Deanol
| |
| |
| Names | |
|---|---|
| IUPAC name
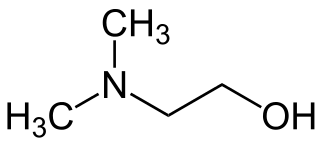
2-(Dimethylamino)ethanol
| |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | Lua error in Module:Wikidata at line 879: attempt to index field 'wikibase' (a nil value). Lua error in Module:Wikidata at line 879: attempt to index field 'wikibase' (a nil value). |
| KEGG | |
| MeSH | Deanol |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C4H11NO | |
| Molar mass | 89.14 g·mol−1 |
| Hazards | |
| Related compounds | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
|
WikiDoc Resources for Deanol |
|
Articles |
|---|
|
Media |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Deanol at Clinical Trials.gov Clinical Trials on Deanol at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Deanol
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Directions to Hospitals Treating Deanol Risk calculators and risk factors for Deanol
|
|
Healthcare Provider Resources |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Dimethylaminoethanol, also known as dimethylethanolamine (DMAE and DMEA respectively), is a primary alcohol. This compound also goes by the names of N,N-dimethyl-2-aminoethanol, beta-dimethylaminoethyl alcohol, beta-hydroxyethyldimethylamine and Deanol. It is a transparent, pale-yellow liquid.
Biochemical significance
Dimethylaminoethanol is related to choline and may be a biochemical precursor to the neurotransmitter acetylcholine, although this conclusion has been disputed based on a 1977 rat experiment.[2] It is commonly believed that dimethylaminoethanol is methylated to produce choline in the brain, but this has been shown not to be the case (in a rat experiment).[2] It is known that dimethylaminoethanol is processed by the liver into choline; however, in a rat experiment the choline molecule is charged and cannot pass the blood–brain barrier.[2] In the brain, DMAE is instead bound to phospholipids in place of choline to produce phosphatidyl-dimethylaminoethanol. This is then incorporated into nerve membranes, increasing fluidity and permeability, and acting as an antioxidant.[3]
Uses
Industrial uses
Dimethylaminoethanol is used as a curing agent for polyurethanes and epoxy resins. It is also used in mass quantities for water treatment, and to some extent in the coatings industry. It is used in the synthesis of dyestuffs, textile auxiliaries, pharmaceuticals, emulsifiers, and corrosion inhibitors. It is also an additive to paint removers, boiler water and amino resins. It forms a number of salts with melting points below room temperature (ionic liquids) such as N,N-dimethylethanolammonium acetate and N,N-dimethylethanolammonium octanoate, which have been used as alternatives to conventional solvents.[4]
2-Dimethylaminoethyl chloride hydrochloride is an intermediate made from dimethylaminoethanol that is widely used for the manufacture of pharmaceuticals.[5]
Biomedical research
Short-term studies have shown an increase in vigilance and alertness with a positive influence on mood following administration of DMAE, vitamins, and minerals in individuals suffering from borderline emotional disturbance.[6] Research for ADHD has been promising, though inconclusive.[7] A study showed dimethylaminoethanol to decrease the average life span of aged quail.[8] Three other studies showed an increase in lifespan of mice [9]
The bitartrate salt of DMAE, i.e. 2-dimethylaminoethanol (+)-bitartrate, is sold as a dietary supplement.[10] It is a white powder providing 37% DMAE.[11]
See also
References
- ↑ Littel, RJ, Bos, M, Knoop, GJ (1990). "Dissociation constants of some alkanolamines at 293, 303, 318, and 333 K". J. Chem. Eng. Data. 35: 276–277. doi:10.1021/je00061a014.
- ↑ 2.0 2.1 2.2 Zahniser NR, Chou D, Hanin I (March 1977). "Is 2-dimethylaminoethanol (deanol) indeed a precursor of brain acetylcholine? A gas chromatographic evaluation". J. Pharmacol. Exp. Ther. 200 (3): 545–59. PMID 850128.
- ↑ Zs-Nagy, I (1989). "On the role of intracellular physicochemistry in quantitative gene expression during aging and the effect of centrophenoxine. A review". Archives of gerontology and geriatrics. 9 (3): 215–229.
- ↑ Sanders MW, Wright L, Tate L, Fairless G, Crowhurst L, Bruce NC, Walker AJ, Hembury GA, Shimizu S (September 2009). "Unexpected preferential dehydration of artemisinin in ionic liquids". J. Phys. Chem. A. 113 (38): 10143–45. doi:10.1021/jp906436e. PMID 19722599.
- ↑ Ashford's Dictionary of Industrial Chemicals, 3rd edition, 2011, ISBN 978-0-9522674-3-0, page 3294
- ↑ Dimpfel W, Wedekind W, Keplinger I (May 2003). "Efficacy of dimethylaminoethanol (DMAE) containing vitamin-mineral drug combination on EEG patterns in the presence of different emotional states". Eur. J. Med. Res. 8 (5): 183–91. PMID 12844472.
- ↑ Knobel M (1974). "Approach to a combined pharmacologic therapy of childhood hyperkinesis". Behav Neuropsychiatry. 6 (1–12): 87–90. PMID 4619768.
- ↑ Cherkin A, Exkardt MJ (January 1977). "Effects of dimethylaminoethanol upon life-span and behavior of aged Japanese quail". J Gerontol. 32 (1): 38–45. doi:10.1093/geronj/32.1.38. PMID 830732.
- ↑ Cherkin A, Exkardt MJ (January 1977). "Effects of dimethylaminoethanol upon life-span and behavior of aged Japanese quail". J Gerontol. 32 (1): 38–45. doi:10.1093/geronj/32.1.38. PMID 830732.
- ↑ "Dimethylethanolamine (DMAE) [108-01-0] and Selected Salts and Esters" (PDF). National Institute of Environmental Health Sciences. November 2002. Retrieved 8 October 2014.
- ↑ Sigma Aldrich: Safety Data Sheet: 2-Dimethylaminoethanol (+)-bitartrate
Studies
- Earliest research
- Pfeiffer CC, Jenney EH, Gallagher W; et al. (September 1957). "Stimulant effect of 2-dimethylaminoethanol; possible precursor of brain acetylcholine". Science. 126 (3274): 610–1. doi:10.1126/science.126.3274.610. PMID 13467254.
- As a treatment for tardive dyskinesia
- Haug BA, Holzgraefe M (1991). "Orofacial and respiratory tardive dyskinesia: potential side effects of 2-dimethylaminoethanol (deanol)?". Eur. Neurol. 31 (6): 423–5. doi:10.1159/000116708. PMID 1756771.
- As an unsuccessful treatment for Alzheimer's disease
- Fisman M, Mersky H, Helmes E (July 1981). "Double-blind trial of 2-dimethylaminoethanol in Alzheimer's disease". Am J Psychiatry. 138 (7): 970–2. doi:10.1176/ajp.138.7.970. PMID 7020434.
- Pages with script errors
- CS1 maint: Multiple names: authors list
- Articles without InChI source
- Chemical articles with unknown parameter in Chembox
- Articles with changed EBI identifier
- Articles with changed ChemSpider identifier
- ECHA InfoCard ID from Wikidata
- Articles with changed KEGG identifier
- Articles with changed InChI identifier
- Chembox having DSD data
- Chembox having GHS data
- Articles containing unverified chemical infoboxes
- Chembox image size set
- CS1 maint: Explicit use of et al.
- Amines
- Alcohols
- Nootropics
- Corrosion inhibitors
- Drug