Allobarbital: Difference between revisions
m (Robot: Automated text replacement (-{{WikiDoc Cardiology Network Infobox}} +, -<references /> +{{reflist|2}}, -{{reflist}} +{{reflist|2}})) |
No edit summary |
||
| Line 1: | Line 1: | ||
{{ | {{Drugbox | ||
| IUPAC_name | | verifiedrevid = 477317940 | ||
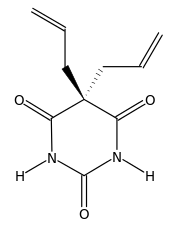
| IUPAC_name = 5,5-diprop-2-enyl-1,3-diazinane-2,4,6-trione | |||
| image = Allobarbital.png | |||
| width = 120 | |||
| image2 = Allobarbital.gif | |||
| width2 = 160 | |||
<!--Clinical data--> | |||
| tradename = | |||
| pregnancy_AU = <!-- A / B1 / B2 / B3 / C / D / X --> | |||
| pregnancy_US = <!-- A / B / C / D / X --> | |||
| pregnancy_category = | |||
| legal_AU = <!-- Unscheduled / S2 / S3 / S4 / S5 / S6 / S7 / S8 / S9 --> | |||
| legal_CA = Schedule IV | |||
| legal_UK = <!-- GSL / P / POM / CD / Class A, B, C --> | |||
| legal_US = Schedule IV | |||
| legal_status = | |||
| routes_of_administration = | |||
<!--Pharmacokinetic data--> | |||
| bioavailability = | |||
| protein_bound = | |||
| metabolism = | |||
| elimination_half-life = | |||
| excretion = | |||
<!--Identifiers--> | |||
| CASNo_Ref = {{cascite|correct|CAS}} | |||
| CAS_number_Ref = {{cascite|correct|??}} | |||
| CAS_number = 52-43-7 | |||
| ATC_prefix = N05 | |||
| ATC_suffix = CA21 | |||
| PubChem = 5842 | |||
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} | |||
| DrugBank = | |||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| ChemSpiderID = 5635 | |||
| UNII_Ref = {{fdacite|correct|FDA}} | |||
| UNII = 8NT43GG2HA | |||
| KEGG_Ref = {{keggcite|correct|kegg}} | |||
| KEGG = D02817 | |||
| ChEMBL_Ref = {{ebicite|correct|EBI}} | |||
| ChEMBL = 267719 | |||
<!--Chemical data--> | |||
| C=10 | H=12 | N=2 | O=3 | |||
| molecular_weight = 208.214 g/mol | |||
| smiles = O=C1NC(=O)NC(=O)C1(C\C=C)C\C=C | |||
| InChI = 1/C10H12N2O3/c1-3-5-10(6-4-2)7(13)11-9(15)12-8(10)14/h3-4H,1-2,5-6H2,(H2,11,12,13,14,15) | |||
| InChIKey = FDQGNLOWMMVRQL-UHFFFAOYAS | |||
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChI = 1S/C10H12N2O3/c1-3-5-10(6-4-2)7(13)11-9(15)12-8(10)14/h3-4H,1-2,5-6H2,(H2,11,12,13,14,15) | |||
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChIKey = FDQGNLOWMMVRQL-UHFFFAOYSA-N | |||
| synonyms = 5,5-Diallylbarbituric acid, Allobarbital | | synonyms = 5,5-Diallylbarbituric acid, Allobarbital | ||
}} | }} | ||
'''Allobarbital''' is a [[barbiturate]] derivative invented in 1912 by Ernst Preiswerk and Ernst Grether working for [[Ciba Specialty Chemicals|CIBA]]. It was used primarily as an [[anticonvulsant]] <ref> Chocholova L | '''Allobarbital''', also known as '''allobarbitone''' and branded as '''Cibalgine''' or '''Dial-Ciba''' (in combination with [[ethyl carbamate]]), is a [[barbiturate]] derivative invented in 1912 by Ernst Preiswerk and Ernst Grether working for [[Ciba Specialty Chemicals|CIBA]]. It was used primarily as an [[anticonvulsant]]<ref>{{cite journal|last1=Chocholova L. |last2=Radil-Weiss |first2=T. |title=Effect of allobarbital on focal epilepsy in rats |journal=Physiologia Bohemoslovaca |year=1971 |volume=20 |issue=4 |pages=325–34 |pmid=4335127}}</ref> although it has now largely been replaced by newer drugs with improved safety profiles. Other uses for allobarbital included as an adjutant to boost the activity of [[analgesic]] drugs, and use in the treatment of [[insomnia]] and [[anxiety]]. | ||
Allobarbital was never particularly widely used compared to better known barbiturates such as [[phenobarbital]] and [[secobarbital]], although it saw more use in some European countries such as [[Bulgaria]] and [[Slovakia]],<ref>{{cite journal|last1=Getova |first1=D. |last2=Georgiev |first2=V. |title=GABA-ergic mechanisms in the anticonvulsive activity of newly synthesized barbiturates. I. Effects of barbiturates on the convulsive action of GABA-antagonists |journal=Acta Physiologica et Pharmacologica Bulgarica |year=1987 |volume=13 |issue=3 |pages=43–50 |pmid=3439474}}</ref> and is still used in for example Poland, but only as compound.<ref>{{cite web|title=APTECZKA BABUNI - KROPLE ŻOŁĄDKOWE KROPLE 20 G |trans_title=GRANDMA'S FIRST AID KIT - DROPS - STOMACH DROPS 20 G |website=Domzdrowia.pl |language=Polish |url=http://www.domzdrowia.pl/29439,apteczka-babuni-krople-zoladkowe-krople-20-g.html |archiveurl=https://web.archive.org/web/20071031195035/http://www.domzdrowia.pl/29439,apteczka-babuni-krople-zoladkowe-krople-20-g.html |archivedate=October 31, 2007 |accessdate=March 10, 2013 |deadurl=no}}</ref> | |||
== References == | ==References== | ||
{{reflist|2}} | {{reflist|2}} | ||
{{Hypnotics and sedatives}} | {{Hypnotics and sedatives}} | ||
{{GABAAR PAMs}} | |||
[[Category:Barbiturates]] | [[Category:Barbiturates]] | ||
[[Category: | [[Category:GABAA receptor positive allosteric modulators]] | ||
[[Category: | [[Category:Drug]] | ||
Revision as of 13:50, 9 April 2015
 | |
 | |
| Clinical data | |
|---|---|
| Synonyms | 5,5-Diallylbarbituric acid, Allobarbital |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C10H12N2O3 |
| Molar mass | 208.214 g/mol |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Allobarbital, also known as allobarbitone and branded as Cibalgine or Dial-Ciba (in combination with ethyl carbamate), is a barbiturate derivative invented in 1912 by Ernst Preiswerk and Ernst Grether working for CIBA. It was used primarily as an anticonvulsant[1] although it has now largely been replaced by newer drugs with improved safety profiles. Other uses for allobarbital included as an adjutant to boost the activity of analgesic drugs, and use in the treatment of insomnia and anxiety.
Allobarbital was never particularly widely used compared to better known barbiturates such as phenobarbital and secobarbital, although it saw more use in some European countries such as Bulgaria and Slovakia,[2] and is still used in for example Poland, but only as compound.[3]
References
- ↑ Chocholova L.; Radil-Weiss, T. (1971). "Effect of allobarbital on focal epilepsy in rats". Physiologia Bohemoslovaca. 20 (4): 325–34. PMID 4335127.
- ↑ Getova, D.; Georgiev, V. (1987). "GABA-ergic mechanisms in the anticonvulsive activity of newly synthesized barbiturates. I. Effects of barbiturates on the convulsive action of GABA-antagonists". Acta Physiologica et Pharmacologica Bulgarica. 13 (3): 43–50. PMID 3439474.
- ↑ "APTECZKA BABUNI - KROPLE ŻOŁĄDKOWE KROPLE 20 G". Domzdrowia.pl (in Polish). Archived from the original on October 31, 2007. Retrieved March 10, 2013. Unknown parameter
|trans_title=ignored (help)
- Pages with script errors
- Pages with citations using unsupported parameters
- CS1 maint: Unrecognized language
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Infobox drug
- Chemical pages without DrugBank identifier
- Barbiturates
- GABAA receptor positive allosteric modulators
- Drug