Citicoline: Difference between revisions
m (Bot: Automated text replacement (-{{SIB}} + & -{{EH}} + & -{{EJ}} + & -{{Editor Help}} + & -{{Editor Join}} +)) |
Kiran Singh (talk | contribs) No edit summary |
||
| Line 1: | Line 1: | ||
{{ | __Notoc__ | ||
| IUPAC_name | {{CMG}} | ||
| image | {{Drugbox | ||
| Verifiedfields = changed | |||
| Watchedfields = changed | |||
| | | verifiedrevid = 449576702 | ||
| | | IUPAC_name = 5'-''O''-[hydroxy({hydroxy[2-(trimethylammonio)ethoxy]<br />phosphoryl}oxy)phosphoryl]cytidine | ||
| image = Citicoline.svg | |||
<!--Clinical data--> | |||
| tradename = | |||
| | | Drugs.com = {{drugs.com|international|citicoline}} | ||
| | | pregnancy_AU = <!-- A / B1 / B2 / B3 / C / D / X --> | ||
| pregnancy_US = <!-- A / B / C / D / X --> | |||
| pregnancy_category = | |||
| legal_AU = <!-- Unscheduled / S2 / S3 / S4 / S5 / S6 / S7 / S8 / S9 --> | |||
| legal_CA = <!-- / Schedule I, II, III, IV, V, VI, VII, VIII --> | |||
| legal_UK = <!-- GSL / P / POM / CD / Class A, B, C --> | |||
| pregnancy_AU | | legal_US = <!-- OTC / Rx-only / Schedule I, II, III, IV, V --> | ||
| pregnancy_US | | legal_status = | ||
| pregnancy_category= | | routes_of_administration = | ||
| legal_AU | |||
| legal_CA | <!--Pharmacokinetic data--> | ||
| legal_UK | | bioavailability = | ||
| legal_US | | protein_bound = | ||
| legal_status | | metabolism = | ||
| routes_of_administration = | | elimination_half-life = | ||
| excretion = | |||
<!--Identifiers--> | |||
| CASNo_Ref = {{cascite|correct|CAS}} | |||
| CAS_number_Ref = {{cascite|correct|??}} | |||
| CAS_number = 987-78-0 | |||
| ATC_prefix = N06 | |||
| ATC_suffix = BX06 | |||
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} | |||
| DrugBank = | |||
| ChEBI_Ref = {{ebicite|changed|EBI}} | |||
| ChEBI = 16436 | |||
| ChEMBL_Ref = {{ebicite|changed|EBI}} | |||
| ChEMBL = 1618340 | |||
| UNII_Ref = {{fdacite|correct|FDA}} | |||
| UNII = 536BQ2JVC7 | |||
| KEGG_Ref = {{keggcite|correct|kegg}} | |||
| KEGG = D00057 | |||
| PubChem = 11583971 | |||
| ChemSpiderID_Ref = {{chemspidercite|changed|chemspider}} | |||
| ChemSpiderID = 13207 | |||
<!--Chemical data--> | |||
| C=14 | H=27 | N=4 | O=11 | P=2 | charge = + | |||
| molecular_weight = 489.332 g/mol | |||
| smiles = C[N+](C)(C)CCOP(=O)([O-])OP(=O)(O)OC[C@@H]1[C@H]([C@H]([C@@H](O1)N2C=CC(=NC2=O)N)O)O | |||
| InChI = 1/C14H26N4O11P2/c1-18(2,3)6-7-26-30(22,23)29-31(24,25)27-8-9-11(19)12(20)13(28-9)17-5-4-10(15)16-14(17)21/h4-5,9,11-13,19-20H,6-8H2,1-3H3,(H3-,15,16,21,22,23,24,25)/t9-,11-,12-,13-/m1/s1 | |||
| InChIKey = RZZPDXZPRHQOCG-OJAKKHQRBJ | |||
| StdInChI_Ref = {{stdinchicite|changed|chemspider}} | |||
| StdInChI = 1S/C14H26N4O11P2/c1-18(2,3)6-7-26-30(22,23)29-31(24,25)27-8-9-11(19)12(20)13(28-9)17-5-4-10(15)16-14(17)21/h4-5,9,11-13,19-20H,6-8H2,1-3H3,(H3-,15,16,21,22,23,24,25)/t9-,11-,12-,13-/m1/s1 | |||
| StdInChIKey_Ref = {{stdinchicite|changed|chemspider}} | |||
| StdInChIKey = RZZPDXZPRHQOCG-OJAKKHQRSA-N | |||
| synonyms = Cytidine diphosphate choline | |||
}} | }} | ||
'''Citicoline''' ([[International Nonproprietary Name|INN]]), also known as '''[[cytidine diphosphate]]-choline''' ('''CDP-Choline''') & '''cytidine 5'-diphosphocholine''' is a [[psychostimulant]]/[[nootropic]]. It is an intermediate in the generation of [[phosphatidylcholine]] from [[choline]]. | |||
Studies suggest that CDP-choline supplements increase dopamine receptor densities,<ref>{{cite journal |author=Giménez R, Raïch J, Aguilar J |title=Changes in brain striatum dopamine and acetylcholine receptors induced by chronic CDP-choline treatment of aging mice |journal=[[British Journal of Pharmacology]] |volume=104 |issue=3 |pages=575–8 |date=November 1991 |pmid=1839138 |pmc=1908237 |doi=10.1111/j.1476-5381.1991.tb12471.x}}</ref> and suggest that CDP-choline supplementation helps prevent memory impairment resulting from poor environmental conditions.<ref>{{cite journal |author=Teather LA, Wurtman RJ |title=Dietary CDP-choline supplementation prevents memory impairment caused by impoverished environmental conditions in rats |journal=Learning & Memory |volume=12 |issue=1 |pages=39–43 |year=2005 |pmid=15647594 |pmc=548494 |doi=10.1101/lm.83905}}</ref> Preliminary research has found that citicoline supplements help improve focus and mental energy and may possibly be useful in the treatment of attention deficit disorder.<ref>{{cite news |url=http://www.smh.com.au/articles/2008/02/24/1203788130776.html |title=Supplement naturally boosts ageing brain power |date=2008-02-25 |work=[[Sydney Morning Herald]] |accessdate=2009-07-28}}</ref><ref>{{cite journal |author=Silveri MM, Dikan J, Ross AJ, et al. |title=Citicoline enhances frontal lobe bioenergetics as measured by phosphorus magnetic resonance spectroscopy |journal=NMR in Biomedicine |volume=21 |issue=10 |pages=1066–75 |date=November 2008 |pmid=18816480 |doi=10.1002/nbm.1281}}</ref> Citicoline has also been shown to elevate [[ACTH]] independently from [[Corticotropin-releasing hormone|CRH]] levels and to amplify the release of other [[HPA axis]] hormones such as [[luteinizing hormone|LH]], [[Follicle-stimulating hormone|FSH]], [[growth hormone|GH]] and [[Thyroid-stimulating hormone|TSH]] in response to hypothalamic releasing factors.<ref>{{cite journal |author=Cavun S, Savci V |title=CDP-choline increases plasma ACTH and potentiates the stimulated release of GH, TSH and LH: the cholinergic involvement |journal=Fundamental & Clinical Pharmacology |volume=18 |issue=5 |pages=513–23 |date=October 2004 |pmid=15482372 |doi=10.1111/j.1472-8206.2004.00272.x}}</ref> | |||
These effects on HPA hormone levels may be beneficial for some individuals but may have undesirable effects in those with medical conditions featuring ACTH or cortisol hypersecretion including, but not limited to, [[PCOS]], [[type II diabetes]] and [[major depressive disorder]].<ref>{{cite journal |author=Benson S, Arck PC, Tan S, et al. |title=Disturbed stress responses in women with polycystic ovary syndrome |journal=Psychoneuroendocrinology |volume=34 |issue=5 |pages=727–35 |date=June 2009 |pmid=19150179 |doi=10.1016/j.psyneuen.2008.12.001}}</ref><ref>{{cite journal |author=Florio P, Zatelli MC, Reis FM, degli Uberti EC, Petraglia F |title=Corticotropin releasing hormone: a diagnostic marker for behavioral and reproductive disorders? |journal=Frontiers in Bioscience |volume=12 |issue= |pages=551–60 |year=2007 |pmid=17127316 |doi=10.2741/2081}}</ref> | |||
==Medical uses== | |||
Citicoline is available as a supplement online and in stores. It is sold in over 70 countries under a variety of brand names: Ceraxon, Cognizin, NeurAxon, Somazina, Synapsine, etc. When taken as a supplement citicoline is hydrolyzed into [[choline]] and cytidine in the [[intestine]].<ref>{{cite journal|last=Wurtman|first=RJ|author2=Regan, M |author3=Ulus, I |author4= Yu, L |title=Effect of oral CDP-choline on plasma choline and uridine levels in humans.|journal=[[Biochemical pharmacology]]|date=Oct 1, 2000|volume=60|issue=7|pages=989–92|pmid=10974208|doi=10.1016/S0006-2952(00)00436-6}}</ref> Once these cross the [[blood–brain barrier]] it is reformed into citicoline by the rate-limiting enzyme in [[phosphatidylcholine]] synthesis, [[Choline-phosphate cytidylyltransferase|CTP-phosphocholine cytidylyltransferase]].<ref name="Alvarez 1999">{{cite journal|last=Alvarez|first=XA|author2=Sampedro, C |author3=Lozano, R |author4= Cacabelos, R |title=Citicoline protects hippocampal neurons against apoptosis induced by brain beta-amyloid deposits plus cerebral hypoperfusion in rats.|journal=Methods and findings in experimental and clinical pharmacology|date=October 1999|volume=21|issue=8|pages=535–40|pmid=10599052|doi=10.1358/mf.1999.21.8.794835}}</ref><ref>{{cite journal|last=Carlezon|first=WA|author2=Pliakas, AM |author3=Parow, AM |author4=Detke, MJ |author5=Cohen, BM |author6= Renshaw, PF |title=Antidepressant-like effects of cytidine in the forced swim test in rats.|journal=Biological Psychiatry|date=Jun 1, 2002|volume=51|issue=11|pages=882–9|pmid=12022961|doi=10.1016/s0006-3223(01)01344-0}}</ref> | |||
===Memory disorders=== | |||
In the hippocampi of rats with induced [[Alzheimer’s Disease]], citicoline counteracts neuronal degeneration and reduces the number of [[apoptotic]] cells present. Citicoline supplementation also improves memory retention.<ref name="Alvarez 1999" /> | |||
===Ischemic stroke=== | |||
Citicoline is approved for treatment in cases of [[head trauma]], [[stroke]], and [[neurodegenerative disease]] in [[Japan]] and [[Europe]]. Citicoline improves the clinical outcome following an [[ischemic stroke]], as evidenced by the reduction in size of lesions caused by [[ischemic strokes]] after supplementation.<ref>{{cite journal|last=Warach|first=S|author2=Pettigrew, LC |author3=Dashe, JF |author4=Pullicino, P |author5=Lefkowitz, DM |author6=Sabounjian, L |author7=Harnett, K |author8=Schwiderski, U |author9= Gammans, R |title=Effect of citicoline on ischemic lesions as measured by diffusion-weighted magnetic resonance imaging. Citicoline 010 Investigators.|journal=Annals of neurology|date=November 2000|volume=48|issue=5|pages=713–22|pmid=11079534|doi=10.1002/1531-8249(200011)48:5<713::aid-ana4>3.0.co;2-#}}</ref> It has been claimed that citicoline reduces rates of death and disability following an [[ischemic stroke]].<ref>{{cite journal|last=Saver|first=JL|title=Citicoline: update on a promising and widely available agent for neuroprotection and neurorepair.|journal=Reviews in neurological diseases|date=Fall 2008|volume=5|issue=4|pages=167–77|pmid=19122569}}</ref> | |||
However, the largest trial to date, a randomised, placebo-controlled, sequential trial in patients with moderate-to-severe acute ischaemic stroke in Europe, enrolling 2298 patients, found no benefit of administering citicoline on survival or recovery from stroke.<ref>{{cite journal|last=Davalos|journal=Lancet|year=2012|volume=380|issue=9839}}</ref> | |||
It should be noted that Citicoline is the only substance that ever showed any significant neuroprotective effect at least in patients with less severe [[stroke]] events.<ref>{{cite journal|journal=Journal of stroke and cerebrovascular diseases : the official journal of National Stroke Association|last=Overgaard|first=K|title=The effects of citicoline on acute ischemic stroke: a review.|year=2014|volume=23(7)|pages=1764-9|pmid=24739589|doi=10.1016/j.jstrokecerebrovasdis.2014.01.020}}</ref> | |||
===Vision=== | |||
Citicoline improves visual function in patients with [[glaucoma]], [[amblyopia]], and [[non-arteritic ischaemic optic neuropathy]].<ref>{{cite journal|last=Parisi|first=V|author2=Coppola, G |author3=Centofanti, M |author4=Oddone, F |author5=Angrisani, AM |author6=Ziccardi, L |author7=Ricci, B |author8=Quaranta, L |author9= Manni, G |title=Evidence of the neuroprotective role of citicoline in glaucoma patients.|journal=Progress in brain research|year=2008|volume=173|pages=541–54|pmid=18929133|doi=10.1016/S0079-6123(08)01137-0}}</ref><ref>{{cite journal|last=Parisi|first=V.|author2=Coppola, G. |author3=Ziccardi, L. |author4=Gallinaro, G. |author5= Falsini, B. |title=Cytidine-5'-diphosphocholine (Citicoline): a pilot study in patients with non-arteritic ischaemic optic neuropathy|journal=European Journal of Neurology|date=1 May 2008|volume=15|issue=5|pages=465–474|doi=10.1111/j.1468-1331.2008.02099.x}}</ref> | |||
===Satiety=== | |||
[[Cocaine]] dependence is associated with depleted [[dopamine]] levels in the [[central nervous system]]. In cocaine-dependent individuals citicoline increases brain [[dopamine]] levels and reduces cravings.<ref>{{cite journal|last=Renshaw|first=PF|author2=Daniels, S |author3=Lundahl, LH |author4=Rogers, V |author5= Lukas, SE |title=Short-term treatment with citicoline (CDP-choline) attenuates some measures of craving in cocaine-dependent subjects: a preliminary report.|journal=Psychopharmacology|date=February 1999|volume=142|issue=2|pages=132–8|pmid=10102764|doi=10.1007/s002130050871}}</ref> In the general population citicoline increases brain responses to food stimuli, specifically in the [[amygdala]], [[insular cortex|insula]], and [[lateral orbitofrontal cortex]], which correlate with decreased appetite.<ref>{{cite journal|last=Killgore|first=WD|author2=Ross, AJ |author3=Kamiya, T |author4=Kawada, Y |author5=Renshaw, PF |author6= Yurgelun-Todd, DA |title=Citicoline affects appetite and cortico-limbic responses to images of high-calorie foods.|journal=The International Journal of Eating Disorders|date=January 2010|volume=43|issue=1|pages=6–13|pmid=19260039|doi=10.1002/eat.20658|pmc=3378241}}</ref> | |||
==Mechanism of action== | |||
[[File:Synthesis of choline from citicoline.png|thumb|Enzymes involved in reactions are identified by numbers. See file description.]] | |||
===Neuroprotective effects=== | |||
The neuroprotective effects exhibited by citicoline may be due to its preservation of [[cardiolipin]] and [[sphingomyelin]], preservation of [[arachidonic acid]] content of [[phosphatidylcholine]] and [[phosphatidylethanolamine]], partial restoration of [[phosphatidylcholine]] levels, and stimulation of [[glutathione]] synthesis and [[glutathione reductase]] activity. Citicoline’s effects may also be explained by the reduction of [[phospholipase A2]] activity.<ref name="Adibhatla 2002">{{cite journal|last=Adibhatla|first=RM|author2=Hatcher, JF |author3=Dempsey, RJ |title=Citicoline: neuroprotective mechanisms in cerebral ischemia.|journal=Journal of Neurochemistry|date=January 2002|volume=80|issue=1|pages=12–23|pmid=11796739|doi=10.1046/j.0022-3042.2001.00697.x}}</ref> | |||
Citicoline increases [[phosphatidylcholine]] synthesis.<ref>{{cite journal|last=López-Coviella|first=I|author2=Agut, J |author3=Savci, V |author4=Ortiz, JA |author5= Wurtman, RJ |title=Evidence that 5'-cytidinediphosphocholine can affect brain phospholipid composition by increasing choline and cytidine plasma levels.|journal=Journal of Neurochemistry|date=August 1995|volume=65|issue=2|pages=889–94|pmid=7616250 |doi=10.1046/j.1471-4159.1995.65020889.x}}</ref><ref name="Conant 2004">{{cite journal|last=Conant|first=R|author2=Schauss, AG|title=Therapeutic applications of citicoline for stroke and cognitive dysfunction in the elderly: a review of the literature.|journal=Alternative medicine review : a journal of clinical therapeutic|date=March 2004|volume=9|issue=1|pages=17–31|pmid=15005642}}</ref><ref>{{cite journal|last=Babb|first=SM|author2=Wald, LL |author3=Cohen, BM |author4=Villafuerte, RA |author5=Gruber, SA |author6=Yurgelun-Todd, DA |author7= Renshaw, PF |title=Chronic citicoline increases phosphodiesters in the brains of healthy older subjects: an in vivo phosphorus magnetic resonance spectroscopy study.|journal=Psychopharmacology|date=May 2002|volume=161|issue=3|pages=248–54|pmid=12021827|doi=10.1007/s00213-002-1045-y}}</ref> The mechanism for this may be: | |||
* By converting 1, 2-diacylglycerol into [[phosphatidylcholine]] | |||
* Stimulating the synthesis of [[SAMe]], which aids in membrane stabilization and reduces levels of [[arachidonic acid]]. This is especially important after an [[ischemia]], when [[arachidonic acid]] levels are elevated.<ref>{{cite journal|last=Rao|first=AM|author2=Hatcher, JF |author3=Dempsey, RJ |title=CDP-choline: neuroprotection in transient forebrain ischemia of gerbils.|journal=Journal of neuroscience research|date=Dec 1, 1999|volume=58|issue=5|pages=697–705|pmid=10561698|doi=10.1002/(sici)1097-4547(19991201)58:5<697::aid-jnr11>3.0.co;2-b}}</ref> | |||
===Neuronal membrane=== | |||
The brain prefers to use [[choline]] to synthesize [[acetylcholine]]. This limits the amount of [[choline]] available to synthesize [[phosphatidylcholine]]. When the availability of [[choline]] is low or the need for [[acetylcholine]] increases, phospholipids containing [[choline]] can be catabolized from neuronal membranes. These phospholipids include sphingomyelin and [[phosphatidylcholine]].<ref name="Adibhatla 2002" /> Supplementation with citicoline can increase the amount of [[choline]] available for [[acetylcholine]] synthesis and aid in rebuilding membrane [[phospholipid]] stores after depletion.<ref name="D’Orlando 1995">{{cite journal|last=D'Orlando|first=KJ|coauthors=Sandage BW, Jr|title=Citicoline (CDP-choline): mechanisms of action and effects in ischemic brain injury.|journal=Neurological research|date=August 1995|volume=17|issue=4|pages=281–4|pmid=7477743}}</ref> | |||
Citicoline decreases [[phospholipase]] stimulation. This can lower levels of [[hydroxyl radicals]] produced after an [[ischemia]] and prevent [[cardiolipin]] from being catabolized by [[phospholipase A2]].<ref name="Rao 2001">{{cite journal|last=Rao|first=AM|author2=Hatcher, JF |author3=Dempsey, RJ |title=Does CDP-choline modulate phospholipase activities after transient forebrain ischemia?|journal=Brain Research|date=Mar 2, 2001|volume=893|issue=1-2|pages=268–72|pmid=11223016|doi=10.1016/S0006-8993(00)03280-7}}</ref><ref>{{cite journal|last=Adibhatla|first=RM|author2=Hatcher, JF|title=Citicoline decreases phospholipase A2 stimulation and hydroxyl radical generation in transient cerebral ischemia.|journal=Journal of neuroscience research|date=Aug 1, 2003|volume=73|issue=3|pages=308–15|pmid=12868064|doi=10.1002/jnr.10672}}</ref> It can also work to restore [[cardiolipin]] levels in the [[inner mitochondrial membrane]].<ref name="Rao 2001" /> | |||
===Cell signalling=== | |||
Citicoline enhances cellular communication by increasing the availability of neurotransmitters, including [[acetylcholine]], [[norepinephrine]], and [[dopamine]].<ref>{{cite journal|last=Secades|first=JJ|author2=Lorenzo, JL|title=Citicoline: pharmacological and clinical review, 2006 update.|journal=Methods and findings in experimental and clinical pharmacology|date=September 2006|volume=28 Suppl B|pages=1–56|pmid=17171187}}</ref> | |||
===Blood flow=== | |||
Citicoline increases glucose metabolism in the brain and cerebral blood flow.<ref>{{cite journal|last=Watanabe|first=S|author2=Kono, S |author3=Nakashima, Y |author4=Mitsunobu, K |author5= Otsuki, S |title=Effects of various cerebral metabolic activators on glucose metabolism of brain.|journal=Folia psychiatrica et neurologica japonica|year=1975|volume=29|issue=1|pages=67–76|pmid=1098982}}</ref> | |||
===Inflammation and stress=== | |||
Citicoline reduces [[oxidative stress]]. It also prevents excessive inflammatory response in the brain by inhibiting the release of free [[fatty acids]] and decreasing [[blood–brain barrier]] breakdown.<ref name="Conant 2004" /> | |||
===Glutamate transport=== | |||
Citicoline lowers increased [[glutamate]] concentrations and raises decreased [[Adenosine triphosphate|ATP]] concentrations induced by [[ischemia]]. Citicoline also increases [[glutamate]] uptake by increasing expression of [[EAAT2]], a [[glutamate transporter]], in vitro in rat astrocytes. It is suggested that the neuroprotective effects of citicoline after a [[stroke]] are due in part to citicoline’s ability to decrease levels of [[glutamate]] in the brain.<ref>{{cite journal|last=Hurtado|first=Olivia|coauthors=Moro, María A.; Cárdenas, Antonio; Sánchez, Verónica; Fernández-Tomé, Paz; Leza, Juan C.; Lorenzo, Pedro; Secades, Julio J.; Lozano, Rafael; Dávalos, Antoni; Castillo, José; Lizasoain, Ignacio|title=Neuroprotection afforded by prior citicoline administration in experimental brain ischemia: effects on glutamate transport|journal=Neurobiology of Disease|volume=18|issue=2|pages=336–345|doi=10.1016/j.nbd.2004.10.006}}</ref> | |||
==Pharmacokinetics== | |||
Citicoline is water-soluble, with more than 90% oral bioavailability.<ref name="D’Orlando 1995" /> Plasma levels peak one hour after oral ingestion, and a majority of the citicoline is excreted as [[CO2]] in respiration, and again 24 hours after ingestion, where the remaining citicoline is excreted through [[urine]].<ref>{{cite journal|last=Dinsdale|first=JR|author2=Griffiths, GK |author3=Rowlands, C |author4=Castelló, J |author5=Ortiz, JA |author6=Maddock, J |author7= Aylward, M |title=Pharmacokinetics of 14C CDP-choline.|journal=Arzneimittel-Forschung|year=1983|volume=33|issue=7A|pages=1066–70|pmid=6412727}}</ref> | |||
===Side effects=== | |||
Citicoline has a very low toxicity profile in animals and humans. Clinically, doses of 2000 mg per day have been observed and approved. Minor transient adverse effects are rare and most commonly include stomach pain and diarrhea.<ref name="Conant 2004" /> | |||
==Synthesis== | |||
===In vivo=== | |||
[[phosphatidylcholine]] is a major phospholipid in eukaryotic cell membranes. Close regulation of its biosynthesis, degradation, and distribution is essential to proper cell function. [[phosphatidylcholine]] is synthesized [[in vivo]] by two pathways | |||
* The [[Kennedy pathway]], which includes the transformation of [[choline]] to citicoline, by way of [[phosphorylcholine]], to produce [[phosphatidylcholine]] when condensed with [[diacylglycerol]]. | |||
* [[Phosphatidylcholine]] can also be produced by the methylation pathway, where [[phosphatidylethanolamine]] is sequentially [[methylated]].<ref>{{cite journal|last=Fernández-Murray|first=JP|author2=McMaster, CR|title=Glycerophosphocholine catabolism as a new route for choline formation for phosphatidylcholine synthesis by the Kennedy pathway.|journal=The Journal of Biological Chemistry|date=Nov 18, 2005|volume=280|issue=46|pages=38290–6|pmid=16172116|doi=10.1074/jbc.M507700200}}</ref> | |||
==See also== | |||
<!-- keep alphabetical --> | |||
{{Refbegin|30em}} | |||
* [[1-alkenyl-2-acylglycerol choline phosphotransferase]] | |||
* [[Ceramide cholinephosphotransferase]] | |||
* [[Choline-phosphate cytidylyltransferase]] | |||
* [[Diacylglycerol cholinephosphotransferase]] | |||
* [[Sphingosine cholinephosphotransferase]] | |||
{{Refend}} | |||
<!-- keep alphabetical --> | |||
== | == References == | ||
{{Reflist|20em}} | |||
{{ | {{Dietary_supplements}} | ||
{{Nootropics}} | |||
{{Cholinergics}} | |||
{{Phospholipids}} | |||
[[Category:Nootropics]] | [[Category:Nootropics]] | ||
[[Category:Nucleotides]] | [[Category:Nucleotides]] | ||
[[Category:Quaternary ammonium compounds]] | [[Category:Quaternary ammonium compounds]] | ||
[[Category:Choline esters]] | |||
Revision as of 18:48, 6 April 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
| Error creating thumbnail: File missing | |
| Clinical data | |
|---|---|
| Synonyms | Cytidine diphosphate choline |
| AHFS/Drugs.com | International Drug Names |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C14H27N4O11P2+ |
| Molar mass | 489.332 g/mol |
| 3D model (JSmol) | |
| |
| |
| | |
Citicoline (INN), also known as cytidine diphosphate-choline (CDP-Choline) & cytidine 5'-diphosphocholine is a psychostimulant/nootropic. It is an intermediate in the generation of phosphatidylcholine from choline.
Studies suggest that CDP-choline supplements increase dopamine receptor densities,[1] and suggest that CDP-choline supplementation helps prevent memory impairment resulting from poor environmental conditions.[2] Preliminary research has found that citicoline supplements help improve focus and mental energy and may possibly be useful in the treatment of attention deficit disorder.[3][4] Citicoline has also been shown to elevate ACTH independently from CRH levels and to amplify the release of other HPA axis hormones such as LH, FSH, GH and TSH in response to hypothalamic releasing factors.[5] These effects on HPA hormone levels may be beneficial for some individuals but may have undesirable effects in those with medical conditions featuring ACTH or cortisol hypersecretion including, but not limited to, PCOS, type II diabetes and major depressive disorder.[6][7]
Medical uses
Citicoline is available as a supplement online and in stores. It is sold in over 70 countries under a variety of brand names: Ceraxon, Cognizin, NeurAxon, Somazina, Synapsine, etc. When taken as a supplement citicoline is hydrolyzed into choline and cytidine in the intestine.[8] Once these cross the blood–brain barrier it is reformed into citicoline by the rate-limiting enzyme in phosphatidylcholine synthesis, CTP-phosphocholine cytidylyltransferase.[9][10]
Memory disorders
In the hippocampi of rats with induced Alzheimer’s Disease, citicoline counteracts neuronal degeneration and reduces the number of apoptotic cells present. Citicoline supplementation also improves memory retention.[9]
Ischemic stroke
Citicoline is approved for treatment in cases of head trauma, stroke, and neurodegenerative disease in Japan and Europe. Citicoline improves the clinical outcome following an ischemic stroke, as evidenced by the reduction in size of lesions caused by ischemic strokes after supplementation.[11] It has been claimed that citicoline reduces rates of death and disability following an ischemic stroke.[12] However, the largest trial to date, a randomised, placebo-controlled, sequential trial in patients with moderate-to-severe acute ischaemic stroke in Europe, enrolling 2298 patients, found no benefit of administering citicoline on survival or recovery from stroke.[13]
It should be noted that Citicoline is the only substance that ever showed any significant neuroprotective effect at least in patients with less severe stroke events.[14]
Vision
Citicoline improves visual function in patients with glaucoma, amblyopia, and non-arteritic ischaemic optic neuropathy.[15][16]
Satiety
Cocaine dependence is associated with depleted dopamine levels in the central nervous system. In cocaine-dependent individuals citicoline increases brain dopamine levels and reduces cravings.[17] In the general population citicoline increases brain responses to food stimuli, specifically in the amygdala, insula, and lateral orbitofrontal cortex, which correlate with decreased appetite.[18]
Mechanism of action
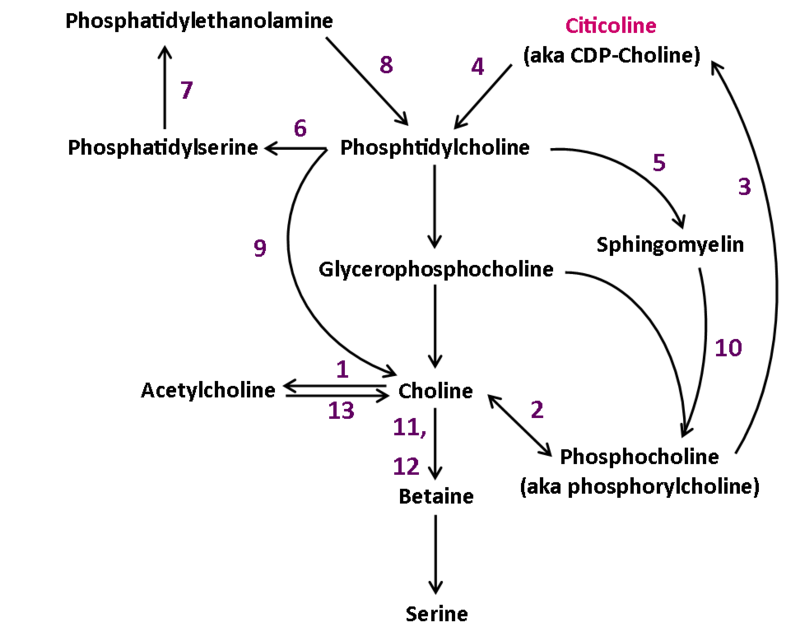
Neuroprotective effects
The neuroprotective effects exhibited by citicoline may be due to its preservation of cardiolipin and sphingomyelin, preservation of arachidonic acid content of phosphatidylcholine and phosphatidylethanolamine, partial restoration of phosphatidylcholine levels, and stimulation of glutathione synthesis and glutathione reductase activity. Citicoline’s effects may also be explained by the reduction of phospholipase A2 activity.[19] Citicoline increases phosphatidylcholine synthesis.[20][21][22] The mechanism for this may be:
- By converting 1, 2-diacylglycerol into phosphatidylcholine
- Stimulating the synthesis of SAMe, which aids in membrane stabilization and reduces levels of arachidonic acid. This is especially important after an ischemia, when arachidonic acid levels are elevated.[23]
Neuronal membrane
The brain prefers to use choline to synthesize acetylcholine. This limits the amount of choline available to synthesize phosphatidylcholine. When the availability of choline is low or the need for acetylcholine increases, phospholipids containing choline can be catabolized from neuronal membranes. These phospholipids include sphingomyelin and phosphatidylcholine.[19] Supplementation with citicoline can increase the amount of choline available for acetylcholine synthesis and aid in rebuilding membrane phospholipid stores after depletion.[24] Citicoline decreases phospholipase stimulation. This can lower levels of hydroxyl radicals produced after an ischemia and prevent cardiolipin from being catabolized by phospholipase A2.[25][26] It can also work to restore cardiolipin levels in the inner mitochondrial membrane.[25]
Cell signalling
Citicoline enhances cellular communication by increasing the availability of neurotransmitters, including acetylcholine, norepinephrine, and dopamine.[27]
Blood flow
Citicoline increases glucose metabolism in the brain and cerebral blood flow.[28]
Inflammation and stress
Citicoline reduces oxidative stress. It also prevents excessive inflammatory response in the brain by inhibiting the release of free fatty acids and decreasing blood–brain barrier breakdown.[21]
Glutamate transport
Citicoline lowers increased glutamate concentrations and raises decreased ATP concentrations induced by ischemia. Citicoline also increases glutamate uptake by increasing expression of EAAT2, a glutamate transporter, in vitro in rat astrocytes. It is suggested that the neuroprotective effects of citicoline after a stroke are due in part to citicoline’s ability to decrease levels of glutamate in the brain.[29]
Pharmacokinetics
Citicoline is water-soluble, with more than 90% oral bioavailability.[24] Plasma levels peak one hour after oral ingestion, and a majority of the citicoline is excreted as CO2 in respiration, and again 24 hours after ingestion, where the remaining citicoline is excreted through urine.[30]
Side effects
Citicoline has a very low toxicity profile in animals and humans. Clinically, doses of 2000 mg per day have been observed and approved. Minor transient adverse effects are rare and most commonly include stomach pain and diarrhea.[21]
Synthesis
In vivo
phosphatidylcholine is a major phospholipid in eukaryotic cell membranes. Close regulation of its biosynthesis, degradation, and distribution is essential to proper cell function. phosphatidylcholine is synthesized in vivo by two pathways
- The Kennedy pathway, which includes the transformation of choline to citicoline, by way of phosphorylcholine, to produce phosphatidylcholine when condensed with diacylglycerol.
- Phosphatidylcholine can also be produced by the methylation pathway, where phosphatidylethanolamine is sequentially methylated.[31]
See also
References
- ↑ Giménez R, Raïch J, Aguilar J (November 1991). "Changes in brain striatum dopamine and acetylcholine receptors induced by chronic CDP-choline treatment of aging mice". British Journal of Pharmacology. 104 (3): 575–8. doi:10.1111/j.1476-5381.1991.tb12471.x. PMC 1908237. PMID 1839138.
- ↑ Teather LA, Wurtman RJ (2005). "Dietary CDP-choline supplementation prevents memory impairment caused by impoverished environmental conditions in rats". Learning & Memory. 12 (1): 39–43. doi:10.1101/lm.83905. PMC 548494. PMID 15647594.
- ↑ "Supplement naturally boosts ageing brain power". Sydney Morning Herald. 2008-02-25. Retrieved 2009-07-28.
- ↑ Silveri MM, Dikan J, Ross AJ; et al. (November 2008). "Citicoline enhances frontal lobe bioenergetics as measured by phosphorus magnetic resonance spectroscopy". NMR in Biomedicine. 21 (10): 1066–75. doi:10.1002/nbm.1281. PMID 18816480.
- ↑ Cavun S, Savci V (October 2004). "CDP-choline increases plasma ACTH and potentiates the stimulated release of GH, TSH and LH: the cholinergic involvement". Fundamental & Clinical Pharmacology. 18 (5): 513–23. doi:10.1111/j.1472-8206.2004.00272.x. PMID 15482372.
- ↑ Benson S, Arck PC, Tan S; et al. (June 2009). "Disturbed stress responses in women with polycystic ovary syndrome". Psychoneuroendocrinology. 34 (5): 727–35. doi:10.1016/j.psyneuen.2008.12.001. PMID 19150179.
- ↑ Florio P, Zatelli MC, Reis FM, degli Uberti EC, Petraglia F (2007). "Corticotropin releasing hormone: a diagnostic marker for behavioral and reproductive disorders?". Frontiers in Bioscience. 12: 551–60. doi:10.2741/2081. PMID 17127316.
- ↑ Wurtman, RJ; Regan, M; Ulus, I; Yu, L (Oct 1, 2000). "Effect of oral CDP-choline on plasma choline and uridine levels in humans". Biochemical pharmacology. 60 (7): 989–92. doi:10.1016/S0006-2952(00)00436-6. PMID 10974208.
- ↑ 9.0 9.1 Alvarez, XA; Sampedro, C; Lozano, R; Cacabelos, R (October 1999). "Citicoline protects hippocampal neurons against apoptosis induced by brain beta-amyloid deposits plus cerebral hypoperfusion in rats". Methods and findings in experimental and clinical pharmacology. 21 (8): 535–40. doi:10.1358/mf.1999.21.8.794835. PMID 10599052.
- ↑ Carlezon, WA; Pliakas, AM; Parow, AM; Detke, MJ; Cohen, BM; Renshaw, PF (Jun 1, 2002). "Antidepressant-like effects of cytidine in the forced swim test in rats". Biological Psychiatry. 51 (11): 882–9. doi:10.1016/s0006-3223(01)01344-0. PMID 12022961.
- ↑ Warach, S; Pettigrew, LC; Dashe, JF; Pullicino, P; Lefkowitz, DM; Sabounjian, L; Harnett, K; Schwiderski, U; Gammans, R (November 2000). "Effect of citicoline on ischemic lesions as measured by diffusion-weighted magnetic resonance imaging. Citicoline 010 Investigators". Annals of neurology. 48 (5): 713–22. doi:10.1002/1531-8249(200011)48:5<713::aid-ana4>3.0.co;2-#. PMID 11079534.
- ↑ Saver, JL (Fall 2008). "Citicoline: update on a promising and widely available agent for neuroprotection and neurorepair". Reviews in neurological diseases. 5 (4): 167–77. PMID 19122569.
- ↑ Davalos (2012). Lancet. 380 (9839). Missing or empty
|title=(help) - ↑ Overgaard, K (2014). "The effects of citicoline on acute ischemic stroke: a review". Journal of stroke and cerebrovascular diseases : the official journal of National Stroke Association. 23(7): 1764–9. doi:10.1016/j.jstrokecerebrovasdis.2014.01.020. PMID 24739589.
- ↑ Parisi, V; Coppola, G; Centofanti, M; Oddone, F; Angrisani, AM; Ziccardi, L; Ricci, B; Quaranta, L; Manni, G (2008). "Evidence of the neuroprotective role of citicoline in glaucoma patients". Progress in brain research. 173: 541–54. doi:10.1016/S0079-6123(08)01137-0. PMID 18929133.
- ↑ Parisi, V.; Coppola, G.; Ziccardi, L.; Gallinaro, G.; Falsini, B. (1 May 2008). "Cytidine-5'-diphosphocholine (Citicoline): a pilot study in patients with non-arteritic ischaemic optic neuropathy". European Journal of Neurology. 15 (5): 465–474. doi:10.1111/j.1468-1331.2008.02099.x.
- ↑ Renshaw, PF; Daniels, S; Lundahl, LH; Rogers, V; Lukas, SE (February 1999). "Short-term treatment with citicoline (CDP-choline) attenuates some measures of craving in cocaine-dependent subjects: a preliminary report". Psychopharmacology. 142 (2): 132–8. doi:10.1007/s002130050871. PMID 10102764.
- ↑ Killgore, WD; Ross, AJ; Kamiya, T; Kawada, Y; Renshaw, PF; Yurgelun-Todd, DA (January 2010). "Citicoline affects appetite and cortico-limbic responses to images of high-calorie foods". The International Journal of Eating Disorders. 43 (1): 6–13. doi:10.1002/eat.20658. PMC 3378241. PMID 19260039.
- ↑ 19.0 19.1 Adibhatla, RM; Hatcher, JF; Dempsey, RJ (January 2002). "Citicoline: neuroprotective mechanisms in cerebral ischemia". Journal of Neurochemistry. 80 (1): 12–23. doi:10.1046/j.0022-3042.2001.00697.x. PMID 11796739.
- ↑ López-Coviella, I; Agut, J; Savci, V; Ortiz, JA; Wurtman, RJ (August 1995). "Evidence that 5'-cytidinediphosphocholine can affect brain phospholipid composition by increasing choline and cytidine plasma levels". Journal of Neurochemistry. 65 (2): 889–94. doi:10.1046/j.1471-4159.1995.65020889.x. PMID 7616250.
- ↑ 21.0 21.1 21.2 Conant, R; Schauss, AG (March 2004). "Therapeutic applications of citicoline for stroke and cognitive dysfunction in the elderly: a review of the literature". Alternative medicine review : a journal of clinical therapeutic. 9 (1): 17–31. PMID 15005642.
- ↑ Babb, SM; Wald, LL; Cohen, BM; Villafuerte, RA; Gruber, SA; Yurgelun-Todd, DA; Renshaw, PF (May 2002). "Chronic citicoline increases phosphodiesters in the brains of healthy older subjects: an in vivo phosphorus magnetic resonance spectroscopy study". Psychopharmacology. 161 (3): 248–54. doi:10.1007/s00213-002-1045-y. PMID 12021827.
- ↑ Rao, AM; Hatcher, JF; Dempsey, RJ (Dec 1, 1999). "CDP-choline: neuroprotection in transient forebrain ischemia of gerbils". Journal of neuroscience research. 58 (5): 697–705. doi:10.1002/(sici)1097-4547(19991201)58:5<697::aid-jnr11>3.0.co;2-b. PMID 10561698.
- ↑ 24.0 24.1 D'Orlando, KJ (August 1995). "Citicoline (CDP-choline): mechanisms of action and effects in ischemic brain injury". Neurological research. 17 (4): 281–4. PMID 7477743. Unknown parameter
|coauthors=ignored (help) - ↑ 25.0 25.1 Rao, AM; Hatcher, JF; Dempsey, RJ (Mar 2, 2001). "Does CDP-choline modulate phospholipase activities after transient forebrain ischemia?". Brain Research. 893 (1–2): 268–72. doi:10.1016/S0006-8993(00)03280-7. PMID 11223016.
- ↑ Adibhatla, RM; Hatcher, JF (Aug 1, 2003). "Citicoline decreases phospholipase A2 stimulation and hydroxyl radical generation in transient cerebral ischemia". Journal of neuroscience research. 73 (3): 308–15. doi:10.1002/jnr.10672. PMID 12868064.
- ↑ Secades, JJ; Lorenzo, JL (September 2006). "Citicoline: pharmacological and clinical review, 2006 update". Methods and findings in experimental and clinical pharmacology. 28 Suppl B: 1–56. PMID 17171187.
- ↑ Watanabe, S; Kono, S; Nakashima, Y; Mitsunobu, K; Otsuki, S (1975). "Effects of various cerebral metabolic activators on glucose metabolism of brain". Folia psychiatrica et neurologica japonica. 29 (1): 67–76. PMID 1098982.
- ↑ Hurtado, Olivia. "Neuroprotection afforded by prior citicoline administration in experimental brain ischemia: effects on glutamate transport". Neurobiology of Disease. 18 (2): 336–345. doi:10.1016/j.nbd.2004.10.006. Unknown parameter
|coauthors=ignored (help) - ↑ Dinsdale, JR; Griffiths, GK; Rowlands, C; Castelló, J; Ortiz, JA; Maddock, J; Aylward, M (1983). "Pharmacokinetics of 14C CDP-choline". Arzneimittel-Forschung. 33 (7A): 1066–70. PMID 6412727.
- ↑ Fernández-Murray, JP; McMaster, CR (Nov 18, 2005). "Glycerophosphocholine catabolism as a new route for choline formation for phosphatidylcholine synthesis by the Kennedy pathway". The Journal of Biological Chemistry. 280 (46): 38290–6. doi:10.1074/jbc.M507700200. PMID 16172116.
- Pages with script errors
- CS1 maint: Multiple names: authors list
- CS1 maint: Explicit use of et al.
- Pages with citations lacking titles
- Pages with citations using unsupported parameters
- Template:drugs.com link with non-standard subpage
- Articles with changed ChemSpider identifier
- Articles with changed EBI identifier
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Articles with changed InChI identifier
- Chemical articles with unknown parameter in Infobox drug
- Chemical pages without DrugBank identifier
- Drugs with no legal status
- Drugboxes which contain changes to verified fields
- Drugboxes which contain changes to watched fields
- Nootropics
- Nucleotides
- Quaternary ammonium compounds
- Choline esters