Semaglutide
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Sonya Gelfand
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
RISK OF THYROID C-CELL TUMORS
See full prescribing information for complete Boxed Warning.
|
Overview
Semaglutide is a glucagon-like peptide 1 (GLP-1) receptor agonist that is FDA approved for the adjunction of diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus. There is a Black Box Warning for this drug as shown here. Common adverse reactions include nausea, vomiting, diarrhea, abdominal pain and constipation.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
- Semaglutide is indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus.
Limitations of Use
- Semaglutide is not recommended as a first-line therapy for patients who have inadequate glycemic control on diet and exercise because of the uncertain relevance of rodent C-cell tumor findings to humans.
- Semaglutide has not been studied in patients with a history of pancreatitis. Consider other antidiabetic therapies in patients with a history of pancreatitis.
- Semaglutide is not a substitute for insulin. Semaglutide is not indicated for use in patients with type 1 diabetes mellitus or for the treatment of patients with diabetic ketoacidosis, as it would not be effective in these settings.
Recommended Dosage
- Start semaglutide with a 0.25 mg subcutaneous injection once weekly for 4 weeks. The 0.25 mg dose is intended for treatment initiation and is not effective for glycemic control.
- After 4 weeks on the 0.25 mg dose, increase the dosage to 0.5 mg once weekly.
- If additional glycemic control is needed after at least 4 weeks on the 0.5 mg dose, the dosage may be increased to 1 mg once weekly. The maximum recommended dosage is 1 mg once weekly.
- Administer semaglutide once weekly, on the same day each week, at any time of the day, with or without meals.
- The day of weekly administration can be changed if necessary as long as the time between two doses is at least 2 days (>48 hours).
- If a dose is missed, administer semaglutide as soon as possible within 5 days after the missed dose. If more than 5 days have passed, skip the missed dose and administer the next dose on the regularly scheduled day. In each case, patients can then resume their regular once weekly dosing schedule.
Dosage Forms and Strengths
- Injection: 2 mg/1.5 mL (1.34 mg/mL) of semaglutide as a clear, colorless solution available in:
- Pre-filled, disposable, single-patient-use pen that delivers 0.25 mg (for treatment initiation) or 0.5 mg (for maintenance treatment) per injection.
- Pre-filled, disposable, single-patient-use pen that delivers 1 mg (for maintenance treatment) per injection.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding semaglutide Off-Label Guideline-Supported Use and Dosage (Adult) in the drug label.
Non–Guideline-Supported Use
There is limited information regarding semaglutide Off-Label Non-Guideline-Supported Use and Dosage (Adult) in the drug label.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Semaglutide FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding semaglutide Off-Label Guideline-Supported Use and Dosage (Pediatric) in the drug label.
Non–Guideline-Supported Use
There is limited information regarding semaglutide Off-Label Non-Guideline-Supported Use and Dosage (Pediatric) in the drug label.
Contraindications
- Semaglutide is contraindicated in patients with:
- A personal or family history of medullary thyroid carcinoma (MTC) or in patients with Multiple Endocrine Neoplasia syndrome type 2 (MEN 2).
- Known hypersensitivity to semaglutide or to any of the product components.
Warnings
|
RISK OF THYROID C-CELL TUMORS
See full prescribing information for complete Boxed Warning.
|
Risk of Thyroid C-Cell Tumors
- In mice and rats, semaglutide caused a dose-dependent and treatment-duration-dependent increase in the incidence of thyroid C-cell tumors (adenomas and carcinomas) after lifetime exposure at clinically relevant plasma exposures. It is unknown whether semaglutide causes thyroid C-cell tumors, including medullary thyroid carcinoma (MTC), in humans as human relevance of semaglutide-induced rodent thyroid C-cell tumors has not been determined.
- Cases of MTC in patients treated with liraglutide, another GLP-1 receptor agonist, have been reported in the postmarketing period; the data in these reports are insufficient to establish or exclude a causal relationship between MTC and GLP-1 receptor agonist use in humans.
- Semaglutide is contraindicated in patients with a personal or family history of MTC or in patients with MEN 2. Counsel patients regarding the potential risk for MTC with the use of semaglutide and inform them of symptoms of thyroid tumors (e.g. a mass in the neck, dysphagia, dyspnea, persistent hoarseness).
- Routine monitoring of serum calcitonin or using thyroid ultrasound is of uncertain value for early detection of MTC in patients treated with semaglutide. Such monitoring may increase the risk of unnecessary procedures, due to the low test specificity for serum calcitonin and a high background incidence of thyroid disease. Significantly elevated serum calcitonin value may indicate MTC and patients with MTC usually have calcitonin values >50 ng/L. If serum calcitonin is measured and found to be elevated, the patient should be further evaluated. Patients with thyroid nodules noted on physical examination or neck imaging should also be further evaluated.
Pancreatitis
- In glycemic control trials, acute pancreatitis was confirmed by adjudication in 7 semaglutide-treated patients (0.3 cases per 100 patient years) versus 3 in comparator-treated patients (0.2 cases per 100 patient years). One case of chronic pancreatitis was confirmed in an semaglutide-treated patient. In a 2-year trial, acute pancreatitis was confirmed by adjudication in 8 semaglutide-treated patients (0.27 cases per 100 patient years) and 10 placebo-treated patients (0.33 cases per 100 patient years), both on a background of standard of care.
- After initiation of semaglutide, observe patients carefully for signs and symptoms of pancreatitis (including persistent severe abdominal pain, sometimes radiating to the back and which may or may not be accompanied by vomiting). If pancreatitis is suspected, semaglutide should be discontinued and appropriate management initiated; if confirmed, semaglutide should not be restarted.
Diabetic Retinopathy Complications
- In a 2-year trial involving patients with type 2 diabetes and high cardiovascular risk, more events of diabetic retinopathy complications occurred in patients treated with semaglutide (3.0%) compared to placebo (1.8%). The absolute risk increase for diabetic retinopathy complications was larger among patients with a history of diabetic retinopathy at baseline (semaglutide 8.2%, placebo 5.2%) than among patients without a known history of diabetic retinopathy (semaglutide 0.7%, placebo 0.4%).
- Rapid improvement in glucose control has been associated with a temporary worsening of diabetic retinopathy. The effect of long-term glycemic control with semaglutide on diabetic retinopathy complications has not been studied. Patients with a history of diabetic retinopathy should be monitored for progression of diabetic retinopathy.
- Semaglutide pens must never be shared between patients, even if the needle is changed. Pen-sharing poses a risk for transmission of blood-borne pathogens.
Hypoglycemia with Concomitant Use of Insulin Secretagogues or Insulin
- The risk of hypoglycemia is increased when semaglutide is used in combination with insulin secretagogues (e.g., sulfonylureas) or insulin. Patients may require a lower dose of the secretagogue or insulin to reduce the risk of hypoglycemia in this setting.
Acute Kidney Injury
- There have been postmarketing reports of acute kidney injury and worsening of chronic renal failure, which may sometimes require hemodialysis, in patients treated with GLP-1 receptor agonists. Some of these events have been reported in patients without known underlying renal disease. A majority of the reported events occurred in patients who had experienced nausea, vomiting, diarrhea, or dehydration. Monitor renal function when initiating or escalating doses of semaglutide in patients reporting severe adverse gastrointestinal reactions.
Hypersensitivity
- Serious hypersensitivity reactions (e.g., anaphylaxis, angioedema) have been reported with GLP-1 receptor agonists. If hypersensitivity reactions occur, discontinue use of semaglutide; treat promptly per standard of care, and monitor until signs and symptoms resolve. Do not use in patients with a previous hypersensitivity to semaglutide.
- Anaphylaxis and angioedema have been reported with other GLP-1 receptor agonists. Use caution in a patient with a history of angioedema or anaphylaxis with another GLP-1 receptor agonist because it is unknown whether such patients will be predisposed to anaphylaxis with semaglutide.
Macrovascular Outcomes
- There have been no clinical studies establishing conclusive evidence of macrovascular risk reduction with semaglutide.
Adverse Reactions
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Pool of Placebo-Controlled Trials
- The data in Table 1 are derived from 2 placebo-controlled trials (1 monotherapy trial and 1 trial in combination with basal insulin) in patients with type 2 diabetes. These data reflect exposure of 521 patients to semaglutide and a mean duration of exposure to semaglutide of 32.9 weeks. Across the treatment arms, the mean age of patients was 56 years, 3.4% were 75 years or older and 55% were male. In these trials 71% were White, 7% were Black or African American, and 19% were Asian; 21% identified as Hispanic or Latino ethnicity. At baseline, patients had type 2 diabetes for an average of 8.8 years and had a mean HbA1c of 8.2%. At baseline, 8.9% of the population reported retinopathy. Baseline estimated renal function was normal (eGFR ≥90 mL/min/1.73m2) in 57.2%, mildly impaired (eGFR 60 to 90 mL/min/1.73m2) in 35.9% and moderately impaired (eGFR 30 to 60 mL/min/1.73m2) in 6.9% of patients.
Pool of Placebo- and Active-Controlled Trials
- The occurrence of adverse reactions was also evaluated in a larger pool of patients with type 2 diabetes participating in 7 placebo- and active-controlled glycemic control trials including two trials in Japanese patients evaluating the use of semaglutide as monotherapy and add-on therapy to oral medications or insulin. In this pool, a total of 3150 patients with type 2 diabetes were treated with semaglutide for a mean duration of 44.9 weeks. Across the treatment arms, the mean age of patients was 57 years, 3.2% were 75 years or older and 57% were male. In these trials, 60% were White, 6% were Black or African American, and 31% were Asian; 16% identified as Hispanic or Latino ethnicity. At baseline, patients had type 2 diabetes for an average of 8.2 years and had a mean HbA1c of 8.2%. At baseline, 7.8% of the population reported retinopathy. Baseline estimated renal function was normal (eGFR ≥90 mL/min/1.73m2) in 63.1%, mildly impaired (eGFR 60 to 90 mL/min/1.73m2) in 34.3%, and moderately impaired (eGFR 30 to 60 mL/min/1.73m2) in 2.5% of the patients.
Common Adverse Reactions
- Table 1 shows common adverse reactions, excluding hypoglycemia, associated with the use of semaglutide in the pool of placebo-controlled trials. These adverse reactions occurred more commonly on semaglutide than on placebo, and occurred in at least 5% of patients treated with semaglutide.

- In the pool of placebo- and active-controlled trials and in the 2-year cardiovascular outcomes trial, the types and frequency of common adverse reactions, excluding hypoglycemia, were similar to those listed in Table 1.
Gastrointestinal Adverse Reactions
- In the pool of placebo-controlled trials, gastrointestinal adverse reactions occurred more frequently among patients receiving semaglutide than placebo (placebo 15.3%, semaglutide 0.5 mg 32.7%, semaglutide 1 mg 36.4%). The majority of reports of nausea, vomiting, and/or diarrhea occurred during dose escalation. More patients receiving semaglutide 0.5 mg (3.1%) and semaglutide 1 mg (3.8%) discontinued treatment due to gastrointestinal adverse reactions than patients receiving placebo (0.4%).
- In addition to the reactions in Table 1, the following gastrointestinal adverse reactions with a frequency of <5% were associated with semaglutide (frequencies listed, respectively, as: placebo; 0.5 mg; 1 mg): dyspepsia (1.9%, 3.5%, 2.7%), eructation (0%, 2.7%, 1.1%), flatulence (0.8%, 0.4%, 1.5%), gastroesophageal reflux disease (0%, 1.9%, 1.5%), and gastritis (0.8%, 0.8%, 0.4%).
Other Adverse Reactions
Hypoglycemia
- Table 2 summarizes the incidence of events related to hypoglycemia by various definitions in the placebo-controlled trials.
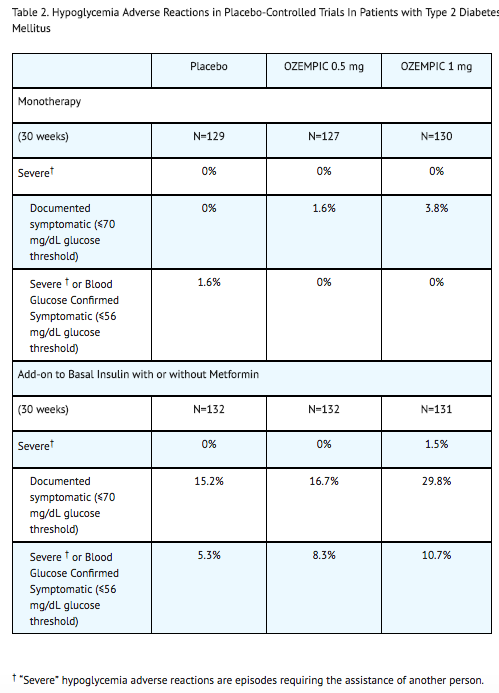
- Hypoglycemia was more frequent when semaglutide was used in combination with a sulfonylurea. Severe hypoglycemia occurred in 0.8% and 1.2% of patients when semaglutide 0.5 mg and 1 mg, respectively, was co-administered with a sulfonylurea. Documented symptomatic hypoglycemia occurred in 17.3% and 24.4% of patients when semaglutide 0.5 mg and 1 mg, respectively, was co-administered with a sulfonylurea. Severe or blood glucose confirmed symptomatic hypoglycemia occurred in 6.5% and 10.4% of patients when semaglutide 0.5 mg and 1 mg, respectively, was co-administered with a sulfonylurea.
Injection Site Reactions
- In placebo-controlled trials, injection site reactions (e.g., injection-site discomfort, erythema) were reported in 0.2% of semaglutide-treated patients.
Increases in Amylase and Lipase
- In placebo-controlled trials, patients exposed to semaglutide had a mean increase from baseline in amylase of 13% and lipase of 22%. These changes were not observed in placebo-treated patients.
Cholelithiasis
- In placebo-controlled trials, cholelithiasis was reported in 1.5% and 0.4% of patients-treated with semaglutide 0.5 mg and 1 mg, respectively. Cholelithiasis was not reported in placebo-treated patients.
Increases in Heart Rate
- In placebo-controlled trials, semaglutide 0.5 mg and 1 mg resulted in a mean increase in heart rate of 2 to 3 beats per minute. There was a mean decrease in heart rate of 0.3 beats per minute in placebo-treated patients.
Fatigue, Dysgeusia and Dizziness
- Other adverse reactions with a frequency of >0.4% were associated with semaglutide include fatigue, dysgeusia and dizziness.
Postmarketing Experience
There is limited information regarding Semaglutide Postmarketing Experience in the drug label.
Drug Interactions
- Concomitant Use with an Insulin Secretagogue (e.g., Sulfonylurea) or with Insulin
- Oral Medications
Concomitant Use with an Insulin Secretagogue (e.g., Sulfonylurea) or with Insulin
- The risk of hypoglycemia is increased when semaglutide is used in combination with insulin secretagogues (e.g., sulfonylureas) or insulin. The risk of hypoglycemia may be lowered by a reduction in the dose of sulfonylurea (or other concomitantly administered insulin secretagogues) or insulin.
Oral Medications
- Semaglutide causes a delay of gastric emptying, and thereby has the potential to impact the absorption of concomitantly administered oral medications. In clinical pharmacology trials, semaglutide did not affect the absorption of orally administered medications to any clinically relevant degree. Nonetheless, caution should be exercised when oral medications are concomitantly administered with semaglutide.
Use in Specific Populations
Pregnancy
Risk Summary
- There are limited data with semaglutide use in pregnant women to inform a drug-associated risk for adverse developmental outcomes. There are clinical considerations regarding the risks of poorly controlled diabetes in pregnancy. Based on animal reproduction studies, there may be potential risks to the fetus from exposure to semaglutide during pregnancy. Semaglutide should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
- In pregnant rats administered semaglutide during organogenesis, embryofetal mortality, structural abnormalities and alterations to growth occurred at maternal exposures below the maximum recommended human dose (MRHD) based on AUC. In rabbits and cynomolgus monkeys administered semaglutide during organogenesis, early pregnancy losses and structural abnormalities were observed at below the MRHD (rabbit) and ≥5-fold the MRHD (monkey). These findings coincided with a marked maternal body weight loss in both animal species.
- The estimated background risk of major birth defects is 6–10% in women with pre-gestational diabetes with an HbA1c >7 and has been reported to be as high as 20–25% in women with a HbA1c >10. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Clinical Considerations
Disease Associated Maternal and Fetal Risk
- Poorly controlled diabetes during pregnancy increases the maternal risk for diabetic ketoacidosis, pre- eclampsia, spontaneous abortions, preterm delivery, stillbirth and delivery complications. Poorly controlled diabetes increases the fetal risk for major birth defects, stillbirth, and macrosomia related morbidity.
Data (Animal)
- In a combined fertility and embryofetal development study in rats, subcutaneous doses of 0.01, 0.03 and 0.09 mg/kg/day (0.1-, 0.4-, and 1.1-fold the MRHD) were administered to males for 4 weeks prior to and throughout mating and to females for 2 weeks prior to mating, and throughout organogenesis to Gestation Day 17. In parental animals, pharmacologically mediated reductions in body weight gain and food consumption were observed at all dose levels. In the offspring, reduced growth and fetuses with visceral (heart blood vessels) and skeletal (cranial bones, vertebra, ribs) abnormalities were observed at the human exposure.
- In an embryofetal development study in pregnant rabbits, subcutaneous doses of 0.0010, 0.0025 or 0.0075 mg/kg/day (0.03-, 0.3-, and 2.3-fold the MRHD) were administered throughout organogenesis from Gestation Day 6 to 19. Pharmacologically mediated reductions in maternal body weight gain and food consumption were observed at all dose levels. Early pregnancy losses and increased incidences of minor visceral (kidney, liver) and skeletal (sternebra) fetal abnormalities were observed at ≥0.0025 mg/kg/day, at clinically relevant exposures.
- In an embryofetal development study in pregnant cynomolgus monkeys, subcutaneous doses of 0.015, 0.075, and 0.15 mg/kg twice weekly (1.0-, 5.2-, and 14.9-fold the MRHD) were administered throughout organogenesis, from Gestation Day 16 to 50. Pharmacologically mediated, marked initial maternal body weight loss and reductions in body weight gain and food consumption coincided with the occurrence of sporadic abnormalities (vertebra, sternebra, ribs) at ≥0.075 mg/kg twice weekly (>5X human exposure).
- In a pre- and postnatal development study in pregnant cynomolgus monkeys, subcutaneous doses of 0.015, 0.075, and 0.15 mg/kg twice weekly (0.7-, 3.3-, and 7.2-fold the MRHD) were administered from Gestation Day 16 to 140. Pharmacologically mediated marked initial maternal body weight loss and reductions in body weight gain and food consumption coincided with an increase in early pregnancy losses and led to delivery of slightly smaller offspring at ≥0.075 mg/kg twice weekly (>3X human exposure).
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Semaglutide in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Semaglutide during labor and delivery.
Nursing Mothers
Risk Summary
- There are no data on the presence of semaglutide in human milk, the effects on the breastfed infant, or the effects on milk production. Semaglutide was present in the milk of lactating rats, however, due to species-specific differences in lactation physiology, the clinical relevance of these data are not clear. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for semaglutide and any potential adverse effects on the breastfed infant from semaglutide or from the underlying maternal condition.
Data
- In lactating rats, semaglutide was detected in milk at levels 3-12 fold lower than in maternal plasma.
Pediatric Use
- Safety and efficacy of semaglutide have not been established in pediatric patients (younger than 18 years).
Geriatic Use
- In the pool of placebo- and active-controlled glycemic control trials, 744 (23.6%) semaglutide-treated patients were 65 years of age and over and 102 semaglutide-treated patients (3.2%) patients were 75 years of age and over. In SUSTAIN 6, the cardiovascular outcome trial, 788 (48.0%) semaglutide-treated patients were 65 years of age and over and 157 semaglutide-treated patients (9.6%) patients were 75 years of age and over.
- No overall differences in safety or efficacy were detected between these patients and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
Gender
There is no FDA guidance on the use of Semaglutide with respect to specific gender populations.
Race
There is no FDA guidance on the use of Semaglutide with respect to specific racial populations.
Renal Impairment
- No dose adjustment of semaglutide is recommended for patients with renal impairment. In subjects with renal impairment including end-stage renal disease (ESRD), no clinically relevant change in semaglutide pharmacokinetics (PK) was observed.
Hepatic Impairment
- No dose adjustment of semaglutide is recommended for patients with hepatic impairment. In a study in subjects with different degrees of hepatic impairment, no clinically relevant change in semaglutide pharmacokinetics (PK) was observed.
Females of Reproductive Potential and Males
- Discontinue semaglutide in women at least 2 months before a planned pregnancy due to the long washout period for semaglutide.
Immunocompromised Patients
There is no FDA guidance one the use of Semaglutide in patients who are immunocompromised.
Administration and Monitoring
Administration
- Administer semaglutide subcutaneously to the abdomen, thigh, or upper arm. Instruct patients to use a different injection site each week when injecting in the same body region.
- Inspect semaglutide visually before use. It should appear clear and colorless. Do not use semaglutide if particulate matter and coloration is seen.
- When using semaglutide with insulin, instruct patients to administer as separate injections and to never mix the products. It is acceptable to inject semaglutide and insulin in the same body region but the injections should not be adjacent to each other.
Monitoring
- Achieving glycemic control, including meeting HbA1c goal is indicative of efficacy.
- HbA1c: Twice yearly in patients who are meeting treatment goals; every 3 months in patients whose therapy has changed and/or who are not meeting glycemic goals; more frequently as clinically warranted.
- Renal function: When initiating or escalating doses and in patients reporting severe adverse reactions, especially in patients with renal impairment.
- Progression of diabetic retinopathy: In patients with a history of disease.
- Signs and symptoms of pancreatitis, including persistent severe abdominal pain, sometimes radiating to the back with or without vomiting.
IV Compatibility
There is limited information regarding the compatibility of Semaglutide and IV administrations.
Overdosage
- In the event of overdose, appropriate supportive treatment should be initiated according to the patient’s clinical signs and symptoms. A prolonged period of observation and treatment for these symptoms may be necessary, taking into account the long half-life of semaglutide of approximately 1 week.
Pharmacology
| |
Semaglutide
| |
| Systematic (IUPAC) name | |
| ? | |
| Identifiers | |
| CAS number | |
| ATC code | A10 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | ? |
| Pharmacokinetic data | |
| Bioavailability | 89% |
| Metabolism | Proteolysis |
| Half life | 1 week |
| Excretion | Urine and faeces |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status |
[[Prescription drug|Template:Unicode-only]](US) |
| Routes | ? |
Mechanism of Action
- Semaglutide is a GLP-1 analogue with 94% sequence homology to human GLP-1. Semaglutide acts as a GLP-1 receptor agonist that selectively binds to and activates the GLP-1 receptor, the target for native GLP-1.
- GLP-1 is a physiological hormone that has multiple actions on glucose, mediated by the GLP-1 receptors.
- The principal mechanism of protraction resulting in the long half-life of semaglutide is albumin binding, which results in decreased renal clearance and protection from metabolic degradation. Furthermore, semaglutide is stabilized against degradation by the DPP-4 enzyme.
- Semaglutide reduces blood glucose through a mechanism where it stimulates insulin secretion and lowers glucagon secretion, both in a glucose-dependent manner. Thus, when blood glucose is high, insulin secretion is stimulated and glucagon secretion is inhibited. The mechanism of blood glucose lowering also involves a minor delay in gastric emptying in the early postprandial phase.
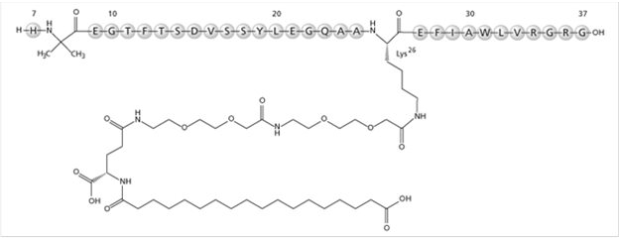
Structure
Pharmacodynamics
- Semaglutide lowers fasting and postprandial blood glucose and reduces body weight. All pharmacodynamic evaluations were performed after 12 weeks of treatment (including dose escalation) at steady state with semaglutide 1 mg.
Fasting and Postprandial Glucose
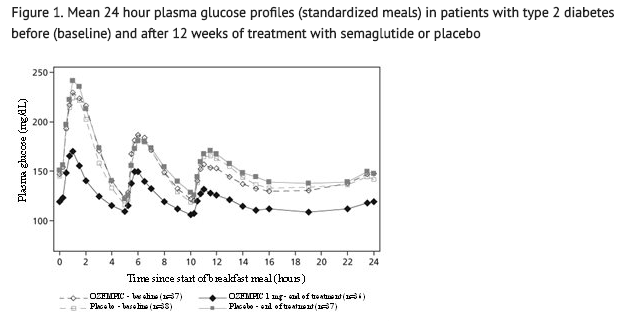
- Semaglutide reduces fasting and postprandial glucose concentrations. In patients with type 2 diabetes, treatment with semaglutide 1 mg resulted in reductions in glucose in terms of absolute change from baseline and relative reduction compared to placebo of 29 mg/dL (22%) for fasting glucose, 74 mg/dL (36%) for 2 hour postprandial glucose, and 30 mg/dL (22%) for mean 24 hour glucose concentration (see Figure 1).
Insulin Secretion
- Both first-and second-phase insulin secretion are increased in patients with type 2 diabetes treated with semaglutide compared with placebo.
Glucagon Secretion
- Semaglutide lowers the fasting and postprandial glucagon concentrations. In patients with type 2 diabetes, treatment with semaglutide resulted in the following relative reductions in glucagon compared to placebo, fasting glucagon (8%), postprandial glucagon response (14-15%), and mean 24 hour glucagon concentration (12%).
Glucose dependent insulin and glucagon secretion
- Semaglutide lowers high blood glucose concentrations by stimulating insulin secretion and lowering glucagon secretion in a glucose-dependent manner. With semaglutide, the insulin secretion rate in patients with type 2 diabetes was similar to that of healthy subjects (see Figure 2).
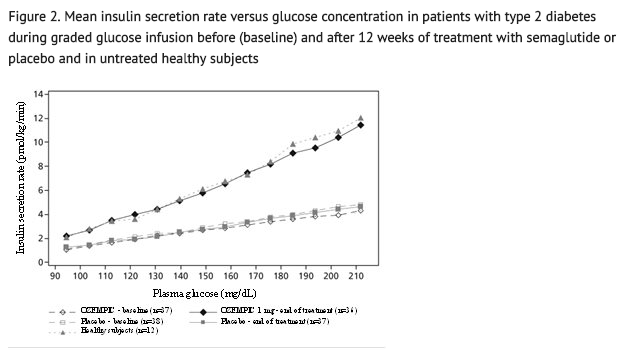
- During induced hypoglycemia, semaglutide did not alter the counter regulatory responses of increased glucagon compared to placebo, and did not impair the decrease of C-peptide in patients with type 2 diabetes.
Gastric Emptying
- Semaglutide causes a delay of early postprandial gastric emptying, thereby reducing the rate at which glucose appears in the circulation postprandially.
Cardiac electrophysiology (QTc)
- The effect of semaglutide on cardiac repolarization was tested in a thorough QTc trial. At a dose 1.5 times the maximum recommended dose, semaglutide does not prolong QTc intervals to any clinically relevant extent.
Pharmacokinetics
Absorption
- Absolute bioavailability of semaglutide is 89%. Maximum concentration of semaglutide is reached 1 to 3 days post dose.
- Similar exposure is achieved with subcutaneous administration of semaglutide in the abdomen, thigh, or upper arm.
- In patients with type 2 diabetes, semaglutide exposure increases in a dose-proportional manner for once-weekly doses of 0.5 mg and 1 mg. Steady-state exposure is achieved following 4-5 weeks of once-weekly administration. In patients with type 2 diabetes, the mean population-PK estimated steady-state concentrations following once weekly subcutaneous administration of 0.5 mg and 1 mg semaglutide were approximately 65.0 ng/mL and 123.0 ng/mL, respectively.
Distribution
- The mean apparent volume of distribution of semaglutide following subcutaneous administration in patients with type 2 diabetes is approximately 12.5 L. Semaglutide is extensively bound to plasma albumin (>99%).
Elimination
- The apparent clearance of semaglutide in patients with type 2 diabetes is approximately 0.05 L/h. With an elimination half-life of approximately 1 week, semaglutide will be present in the circulation for about 5 weeks after the last dose.
Metabolism
- The primary route of elimination for semaglutide is metabolism following proteolytic cleavage of the peptide backbone and sequential beta-oxidation of the fatty acid sidechain.
Excretion
- The primary excretion routes of semaglutide-related material is via the urine and feces. Approximately 3% of the dose is excreted in the urine as intact semaglutide.
Specific Populations
- Based on a population pharmacokinetic analysis, age, sex, race, and ethnicity, and renal impairment do not have a clinically meaningful effect on the pharmacokinetics of semaglutide. The exposure of semaglutide decreases with an increase in body weight. However, semaglutide doses of 0.5 mg and 1 mg provide adequate systemic exposure over the body weight range of 40-198 kg evaluated in the clinical trials. The effects of intrinsic factors on the pharmacokinetics of semaglutide are shown in Figure 3.
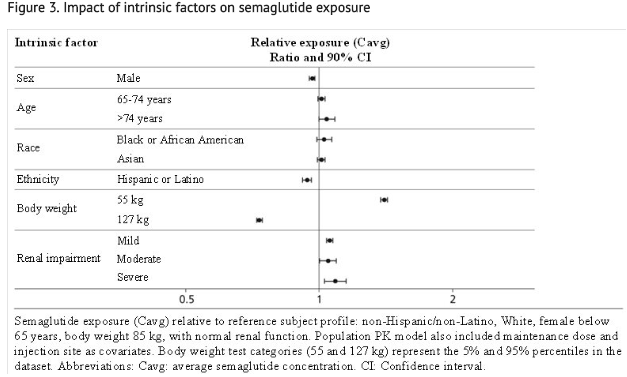
Patients with Renal Impairment
- Renal impairment does not impact the pharmacokinetics of semaglutide in a clinically relevant manner. This was shown in a study with a single dose of 0.5 mg semaglutide in patients with different degrees of renal impairment (mild, moderate, severe, ESRD) compared with subjects with normal renal function. This was also shown for subjects with both type 2 diabetes and renal impairment based on data from clinical studies (Figure 3).
Patients with Hepatic Impairment
- Hepatic impairment does not have any impact on the exposure of semaglutide. The pharmacokinetics of semaglutide were evaluated in patients with different degrees of hepatic impairment (mild, moderate, severe) compared with subjects with normal hepatic function in a study with a single-dose of 0.5 mg semaglutide.
Pediatric Patients
- Semaglutide has not been studied in pediatric patients.
Drug Interaction Studies
- In vitro studies have shown very low potential for semaglutide to inhibit or induce CYP enzymes, and to inhibit drug transporters.
- The delay of gastric emptying with semaglutide may influence the absorption of concomitantly administered oral medicinal products. The potential effect of semaglutide on the absorption of co-administered oral medications was studied in trials at semaglutide 1 mg steady-state exposure.
- No clinically relevant drug-drug interaction with semaglutide (Figure 4) was observed based on the evaluated medications; therefore, no dose adjustment is required when co-administered with semaglutide.
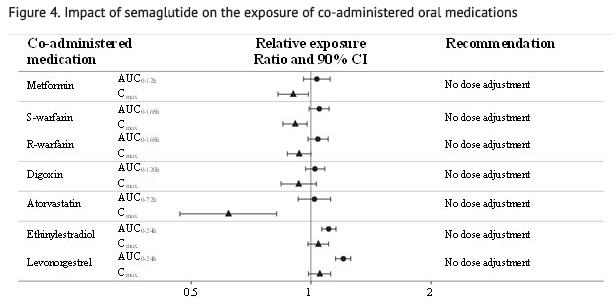
- Relative exposure in terms of AUC and Cmax for each medication when given with semaglutide compared to without semaglutide. Metformin and oral contraceptive drug (ethinylestradiol/levonorgestrel) were assessed at steady state. Warfarin (S-warfarin/R-warfarin), digoxin and atorvastatin were assessed after a single dose.
- Abbreviations: AUC: area under the curve. Cmax: maximum concentration. CI: confidence interval.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- In a 2-year carcinogenicity study in CD-1 mice, subcutaneous doses of 0.3, 1 and 3 mg/kg/day [5-, 17-, and 59-fold the maximum recommended human dose (MRHD) of 1 mg/week, based on AUC] were administered to the males, and 0.1, 0.3 and 1 mg/kg/day (2-, 5-, and 17-fold MRHD) were administered to the females. A statistically significant increase in thyroid C-cell adenomas and a numerical increase in C-cell carcinomas were observed in males and females at all dose levels (>2X human exposure).
- In a 2-year carcinogenicity study in Sprague Dawley rats, subcutaneous doses of 0.0025, 0.01, 0.025 and 0.1 mg/kg/day were administered (below quantification, 0.4-, 1-, and 6-fold the exposure at the MRHD). A statistically significant increase in thyroid C-cell adenomas was observed in males and females at all dose levels, and a statistically significant increase in thyroid C-cell carcinomas was observed in males at ≥0.01 mg/kg/day, at clinically relevant exposures.
- Human relevance of thyroid C-cell tumors in rats is unknown and could not be determined by clinical studies or nonclinical studies.
- Semaglutide was not mutagenic or clastogenic in a standard battery of genotoxicity tests (bacterial mutagenicity (Ames), human lymphocyte chromosome aberration, rat bone marrow micronucleus).
- In a combined fertility and embryo-fetal development study in rats, subcutaneous doses of 0.01, 0.03 and 0.09 mg/kg/day (0.1-, 0.4-, and 1.1-fold the MRHD) were administered to male and female rats. Males were dosed for 4 weeks prior to mating, and females were dosed for 2 weeks prior to mating and throughout organogenesis until Gestation Day 17. No effects were observed on male fertility. In females, an increase in oestrus cycle length was observed at all dose levels, together with a small reduction in numbers of corpora lutea at ≥0.03 mg/kg/day. These effects were likely an adaptive response secondary to the pharmacological effect of semaglutide on food consumption and body weight.
Clinical Studies
Overview of Clinical Studies
- Semaglutide has been studied as monotherapy and in combination with metformin, metformin and sulfonylureas, metformin and/or thiazolidinedione, and basal insulin in patients with type 2 diabetes mellitus. The efficacy of semaglutide was compared with placebo, sitagliptin, exenatide extended-release (ER), and insulin glargine.
- Most trials evaluated the use of semaglutide 0.5 mg, and 1 mg, with the exception of the trial comparing semaglutide and exenatide ER where only the 1 mg dose was studied.
- In patients with type 2 diabetes mellitus, semaglutide produced clinically relevant reduction from baseline in HbA1c compared with placebo.
- The efficacy of semaglutide was not impacted by age, gender, race, ethnicity, BMI at baseline, body weight (kg) at baseline, diabetes duration and level of renal function impairment.
Monotherapy Use of Semaglutide in Patients with Type 2 Diabetes Mellitus
- In a 30-week double-blind trial (NCT02054897), 388 patients with type 2 diabetes mellitus inadequately controlled with diet and exercise were randomized to semaglutide 0.5 mg or semaglutide 1 mg once weekly or placebo. Patients had a mean age of 54 years and 54% were men. The mean duration of type 2 diabetes was 4.2 years, and the mean BMI was 33 kg/m2. Overall, 64% were White, 8% were Black or African American, and 21% were Asian; 30% identified as Hispanic or Latino ethnicity.
- Monotherapy with semaglutide 0.5 mg and 1 mg once weekly for 30 weeks resulted in a statistically significant reduction in HbA1c compared with placebo (see Table 3).
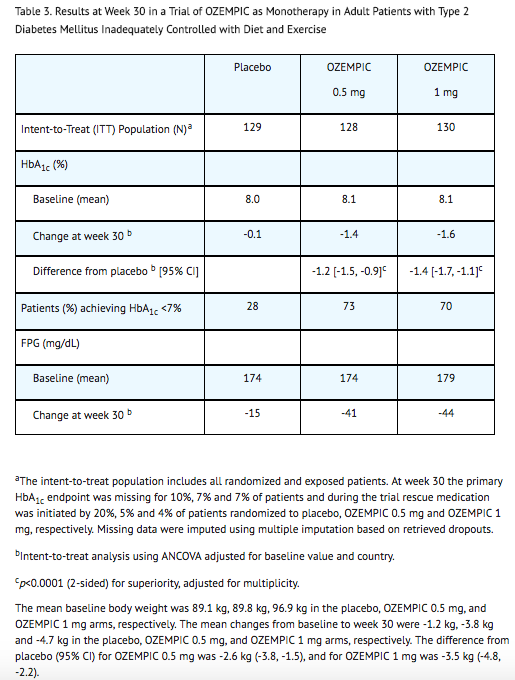
Combination Therapy Use of Semaglutide in Patients with Type 2 Diabetes Mellitus
Combination with Metformin and/or Thiazolidinediones
- In a 56-week, double-blind trial (NCT01930188), 1231 patients with type 2 diabetes mellitus were randomized to semaglutide 0.5 mg once weekly, semaglutide 1 mg once weekly, or sitagliptin 100 mg once daily, all in combination with metformin (94%) and/or thiazolidinediones (6%). Patients had a mean age of 55 years and 51% were men. The mean duration of type 2 diabetes was 6.6 years, and the mean BMI was 32 kg/m2. Overall, 68% were White, 5% were Black or African American, and 25% were Asian; 17% identified as Hispanic or Latino ethnicity.
- Treatment with semaglutide 0.5 mg and 1 mg once weekly for 56 weeks resulted in a statistically significant reduction in HbA1c compared to sitagliptin (see Table 4 and Figure 5).
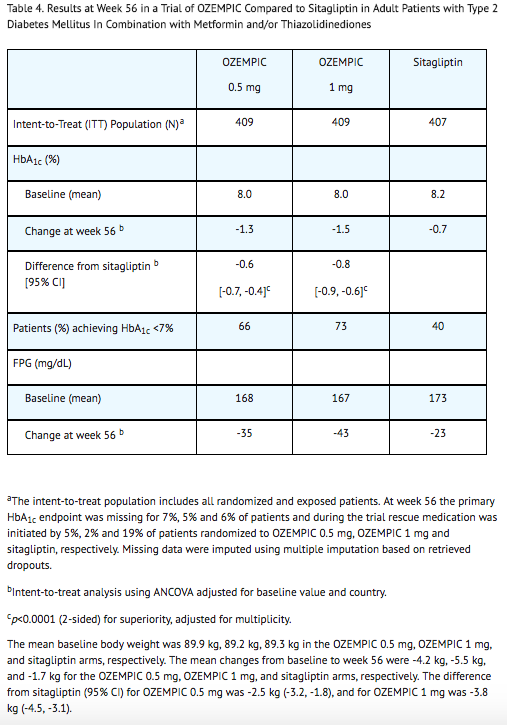
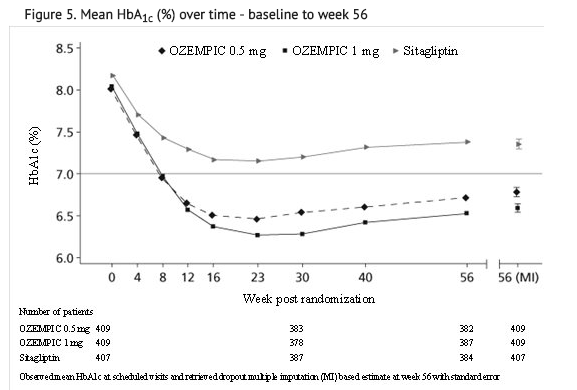
Combination with Metformin or Metformin with Sulfonylurea
- In a 56-week, open-label trial (NCT01885208), 813 patients with type 2 diabetes mellitus on metformin alone (49%), metformin with sulfonylurea (45%), or other (6%) were randomized to semaglutide 1 mg once weekly or exenatide 2 mg once weekly. Patients had a mean age of 57 years and 55% were men. The mean duration of type 2 diabetes was 9 years, and the mean BMI was 34 kg/m2. Overall, 84% were White, 7% were Black or African American, and 2% were Asian; 24% identified as Hispanic or Latino ethnicity.
- Treatment with semaglutide 1 mg once weekly for 56 weeks resulted in a statistically significant reduction in HbA1c compared to exenatide 2 mg once weekly (see Table 5).
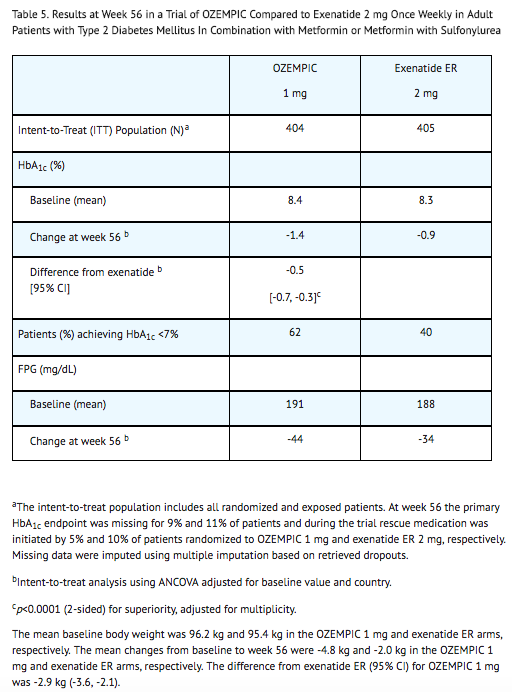
Combination with Metformin or Metformin with Sulfonylurea
- In a 30-week, open-label trial (NCT02128932), 1089 patients with type 2 diabetes mellitus were randomized to semaglutide 0.5 mg once weekly, semaglutide 1 mg once weekly, or insulin glargine once daily on a background of metformin (48%) or metformin and sulfonylurea (51%). Patients had a mean age of 57 years and 53% were men. The mean duration of type 2 diabetes was 8.6 years, and the mean BMI was 33 kg/m2. Overall, 77% were White, 9% were Black or African American, and 11% were Asian; 20% identified as Hispanic or Latino ethnicity.
- Patients assigned to insulin glargine had a baseline mean HbA1c of 8.1% and were started on a dose of 10 U once daily. Insulin glargine dose adjustments occurred throughout the trial period based on self-measured fasting plasma glucose before breakfast, targeting 71 to <100 mg/dL. In addition, investigators could titrate insulin glargine at their discretion between study visits. Only 26% of patients had been titrated to goal by the primary endpoint at week 30, at which time the mean daily insulin dose was 29 U per day.
- Treatment with semaglutide 0.5 mg and 1 mg once weekly for 30 weeks resulted in a statistically significant reduction in HbA1c compared with the insulin glargine titration implemented in this study protocol (see Table 6).
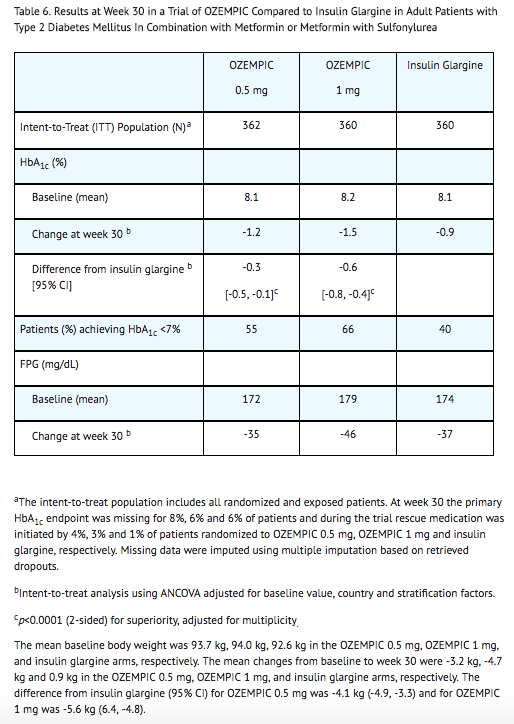
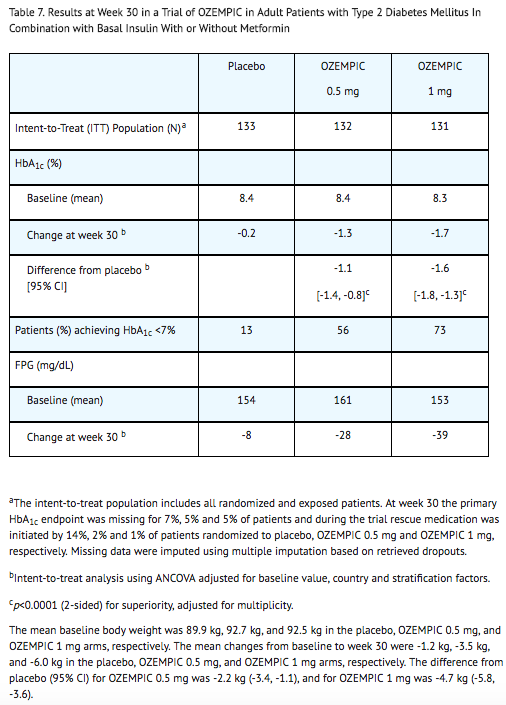
Combination with Basal Insulin
- In a 30-week, double-blind trial (NCT02305381), 397 patients with type 2 diabetes mellitus inadequately controlled with basal insulin, with or without metformin, were randomized to semaglutide 0.5 mg once weekly, semaglutide 1 mg once weekly, or placebo. Patients with HbA1c ≤ 8.0% at screening reduced their insulin dose by 20% at start of the trial to reduce the risk of hypoglycemia. Patients had a mean age of 59 years and 56% were men. The mean duration of type 2 diabetes was 13 years, and the mean BMI was 32 kg/m2. Overall, 78% were White, 5% were Black or African American, and 17% were Asian; 12% identified as Hispanic or Latino ethnicity.
- Treatment with semaglutide resulted in a statistically significant reduction in HbA1c after 30 weeks of treatment compared to placebo (see Table 7).
Cardiovascular Outcomes Trial of Semaglutide in Patients with Type 2 Diabetes Mellitus
- SUSTAIN 6 (NCT01720446) was a 104-week, double-blind trial in which 3,297 patients with type 2 diabetes and high risk of cardiovascular events were randomized to semaglutide 0.5 mg once weekly, semaglutide 1 mg once weekly, or placebo in addition to standard-of-care. In total, 2,735 (83%) of the patients had a history of cardiovascular disease and 562 (17%) were at high risk but without known cardiovascular disease. The mean age at baseline was 65 years, and 61% were men. The mean duration of diabetes was 13.9 years, and mean BMI was 33 kg/m2. Overall, 83% were White, 7% were Black or African American, and 8% were Asian; 16% identified as Hispanic or Latino ethnicity. Concomitant diseases of patients in this trial included, but were not limited to, heart failure (24%), hypertension (93%), history of ischemic stroke (12%) and history of a myocardial infarction (33%).
- In total, 98.0% of the patients completed the trial and the vital status was known at the end of the trial for 99.6%. The primary composite endpoint was the time from randomization to first occurrence of a major adverse cardiovascular event (MACE): cardiovascular death, non-fatal myocardial infarction or non-fatal stroke. The secondary endpoint was time from randomization to first occurrence of an expanded composite cardiovascular outcome, defined as MACE, revascularization (coronary and peripheral), unstable angina requiring hospitalization or hospitalization for heart failure. The total number of primary component MACE endpoints was 254 (108 [6.6%] with semaglutide and 146 [8.9%] with placebo). No increased risk for MACE was observed with semaglutide.
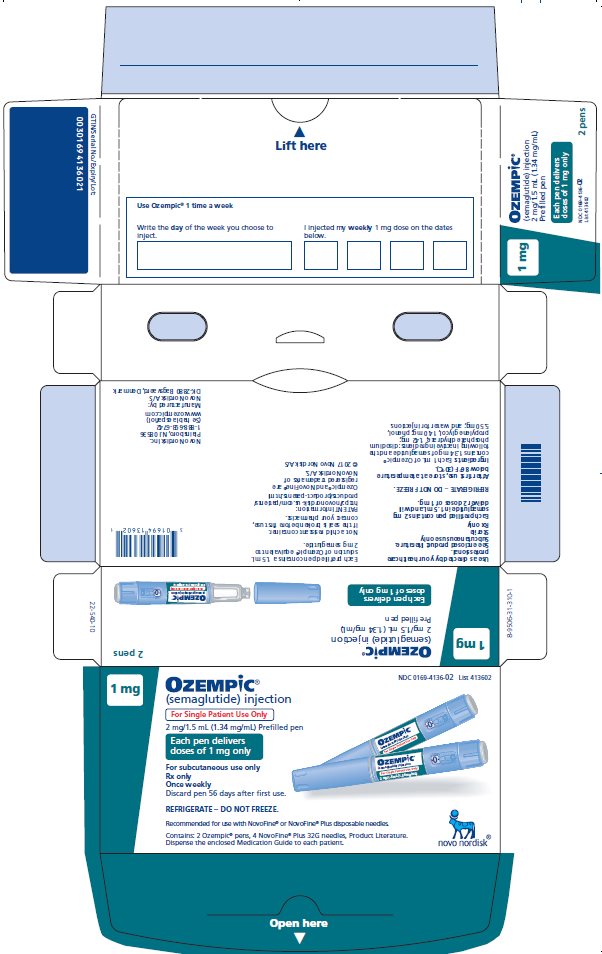
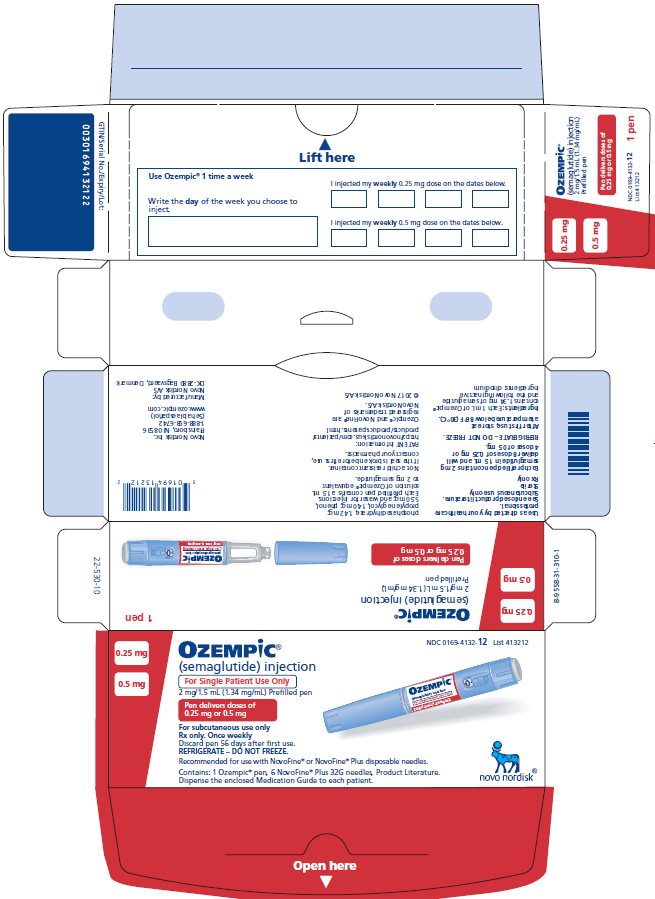
How Supplied
- Semaglutide injection is supplied as a clear, colorless solution that contains 2 mg of semaglutide in a 1.5 mL (1.34 mg/mL) pre-filled, disposable, single-patient-use pen injector in the following packaging configurations:
Carton of 1 Pen (NDC 0169-4132-12)
- Pen delivers doses of 0.25 mg or 0.5 mg per injection.
- 6 NovoFine ® Plus needles.
- Intended for treatment initiation at the 0.25 mg dose and maintenance treatment at the 0.5 mg dose.
Carton of 2 Pens (NDC 0169-4136-02)
- Pen delivers doses of 1 mg per injection.
- 4 NovoFine ® Plus needles.
- Intended for maintenance treatment at the 1 mg dose only.
- Each semaglutide pen is for use by a single patient. An semaglutide pen must never be shared between patients, even if the needle is changed.
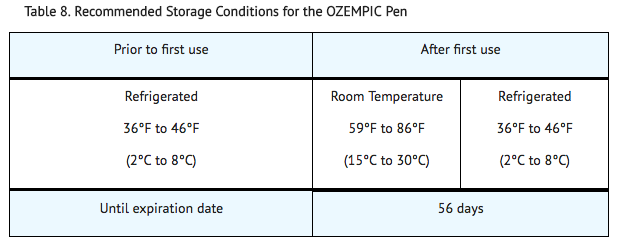
Storage
- Prior to first use, semaglutide should be stored in a refrigerator between 36ºF to 46ºF (2ºC to 8ºC) (Table 8). Do not store in the freezer or directly adjacent to the refrigerator cooling element. Do not freeze semaglutide and do not use semaglutide if it has been frozen.
- After first use of the semaglutide pen, the pen can be stored for 56 days at controlled room temperature (59°F to 86°F; 15°C to 30°C) or in a refrigerator (36°F to 46°F; 2°C to 8°C). Do not freeze. Keep the pen cap on when not in use. Semaglutide should be protected from excessive heat and sunlight.
- Always remove and safely discard the needle after each injection and store the semaglutide pen without an injection needle attached. Always use a new needle for each injection.
- The storage conditions are summarized in Table 8:
Images
Drug Images
{{#ask: Page Name::Semaglutide |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Semaglutide |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Advise the patient to read the FDA-approved patient labeling.
Risk of Thyroid C-cell Tumors
- Inform patients that semaglutide causes thyroid C-cell tumors in rodents and that the human relevance of this finding has not been determined. Counsel patients to report symptoms of thyroid tumors (e.g., a lump in the neck, hoarseness, dysphagia, or dyspnea) to their physician.
Pancreatitis
- Inform patients of the potential risk for pancreatitis. Instruct patients to discontinue semaglutide promptly and contact their physician if pancreatitis is suspected (severe abdominal pain that may radiate to the back, and which may or may not be accompanied by vomiting).
Diabetic Retinopathy Complications
- Inform patients to contact their physician if changes in vision are experienced during treatment with semaglutide.
- Advise patients that they must never share an semaglutide pen with another person, even if the needle is changed, because doing so carries a risk for transmission of blood-borne pathogens.
Dehydration and Renal Failure
- Advise patients treated with semaglutide of the potential risk of dehydration due to gastrointestinal adverse reactions and take precautions to avoid fluid depletion. Inform patients of the potential risk for worsening renal function and explain the associated signs and symptoms of renal impairment, as well as the possibility of dialysis as a medical intervention if renal failure occurs.
Hypersensitivity Reactions
- Inform patients to stop taking semaglutide and seek medical advice promptly if symptoms of hypersensitivity reactions occur.
Pregnancy
- Advise a pregnant woman of the potential risk to a fetus. Advise women to inform their healthcare provider if they are pregnant or intend to become pregnant.
Instructions
- Inform patients of the potential risks and benefits of semaglutide and of alternative modes of therapy. Inform patients about the importance of adherence to dietary instructions, regular physical activity, periodic blood glucose monitoring and A1c testing, recognition and management of hypoglycemia and hyperglycemia, and assessment for diabetes complications. Advise patients to seek medical advice promptly during periods of stress such as fever, trauma, infection, or surgery as medication requirements may change.
- Advise patients that the most common side effects of semaglutide are nausea, vomiting, diarrhea, abdominal pain and constipation. Inform patients that nausea, vomiting and diarrhea are most common when first starting semaglutide, but decreases over time in the majority of patients.
- Instruct patients to reread the Medication Guide each time the prescription is renewed.
- Inform patients if a dose is missed, it should be administered as soon as possible within 5 days after the missed dose. If more than 5 days have passed, the missed dose should be skipped and the next dose should be administered on the regularly scheduled day. In each case, inform patients to resume their regular once weekly dosing schedule.
- Semaglutide (oh-ZEM-pick)
- (Semaglutide)
- Injection, for subcutaneous use
- Do not share your semaglutide pen with other people, even if the needle has been changed. You may give other people a serious infection, or get a serious infection from them.
- Read this Medication Guide before you start using semaglutide and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or your treatment.
- What is the most important information I should know about semaglutide?
- Semaglutide may cause serious side effects, including:
- Possible thyroid tumors, including cancer. Tell your healthcare provider if you get a lump or swelling in your neck, hoarseness, trouble swallowing, or shortness of breath. These may be symptoms of thyroid cancer. In studies with rodents, semaglutide and medicines that work like semaglutide caused thyroid tumors, including thyroid cancer. It is not known if semaglutide will cause thyroid tumors or a type of thyroid cancer called medullary thyroid carcinoma (MTC) in people.
- Do not use semaglutide if you or any of your family have ever had a type of thyroid cancer called medullary thyroid carcinoma (MTC), or if you have an endocrine system condition called Multiple Endocrine Neoplasia syndrome type 2 (MEN 2).
- What is semaglutide?
- Semaglutide is an injectable prescription medicine for adults with type 2 diabetes mellitus that:
- Along with diet and exercise may improve blood sugar (glucose).
- Semaglutide is not recommended as the first choice of medicine for treating diabetes.
- It is not known if semaglutide can be used in people who have had pancreatitis.
- Semaglutide is not a substitute for insulin and is not for use in people with type 1 diabetes or people with diabetic ketoacidosis.
- It is not known if semaglutide is safe and effective for use in children under 18 years of age.
- Do not use semaglutide if:
- You or any of your family have ever had a type of thyroid cancer called medullary thyroid carcinoma (MTC) or if you have an endocrine system condition called Multiple Endocrine Neoplasia syndrome type 2 (MEN 2).
- You are allergic to semaglutide or any of the ingredients in semaglutide. See the end of this Medication Guide for a complete list of ingredients in semaglutide.
- Before using semaglutide, tell your healthcare provider if you have any other medical conditions, including if you:
- Have or have had problems with your pancreas or kidneys.
- Have a history of diabetic retinopathy.
- Are pregnant or plan to become pregnant. It is not known if semaglutide will harm your unborn baby. You should stop using semaglutide 2 months before you plan to become pregnant. Talk to your healthcare provider about the best way to control your blood sugar if you plan to become pregnant or while you are pregnant.
- Are breastfeeding or plan to breastfeed. It is not known if semaglutide passes into your breast milk. You should talk with your healthcare provider about the best way to feed your baby while using semaglutide.
- Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Semaglutide may affect the way some medicines work and some medicines may affect the way semaglutide works.
- Before using semaglutide, talk to your healthcare provider about low blood sugar and how to manage it. Tell your healthcare provider if you are taking other medicines to treat diabetes, including insulin or sulfonylureas.
- Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine.
- How should I use semaglutide?
- Read the Instructions for Use that comes with semaglutide.
- Use semaglutide exactly as your healthcare provider tells you to.
- Your healthcare provider should show you how to use semaglutide before you use it for the first time.
- Semaglutide is injected under the skin (subcutaneously) of your stomach (abdomen), thigh, or upper arm. Do not inject semaglutide into a muscle (intramuscularly) or vein (intravenously).
- Use semaglutide 1 time each week, on the same day each week, at any time of the day.
- You may change the day of the week you use semaglutide as long as your last dose was given 2 or more days before.
- If you miss a dose of semaglutide, take the missed dose as soon as possible within 5 days after the missed dose. If more than 5 days have passed, skip the missed dose and take your next dose on the regularly scheduled day.
- Semaglutide may be taken with or without food.
- Do not mix insulin and semaglutide together in the same injection.
- You may give an injection of semaglutide and insulin in the same body area (such as your stomach area), but not right next to each other.
- Change (rotate) your injection site with each injection. Do not use the same site for each injection.
- Check your blood sugar as your healthcare provider tells you to.
- Stay on your prescribed diet and exercise program while using semaglutide.
- Talk to your healthcare provider about how to prevent, recognize and manage low blood sugar (hypoglycemia), high blood sugar (hyperglycemia), and problems you have because of your diabetes.
- Your healthcare provider will check your diabetes with regular blood tests, including your blood sugar levels and your hemoglobin A1C.
- Do not share your semaglutide pen with other people, even if the needle has been changed. You may give other people a serious infection, or get a serious infection from them.
- Your dose of semaglutide and other diabetes medicines may need to change because of:
- Change in level of physical activity or exercise, weight gain or loss, increased stress, illness, change in diet, fever, trauma, infection, surgery or because of other medicines you take.
- What are the possible side effects of semaglutide?
- Semaglutide may cause serious side effects, including:
- See “What is the most important information I should know about semaglutide?”
- Inflammation of your pancreas (pancreatitis). Stop using semaglutide and call your healthcare provider right away if you have severe pain in your stomach area (abdomen) that will not go away, with or without vomiting. You may feel the pain from your abdomen to your back.
- Changes in vision. Tell your healthcare provider if you have changes in vision during treatment with semaglutide.
- Low blood sugar (hypoglycemia). Your risk for getting low blood sugar may be higher if you use semaglutide with another medicine that can cause low blood sugar, such as a sulfonylurea or insulin. Signs and symptoms of low blood sugar may include:
- Dizziness or light-headedness
- Blurred vision
- Anxiety, irritability, or mood changes
- Sweating
- Slurred speech
- Hunger
- Confusion or drowsiness
- Shakiness
- Weakness
- Headache
- Fast heartbeat
- Feeling jittery
- Kidney problems (kidney failure). In people who have kidney problems, diarrhea, nausea, and vomiting may cause a loss of fluids (dehydration) which may cause kidney problems to get worse. It is important for you to drink fluids to help reduce your chance of dehydration.
- Serious allergic reactions. Stop using semaglutide and get medical help right away, if you have any symptoms of a serious allergic reaction including itching, rash, or difficulty breathing.
- The most common side effects of semaglutide may include nausea, vomiting, diarrhea, stomach (abdominal) pain and constipation.
- Talk to your healthcare provider about any side effect that bothers you or does not go away. These are not all the possible side effects of semaglutide.
- Call your doctor for medical advice about side effects. You may report side effects to FDA at 1‑800‑FDA‑1088.
- General information about the safe and effective use of semaglutide.
- Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use semaglutide for a condition for which it was not prescribed. Do not give semaglutide to other people, even if they have the same symptoms that you have. It may harm them.
- You can ask your pharmacist or healthcare provider for information about semaglutide that is written for health professionals.
- For more information, go to OZEMPIC.com or call 1-888-693-6742.
- What are the ingredients in OZEMPIC?
- Active Ingredient: semaglutide.
- Inactive Ingredients: disodium phosphate dihydrate, propylene glycol, phenol and water for injection.


Precautions with Alcohol
Alcohol-Semaglutide interaction has not been established. Talk to your doctor regarding the effects of taking alcohol with this medication.
Brand Names
- Ozempic
Look-Alike Drug Names
There is limited information regarding Semaglutide Look-Alike Drug Names in the drug label.
Drug Shortage Status
Drug Shortage
Price
References
The contents of this FDA label are provided by the National Library of Medicine.