Cefepime
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Alberto Plate [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Cefepime is a antibiotic (cephalosporin) that is FDA approved for the treatment of pneumonia, febrile neutropenia, uncomplicated UTI, uncomplicated skin infection and complicated intraabdominal infections. Common adverse reactions include rash, hypophosphatemia, diarrhea, Direct Coombs test positive, ALT/SGPT level raised, AST/SGOT level raised.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
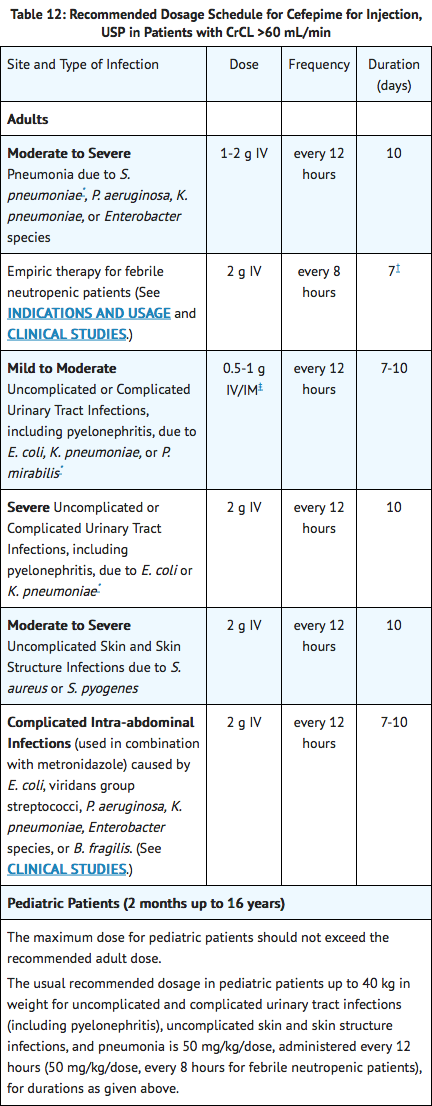
Cefepime for injection, USP is indicated in the treatment of the following infections caused by susceptible strains of the designated microorganisms:
Pneumonia (moderate to severe)
- Caused by Streptococcus pneumoniae, including cases associated with concurrent bacteremia, Pseudomonas aeruginosa, Klebsiella pneumoniae, or Enterobacter species.
Empiric Therapy for Febrile Neutropenic Patients
- Cefepime as monotherapy is indicated for empiric treatment of febrile neutropenic patients. In patients at high risk for severe infection (including patients with a history of recent bone marrow transplantation, with hypotension at presentation, with an underlying hematologic malignancy, or with severe or prolonged neutropenia), antimicrobial monotherapy may not be appropriate. Insufficient data exist to support the efficacy of cefepime monotherapy in such patients.
Uncomplicated and Complicated Urinary Tract Infections (Including Pyelonephritis)
- Caused by Escherichia coli or Klebsiella pneumoniae, when the infection is severe, or caused by Escherichia coli, Klebsiella pneumoniae, or Proteus mirabilis, when the infection is mild to moderate, including cases associated with concurrent bacteremia with these microorganisms.
Uncomplicated Skin and Skin Structure Infections
- Caused by Staphylococcus aureus (methicillin-susceptible strains only) or Streptococcus pyogenes.
Complicated Intra-abdominal Infections
- Its used in combination with metronidazole. Caused by Escherichia coli, viridans group streptococci, Pseudomonas aeruginosa, Klebsiella pneumoniae, Enterobacter species, or Bacteroides fragilis.
- To reduce the development of drug-resistant bacteria and maintain the effectiveness of cefepime for injection, USP and other antibacterial drugs, cefepime for injection, USP should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Prevention and Treatment of Respiratory Infections in HIV Patients
- Community Acquired Pneumonia - Empiric Therapy for Patients at Risk for Pseudomonas Pneumonia
- Dosage: IV beta-lactam (piperacillin-tazobactam, cefepime, imipenem, or meropenem) + IV ciprofloxacin (400 mg q8h-q12h) or IV levofloxacin (750 mg q24h)[1]
Culture-Negative Endocarditis Including Bartonella Endocarditis
- Dosage: Multidrug Scheme
- Vancomycin hydrochloride 15 mg/kg IV q12h for 6 weeks
- Gentamicin sulfate (Can be either IM or IV)
- 1 mg/kg IV
- IM every 8 hours for 2 weeks.
- Cefepime 2 g IV q8h for 6 weeks[2]
- Rifampin 300 mg IV or orally q8h for 6 weeks
Non–Guideline-Supported Use
Peritoneal dialysis-associated peritonitis
- Dosage[3]
- Loading dose: Cefepime 2g
- Maintenance dose: 1g IP for 9 consecutive days
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)

Cefepime for injection, USP is indicated in the treatment of the following infections caused by susceptible strains of the designated microorganisms:
Pneumonia (moderate to severe)
- Caused by Streptococcus pneumoniae, including cases associated with concurrent bacteremia, Pseudomonas aeruginosa, Klebsiella pneumoniae, or Enterobacter species.
Empiric Therapy for Febrile Neutropenic Patients
- Cefepime as monotherapy is indicated for empiric treatment of febrile neutropenic patients. In patients at high risk for severe infection (including patients with a history of recent bone marrow transplantation, with hypotension at presentation, with an underlying hematologic malignancy, or with severe or prolonged neutropenia), antimicrobial monotherapy may not be appropriate. Insufficient data exist to support the efficacy of cefepime monotherapy in such patients.
Uncomplicated and Complicated Urinary Tract Infections (Including Pyelonephritis)
- Caused by Escherichia coli or Klebsiella pneumoniae, when the infection is severe, or caused by Escherichia coli, Klebsiella pneumoniae, or Proteus mirabilis, when the infection is mild to moderate, including cases associated with concurrent bacteremia with these microorganisms.
Uncomplicated Skin and Skin Structure Infections
- Caused by Staphylococcus aureus (methicillin-susceptible strains only) or Streptococcus pyogenes.
Complicated Intra-abdominal Infections
- Its used in combination with metronidazole. Caused by Escherichia coli, viridans group streptococci, Pseudomonas aeruginosa, Klebsiella pneumoniae, Enterobacter species, or Bacteroides fragilis.
- To reduce the development of drug-resistant bacteria and maintain the effectiveness of cefepime for injection, USP and other antibacterial drugs, cefepime for injection, USP should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
Prevention and Treatment of Respiratory Infections in HIV Patients
- Community Acquired Pneumonia - Empiric Therapy for Patients at Risk for Pseudomonas Pneumonia
- Dosage: IV beta-lactam (piperacillin-tazobactam, cefepime, imipenem, or meropenem) + IV ciprofloxacin (400 mg q8h-q12h) or IV levofloxacin (750 mg q24h)[4]
Culture-Negative Endocarditis Including Bartonella Endocarditis
- Dosage: Multidrug Scheme
- Vancomycin hydrochloride 15 mg/kg IV q12h for 6 weeks
- Gentamicin sulfate (Can be either IM or IV)
- 1 mg/kg IV
- IM every 8 hours for 2 weeks.
- Cefepime 2 g IV q8h for 6 weeks[5]
- Rifampin 300 mg IV or orally q8h for 6 weeks
Non–Guideline-Supported Use
Bacterial Meningitis
Osteomyelitis and Septic Arthritis
- Dosage: 2g IV q12h[8]
Contraindications
- Cefepime for injection is contraindicated in patients who have shown immediate hypersensitivity reactions to cefepime or the cephalosporin class of antibiotics, penicillins or other beta-lactam antibiotics.
Warnings
Hypersensitivity
- Before therapy with cefepime for injection is instituted, careful inquiry should be made to determine whether the patient has had previous immediate hypersensitivity reactions to cefepime, cephalosporins, penicillins, or other drugs. If this product is to be given to penicillin-sensitive patients, caution should be exercised because cross-hypersensitivity among beta-lactam antibiotics has been clearly documented and may occur in up to 10% of patients with a history of penicillin allergy. If an allergic reaction to cefepime for injection occurs, discontinue the drug. Serious acute hypersensitivity reactions may require treatment with epinephrine and other emergency measures including oxygen, corticosteroids, intravenous fluids, intravenous antihistamines, pressor amines, and airway management, as clinically indicated.
Renal Impairment
- In patients with creatinine clearance less than or equal to 60 mL/min, the dose of cefepime for injection (cefepime hydrochloride) should be adjusted to compensate for the slower rate of renal elimination. Because high and prolonged serum antibiotic concentrations can occur from usual dosages in patients with renal impairment or other conditions that may compromise renal function, the maintenance dosage should be reduced when cefepime is administered to such patients. Continued dosage should be determined by degree of renal impairment, severity of infection, and susceptibility of the causative organisms.
Clostridium Difficile Associated Diarrhea
- Clostridium difficile associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including cefepime for injection, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile.
- C. difficile produces toxins A and B, which contribute to the development of CDAD. Hypertoxin-producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibiotic use. Careful medical history is necessary since [CDAD]] has been reported to occur over two months after the administration of antibacterial agents.
- If CDAD is suspected or confirmed, ongoing antibiotic use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibiotic treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
During postmarketing surveillance, serious adverse events have been reported including life-threatening or fatal occurrences of the following: encephalopathy (disturbance of consciousness including confusion, hallucinations, stupor, and coma), myoclonus, and seizures. Most cases occurred in patients with renal impairment who received doses of cefepime that exceeded the recommended dosage schedules. However, some cases of encephalopathy occurred in patients receiving a dosage adjustment for their renal function. In the majority of cases, symptoms of neurotoxicity were reversible and resolved after discontinuation of cefepime and/or after hemodialysis.
Adverse Reactions
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
- In clinical trials using multiple doses of cefepime, 4137 patients were treated with the recommended dosages of cefepime (500 mg to 2 g intravenous every 12 hours). There were no deaths or permanent disabilities thought related to drug toxicity. Sixty-four (1.5%) patients discontinued medication due to adverse events thought by the investigators to be possibly, probably, or almost certainly related to drug toxicity. Thirty-three (51%) of these 64 patients who discontinued therapy did so because of rash. The percentage of cefepime-treated patients who discontinued study drug because of drug-related adverse events was very similar at daily doses of 500 mg, 1 g, and 2 g every 12 hours (0.8%, 1.1%, and 2.0%, respectively). However, the incidence of discontinuation due to rash increased with the higher recommended doses. The following adverse events were thought to be probably related to cefepime during evaluation of the drug in clinical trials conducted in North America (n=3125 cefepime-treated patients).
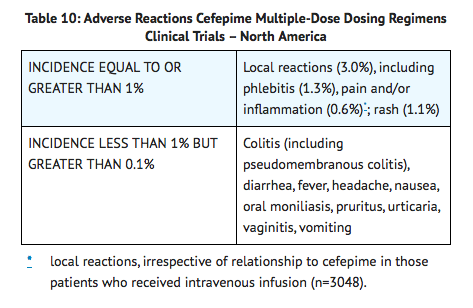
- At the higher dose of 2 g every 8 hours, the incidence of probably-related adverse events was higher among the 795 patients who received this dose of cefepime. They consisted of rash (4%), diarrhea (3%), nausea (2%), vomiting (1%), pruritus (1%), fever (1%), and headache (1%). The following adverse laboratory changes, irrespective of relationship to therapy with Cefepime, were seen during clinical trials conducted in North America.
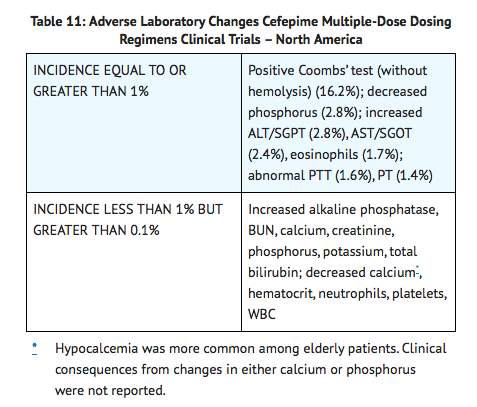
A similar safety profile was seen in clinical trials of pediatric patients.
Postmarketing Experience
In addition to the events reported during North American clinical trials with cefepime, the following adverse experiences have been reported during worldwide postmarketing experience.
- As with some other drugs in this class, encephalopathy (disturbance of consciousness including confusion, hallucinations, stupor, and coma), myoclonus, and seizures have been reported. Although most cases occurred in patients with renal impairment who received doses of cefepime that exceeded the recommended dosage schedules, some cases of encephalopathy occurred in patients receiving a dosage adjustment for their renal function. If seizures associated with drug therapy occur, the drug should be discontinued. Anticonvulsant therapy can be given if clinically indicated. Precautions should be taken to adjust daily dosage in patients with renal insufficiency or other conditions that may compromise renal function to reduce antibiotic concentrations that can lead or contribute to these and other serious adverse events, including renal failure. As with other cephalosporins, anaphylaxis including anaphylactic shock, transient leukopenia, neutropenia, agranulocytosis and thrombocytopenia have been reported.
Cephalosporin-Class Adverse Reactions
- In addition to the adverse reactions listed above that have been observed in patients treated with cefepime, the following adverse reactions and altered laboratory tests have been reported for cephalosporin-class antibiotics: Stevens-Johnson syndrome, erythema multiforme, toxic epidermal necrolysis, renal dysfunction, toxic nephropathy, aplastic anemia, hemolytic anemia, hemorrhage, hepatic dysfunction including cholestasis, and pancytopenia.
Drug Interactions
- Renal function should be monitored carefully if high doses of aminoglycosides are to be administered with cefepime for injection because of the increased potential of nephrotoxicity and ototoxicity of aminoglycoside antibiotics. Nephrotoxicity has been reported following concomitant administration of other cephalosporins with potent diuretics such as furosemide.
Use in Specific Populations
Pregnancy
- Cefepime was not teratogenic or embryocidal when administered during the period of organogenesis to rats at doses up to 1000 mg/kg/day (1.6 times the recommended maximum human dose calculated on a mg/m2 basis) or to mice at doses up to 1200 mg/kg (approximately equal to the recommended maximum human dose calculated on a mg/m2 basis) or to rabbits at a dose level of 100 mg/kg (0.3 times the recommended maximum human dose calculated on a mg/m2 basis).
- There are, however, no adequate and well-controlled studies of cefepime use in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Cefepime in women who are pregnant.
Labor and Delivery
- Cefepime has not been studied for use during labor and delivery. Treatment should only be given if clearly indicated.
Nursing Mothers
- Cefepime is excreted in human breast milk in very low concentrations (0.5 mcg/mL). Caution should be exercised when cefepime is administered to a nursing woman.
Pediatric Use
- The safety and effectiveness of cefepime in the treatment of uncomplicated and complicated urinary tract infections (including pyelonephritis), uncomplicated skin and skin structure infections, pneumonia, and as empiric therapy for febrile neutropenic patients have been established in the age groups 2 months up to 16 years. Use of cefepime for injection in these age groups is supported by evidence from adequate and well-controlled studies of cefepime in adults with additional pharmacokinetic and safety data from pediatric trials.
- Safety and effectiveness in pediatric patients below the age of 2 months have not been established. There are insufficient clinical data to support the use of cefepime for injection in pediatric patients under 2 months of age or for the treatment of serious infections in the pediatric population where the suspected or proven pathogen is Haemophilus influenzae type b.
- In those patients in whom meningeal seeding from a distant infection site or in whom meningitis is suspected or documented, an alternate agent with demonstrated clinical efficacy in this setting should be used.
Geriatic Use
- Of the more than 6400 adults treated with cefepime for injection in clinical studies, 35% were 65 years or older while 16% were 75 years or older. When geriatric patients received the usual recommended adult dose, clinical efficacy and safety were comparable to clinical efficacy and safety in nongeriatric adult patients.
- Serious adverse events have occurred in geriatric patients with renal insufficiency given unadjusted doses of cefepime, including life-threatening or fatal occurrences of the following: encephalopathy, myoclonus, and seizures. This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and renal function should be monitored.
Gender
There is no FDA guidance on the use of Cefepime with respect to specific gender populations.
Race
There is no FDA guidance on the use of Cefepime with respect to specific racial populations.
Renal Impairment
- Cefepime pharmacokinetics have been investigated in patients with various degrees of renal impairment (n=30). The average half-life in patients requiring hemodialysis was 13.5 (±2.7) hours and in patients requiring continuous peritoneal dialysis was 19.0 (±2.0) hours. Cefepime total body clearance decreased proportionally with creatinine clearance in patients with abnormal renal function, which serves as the basis for dosage adjustment recommendations in this group of patients.
Hepatic Impairment
The pharmacokinetics of cefepime were unaltered in patients with hepatic impairment who received a single 1 g dose (n=11).
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Cefepime in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Cefepime in patients who are immunocompromised.
Administration and Monitoring
Administration
For Intravenous Infusion
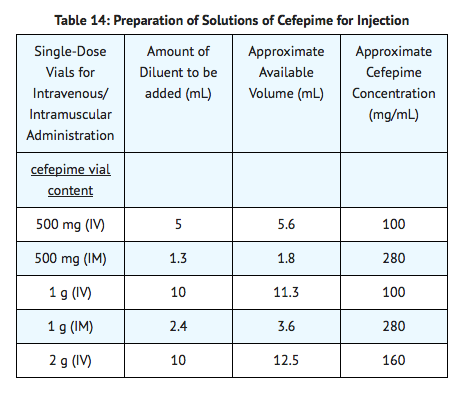
- Constitute the 500 mg, 1 g, or 2 g vial, and add an appropriate quantity of the resulting solution to an intravenous container with one of the compatible intravenous fluids listed in the compatibility and stability subsection. The resulting solution should be administered over approximately 30 minutes.
- Intermittent intravenous infusion with a Y-type administration set can be accomplished with compatible solutions. However, during infusion of a solution containing cefepime, it is desirable to discontinue the other solution.
Intramuscular Administration
- For intramuscular administration, cefepime for injection (cefepime hydrochloride) should be constituted with one of the following diluents: Sterile Water for Injection, 0.9% Sodium Chloride, 5% Dextrose Injection, 0.5% or 1.0% Lidocaine Hydrochloride, or Sterile Bacteriostatic Water for Injection with Parabens or Benzyl Alcohol.
Monitoring
There is limited information regarding Cefepime Monitoring in the drug label.
IV Compatibility
- Cefepime for injection is compatible at concentrations between 1 mg/mL and 40 mg/mL with the following intravenous infusion fluids: 0.9% Sodium Chloride Injection, 5% and 10% Dextrose Injection, M/6 Sodium Lactate Injection, 5% Dextrose and 0.9% Sodium Chloride Injection, Lactated Ringers and 5% Dextrose Injection, Normosol-R™, and Normosol-M™ in 5% Dextrose Injection. These solutions may be stored up to 24 hours at controlled room temperature 20° to 25° C (68° to 77° F) or 7 days in a refrigerator 2° to 8° C (36° to 46° F).
- Cefepime for injection admixture compatibility information is summarized in the following table:
- Solutions of cefepime for injection, like those of most beta-lactam antibiotics, should not be added to solutions of ampicillin at a concentration greater than 40 mg/mL, and should not be added to metronidazole, vancomycin, gentamicin, tobramycin, netilmicin sulfate or aminophylline because of potential interaction. However, if concurrent therapy with cefepime for injection is indicated, each of these antibiotics can be administered separately.
IM Administration
- Cefepime for injection (cefepime hydrochloride) constituted as directed is stable for 24 hours at controlled room temperature 20° to 25° C (68° to 77° F) or for 7 days in a refrigerator 2° to 8° C (36° to 46° F) with the following diluents: Sterile Water for Injection, 0.9% Sodium Chloride Injection, 5% Dextrose Injection, Sterile Bacteriostatic Water for Injection with Parabens or Benzyl Alcohol, or 0.5% or 1% Lidocaine Hydrochloride.
Overdosage
- Patients who receive an overdose should be carefully observed and given supportive treatment. In the presence of renal insufficiency, hemodialysis, not peritoneal dialysis, is recommended to aid in the removal of cefepime from the body. Accidental overdosing has occurred when large doses were given to patients with impaired renal function. Symptoms of overdose include encephalopathy (disturbance of consciousness including confusion, hallucinations, stupor, and coma), myoclonus, seizures, and neuromuscular excitability.
Pharmacology
Mechanism of Action
- Cefepime is a bactericidal agent that acts by inhibition of bacterial cell wall synthesis. Cefepime has a broad spectrum of in vitro activity that encompasses a wide range of Gram-positive and Gram-negative bacteria. Cefepime has a low affinity for chromosomally-encoded beta-lactamases. Cefepime is highly resistant to hydrolysis by most beta-lactamases and exhibits rapid penetration into gram-negative bacterial cells. Within bacterial cells, the molecular targets of cefepime are the penicillin binding proteins (PBP).
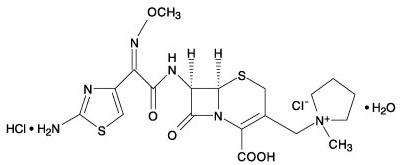
Structure
- Cefepime for injection, USP (cefepime hydrochloride, USP) is a semi-synthetic, broad spectrum, cephalosporin antibiotic for parenteral administration. The chemical name is 1-[[(6R,7R)-7-[2-(2-amino-4-thiazolyl)-glyoxylamido]-2-carboxy-8-oxo-5-thia-1-azabicyclo [4.2.0]oct-2-en-3-yl]methyl]-1-methylpyrrolidinium chloride, 72-(Z)-(O-methyloxime), monohydrochloride, monohydrate]], which corresponds to the following structural formula:
Pharmacodynamics
There is limited information regarding Cefepime Pharmacodynamics in the drug label.
Pharmacokinetics
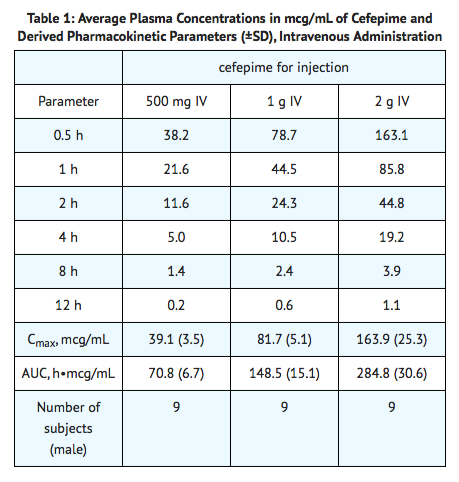
- The average plasma concentrations of cefepime observed in healthy adult male volunteers (n=9) at various times following single 30-minute infusions (IV) of cefepime 500 mg, 1 g, and 2 g are summarized in Table 1. Elimination of cefepime is principally via renal excretion with an average (±SD) half-life of 2.0 (±0.3) hours and total body clearance of 120.0 (±8.0) mL/min in healthy volunteers. Cefepime pharmacokinetics are linear over the range 250 mg to 2 g. There is no evidence of accumulation in healthy adult male volunteers (n=7) receiving clinically relevant doses for a period of 9 days.
Absorption
The average plasma concentrations of cefepime and its derived pharmacokinetic parameters after intravenous (IV) administration are portrayed in Table 1.
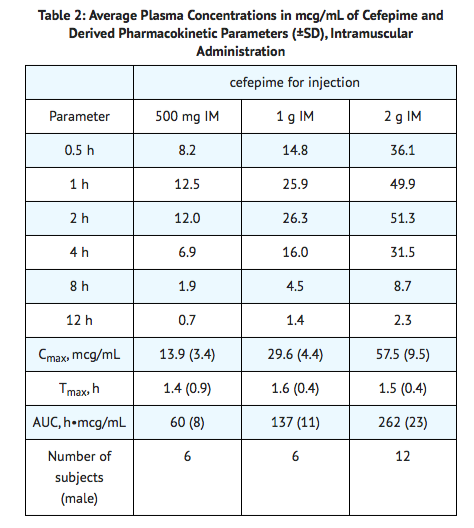
Following intramuscular (IM) administration, cefepime is completely absorbed. The average plasma concentrations of cefepime at various times following a single intramuscular injection are summarized in Table 2. The pharmacokinetics of cefepime are linear over the range of 500 mg to 2 g intramuscularly and do not vary with respect to treatment duration.
Distribution
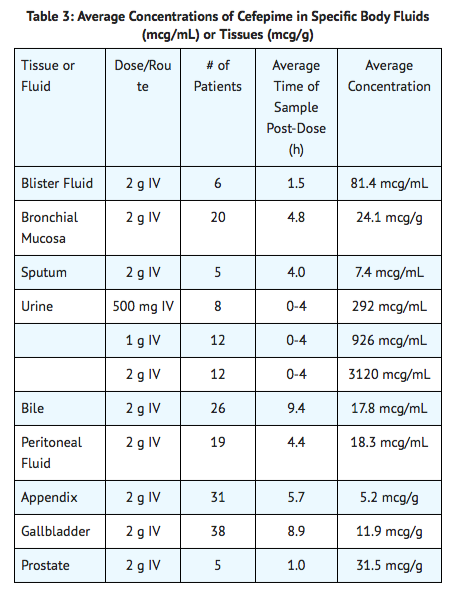
- The average steady-state volume of distribution of cefepime is 18.0 (±2.0) L. The serum protein binding of cefepime is approximately 20% and is independent of its concentration in serum. Cefepime is excreted in human milk. A nursing infant consuming approximately 1000 mL of human milk per day would receive approximately 0.5 mg of cefepime per day. Concentrations of cefepime achieved in specific tissues and body fluids are listed in Table 3.
- Data suggest that cefepime does cross the inflamed blood-brain barrier. The clinical relevance of these data is uncertain at this time.
Metabolismo and Excretion
- Cefepime is metabolized to N-methylpyrrolidine (NMP) which is rapidly converted to the N-oxide (NMP-N-oxide). Urinary recovery of unchanged cefepime accounts for approximately 85% of the administered dose. Less than 1% of the administered dose is recovered from urine as NMP, 6.8% as NMP-N-oxide, and 2.5% as an epimer of Cefepime. Because renal excretion is a significant pathway of elimination, patients with renal dysfunction and patients undergoing hemodialysis require dosage adjustment.
Nonclinical Toxicology
There is limited information regarding Cefepime Nonclinical Toxicology in the drug label.
Clinical Studies
Febrile Neutropenic Patients
- The safety and efficacy of empiric cefepime monotherapy of febrile neutropenic patients have been assessed in two multicenter, randomized trials, comparing cefepime monotherapy (at a dose of 2 g intravenously every 8 hours) to ceftazidime monotherapy (at a dose of 2 g intravenously every 8 hours). These studies comprised 317 evaluable patients. Table 8 describes the characteristics of the evaluable patient population.
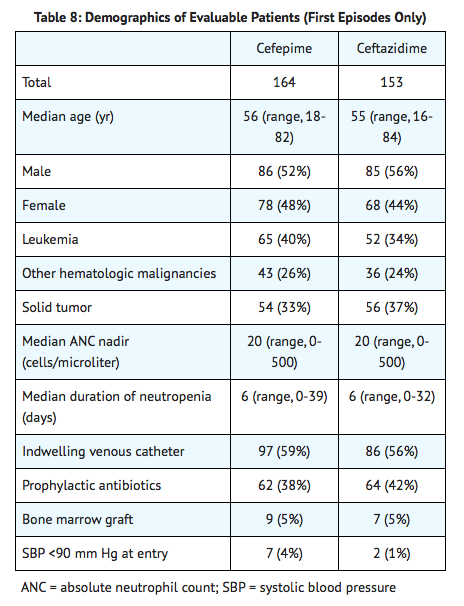
Table 9 describes the clinical response rates observed. For all outcome measures, cefepime was therapeutically equivalent to ceftazidime.
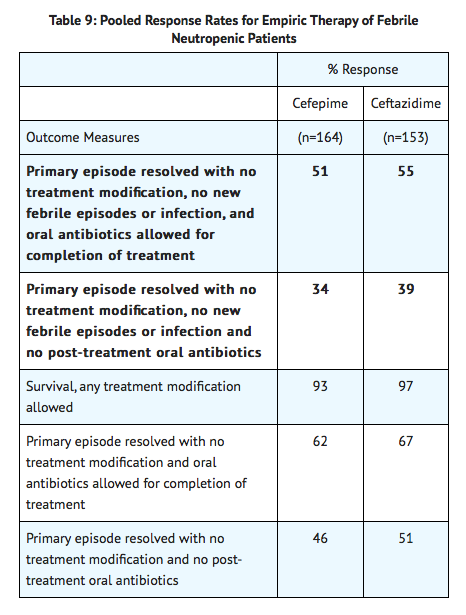
- Insufficient data exist to support the efficacy of cefepime monotherapy in patients at high risk for severe infection (including patients with a history of recent bone marrow transplantation, with hypotension at presentation, with an underlying hematologic malignancy, or with severe or prolonged neutropenia). No data are available in patients with septic shock.
Complicated Abdominal Infections
- Patients hospitalized with complicated intra-abdominal infections participated in a randomized, double-blind, multicenter trial comparing the combination of cefepime (2 g every 12 hours) plus intravenous metronidazole (500 mg every 6 hours) versus imipenem/cilastatin (500 mg every 6 hours) for a maximum duration of 14 days of therapy. The study was designed to demonstrate equivalence of the two therapies. The primary analyses were conducted on the protocol-valid population, which consisted of those with a surgically confirmed complicated infection, at least one pathogen isolated pretreatment, at least 5 days of treatment, and a 4 to 6 week follow-up assessment for cured patients. Subjects in the imipenem/cilastatin arm had higher APACHE II scores at baseline. The treatment groups were otherwise generally comparable with regard to their pretreatment characteristics. The overall clinical cure rate among the protocol-valid patients was 81% (51 cured/63 evaluable patients) in the cefepime plus metronidazole group and 66% (62/94) in the imipenem/cilastatin group. The observed differences in efficacy may have been due to a greater proportion of patients with high APACHE II scores in the imipenem/cilastatin group.
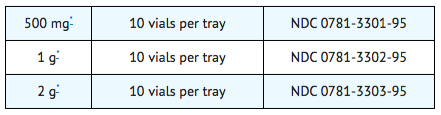
How Supplied
- Cefepime for injection, USP (cefepime hydrochloride, USP) for injection is supplied as follows:
Storage
- Store dry powder between 2° to 25°C (36° to 77°F). Protect from light.
Images
Drug Images
{{#ask: Page Name::Cefepime |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
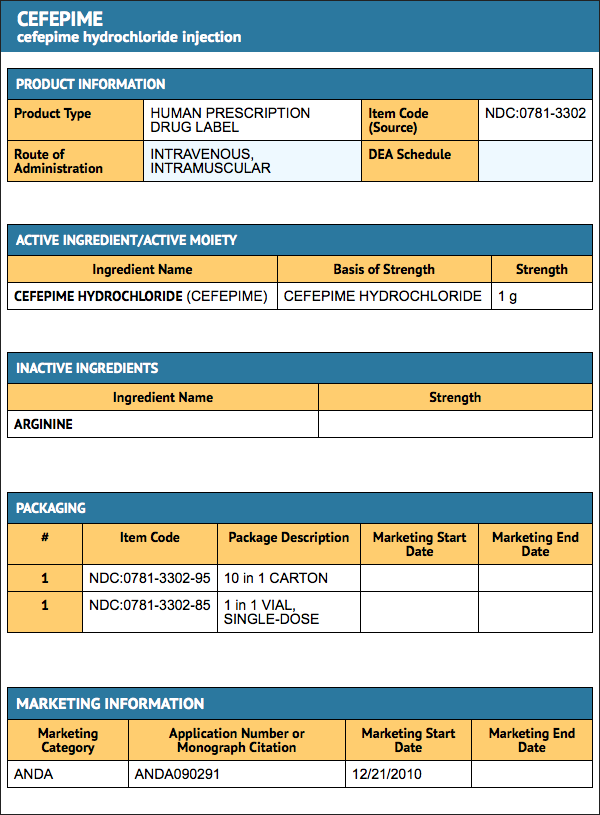
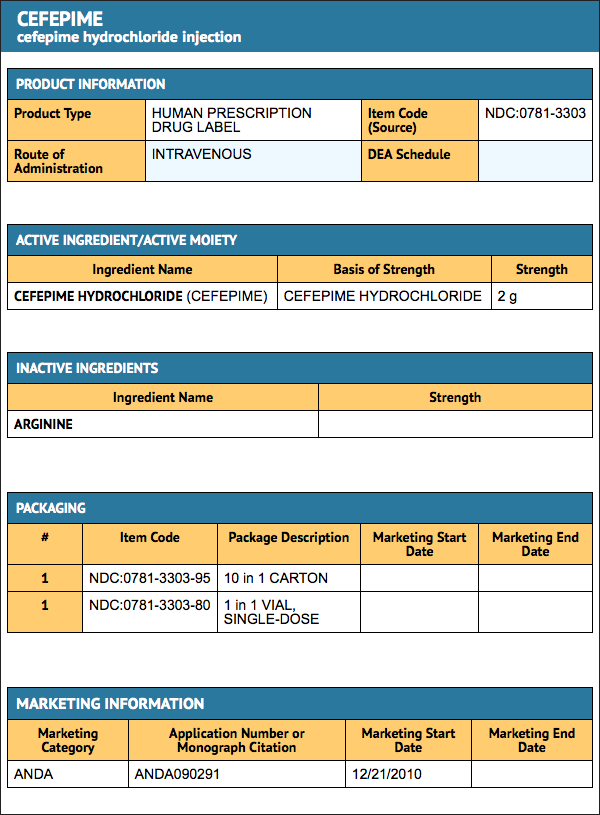
{{#ask: Label Page::Cefepime |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Cefepime Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Cefepime interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
Look-Alike Drug Names
There is limited information regarding Cefepime Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ "Guidelines for Prevention and Treatment of Opportunistic Infections in HIV-Infected Adults and Adolescents" (PDF).
- ↑ http://www.micromedexsolutions.com/micromedex2/librarian/ND_T/evidencexpert/ND_PR/evidencexpert/CS/D7ABBA/ND_AppProduct/evidencexpert/DUPLICATIONSHIELDSYNC/D074EC/ND_PG/evidencexpert/ND_B/evidencexpert/ND_P/evidencexpert/PFActionId/evidencexpert.IntermediateToDocumentLink?docId=1175&contentSetId=50. Unknown parameter
|Title=ignored (|title=suggested) (help); Missing or empty|title=(help) - ↑ Wong KM, Chan WK, Chan YH, Li CS (1999). "Cefepime-related neurotoxicity in a haemodialysis patient". Nephrol Dial Transplant. 14 (9): 2265–6. PMID 10489256.
- ↑ "Guidelines for Prevention and Treatment of Opportunistic Infections in HIV-Infected Adults and Adolescents" (PDF).
- ↑ http://www.micromedexsolutions.com/micromedex2/librarian/ND_T/evidencexpert/ND_PR/evidencexpert/CS/D7ABBA/ND_AppProduct/evidencexpert/DUPLICATIONSHIELDSYNC/D074EC/ND_PG/evidencexpert/ND_B/evidencexpert/ND_P/evidencexpert/PFActionId/evidencexpert.IntermediateToDocumentLink?docId=1175&contentSetId=50. Unknown parameter
|Title=ignored (|title=suggested) (help); Missing or empty|title=(help) - ↑ Sáez-Llorens X, O'Ryan M (2001). "Cefepime in the empiric treatment of meningitis in children". Pediatr Infect Dis J. 20 (3): 356–61. PMID 11303850.
- ↑ Tunkel AR, Hartman BJ, Kaplan SL, Kaufman BA, Roos KL, Scheld WM; et al. (2004). "Practice guidelines for the management of bacterial meningitis". Clin Infect Dis. 39 (9): 1267–84. doi:10.1086/425368. PMID 15494903.
- ↑ Jauregui L, Matzke D, Scott M, Minns P, Hageage G (1993). "Cefepime as treatment for osteomyelitis and other severe bacterial infections". J Antimicrob Chemother. 32 Suppl B: 141–9. PMID 8150758.
{{#subobject:
|Label Page=Cefepime |Label Name=Cefepime 500mg.png
}}
{{#subobject:
|Label Page=Cefepime |Label Name=Cefepime 1g.png
}}
{{#subobject:
|Label Page=Cefepime |Label Name=Efepime2g.png
}}