Cefquinome: Difference between revisions
m (Bot: Automated text replacement (-{{SIB}} + & -{{EH}} + & -{{EJ}} + & -{{Editor Help}} + & -{{Editor Join}} +)) |
No edit summary |
||
| Line 1: | Line 1: | ||
{{drugbox | {{refimprove|date=March 2014}} | ||
| IUPAC_name = 1-<nowiki>[[</nowiki>(6''R'',7''R'')-7-<nowiki>[[</nowiki>(2''Z'')-(2-Amino-4-thiazolyl)- | {{drugbox | ||
| Watchedfields = changed | |||
| verifiedrevid = 443511465 | |||
| UNII_Ref = {{fdacite|correct|FDA}} | |||
| UNII = Z74S078CWP | |||
| IUPAC_name = 1-<nowiki>[[</nowiki>(6''R'',7''R'')-7-<nowiki>[[</nowiki>(2''Z'')-(2-Amino-4-thiazolyl)-(methoxyimino)acetyl]amino]-2-carboxy-8-oxo-5-thia-1-azabicyclo[4.2.0-oct-2-en-3-yl]methyl]-5,6,7,8-tetrahydroquinolinium inner salt | |||
| image = Cefquinome.png | | image = Cefquinome.png | ||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| ChemSpiderID = 16736863 | |||
| InChI = 1/C23H24N6O5S2/c1-34-27-16(14-11-36-23(24)25-14)19(30)26-17-20(31)29-18(22(32)33)13(10-35-21(17)29)9-28-8-4-6-12-5-2-3-7-15(12)28/h4,6,8,11,17,21H,2-3,5,7,9-10H2,1H3,(H3-,24,25,26,30,32,33)/b27-16+/t17-,21-/m1/s1 | |||
| smiles = O=C4[C@@H](NC(=O)C(=N\OC)/c1csc(N)n1)[C@H]5SC\C(C[n+]3cccc2CCCCc23)=C(/N45)C([O-])=O | |||
| InChIKey = YWKJNRNSJKEFMK-KJXIDEHUBC | |||
| InChI1 = 1/C23H24N6O5S2/c1-34-27-16(14-11-36-23(24)25-14)19(30)26-17-20(31)29-18(22(32)33)13(10-35-21(17)29)9-28-8-4-6-12-5-2-3-7-15(12)28/h4,6,8,11,17,21H,2-3,5,7,9-10H2,1H3,(H3-,24,25,26,30,32,33)/b27-16-/t17-,21-/m1/s1 | |||
| CAS_number_Ref = {{cascite|correct|??}} | |||
| CAS_number = 84957-30-2 | | CAS_number = 84957-30-2 | ||
| ATC_prefix = | | ChEMBL = 2103931 | ||
| ATC_suffix = | | ATCvet = yes | ||
| ATC_supplemental = | | ATC_prefix = G51 | ||
| ATC_suffix = AA07 | |||
| ATC_supplemental = {{ATCvet|J01|DE90}} {{ATCvet|J51|DE90}} | |||
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChI = 1S/C23H24N6O5S2/c1-34-27-16(14-11-36-23(24)25-14)19(30)26-17-20(31)29-18(22(32)33)13(10-35-21(17)29)9-28-8-4-6-12-5-2-3-7-15(12)28/h4,6,8,11,17,21H,2-3,5,7,9-10H2,1H3,(H3-,24,25,26,30,32,33)/b27-16-/t17-,21-/m1/s1 | |||
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChIKey = YWKJNRNSJKEFMK-PQFQYKRASA-N | |||
| PubChem = | | PubChem = | ||
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} | |||
| DrugBank = | | DrugBank = | ||
| KEGG_Ref = {{keggcite|correct|kegg}} | |||
| KEGG = D07652 | |||
| C = 23 | H = 24 | N = 6 | O = 5 | S = 2 | | C = 23 | H = 24 | N = 6 | O = 5 | S = 2 | ||
| molecular_weight = 528.60 g/mol | | molecular_weight = 528.60 g/mol | ||
| Line 20: | Line 41: | ||
| legal_AU = <!-- Unscheduled / S2 / S4 / S8 --> | | legal_AU = <!-- Unscheduled / S2 / S4 / S8 --> | ||
| legal_UK = <!-- GSL / P / POM / CD --> | | legal_UK = <!-- GSL / P / POM / CD --> | ||
| legal_US = | | legal_US = Rx-only, Unscheduled | ||
| legal_status = | | legal_status = | ||
| routes_of_administration = | | routes_of_administration = | ||
}} | }} | ||
__NOTOC__ | |||
{{SI}} | {{SI}} | ||
{{CMG}} | |||
'''Cefquinome | ==Overview== | ||
'''Cefquinome''' is a fourth-generation [[cephalosporin]] with pharmacological and antibacterial properties valuable in the treatment of coliform mastitis and other infections. It is only used in veterinary applications. | |||
== Properties == | == Properties == | ||
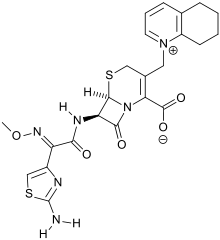
Cefquinome is resistant to [[ | Cefquinome is resistant to [[beta-lactamase]]. Chemically, its [[zwitterion]]ic structure can facilitate rapid penetration across biological membranes, including [[porins]] of bacterial cell walls. Plus, it has a higher affinity to target penicillin-binding proteins. The reactive site is a [[beta-lactam]] nucleus, while the main peripheral functional groups are a quaternary quinolinium, an aminothiazolyl moiety and an unusual ''O''-alkylated [[oxime]]. | ||
Cefquinome acts by inhibition of the cell wall synthesis, but it has a relatively short half-life of about | Cefquinome acts by inhibition of the cell wall synthesis, but it has a relatively short half-life of about 2.5 hours. It is less than 5% protein bound and is excreted unchanged in the urine.<ref name="Intervet">Intervet, "Cephaguard Injection Data Sheet," http://www.intervet.co.uk/Products_Public/Cephaguard_Injection/090_Product_Datasheet.asp</ref> | ||
== Studies == | == Studies == | ||
Many studies have been conducted, mostly for animal use. One such study was conducted by the Pharma Research in Germany. | Many studies have been conducted, mostly for animal use. One such study was conducted by the Pharma Research in Germany. | ||
=== Test | === Test groups === | ||
Groups of albino mice, weighing 191 g, were dosed with 10 and 40 mg of cefquinome per kg. Blood samples were obtained from a cut at the tip of the tail and kept at | Groups of albino mice, weighing 191 g, were dosed with 10 and 40 mg of cefquinome per kg. Blood samples were obtained from a cut at the tip of the tail and kept at 4°C. Urine was collected in metabolism cages. | ||
Three male beagle dogs, weighing about 22 kg each, were dosed with 5, 10, and 20 mg/kg at the [[cephalic vein]]. Blood samples were drawn from the same vein in the opposite leg. Meanwhile, urine was collected by catheterization. | Three male beagle dogs, weighing about 22 kg each, were dosed with 5, 10, and 20 mg/kg at the [[cephalic vein]]. Blood samples were drawn from the same vein in the opposite leg. Meanwhile, urine was collected by catheterization. | ||
Pigs, five or six male and female in each group weighing | Pigs, five or six male and female in each group weighing about 18 kg each, were injected with 10 mg of cefquinome at the venajuglaris in the base of the left ear. Blood samples were withdrawn from the contralateral jugular vein. | ||
Male and female calves | Male and female calves weighing between 110 and 140 kg were dosed with 10 mg of cefquinome per kg through the vera jucular. | ||
Standard solutions were prepared from pooled murine blood and urine taken from untreated dogs, pigs, and calves. | Standard solutions were prepared from pooled murine blood and urine taken from untreated dogs, pigs, and calves. | ||
=== Calculations === | === Calculations === | ||
Cefquinome concentrations were calculated by regression analysis, using the standard curves in which logarithms of the concentration were proportional to the areas of the inhibition zones. Curve fitting was carried out by nonlinear regression with the computer program PHAKOK. Pharmokinetic analysis of the concentration-time data after administration indicated that the best curve fits were usually achieved by using an open two-compartment model. | Cefquinome concentrations were calculated by regression analysis, using the standard curves in which logarithms of the concentration were proportional to the areas of the inhibition zones. [[Curve fitting]] was carried out by nonlinear regression with the computer program PHAKOK. Pharmokinetic analysis of the concentration-time data after administration indicated that the best curve fits were usually achieved by using an open two-compartment model. | ||
=== Conclusion === | === Conclusion === | ||
Data | Data indicate that cefquinome has high antibacterial activity ''in vitro'' against nearly all strains tested. In general, cefquinome is within the same range as cefpirome and [[cefotaxime]]. Against Gram-negative species, cefquinome has very limited ''[[in vitro]]'' activity. The ''in vitro'' activity of cefquinome does not depend on the composition or pH of the test medium. The broad antibacterial spectrum and the high ''in vitro'' activity are reflected by high ''[[in vivo]]'' efficacy in experimental infections. In mouse models of septicemia, cefquinome possessed high therapetic efficacy. All infections were cured. | ||
=== Treatment === | === Treatment === | ||
In cattle, the injection should help against respiratory disease caused by Mannheimia haemolytica and [[Pasteurella multocida]]. It also helps with acute E. coli mastitis, [[ | In cattle, the injection should help against respiratory disease caused by ''Mannheimia haemolytica'' and ''[[Pasteurella multocida]]''. It also helps with acute ''E. coli'' mastitis, [[dermatitis]], infectious ulbar necrosis, and interdigital necrobacillosis. In calves, it is effective against ''E. coli'' septicaemia. | ||
For pigs, it | For pigs, it is used to treat bacterial infections of the lungs and respiratory tract caused by ''[[Pasteurella multocida|P. multocida]]'', ''[[Haemophilus parasuis]]'', ''[[Actinobacillus pleuropneumoniae]]'', and ''[[Streptococcus suis]]''. [[Mastitis-metritis-agalactia syndrome]] involved with ''E. coli'', ''[[Staphylococcus]]'', ''[[Streptococcus]]'', and other cefquinome-sensitive organisms are also treated. In piglets, the mortality rate in cases of meningitis caused by ''Streptococcus sues'' is reduced. It is used in the treatment of mild or moderate lesions caused by ''[[Staphylococcus hyicus]]'' and arthritis caused by ''Streptococcus'' spp. and ''E. coli''. | ||
=== Usage === | <!-- how-to - not encyclopedic === Usage === | ||
Shake the vial well before using. | Shake the vial well before using. | ||
Swab the septum before removing each dose. Use a dry sterile needle and syringe. An appropriately graduated syringe must be used to allow accurate administration of the required dose volume. This is particularly important when injecting small volumes, for example when treating piglets. The cap may be safely punctured up to 25 times. The 50 ml vial should be used for treating small piglets. When treating groups of animals, use a draw-off needle. | Swab the septum before removing each dose. Use a dry sterile needle and syringe. An appropriately graduated syringe must be used to allow accurate administration of the required dose volume. This is particularly important when injecting small volumes, for example when treating piglets. The cap may be safely punctured up to 25 times. The 50 ml vial should be used for treating small piglets. When treating groups of animals, use a draw-off needle. --> | ||
=== Caution/warnings === | |||
=== Caution/ | These are some factors to be aware of before treating: | ||
These are some factors to be aware of before treating | |||
* This product should not be used in animals known to be hypersensitive to β-lactam antibiotics. | * This product should not be used in animals known to be hypersensitive to β-lactam antibiotics. | ||
* | * It should not be administered to animals with a body weight less than 1.25 kg. | ||
* Use of the product may result in localised tissue reaction. Tissue lesions are repaired by 15 days after the last administration of the product. | * Use of the product may result in localised tissue reaction. Tissue lesions are repaired by 15 days after the last administration of the product. | ||
* Hypersensitivity reactions to cephalosporins occur rarely. | * Hypersensitivity reactions to cephalosporins occur rarely. | ||
| Line 77: | Line 98: | ||
* To prevent the claimed infections in piglets, attention should be paid to hygiene and ventilation, and overcrowding should be avoided. When the first piglets are affected, careful examination of all animals in the same pen is recommended to enable an early treatment of any other infected piglets. | * To prevent the claimed infections in piglets, attention should be paid to hygiene and ventilation, and overcrowding should be avoided. When the first piglets are affected, careful examination of all animals in the same pen is recommended to enable an early treatment of any other infected piglets. | ||
== Clinical usage == | |||
=== Human use === | |||
=== Human | Cefquinome is not approved for human use. | ||
However, treatment should be short, meaning a single injection daily for about a week. Treatment should only be given by prescription. Cefquinome should not be used in | === Veterinary medicine === | ||
Conditions of use are limited to therapeutic, parenteral, and individual animal use. Individual parenteral therapy of bovine respiratory disease data on cefquinome-related residues demonstrate only very small amounts are present in the intestinal tract of treated cattle with gastrointestinal activation. However, treatment should be short, meaning a single injection daily for about a week. Treatment should only be given by prescription. Cefquinome should not be used in feed or water. | |||
Since 1994, in Europe, it was allowed to treat cattle by prescription only. In 1999, | Since 1994, in Europe, it was allowed to treat cattle by prescription only. In 1999, swine were included. By 2005, horses were allowed as well. In the United States, approval is pending for treatment of bovine respiratory disease. Even so, this is only available by prescription. | ||
Cefquinome is also used for other illnesses, such as “shipping fever”, a [[pneumonia]]-like illness commonly found in cattle.<ref name="Farmers, doctors">Associated Press, "Farmers, doctors battle over new drug for dairy cows," April 5, 2007, State and Regional</ref> | |||
== Concerns == | == Concerns == | ||
=== Resistance and food-borne transmission === | |||
Of concern, the use of the drug in animals may lead to increases in [[antibiotic resistance]]. Humans can be exposed to bacteria through food-borne transmission, raising chances of becoming exposed to resistant bacterial species, such as ''[[Salmonella]]'' or ''E. coli''. The potential for the development of antibiotic resistance increases as usage increases, by selecting bacteria which have acquired [[beta-lactamase]]s. | |||
=== Salmonella === | === ''Salmonella'' === | ||
The use | The use may cause resistance in ''Salmonella'' present in the intestinal tract of the target animal. Resistant ''Salmonella'' may also contaminate the carcass at slaughter and transfer to humans when used as food. When humans are infected and treated with a fourth-generation cephalosporin, effectiveness may be compromised. | ||
Although | Although fourth-generation cephalosporin resistance is very rare, they are active against bacteria carrying the AmpC-type β-lactamase resistance mechanism. Since the late 1990s, the US and EU have surveyed and gathered data for fourth-generation cephalosporins for both human and veterinary use. Data indicate no changes occur in resistance patterns of relevant food-borne pathogens. | ||
== FDA | == FDA guidelines == | ||
*Administered products will be used in individual animals for short duration and by prescription only. | *Administered products will be used in individual animals for short duration and by prescription only. | ||
*The extent of use is ranked low. | *The extent of use is ranked low. | ||
*Avoid human drug resistance to fourth-generation cephalosporins by authorizing extra-label prohibition. | *Avoid human drug resistance to fourth-generation cephalosporins by authorizing extra-label prohibition. | ||
== See also == | == See also == | ||
* | *''[[Campylobacter]]'' | ||
*[[Cefepime]] | *[[Cefepime]] | ||
*[[Cephalosporin]] | *[[Cephalosporin]] | ||
*[[Enterococcus]] | *[[Enterococcus]] | ||
*[[Zwitterion]] | *[[Zwitterion]] | ||
== | == References == | ||
{{Reflist|2}} | |||
{{Cell wall disruptive antibiotics}} | |||
[[Category:Cephalosporin antibiotics]] | [[Category:Cephalosporin antibiotics]] | ||
[[Category:Quinolines]] | |||
[[Category:Thiazoles]] | |||
[[Category:Oximes]] | |||
[[Category:Drug]] | |||
Revision as of 14:23, 10 April 2015
This article needs additional citations for verification. (March 2014) (Learn how and when to remove this template message) |
 | |
| Clinical data | |
|---|---|
| ATCvet code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 87% |
| Protein binding | <5% |
| Elimination half-life | 2½ hours |
| Excretion | Renal, unchanged |
| Identifiers | |
| |
| CAS Number | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C23H24N6O5S2 |
| Molar mass | 528.60 g/mol |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
|
WikiDoc Resources for Cefquinome |
|
Articles |
|---|
|
Most recent articles on Cefquinome |
|
Media |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Cefquinome at Clinical Trials.gov Clinical Trials on Cefquinome at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Cefquinome
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Cefquinome Discussion groups on Cefquinome Patient Handouts on Cefquinome Directions to Hospitals Treating Cefquinome Risk calculators and risk factors for Cefquinome
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Cefquinome |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Cefquinome is a fourth-generation cephalosporin with pharmacological and antibacterial properties valuable in the treatment of coliform mastitis and other infections. It is only used in veterinary applications.
Properties
Cefquinome is resistant to beta-lactamase. Chemically, its zwitterionic structure can facilitate rapid penetration across biological membranes, including porins of bacterial cell walls. Plus, it has a higher affinity to target penicillin-binding proteins. The reactive site is a beta-lactam nucleus, while the main peripheral functional groups are a quaternary quinolinium, an aminothiazolyl moiety and an unusual O-alkylated oxime.
Cefquinome acts by inhibition of the cell wall synthesis, but it has a relatively short half-life of about 2.5 hours. It is less than 5% protein bound and is excreted unchanged in the urine.[1]
Studies
Many studies have been conducted, mostly for animal use. One such study was conducted by the Pharma Research in Germany.
Test groups
Groups of albino mice, weighing 191 g, were dosed with 10 and 40 mg of cefquinome per kg. Blood samples were obtained from a cut at the tip of the tail and kept at 4°C. Urine was collected in metabolism cages.
Three male beagle dogs, weighing about 22 kg each, were dosed with 5, 10, and 20 mg/kg at the cephalic vein. Blood samples were drawn from the same vein in the opposite leg. Meanwhile, urine was collected by catheterization.
Pigs, five or six male and female in each group weighing about 18 kg each, were injected with 10 mg of cefquinome at the venajuglaris in the base of the left ear. Blood samples were withdrawn from the contralateral jugular vein.
Male and female calves weighing between 110 and 140 kg were dosed with 10 mg of cefquinome per kg through the vera jucular.
Standard solutions were prepared from pooled murine blood and urine taken from untreated dogs, pigs, and calves.
Calculations
Cefquinome concentrations were calculated by regression analysis, using the standard curves in which logarithms of the concentration were proportional to the areas of the inhibition zones. Curve fitting was carried out by nonlinear regression with the computer program PHAKOK. Pharmokinetic analysis of the concentration-time data after administration indicated that the best curve fits were usually achieved by using an open two-compartment model.
Conclusion
Data indicate that cefquinome has high antibacterial activity in vitro against nearly all strains tested. In general, cefquinome is within the same range as cefpirome and cefotaxime. Against Gram-negative species, cefquinome has very limited in vitro activity. The in vitro activity of cefquinome does not depend on the composition or pH of the test medium. The broad antibacterial spectrum and the high in vitro activity are reflected by high in vivo efficacy in experimental infections. In mouse models of septicemia, cefquinome possessed high therapetic efficacy. All infections were cured.
Treatment
In cattle, the injection should help against respiratory disease caused by Mannheimia haemolytica and Pasteurella multocida. It also helps with acute E. coli mastitis, dermatitis, infectious ulbar necrosis, and interdigital necrobacillosis. In calves, it is effective against E. coli septicaemia.
For pigs, it is used to treat bacterial infections of the lungs and respiratory tract caused by P. multocida, Haemophilus parasuis, Actinobacillus pleuropneumoniae, and Streptococcus suis. Mastitis-metritis-agalactia syndrome involved with E. coli, Staphylococcus, Streptococcus, and other cefquinome-sensitive organisms are also treated. In piglets, the mortality rate in cases of meningitis caused by Streptococcus sues is reduced. It is used in the treatment of mild or moderate lesions caused by Staphylococcus hyicus and arthritis caused by Streptococcus spp. and E. coli.
Caution/warnings
These are some factors to be aware of before treating:
- This product should not be used in animals known to be hypersensitive to β-lactam antibiotics.
- It should not be administered to animals with a body weight less than 1.25 kg.
- Use of the product may result in localised tissue reaction. Tissue lesions are repaired by 15 days after the last administration of the product.
- Hypersensitivity reactions to cephalosporins occur rarely.
- The product does not contain an antimicrobial preservative.
- To prevent the claimed infections in piglets, attention should be paid to hygiene and ventilation, and overcrowding should be avoided. When the first piglets are affected, careful examination of all animals in the same pen is recommended to enable an early treatment of any other infected piglets.
Clinical usage
Human use
Cefquinome is not approved for human use.
Veterinary medicine
Conditions of use are limited to therapeutic, parenteral, and individual animal use. Individual parenteral therapy of bovine respiratory disease data on cefquinome-related residues demonstrate only very small amounts are present in the intestinal tract of treated cattle with gastrointestinal activation. However, treatment should be short, meaning a single injection daily for about a week. Treatment should only be given by prescription. Cefquinome should not be used in feed or water.
Since 1994, in Europe, it was allowed to treat cattle by prescription only. In 1999, swine were included. By 2005, horses were allowed as well. In the United States, approval is pending for treatment of bovine respiratory disease. Even so, this is only available by prescription.
Cefquinome is also used for other illnesses, such as “shipping fever”, a pneumonia-like illness commonly found in cattle.[2]
Concerns
Resistance and food-borne transmission
Of concern, the use of the drug in animals may lead to increases in antibiotic resistance. Humans can be exposed to bacteria through food-borne transmission, raising chances of becoming exposed to resistant bacterial species, such as Salmonella or E. coli. The potential for the development of antibiotic resistance increases as usage increases, by selecting bacteria which have acquired beta-lactamases.
Salmonella
The use may cause resistance in Salmonella present in the intestinal tract of the target animal. Resistant Salmonella may also contaminate the carcass at slaughter and transfer to humans when used as food. When humans are infected and treated with a fourth-generation cephalosporin, effectiveness may be compromised.
Although fourth-generation cephalosporin resistance is very rare, they are active against bacteria carrying the AmpC-type β-lactamase resistance mechanism. Since the late 1990s, the US and EU have surveyed and gathered data for fourth-generation cephalosporins for both human and veterinary use. Data indicate no changes occur in resistance patterns of relevant food-borne pathogens.
FDA guidelines
- Administered products will be used in individual animals for short duration and by prescription only.
- The extent of use is ranked low.
- Avoid human drug resistance to fourth-generation cephalosporins by authorizing extra-label prohibition.
See also
References
- ↑ Intervet, "Cephaguard Injection Data Sheet," http://www.intervet.co.uk/Products_Public/Cephaguard_Injection/090_Product_Datasheet.asp
- ↑ Associated Press, "Farmers, doctors battle over new drug for dairy cows," April 5, 2007, State and Regional
- Pages with script errors
- Articles needing additional references from March 2014
- Articles with invalid date parameter in template
- All articles needing additional references
- Drugs with non-standard legal status
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Infobox drug
- Chemical pages without DrugBank identifier
- Drugboxes which contain changes to watched fields
- Cephalosporin antibiotics
- Quinolines
- Thiazoles
- Oximes
- Drug