termini of these factors, they no longer stablely bind to the blood vessel endothelium and cannot activate clotting to allow formation of a clot during tissue injury. As administration of Warfarin to a patient suppresses the clotting response, it must be carefully monitored to avoid over-dosing. See Warfarin.
Gla-proteins
At present, the following human Gla-containing proteins have been characterized to the level of primary structure: the blood coagulation factors II (prothrombin), VII, IX, and X, the anticoagulant proteins C and S, and the Factor X-targeting protein Z. The bone Gla-protein osteocalcin, the calcification inhibiting matrix gla protein (MGP), the cell growth regulating growth arrest specific gene 6 protein (Gas6), and the four transmembrane Gla proteins (TMGPs) the function of which is at present unknown. Gas6 can function as a growth factor that activates the Axl receptor tyrosine kinase and stimulates cell proliferation or prevents apoptosis in some cells. In all cases in which their function was known, the presence of the Gla-residues in these proteins turned out to be essential for functional activity.
Gla-proteins are known to occur in a wide variety of vertebrates: mammals, birds, reptiles, and fish. The venom of a number of Australian snakes acts by activating the human blood clotting system. Remarkably, in some cases activation is accomplished by snake Gla-containing enzymes that bind to the endothelium of human blood vessels and catalyze the conversion of procoagulant clotting factors into activated ones, leading to unwanted and potentially deadly clotting.
Another interesting class of invertebrate Gla-containing proteins is synthesized by the fish-hunting snail Conus geographus.[22] These snails produce a venom containing hundreds of neuro-active peptides, or conotoxins, which is sufficiently toxic to kill an adult human. Several of the conotoxins contain 2-5 Gla residues.[23]
Function in Bacteria
Many bacteria, such as Escherichia coli found in the large intestine, can synthesize Vitamin K2 (menaquinone),[24] but not Vitamin K1 (phylloquinone). In these bacteria, menaquinone will transfer two electrons between two different small molecules, in a process called anaerobic respiration.[25] For example, a small molecule with an excess of electrons (also called an electron donor) such as lactate, formate, or NADH, with the help of an enzyme, will pass two electrons to a menaquinone. The menaquinone, with the help of another enzyme, will in turn transfer these 2 electrons to a suitable oxidant, such fumarate or nitrate (also called an electron acceptor). Adding two electrons to fumarate or nitrate will convert the molecule to succinate or nitrite + water, repectively. Some of these reactions generate a cellular energy source, ATP, in a manner similar to eukaryotic cell aerobic respiration, except that the final electron acceptor is not molecular oxygen, but say fumarate or nitrate (In aerobic respiration, the final oxidant is molecular oxygen (O2) , which accepts four electrons from an electron donor such as NADH to be converted to water.) Escherichia coli can carry out aerobic respiration and menaquninone-mediated anaerobic respiration.
Further reading
- Dam, H., Researches in Vitamin K, In: Pespectives in Biological Chemistry (RE Olson, ed.), Marcel Dekker, 1970. The Nobel Prize winner recounts the history of the discovery of Vitamin K.
- Suttie, J.W., Vitamin K, In: Handbook of Lipid research: The fat-soluble vitamins (HF DeLuca, ed.), Plenum Press, 1978. Outstanding review of Vitamin K research from 1930-1978 by one of the leaders in the field.
- David A. Bender, Nutritional biochemistry of the vitamins, Cambridge University Press, 2003
- G. F. M. Ball, Vitamins: their role in the human body, Blackwell Science, 2004
- Gerald F. Combs, The vitamins: fundamental aspects in nutrition and health, Academic Press, 1998
External links
- Template:Pauling
- Vitamin K: Another Reason to Eat Your Greens
- Vitamin K: Signs of Deficiency
- Vitamin K Deficiency - from the Merck Manual
- An Alternative Perspective on Vitamin K Prophylaxis
- Vitamin K Content - USDA National Nutrient Database for Standard Reference, Release 19
References
- ↑ Furie B, Bouchard BA, Furie BC. Vitamin K-dependent biosynthesis of gamma-carboxyglutamic acid. Blood, 1999, 93(6):1798-808. Review
- ↑ 2.0 2.1 Mann KG. Biochemistry and physiology of blood coagulation. Thrombosis and Haemostasis, 1999, 82(2):165-74. Review. PMID: 10605701
- ↑ 3.0 3.1 Price PA. Role of vitamin-K-dependent proteins in bone metabolism, Annual Review of Nutrition, 1988, 8:565-83. Review. PMID: 3060178
- ↑ 4.0 4.1 Berkner KL, Runge KW. The physiology of vitamin K nutriture and vitamin K-dependent protein function in atherosclerosis, Journal of Thrombosis and Haemostasis, 2004, 2(12):2118-32. Review
- ↑ 5.0 5.1 Weber P (2001). "Vitamin K and bone health". Nutrition. 17 (10): 880–7. PMID 11684396.
- ↑ Luo LZ, Xu L (2003). "[Vitamin K and osteoporosis]". Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 25 (3): 346–9. PMID 12905754.
- ↑ Doyon M, Mathieu P, Moreau P (2012). "Decreased expression of γ-carboxylase in diabetes-associated arterial stiffness: impact on matrix Gla protein". Cardiovasc Res. doi:10.1093/cvr/cvs325. PMID 23118128.
- ↑ http://www.nutritiondata.com/facts-C00001-01c20eX.html
- ↑ Dezee KJ, Shimeall WT, Douglas KM, Shumway NM, O'malley PG (2006). "Treatment of excessive anticoagulation with phytonadione (vitamin K): a meta-analysis". Arch Intern Med. 166 (4): 391–7. doi:10.1001/.391. PMID 16505257.
- ↑ Dam H. The antihemorrhagic vitamin of the chick. Occurrence and chemical nature, Nature, 1935;135:652
- ↑ MacCorquodale, DW, Binkley, SB, Thayer, SA, Doisy, EA, On the constitution of Vitamin K1, Journal of the American Chemical Society, 1939, 61:1928-1929
- ↑ Fieser, LF, Synthesis of Vitamin K1, Journal of the American Chemical Society,1939, 61:3467-3475
- ↑ Warner, ED, Brinkhous, KM, Smith, HP, Proceedings of the Society of Experimental Biology and Medicine, 1938, 37:628
- ↑ Stenflo J, Fernlund P, Egan W, Roepstorff P., Vitamin K-dependent modifications of glutamic acid residues in prothrombin, Proceedings of the National Academy of Sciences, USA, 1974, 71:2730–3. PMID 4528109
- ↑ Nelsestuen GL, Zytkovicz TH, Howard JB., The mode of action of vitamin K. Identification of gamma-carboxyglutamic acid as a component of prothrombin, Journal of Biological Chemistry, 1974, 249(19):6347-50. PMID: 4214105
- ↑ Magnusson S, Sottrup-Jensen L, Petersen TE, Morris HR, Dell A, Primary structure of the vitamin K-dependent part of prothrombin. FEBS Letters, 1974, 44(2):189-93. PMID: 4472513
- ↑ Oldenburg J, Bevans CG, Muller CR, Watzka M, Vitamin K epoxide reductase complex subunit 1 (VKORC1): the key protein of the vitamin K cycle, Antioxidants and Redox Signaling, 2006, 8(3-4):347-53. Review. PMID: 16677080
- ↑ Suttie JW, Vitamin K-dependent carboxylase, Annual Review of Biochemistry,1985, 54:459-77. Review. PMID: 3896125
- ↑ Presnell SR, Stafford DW, The vitamin K-dependent carboxylase, Thrombosis and Haemostasis, 2002, 87(6):937-46. Review. PMID: 12083499
- ↑ Stafford DW, The vitamin K cycle, Journal of Thrombosis Haemostais, 2005, (8):1873-8. Review. PMID: 16102054
- ↑ Whitlon DS, Sadowski JA, Suttie JW, Mechanisms of coumarin action: significance of vitamin K epoxide reductase inhibition, Biochemistry, 1978, 17:1371–7. PMID 646989
- ↑ Terlau H, Olivera BM. Conus venoms: a rich source of novel ion channel-targeted peptides, Physiological Reviews, 2004, 84(1):41-68. Review. PMID: 14715910
- ↑ Buczek O, Bulaj G, Olivera BM, Conotoxins and the posttranslational modification of secreted gene products, Cell and Molecular Life Sciences, 2005, 62(24):3067-79. Review. PMID:16314929
- ↑ Bentley, R, Meganathan, R., Biosynthesis of Vitamin K (menaquinone) in Bacteria, Bacteriological Reviews, 1982, 46(3):241-280. Review.
- ↑ Haddock, BA, Jones, CW, Bacterial Respiration, Bacteriological Reviews, 1977, 41(1):74-99. Review.
ar:فيتامين كي zh-min-nan:Bî-tá-mín K ca:Vitamina K cs:Vitamín K da:Vitamin K de:Vitamin K ko:바이타민 K ml:ജീവകം കെ hr:Vitamin K is:K-vítamín it:Vitamina K he:ויטמין K lt:Vitaminas K nl:Fytomenadion no:Vitamin K simple:Vitamin K sk:Fylochinón sr:Нафтокинон fi:K-vitamiini sv:K-vitamin