Corticorelin
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Rabin Bista, M.B.B.S. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Corticorelin is a diagnostic agent that is FDA approved for the diagnosis of differentiating pituitary and ectopic production of ACTH in patients with ACTH-dependent Cushing's syndrome. Common adverse reactions include flushing, tachycardia, hypotension, dyspnea, and chest compression or tightness.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
- ACTHREL® is indicated for use in differentiating pituitary and ectopic production of ACTH in patients with ACTH-dependent Cushing's syndrome.
Differential Diagnosis
- There are two forms of Cushing's syndrome:
- ACTH-dependent (83%), in which hypercortisolism is due either to pituitary hypersecretion of ACTH (Cushing's disease) resulting from an adenoma (40%, usually microadenomas) or nonadenomatous hyperplasia, possibly of hypothalamic origin (28%), or to hypercortisolism that is secondary to ectopic secretion of ACTH (15%) and,
- ACTH- independent (17%), in which hypercortisolism is due to autonomous cortisol secretion by an adrenal tumor (9% adenomas, 8% carcinomas).
After the establishment of hypercortisolism consistent with the presence of Cushing's syndrome, and following the elimination of autonomous adrenal hyperfunction as its cause, the corticorelin test is used to aid in establishing the source of excessive ACTH secretion.
- The corticorelin stimulation test helps to differentiate between the etiologies of ACTH-dependent hypercortisolism as follows:
- High basal plasma ACTH plus high basal plasma cortisol (20 - 40 mcg/dL). ACTHREL ® injection (1 mcg/kg) results in:
- Increased plasma ACTH levels
- Increased plasma cortisol levels
- Diagnosis: Cushing's disease (ACTH of pituitary origin)
- High basal plasma ACTH (may be very high) plus high basal plasma cortisol (20 - 40 mcg/dL). ACTHREL ® injection (1 mcg/kg) results in:
- Little or no response of plasma ACTH levels
- Little or no response of plasma cortisol levels
- Diagnosis: Ectopic ACTH syndrome
- Test Methodology
- To evaluate the status of the pituitary-adrenal axis in the differentiation of a pituitary source from an ectopic source of excessive ACTH secretion, a corticorelin test procedure requires a minimum of five blood samples.
Procedure
- Venous blood samples should be drawn 15 minutes before and immediately prior to ACTHREL® administration. The ACTH baseline is obtained by averaging the values of the two samples.
- Administer ACTHREL® as an intravenous infusion over a 30 to 60- second interval at a dose of 1 mcg/kg body weight. Higher doses are not recommended.
- Draw venous blood samples at 15, 30, and 60 minutes after administration.
- Blood samples should be handled as recommended by the laboratory that will determine their ACTH content. It is extremely important to recognize that the reliability of the ACTHREL® test is directly related to the inter-assay and intra-assay variability of the laboratory performing the assay.
- Cortisol determinations may be performed on the same blood samples for the same time points as outlined above. The blood sample handling precautions noted for ACTH should be followed for cortisol.
Interpretation of Test Results
- The interpretation of the ACTH and cortisol responses following ACTHREL® administration requires a knowledge of the clinical status of the individual patient, understanding of hypothalamic-pituitary-adrenal physiology, and familiarity with the normal hormonal ranges and the standards used by the laboratory that performs the ACTH and cortisol assays.
Cushing's Disease
- The results of challenge with corticorelin injection have been reported in approximately 300 patients with Cushing's disease. Although the ACTH and cortisol responses were variable, a hyper-response to corticorelin was seen in a majority of patients, despite high basal cortisol levels. This response pattern indicates an impairment of the negative feedback of cortisol on the pituitary. Patients with pituitary-dependent Cushing's disease tested with corticorelin do not show the negative correlation between basal and stimulated levels of ACTH and cortisol that is found in normal subjects. A positive correlation between basal ACTH levels and maximum ACTH increments after corticorelin administration has been found in Cushing's disease patients.
Ectopic ACTH Secretion
- Patients with Cushing's syndrome due to ectopic ACTH secretion (N=32) were found to have very high basal levels of ACTH and cortisol, which were not further stimulated by corticorelin. However, there have been rare instances of patients with ectopic sources of ACTH that have responded to the corticorelin test.
CUSHING'S DISEASE ACTH RESPONSES
- (mean of 181 patients)
- Basal ACTH 63 ± 72 pg/mL (mean ± SD)
- Peak ACTH 189 ± 262 pg/mL (mean ± SD)
- Mean of individual change from baseline + 227%
ECTOPIC ACTH SECRETION RESPONSES
- (mean for 31 patients)
- Basal ACTH 266 ± 464 pg/mL (mean ± SD)
- Peak ACTH 276 ± 466 pg/mL (mean ± SD)
- Mean of individual change from baseline + 15%
- False negative responses to the corticorelin test in Cushing's disease patients occur approximately 5 to 10% of the time, which may lead the clinician to an incorrect diagnosis of ectopic production of ACTH at that frequency.
Dosage
- A single intravenous dose of ACTHREL® at 1 mcg/kg is recommended for the testing of pituitary corticotrophin function. A dose of 1 mcg/kg is the lowest dose that produces maximal cortisol responses and significant (though apparently sub-maximal) ACTH responses. Doses above 1 mcg/kg are not recommended.
- At a dose of 1 mcg/kg, the ACTH and cortisol responses to ACTHREL® are prolonged and remain elevated for up to 2 hours. The maximum increment in plasma ACTH occurs between 15 and 60 minutes after ACTHREL® administration, whereas the maximum increment in plasma cortisol occurs between 30 and 120 minutes. In a clinical study of 30 normal healthy men, the peak plasma ACTH and cortisol responses to ACTHREL® administration in the early afternoon occurred at 42 ± 29 minutes and 65 ± 26 minutes (average ±SD), respectively. If a repeated evaluation using the corticorelin stimulation test with ACTHREL® is needed, it is recommended that the repeat test be carried out at the same time of day as the original test because there are differences in basal levels and peak response levels following a.m. or p.m. administration to normal humans.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Corticorelin in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Corticorelin in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Corticorelin in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Corticorelin in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Corticorelin in pediatric patients.
Contraindications
There is limited information regarding Corticorelin Contraindications in the drug label.
Warnings
Precautions
General
- The severity of adverse effects to a corticorelin injection appear to be dose-dependent. Dosages above 1 mcg/kg are not recommended. While few adverse effects have been observed at the 1 mcg/kg or 100 mcg dose, higher doses have been associated with transient tachycardia, decreased blood pressure, loss of consciousness, and asystole. These symptoms can be substantially reduced by administering the drug as a 30-second intravenous infusion instead of a bolus injection. At a dose of 200 mcg corticorelin, 4 of 60 volunteers and patients with disturbances of the hypothalamic-pituitary-adrenal (HPA) axis were reported to have had decreased blood pressures. One patient had a severe hypotensive reaction with asystole. Three other patients had an "absence-like" loss of consciousness lasting approximately 5 minutes. In subsequent investigations by the same researchers over a 3-year period using 100 mcg of corticorelin, one patient in approximately 150 to 200 experienced a severe drop in blood pressure and loss of sinus rhythm after receiving 55 mcg of corticorelin, which may have been due to interaction with heparin
Adverse Reactions
Clinical Trials Experience
- Adverse effects reported with 1 mcg/kg or 100 mcg/patient include flushing of the face, neck, and upper chest (16%; 45/276), beginning almost immediately and lasting 3 to 5 minutes. Recipients have also reported an urge to take a deep breath (6%; 3/49), which occurs with a timing similar to, but less frequently than, that of flushing. Higher doses (>3 mcg/kg) are associated with more prolonged flushing, tachycardia, hypotension, dyspnea, and "chest compression" or tightness. In addition, at doses of >5 mcg/kg, significant increases in heart rate and decreases in blood pressure were observed. The cardiovascular effects occurred 2-3 minutes after injection and lasted for 30-60 minutes. The facial flushing was more prolonged, lasting up to 4 hours in some subjects. All signs and symptoms could be reduced by administering the drug as a 30-second infusion instead of by bolus injection.
- Total doses of up to 200 mcg of corticorelin were administered as a bolus injection to 60 men and women, including both healthy normal subjects and patients with endocrine disorders. In most cases, only minor adverse effects, such as transient flushing and feelings of dyspnea, were noted. However, a few patients with disorders of the pituitary-adrenal axis had major symptoms. One patient had a precipitous fall in blood pressure and pulse rate and developed asystole, which required resuscitation. In two patients with Cushing's disease and in one with secondary adrenal insufficiency, an "absence-like" loss of consciousness occurred, which started within a few seconds after injection of corticorelin and lasted from 10 seconds to 5 minutes. This was accompanied by a slight fall in blood pressure. One patient with a well documented seizure diathesis experienced a grand mal epileptic seizure following ACTHREL® administration. The patient had discontinued anti-convulsant therapy the day of the procedure.
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Corticorelin in the drug label.
Drug Interactions
- The plasma ACTH response to corticorelin injection is inhibited or blunted in normal subjects pretreated with dexamethasone. The use of a heparin solution to maintain i.v. cannula patency during the corticorelin test is not recommended. A possible interaction between corticorelin and heparin may have been responsible for a major hypotensive reaction that occurred after corticorelin administration
Use in Specific Populations
Pregnancy
- Animal reproduction studies have not been conducted with corticorelin. It is also not known whether corticorelin can cause fetal harm when administered to a pregnant woman or can affect reproductive capacity. ACTHREL® should be given to a pregnant woman only if clearly needed.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Corticorelin in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Corticorelin during labor and delivery.
Nursing Mothers
- It is not known whether corticorelin is secreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when ACTHREL® is administered to a nursing woman.
Pediatric Use
- Only a few tests have been performed on children. Dosages were 1 mcg/kg body weight. Patient studies have involved only children with multiple hypothalamic and/or pituitary hormone deficiencies, or tumors. Only two studies with normal pediatric subjects have been conducted. No differences in response to the corticorelin test have been reported in the children studied.
Geriatic Use
There is no FDA guidance on the use of Corticorelin with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Corticorelin with respect to specific gender populations.
Race
There is no FDA guidance on the use of Corticorelin with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Corticorelin in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Corticorelin in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Corticorelin in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Corticorelin in patients who are immunocompromised.
Administration and Monitoring
Administration
- ACTHREL® is to be reconstituted aseptically with 2 mL of Sodium Chloride injection, USP (0.9% sodium chloride), at the time of use by injecting 2 mL of the saline diluent into the lyophilized drug product cake. To avoid bubble formation, DO NOT SHAKE the vial; instead, roll the vial to dissolve the product. The sterile solution containing 50 mcg corticorelin/mL is then ready for injection by the intravenous route. The dosage to be administered is determined by the patient's weight (1 mcg corticorelin/kg). Some of the adverse effects can be reduced by administering the drug as an infusion over 30 seconds instead of as a bolus injection.
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
Monitoring
There is limited information regarding Monitoring of Corticorelin in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Corticorelin in the drug label.
Overdosage
- Symptoms of overdose include severe facial flushing, cardiovascular changes, and dyspnea. In the event of toxic overdose, adverse effects should be treated symptomatically.
Pharmacology
| |
Corticorelin
| |
| Systematic (IUPAC) name | |
| ? | |
| Identifiers | |
| CAS number | |
| ATC code | V04 |
| PubChem | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 4670.31 g/mol |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status | |
| Routes | ? |
Mechanism of Action
There is limited information regarding Corticorelin Mechanism of Action in the drug label.
Structure
- ACTHREL® (corticorelin ovine triflutate for injection) is a sterile, nonpyrogenic, lyophilized white cake powder, containing corticorelin ovine triflutate, a trifluoroacetate salt of a synthetic peptide that is used for the determination of pituitary corticotroph responsiveness. Corticorelin ovine has an amino acid sequence identical to ovine corticotropin-releasing hormone (oCRH). Corticorelin ovine is an analogue of the naturally occurring human CRH (hCRH) peptide. Both peptides are potent stimulators of adrenocorticotropic hormone (ACTH) release from the anterior pituitary. ACTH stimulates cortisol production from the adrenal cortex. The structural formula for corticorelin ovine triflutate is described below:
- Ser-Gin-Glu-Pro-Pro-Ile-Ser-Leu-Asp-Leu-Thr-Phe-His-Leu-Leu-Arg-Glu-Val-Leu-Glu-Met-Thr-Lys-Ala-Asp-Gin-Leu-Ala-Gln-Gln-Ala-His-Ser-Asn-Arg-Lys-Leu-Leu-Asp-Ile-Ala-NH2∙xCF2COOH
- whereas x=4 - 8.
- The empirical formula of corticorelin ovine is C205H339N59O63S with a molecular weight of 4670.35 Daltons.
- ACTHREL® for injection is available in vials containing 100 mcg corticorelin ovine (as the trifluoroacetate), 0.88 mg ascorbic acid, 10 mg lactose, and 26 mg cysteine hydrochloride monohydrate. Trace amounts of chloride ion may be present from the manufacturing process. The preparation is intended for intravenous administration.
Pharmacodynamics
- In normal subjects, intravenous administration of corticorelin results in a rapid and sustained increase of plasma ACTH levels and a near parallel increase of plasma cortisol. In addition, intravenous administration of corticorelin to normal subjects causes a concomitant and prolonged release of the related proopiomelanocortin peptides β- and γ-lipotropins (β -and γ-LPH) and β-endorphin (β -END). A number of dose-response studies have been performed on normal subjects using a range of corticorelin doses. In one study, doses of corticorelin ranging from 0.001 to 30 mcg/kg body weight were administered to 29 healthy volunteers. Blood samples were taken over a 2-hour period for determination of plasma ACTH and cortisol concentrations. There was a direct dose-dependent relationship that was more pronounced for ACTH than for cortisol. The threshold dose was 0.03 mcg/kg, the half-maximal dose was 0.3-1.0 mcg/kg and the maximally effective dose was 3-10 mcg/kg.
- Plasma ACTH levels in normal subjects increased 2 minutes after injection of corticorelin doses of >0.3 mcg/kg and reached peak levels after 10-15 minutes. Plasma cortisol levels increased within 10 minutes and reached peak levels at 30 to 60 minutes. As the dose of corticorelin was increased, the rises in plasma ACTH and cortisol were more sustained, showing a biphasic response with a second lower peak at 2-3 hours after injection. Similar results were found in another study using 0.3, 3.0, and 30 mcg/kg doses. The duration of mean plasma ACTH increase after injection of 0.3, 3.0, and 30 mcg/kg was 4, 7, and 8 hours, respectively. The effect on plasma cortisol was similar, but more prolonged. Because there are differences in basal levels and peak response levels following a.m. or p.m. administration, it is recommended that subsequent evaluations in the same patient using the corticorelin stimulation test be carried out at the same time of day as the original evaluation.
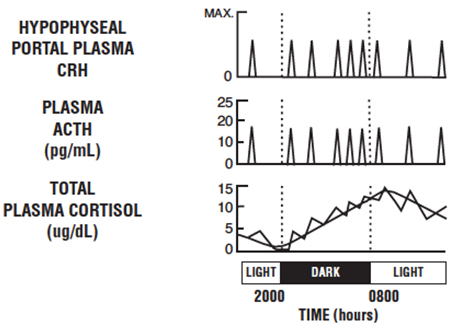
- Baseline ACTH and cortisol levels are usually higher in the morning. Pooled ACTH values from normal unstressed subjects (n=119) were 25 ± 7 pg/mL in the a.m. and 10 ± 3 in the p.m.; similar pooled cortisol values (n=170) were 11 ± 3 mcg/dL in the a.m. and 4 ± 2 mcg/dL in the p.m. The normal unstressed person has about seven to ten secretory episodes of ACTH each day. Most of them occur in the early morning hours and are responsible for the morning plasma cortisol surge. The following figure shows the daily circadian rhythm of ACTH and cortisol secretions in a normal unstressed person. Insulin, plasma resin activity, prolactin, and growth hormone release are not affected by corticorelin administration in humans.
- Continuous 24-hour infusion of corticorelin (0.5, 1.0, and 3.0 mcg/kg/hr) increased plasma ACTH concentrations to a plateau of 15-20 pg/mL by the third hour and urinary-free cortisol reaches 173 ± 43 mcg/dL by 24 hours, comparable to those levels observed in patients with major depression, but less than levels noted in Cushing's disease. Continuous infusion did not abolish the circadian rhythm of plasma ACTH and cortisol, but did appear to desensitize the corticotroph. Intermittent doses of corticorelin (25 mcg every 4 hours for 72 hours), however, continued to elicit the expected ACTH and cortisol responses.
- Intravenous administration of 1 mcg/kg corticorelin in combination with 10 pressor units intramuscular vasopressin had a synergistic effect on ACTH and a less marked synergistic effect on cortisol secretion.
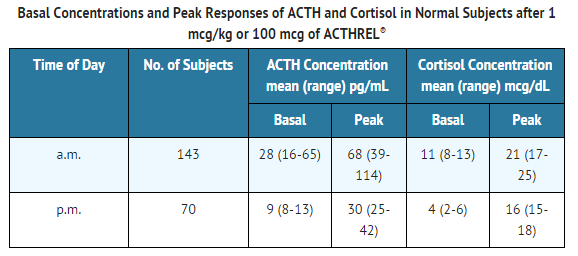
- The basal and peak response levels of ACTH and cortisol to a 1 mcg/kg or 100 mcg dose of corticorelin administered to normal volunteers in the morning and the evening are given below. These values were obtained by combining the results from 9 clinical trials conducted in the a.m. and 4 clinical trials conducted in the p.m.
- The following table is to be used only as a general guide.
Pharmacokinetics
- Following a single intravenous injection of 1 mcg/kg of corticorelin to normal men, the disappearance of immunoreactive corticorelin (IR-corticorelin) from plasma follows a biexponential decay curve. Plasma half-lives for IR-corticorelin are 11.6 ± 1.5 minutes (mean ± SE) for the fast component and 73 ± 8 minutes for the slow component. The mean volume of distribution for IR-corticorelin is 6.2 ± 0.5 L with an approximate metabolic clearance rate of 95 ± 11 L/m2/day. Graded intravenous doses of corticorelin (0.01, 0.03, 0.1, 0.3, 1, 3, 10, 30 mcg/kg) produced a linear increase in plasma IR-corticorelin. Corticorelin does not appear to be bound specifically by a circulating plasma protein.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- Animal studies have not been conducted with corticorelin to evaluate carcinogenic potential, mutagenicity, or effect on fertility.
Clinical Studies
There is limited information regarding Clinical Studies of Corticorelin in the drug label.
How Supplied
- CTHREL® is supplied as a sterile, nonpyrogenic, lyophilized, white cake containing 100 mcg corticorelin ovine (as the trifluoroacetate), 0.88 mg ascorbic acid, 10 mg lactose, and 26 mg cysteine hydrochloride monohydrate. Trace amounts of chloride ion may be present from the manufacturing process. The package provides a single-dose, rubber-capped, 5 mL, brown-glass vial (NDC 55566-0302-1) containing 100 mcg corticorelin ovine (as the trifluoroacetate).
Storage
- ACTHREL® is stable in the lyophilized form when stored refrigerated at 2°C to 8°C (36°F to 46°F) and protected from light. The reconstituted solution is stable up to 8 hours under refrigerated conditions. Discard unused reconstituted solution.
Images
Drug Images
{{#ask: Page Name::Corticorelin |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
PRINCIPAL DISPLAY PANEL - 100 MCG VIAL LABEL
Ingredients and Appearance
{{#ask: Label Page::Corticorelin |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Corticorelin in the drug label.
Precautions with Alcohol
- Alcohol-Corticorelin interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- ACTHREL®[1]
Look-Alike Drug Names
There is limited information regarding Corticorelin Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.