Biotin
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Ammu Susheela, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Biotin is a vitamin that is FDA approved for the treatment of to strengthen the hair follicle and prevents hair loss as well. It is nutrient for the hair root and helps oxygenate the hair turn slowing the loss and provides resilience and resistence to hair. Common adverse reactions include gastrointestinal upset, administration of anticonvulsant medications may impair biotin absorption.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- Biotin is a major component of the formulation acts to strengthen the hair follicle and prevents hair loss as well. It is nutrient for the hair root and helps oxygenate the hair turn slowing the loss. Provides resilience and resistence to hair.
- Apply amount of the hair follicle and massage until absorbed. Use every 12 hours for the fast and sustained effect.
- Suitable for children, adolescents, adults and seniors.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
- BIOTINIDASE DEFICIENCY, oral: 10 to 40 milligrams daily
- BRITTLE NAILS, oral, tablet: 2.5 milligrams daily
- CARBOXYLASE DEFICIENCY, intravenous, solution: 18.4 micromoles daily
- HOLOCARBOXYLASE DEFICIENCY IN PREGNANCY, oral: 10 milligrams daily
- PARENTERAL ALIMENTATION-INDUCED DEFICIENCY, oral, solution or tablet: 0.1 to 1 milligram daily
- RECOMMENDED DIETARY ALLOWANCE (RDA), oral: 30 to 100 micrograms daily
- VAGINAL CANDIDIASIS, oral: 20 milligrams daily
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Biotin in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Biotin in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
- ALOPECIA AREATA, oral: 20 milligrams daily with oral zinc aspartate 100 milligrams and topical clobetasol propionate 0.025%
- BASAL-GANGLIA DISEASE, BIOTIN-responsive, oral or solution: 5 to 10 milligrams/kilogram daily
- BETA-METHYLCROTONYL-GLYCINURIA, solution, oral: 10 milligrams daily
- BIOTINIDASE DEFICIENCY, solution or tablet, oral: 6 to 40 milligrams daily
- PARENTERAL ALIMENTATION-INDUCED DEFICIENCY, solution or tablet, oral: 0.1 to 1 milligram daily
- PARTIAL BIOTINIDASE DEFICIENCY, solution or tablet: 5 to 10 milligrams daily or less.
- PROTEIN-ENERGY MALNUTRITION, solution or tablet, oral: BIOTIN 10 milligrams daily along with standard treatment for malnutrition
- PYRUVATE-CARBOXYLASE DEFICIENCY, solution or tablet, oral: BIOTIN 10 milligrams (mg) daily, thiamine 80 mg daily, pyridoxine 300 mg daily
- RECOMMENDED DIETARY ALLOWANCE, oral, diet or tablet: 50 micrograms daily
- UNCOMBABLE HAIR SYNDROME, solution or tablet, oral: 0.9 milligram daily in three divided doses
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Biotin in pediatric patients.
Contraindications
- Hypersensitivity to Biotin
Warnings
This product is for topical application, if this product to contact with eyes rinse immediately with water and consult your doctor.
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Clinical Trial Experience of Biotin in the drug label.
Postmarketing Experience
- Gastrointestinal upset
- Administration of anticonvulsant medications may impair Biotin absorption
Drug Interactions
There is limited information regarding Biotin Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
- Pregnancy Category
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Biotin in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Biotin during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Biotin with respect to nursing mothers.
Pediatric Use
There is no FDA guidance on the use of Biotin with respect to pediatric patients.
Geriatic Use
There is no FDA guidance on the use of Biotin with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Biotin with respect to specific gender populations.
Race
There is no FDA guidance on the use of Biotin with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Biotin in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Biotin in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Biotin in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Biotin in patients who are immunocompromised.
Administration and Monitoring
Administration
Monitoring
There is limited information regarding Monitoring of Biotin in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Biotin in the drug label.
Overdosage
There is limited information regarding Chronic Overdose of Biotin in the drug label.
Pharmacology
Template:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox E numberTemplate:Chembox AppearanceTemplate:Chembox MeltingPtTemplate:Chembox SolubilityInWaterTemplate:Chembox NFPATemplate:Chembox Supplement| Template:Chembox header2 | Biotin[1] | |
|---|---|
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | Lua error in Module:Wikidata at line 879: attempt to index field 'wikibase' (a nil value). Lua error in Module:Wikidata at line 879: attempt to index field 'wikibase' (a nil value). |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C10H16N2O3S | |
| Molar mass | 244.31 g·mol−1 |
| Hazards | |
| Template:Chembox header2 | Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox disclaimer and references | |
Mechanism of Action
There is limited information regarding Biotin Mechanism of Action in the drug label.
Structure
There is limited information regarding Biotin Structure in the drug label.
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Biotin in the drug label.
Pharmacokinetics
There is limited information regarding Pharmacokinetics of Biotin in the drug label.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Biotin in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of Biotin in the drug label.
How Supplied
Storage
There is limited information regarding Biotin Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Biotin |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel

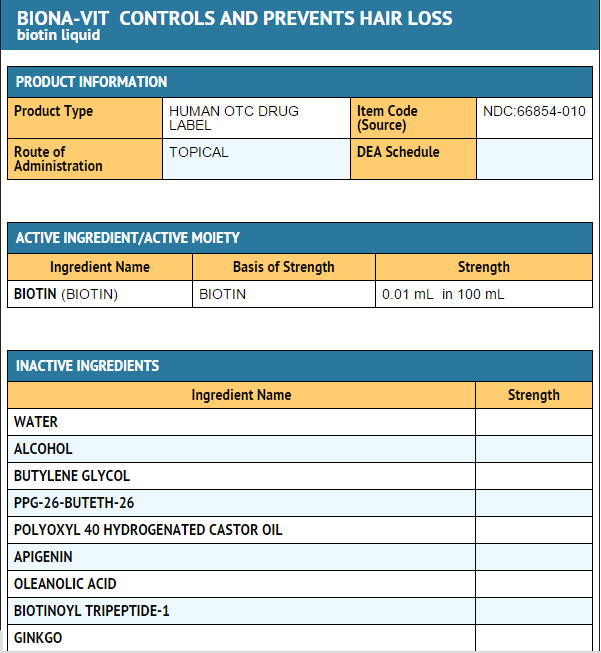
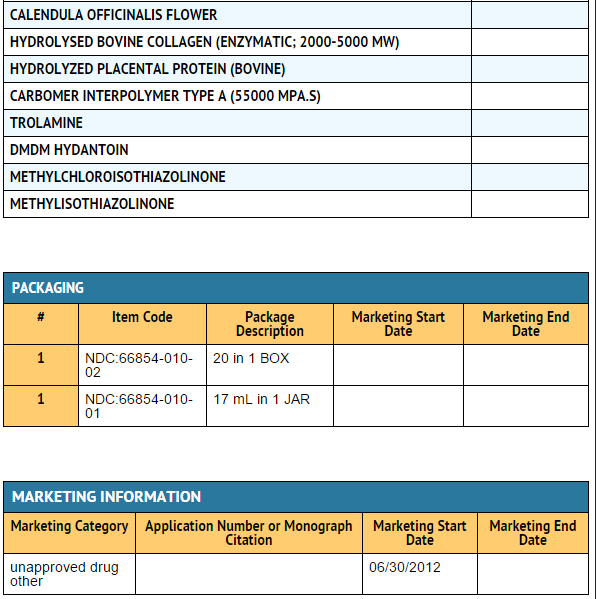
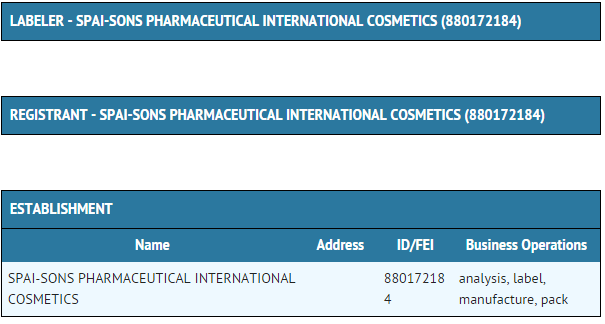
{{#ask: Label Page::Biotin |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Biotin in the drug label.
Precautions with Alcohol
- Alcohol-Biotin interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- BIONA-VIT ®[2]
Look-Alike Drug Names
There is limited information regarding Biotin Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Merck Index, 11th Edition, 1244.
- ↑ "BIONA-VIT CONTROLS AND PREVENTS HAIR LOSS- biotin liquid".