Histidine
Histidine (abbreviated as His or H)[1] is one of the 20 most common natural amino acids present in proteins. In the nutritional sense, in humans, histidine is considered an essential amino acid, but mostly only in children. Its codons are CAU and CAC.
Histidine was first isolated by German physician Albrecht Kossel in 1896.
Chemical properties
The imidazole side chains and the relatively neutral pKa of histidine (ca 6.0) mean that relatively small shifts in cellular pH will change its charge. For this reason, this amino acid side chain finds its way into considerable use as a coordinating ligand in metalloproteins, and also as a catalytic site in certain enzymes. The imidazole side chain has two nitrogens with different properties: One is bound to hydrogen and donates its lone pair to the aromatic ring and as such is slightly acidic, whereas the other one donates only one electron pair to the ring so it has a free lone pair and is basic. These properties are exploited in different ways in proteins. In catalytic triads, the basic nitrogen of histidine is used to abstract a proton from serine, threonine or cysteine to activate it as a nucleophile. In a histidine proton shuttle, histidine is used to quickly shuttle protons, it can do this by abstracting a proton with its basic nitrogen to make a positively-charged intermediate and then use another molecule, a buffer, to extract the proton from its acidic nitrogen. In carbonic anhydrases, a histidine proton shuttle is utilized to rapidly shuttle protons away from a zinc-bound water molecule to quickly regenerate the active form of the enzyme.
Because of histidine's affinity for metal ions, researchers will often add a polyhistidine-tag to a protein of interest. The metal affinity can then be used to purify, detect, or immobilize the protein to be studied.
Metabolism
The amino acid is a precursor for histamine and carnosine biosynthesis.
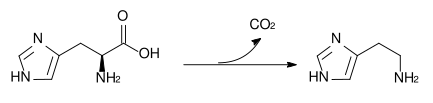
The enzyme histidine ammonia-lyase converts histidine into ammonia and urocanic acid. A deficiency in this enzyme is present in the rare metabolic disorder histidinemia.
Additional images
-
Histidine
-
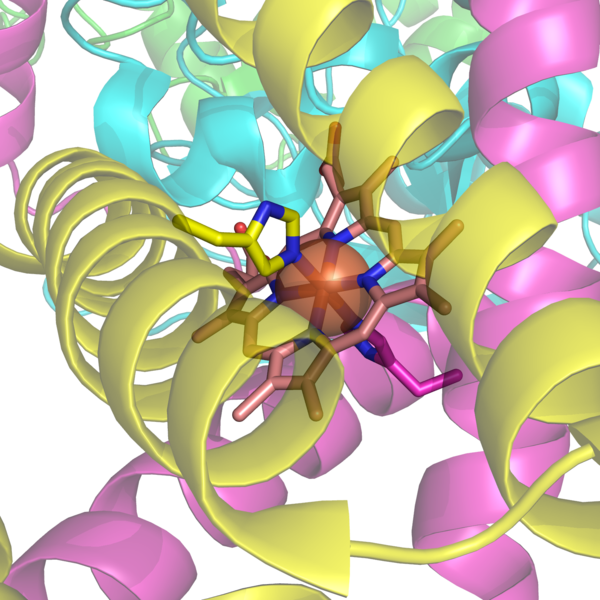
The histidine bound heme group of succinate dehydrogenase, an electron carrier in the mitochondrial electron transfer chain. The large semi-transparent sphere indicates the location of the iron ion. From PDB: 1YQ3.
References
- ↑ IUPAC-IUBMB Joint Commission on Biochemical Nomenclature. "Nomenclature and Symbolism for Amino Acids and Peptides". Recommendations on Organic & Biochemical Nomenclature, Symbols & Terminology etc. Retrieved 2007-05-17.
External links
- Histidine biosynthesis (early stages)
- Histidine biosynthesis (later stages)
- Histidine catabolism
- Computational Chemistry Wiki
| Alanine (dp) | Arginine (dp) | Asparagine (dp) | Aspartic acid (dp) | Cysteine (dp) | Glutamic acid (dp) | Glutamine (dp) | Glycine (dp) | Histidine (dp) | Isoleucine (dp) | Leucine (dp) | Lysine (dp) | Methionine (dp) | Phenylalanine (dp) | Proline (dp) | Serine (dp) | Threonine (dp) | Tryptophan (dp) | Tyrosine (dp) | Valine (dp) |
bs:Histidin ca:Histidina cs:Histidin de:Histidin eo:Histidino ko:히스티딘 hr:Histidin id:Histidin it:Istidina he:היסטידין lv:Histidīns lb:Histidin lt:Histidinas hu:Hisztidin nl:Histidine no:Histidin fi:Histidiini sv:Histidin uk:Гістидин Template:WikiDoc Sources