Vitamin B12
 | |
 | |
| Clinical data | |
|---|---|
| Routes of administration | oral, iv |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | readily absorbed in lower half ileum |
| Protein binding | Very high to specific transcobalamins plasma proteins Binding of hydroxocobalamin is slightly higher than cyanocobalamin. |
| Metabolism | hepatic |
| Elimination half-life | Approximately 6 days (400 days in the liver) |
| Excretion | renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C63H88CoN14O14P |
| Molar mass | 1355.37 g/mol |
|
WikiDoc Resources for Vitamin B12 |
|
Articles |
|---|
|
Most recent articles on Vitamin B12 Most cited articles on Vitamin B12 |
|
Media |
|
Powerpoint slides on Vitamin B12 |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Vitamin B12 at Clinical Trials.gov Clinical Trials on Vitamin B12 at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Vitamin B12
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Vitamin B12 Discussion groups on Vitamin B12 Patient Handouts on Vitamin B12 Directions to Hospitals Treating Vitamin B12 Risk calculators and risk factors for Vitamin B12
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Vitamin B12 |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [2]
Associate Editor-In-Chief: Cafer Zorkun, M.D., Ph.D. [3]
Vitamin B-12 is a vitamin which is important for the normal functioning of the brain and nervous system, and for the formation of blood. It is normally involved in the metabolism of every cell of the body, especially affecting DNA synthesis and regulation, but also fatty acid synthesis and energy production.
Vitamin B-12 is the name for a class of chemically-related compounds, all of which have vitamin activity. It is structurally the most complicated vitamin. Biosynthesis of the basic structure of the vitamin can only be accomplished by bacteria, but conversion between different forms of the vitamin can be accomplished in the human body. A common form of the vitamin, cyanocobalamin, does not occur in nature, but is used as a supplement and food additive, due to its stability. It is converted to other forms of the vitamin which are actually used in chemical reactions in the body.
Historically, vitamin B-12 was discovered from its relationship to the disease pernicious anemia, which was eventually discovered to result from an effective lack of this vitamin due to problems with the mechanisms in the body which normally absorb it. Many other more subtle kinds of biochemical B-12 deficiencies, and biochemical effects from them, have since been elucidated.
A B12 deficiency causes:
- distinctive dyserythropoietic abnormalities in the bone marrow
- megaloblastic erythropoiesis which is associated with abnormally large red cells in the peripheral cells, for example macrocytosis. A severe deficiency in B12 can cause pernicious anemia and funicular myelosis.
Terminology
The name vitamin B-12, known also as vitamin BTemplate:Ssub (or commonly BTemplate:Ssub or B-12 for short) generally refers to all forms of the vitamin. Some medical practitioners have suggested that its use be split into two different categories, however.
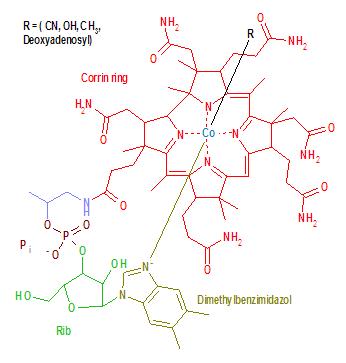
- In a broad sense B-12 still refers to a group of cobalt-containing vitamer compounds known as cobalamins: these include cyanocobalamin (an artifact formed as a result of the use of cyanide in the purification procedures), hydroxocobalamin (another medicinal form), and finally, the two naturally occurring cofactor forms of B-12: 5-deoxyadenosylcobalamin (adenosylcobalamin—AdoB-12), the cofactor of Methylmalonyl Coenzyme A mutase (MUT), and methylcobalamin (MeB-12), the cofactor of 5-methyltetrahydrofolate-homocysteine methyltransferase (MTR).
- The term B-12 may be properly used to refer to cyanocobalamin, the principal B-12 form used for foods and in nutritional supplements. This ordinarily creates no problem, except perhaps in rare cases of eye nerve damage, where the body is only marginally able to use this form due to high cyanide levels in the blood due to cigarette smoking, and thus requires cessation of smoking, or else B-12 given in another form, for the optic symptoms to abate.[1] However, tobacco amblyopia is a rare enough condition that debate continues about whether or not it represents a peculiar B-12 deficiency which is resistant to treatment with cyanocobalamin.
Finally, so-called Pseudo-B-12 refers to B-12-like substances which are found in certain organisms, including spirulina (a cyanobacterium) and some algae. These substances are active in tests of B-12 activity by highly sensitive antibody-binding serum assay tests, which measure levels of B-12 and B-12 like compounds in blood. However, these substances do not have B-12 biological activity for humans, a fact which may pose a theoretical danger to vegans and others on limited diets who do not ingest B-12 producing bacteria, but who nevertheless may show normal "B-12" levels in the standard immunoassay which has become the normal medical method for testing for B-12 deficiency.[2]
Structure
Vitamin B-12 is a collection of cobalt and corrin ring molecules which are defined by their particular vitamin function in the body. All of the substrate cobalt-corrin molecules from which B-12 is made must be synthesized by bacteria. However, after this synthesis is complete, the body has a limited power to convert any form of B-12 to another, by means of enzymatically removing certain prosthetic chemical groups from the cobalt atom.
Cyanocobalamin is one such compound that is a vitamin in this B complex, because it can be metabolized in the body to an active co-enzyme form. However, the cyanocobalamin form of B-12 does not occur in nature normally, but is a byproduct of the fact that other forms of B-12 are avid binders of cyanide (-CN) which they pick up in the process of activated charcoal purification of the vitamin after it is made by bacteria in the commercial process. Since the cyanocobalamin form of B-12 is deeply red colored, easy to crystallize, and is not sensitive to air-oxidation, it is typically used as a form of B-12 for food additives and in many common multivitamins. However, this form is not perfectly synonymous with B-12, inasmuch as a number of substances (vitamers) have B-12 vitamin activity and can properly be labeled vitamin B-12, and cyanocobalamin is but one of them. (Thus, all cyanocobalamin is vitamin B-12, but not all vitamin B-12 is cyanocobalamin).[3]
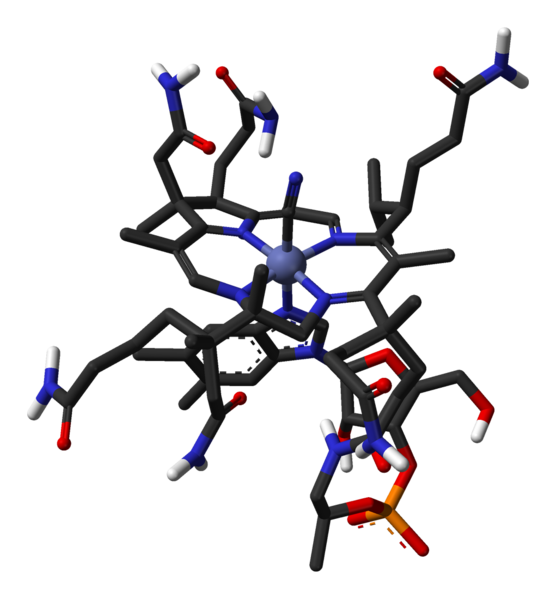
B-12 is the most chemically complex of all the vitamins. The structure of B-12 is based on a corrin ring, which is similar to the porphyrin ring found in heme, chlorophyll, and cytochrome. The central metal ion is Co (cobalt). Four of the six coordination sites are provided by the corrin ring, and a fifth by a dimethylbenzimidazole group. The sixth coordination site, the center of reactivity, is variable, being a cyano group (-CN), a hydroxyl group (-OH), a methyl group (-CH3) or a 5'-deoxyadenosyl group (here the C5' atom of the deoxyribose forms the covalent bond with Co), respectively, to yield the four B-12 forms mentioned above. The covalent C-Co bond is one of first examples of carbon-metal bonds in biology. The hydrogenases and, by necessity, enzymes associated with cobalt utilization, involve metal-carbon bonds.[4]
Synthesis
Vitamin B-12 cannot be made by plants or animals[5] as only bacteria have the enzymes required for its synthesis. The total synthesis of B-12 was reported by Robert Burns Woodward[6][7] and Albert Eschenmoser,[8][9] and remains one of the classic feats of organic synthesis.
Species from the following genera are known to synthesize B-12: Aerobacter, Agrobacterium, Alcaligenes, Azotobacter, Bacillus, Clostridium, Corynebacterium, Flavobacterium, Micromonospora, Mycobacterium, Nocardia, Propionibacterium, Protaminobacter, Proteus, Pseudomonas, Rhizobium, Salmonella, Serratia, Streptomyces, Streptococcus and Xanthomonas. Industrial production of B-12 is through fermentation of selected microorganisms.[10] The species most often used, Pseudomonas denitrificans and Propionibacterium shermanii, are frequently genetically engineered and grown under special conditions to enhance yield.
| Reference Range | |
| Vitamin B12 deficiency | <150pg/ml |
| Adequate cobalamin supply | >250 pg/ml |
| Elevated concentration | >1000 pg/ml |
Functions
Vitamin B-12 is normally involved in the metabolism of every cell of the body, especially affecting the DNA synthesis and regulation but also fatty acid synthesis and energy production. However, many (though not all) of the effects of functions of B-12 can be replaced by sufficient quantities of folic acid (another B vitamin), since B-12 is used to regenerate folate in the body. Most "B-12 deficient symptoms" are actually folate deficient symptoms, since they include all the effects of pernicious anemia and megaloblastosis, which are due to poor synthesis of DNA when the body does not have a proper supply of folic acid for the production of thymine. When sufficient folic acid is available, all known B-12 related deficiency syndromes normalize, save those narrowly connected with the B-12 dependent enzymes Methylmalonyl Coenzyme A mutase (MUT), and 5-methyltetrahydrofolate-homocysteine methyltransferase (MTR), also known as methionine synthase; and the buildup of their respective substrates (methylmalonic acid, MMA) and homocysteine.
Coenzyme B-12's reactive C-Co bond participates in two types of enzyme-catalyzed reactions.[11]
- Rearrangements in which a hydrogen atom is directly transferred between two adjacent atoms with concomitant exchange of the second substituent, X, which may be a carbon atom with substituents, an oxygen atom of an alcohol, or an amine.
- Methyl (-CH3) group transfers between two molecules.
In humans, only two corresponding coenzyme B-12-dependent enzymes are known:
- Methylmalonyl Coenzyme A mutase (MUT) which uses the AdoB-12 form and reaction type 1 to catalyze a carbon skeleton rearrangement (the X group is -COSCoA). MUT's reaction converts MMl-CoA to Su-CoA, an important step in the extraction of energy from proteins and fats (for more see MUT's reaction mechanism). This functionality is lost in vitamin B-12 deficiency, and can be measured clinically as an increased methylmalonic acid (MMA) level. Unfortunately, an elevated MMA, though sensitive to B-12 deficiency, is probably overly sensitive, and not all who have it actually have B-12 deficiency. For example, MMA is elevated in 90-98% of patients with B-12 deficiency; however 25-20% of patients over the age of 70 have elevated levels of MMA, yet 25-33% of them do not have B-12 deficiency. For this reason, MMA is not routinely recommended in the elderly.[12] The "gold standard" test for B-12 deficiency continues to be low blood levels of the vitamin. The MUT function cannot be affected by folate supplementation, and which is necessary for myelin synthesis (see mechanism below) and certain other functions of the central nervous system. Other functions of B-12 related to DNA synthesis related to MTR dysfunction (see below) can often be corrected with supplementation with the vitamin folic acid, but not the elevated levels of homocysteine, which is normally converted to methionine by MTR.
- 5-methyltetrahydrofolate-homocysteine methyltransferase (MTR), also known as methionine synthase. This is a methyl transfer enzyme, which uses the MeB-12 and reaction type 2 to catalyze the conversion of the amino acid Hcy back into Met (for more see MTR's reaction mechanism).[13] This functionality is lost in vitamin B-12 deficiency, and can be measured clinically as an increased homocysteine level in vitro. Increased homocysteine can also be caused by a folic acid deficiency, since B-12 helps to regenerate the tetrahydrofolate (THF) active form of folic acid. Without B-12, folate is trapped as 5-methyl-folate, from which THF cannot be recovered unless a MTR process reacts the 5-methyl-folate with homocysteine to produce methionine and THF, thus decreasing the need for fresh sources of THF from the diet. THF may be produced in the conversion of homocysteine to methionine, or may be obtained in the diet. It is converted by a non-B-12-dependent process to 5,10-methylene-THF, which is involved in the synthesis of thymine. Reduced availability of 5,10-methylene-THF results in problems with DNA synthesis, and ultimately in ineffective production cells with rapid turnover, in particular blood cells, and also intestinal wall cells which are responsible for absorption. The failure of blood cell production results in the once-dreaded and fatal disease, pernicious anemia. All of the DNA synthetic effects, including the megaloblastic anemia of pernicious anemia, resolve if sufficient folate is present (since levels of 5,10-methylene-THF still remain adequate with enough dietary folate). Thus the best known function of B-12 (that which is indirectly involved with DNA synthesis and restoration of cell-division and anemia) is actually a facultative function which is mediated by B-12 conservation of active folate which can be used for DNA production.[14]
If folate is present in quantity, then of the two absolutely B-12 dependent reactions, the MUT reaction shows the most direct and characteristic secondary effects, focusing on the nervous system. Since the late 1990s folic acid has begun to be added to fortify flour in many countries, so that folate deficiency is now more rare. At the same time, since DNA synthetic-sensitive tests for anemia and erythrocyte size are routinely done in even simple medical test clinics (so that these folate mediated-biochemical effects are more often directly detected), the MTR dependent effects of B-12 deficiency are becoming apparent not as anemia (as they were classically), but now mainly as an elevation of homocysteine in the blood and urine (homocysteinuria). This condition may result in long term damage to arteries and in clotting (stroke and heart attack), but is difficult to separate from other processes associated with atherosclerosis and aging.
The B-12 dependent MTR reactions may have neurological effects through an indirect mechanism. Adequate methionine (which must otherwise be obtained in the diet) is needed to make S-adenosyl-methionine, which is in turn necessary for methylation of myelin sheath phospholipids. In addition, SAMe is involved in the manufacture of certain neurotransmitters, catecholamines and in brain metabolism. These neurotransmitters are important for maintaining mood, possibly explaining why depression is associated with B-12 deficiency. Methylation of the myelin sheath phospholipids may also depend on adequate folate, which in turn is dependent on MTR recycling, unless ingested in relatively high amounts.
The specific myelin damage resulting from from B-12 deficiency has also been connected to B-12 reactions related to MUT, which is needed to convert methylmalonyl coenzyme A into succinyl coenzyme A. Failure of this second reaction to occur results in elevated levels of methylmalonic acid (MMA), a myelin destabilizer. Excessive MAA will prevent normal fatty acid synthesis, or it will be incorporated into fatty acid itself rather than normal malonic acid. If this abnormal fatty acid subsequently is incorporated into myelin, the resulting myelin will be too fragile, and demyelination will occur. Although the precise methanism(s) are not known with certainty, the result is subacute combined degeneration of central nervous system and spinal cord. [15] Whatever the cause, it is known that B-12 deficiency causes neuropathies, even if folic acid is present in good supply, and therefore anemia is not present.
Human absorption and distribution
The human physiology of vitamin B-12 is complex, and therefore is prone to mishaps leading to vitamin B-12 deficiency. The vitamin as it occurs in foods enters the digestive tract bound to proteins, known as salivary R-binders. Stomach proteolysis of these proteins requires an acid pH, and also requires proper pancreatic release of proteolytic enzymes. (Even small amounts of B-12 taken in supplements bypasses these steps and thus any need for gastric acid, which may be blocked by antacid drugs).
The free B-12 then attaches to gastric intrinsic factor, which is generated by the gastric parietal cells. If this step fails due to gastric parietal cell atrophy (the problem in pernicious anemia), sufficient B-12 is not absorbed later on, unless administered orally in relatively massive doses (500 to 1000 mcg/day).
The conjugated vitamin B-12-intrinsic factor complex (IF/B-12) is then normally absorbed by the terminal ileum of the small bowel. Absorption of food vitamin B-12 therefore requires an intact and functioning stomach, exocrine pancreas, intrinsic factor, and small bowel. Problems with any one of these organs makes a vitamin B-12 deficiency possible.
Once the IF/B-12 complex is recognized by specialized ileal receptors, it is transported into the portal circulation. The vitamin is then transferred to transcobalamin II (TC-II/B-12), which serves as the plasma transporter of the vitamin. Genetic deficiencies of this protein are known, also leading to functional B-12 deficiency.
For the vitamin to serve inside cells, the TC-II/B-12 complex must bind to a cell receptor, and be endocytosed. The transcobalamin-II is degraded within a lysozyme, and the B-12 is finally released into the cytoplasm, where it may be transformed into the proper coenzyme, by certain cellular enzymes (see above).
Hereditary defects in production of the transcobalamins and their receptors may produce functional deficiencies in B-12 and infantile megaloblastic anemia, and abnormal B-12 related biochemistry, even in some cases with normal blood B-12 levels.[16]
The total amount of vitamin B-12 stored in body is about 2,000-5,000 mcg in adults. Around 80% of this is stored in the liver[4]. 0.1 % of this is lost per day by secretions into the gut as not all these secretions are reabsorbed. How fast B-12 levels change depends on the balance between how much B-12 is obtained from the diet, how much is secreted and how much is absorbed. B-12 deficiency may arise in a year if initial stores are low and genetic factors unfavourable or may not appear for decades. In infants, B-12 deficiency can appear much more quickly[5].
History of B-12 as a treatment for pernicious anemia
B-12 deficiency is the cause of pernicious anemia, a usually-fatal disease of unknown etiology when it was first described in medicine. The cure was discovered by accident. George Whipple had been inducing anemia in dogs by bleeding them, and then conducting experiments in which he fed them various foods to observe which diets allowed them fastest recovery from the anemia produced. In the process, he discovered that ingesting large amounts of liver seemed to most-rapidly cure the anemia of blood loss, and hypothesized that therefore liver ingestion be tried for pernicious anemia, an anemic disease of the time with no known cause or cure. He tried this and reported some signs of success in 1920. After a series of careful clinical studies George Minot and William Murphy set out to partly isolate the substance in liver which cured anemia in dogs, and found that it was iron. They found further that the partly isolated water-soluble liver-substance which cured pernicious anemia in humans, was something else entirely different -- and which had no effect at all on canines under the conditions used. The specific factor treatment for pernicious anemia, found in liver juice, had been found by this coincidence. These experiments were reported by Minot and Murphy in 1926, marking the date of the first real progress with this disease, though for several years, patients were still required to eat large amounts of raw liver or to drink considerable amounts of liver juice.
In 1928, the chemist Edwin Cohn prepared a liver extract that was 50 to 100 times more potent than the natural liver products. The extract was the first workable treatment for the disease. For their initial work in pointing the way to a working treatment, Whipple, Minot, and Murphy shared the 1934 Nobel Prize in Physiology or Medicine.
The active ingredient in liver was not isolated until 1948 by the chemists Karl A. Folkers of the United States and Alexander R. Todd of Great Britain. The substance was a cobalamin called vitamin B-12. It could also be injected directly into muscle, making it possible to treat pernicious anemia more easily.[17]
The chemical structure of the molecule was determined by Dorothy Crowfoot Hodgkin and her team in 1956, based on crystallographic data.
Eventually, methods of producing the vitamin in large quantities from bacteria cultures were developed in the 1950s, and these led to the modern form of treatment for the disease.
Symptoms and damage from deficiency
Vitamin B-12 deficiency can potentially cause severe and irreversible damage, especially to the brain and nervous system. At levels only slightly lower than normal, a range of symptoms such as fatigue, depression, and poor memory may be experienced.[18] However, these symptoms by themselves are too nonspecific to diagnose deficiency of the vitamin.
Vitamin B-12 deficiency has the following pathomorphology and symptoms:[19]
Pathomorphology includes: A spongiform state of neural tissue along with edema of fibers and deficiency of tissue. The myelin decays, along with axial fiber. In later phases, fibric sclerosis of nervous tissues occurs. Those changes apply to dorsal parts of the spinal cord, and to pyramidal tracts in lateral cords.
In the brain itself, changes are less severe: they occur as small sources of nervous fibers decay and accumulation of astrocytes, usually subcortically located, an also round hemorrhages with a torus of glial cells. Pathological changes can be noticed as well in the posterior roots of the cord and, to lesser extent, in peripheral nerves.
Clinical symptoms : The main syndrome of vitamin B-12 deficiency is Addison's disease and Biermer's disease (pernicious anemia). It is characterized by a triad of symptoms:
1) Anemia with bone marrow promegaloblastosis (Megaloblastic anemia)
2) Gastrointestinal symptoms;
3) Neurological symptoms.
Each of those symptoms can occur either alone or along with others. The neurological complex, defined as myelosis funicularis, consists of the following symptoms:
1) Impaired perception of deep touch, pressure and vibration, abolishment of sense of touch, very annoying and persistent paresthesias;
2) Ataxia of dorsal cord type;
3) Decrease or abolishment of deep muscle-tendon reflexes;
4) Pathological reflexes - Babinski, Rossolimo and others, also severe paresis.
During the course of disease, mental disorders can occur: irritablity, focus/concentration problems, depressive state with suicidal tendencies, paraphrenia complex. These symptoms may not reverse after correction of hematological abnormalities, and the chance of complete reversal decreases with the length of time the neurological symptoms have been present.
Differential Diagnosis of Causes of Vitamin B12 Deficiency
In alphabetical order. [20] [21]
- Acute Hepatitis
- Bacterial colonization of the small intestine
- Caustic burn of the stomach mucosa
- Chronic Atrophic Gastritis
- Chronic hepatitis
- Chronic Kidney Disease
- Chronic Liver Disease
- Diseases of the terminal ileum
- Fish tapeworm
- Genuine pernicious anemia
- Hepatic tumor
- Leukemia
- Malnutrition
- Polycythemia
- Pregnancy
- Stomach polyposis
- Stomach resection
- Strict vegetarian diet
- Transcobalamin 2 deficiency
Sources
Vitamin B-12 is naturally found in foods of animal origin including meat (especially liver and shellfish) and milk products. Animals, in turn, must obtain it directly or indirectly from bacteria, and these bacteria may inhabit a section of the gut which is posterior to the section where B-12 is absorbed. Thus, herbivorous animals must either obtain B-12 from bacteria in their rumens, or (if fermenting plant material in the hindgut) by reingestion of cecotrope feces. Eggs are often mentioned as a good B-12 source, but they also contain a factor that blocks absorption.[22] Certain insects such as termites contain B-12 produced by their gut bacteria, in a manner analogous to ruminant animals.[23] An NIH Fact Sheet lists a variety of food sources of vitamin B-12.
Plants only supply B-12 to humans when the soil containing B-12-producing microorganisms has not been washed from them. Vegan humans who eat only washed vegetables must take special care to supplement their diets accordingly. According to the U.K. Vegan Society, the only reliable vegan sources of B-12 are foods fortified with B-12 (including some plant milks, some soy products and some breakfast cereals), and B-12 supplements.[24] Fortified breakfast cereals are a particularly valuable source of vitamin B-12 for vegetarians and vegans.
While lacto-ovo vegetarians usually get enough B-12 through consuming dairy products, vitamin B-12 may be found to be lacking in those practicing vegan diets who do not use multivitamin supplements or eat B-12 fortified foods, such as fortified breakfast cereals, fortified soy-based products, and fortified energy bars. Claimed sources of B-12 that have been shown through direct studies[25] of vegans to be inadequate or unreliable include, laver (a seaweed), barley grass, and human gut bacteria. People on a vegan raw food diet are also susceptible to B-12 deficiency if no supplementation is used[26].
The Vegan Society, the Vegetarian Resource Group, and the Physicians Committee for Responsible Medicine, among others, recommend that vegans either consistently eat foods fortified with B-12 or take a daily or weekly B-12 supplement.[24][27][28]
Cyanocobalamin is converted to its active forms, first hydroxocobalamin and then methylcobalamin and adenosylcobalamin in the liver. The sublingual route, in which B-12 is presumably or supposedly absorbed more directly under the tongue, has not proven to be necessary or helpful. A 2003 study found no significant difference in absorption for serum levels from oral vs. sublingual delivery of 500 micrograms of cobalamin.[29] However, if patient has inborn errors in the methyltransfer pathway (cobalamin C disease, combined methylmalonic aciduria and homocystinuria), treating with intravenous or intramuscular hydroxocobalamin is needed.[30][31][32][33][34]
Vitamin B-12 can be supplemented in healthy subjects also by liquid, strip, nasal spray, or injection. B-12 is available singly or in combination with other supplements.
Injection is sometimes used in cases where digestive absorption is impaired, but there is some evidence that this course of action may not be necessary with modern high potency oral supplements (such as 500 to 1000 mcg or more). [35][36][37] These supplements carry such large doses of the vitamin that the many different components of the B-12 absorption system are not required, and enough of the vitamin (only a few mcg a day) is obtained simply by mass-action transport across the gut. Even pernicious anemia can be treated entirely by the oral route.
For the much lower amounts of B-12 found in food sources, however, oral absorption is complex and requires stomach acid, and also specific intestinal transport proteins (intrinsic factor) produced in the stomach. Lack of function in these systems is the causes of much of the increased risk in many elderly persons who develop B-12 deficiency later in life. However, it can be treated with a simple high dose oral B-12 supplement. Cyanocobalamin is also sometimes added to beverages including Diet Coke Plus and many energy drinks, in some cases with over 80 times the recommended intake. However, 500 mcg would be needed to reverse biochemical signs of vitamin B-12 deficiency in older adults.[38]
Allergies
Vitamin B-12 supplements in theory should be avoided in people sensitive or allergic to cobalamin, cobalt, or any other product ingredients. However, direct allergy to a vitamin or nutrient is extremely rare, and if reported, other causes should be sought.
Side effects, contraindications, and warnings
- Dermatologic: Itching, rash, transitory exanthema, and urticaria have been reported. Vitamin B-12 (20 micrograms/day) and pyridoxine (80mg/day) has been associated with cases of rosacea fulminans, characterized by intense erythema with nodules, papules, and pustules. Symptoms may persist for up to 4 months after the supplement is stopped, and may require treatment with systemic corticosteroids and topical therapy.
- Gastrointestinal: Diarrhea has been reported.
- Hematologic: Peripheral vascular thrombosis has been reported. Treatment of vitamin B-12 deficiency can unmask polycythemia vera, which is characterized by an increase in blood volume and the number of red blood cells. The correction of megaloblastic anemia with vitamin B-12 can result in fatal hypokalemia and gout in susceptible individuals, and it can obscure folate deficiency in megaloblastic anemia. Caution is warranted.
- Leber's disease: Vitamin B-12 in the form of cyanocobalamin is contraindicated in early Leber's disease, which is hereditary optic nerve atrophy. Cyanocobalamin can cause severe and swift optic atrophy, but other forms of vitamin B-12 are available. However, the sources of this statement are not clear, while an opposing view[39] concludes: "The clinical picture of optic neuropathy associated with vitamin B-12 deficiency shows similarity to that of Leber's disease optic neuropathy. Both involve the nerve fibres of the papillomacular bundle. The present case reports suggest that optic neuropathy in patients carrying a primary LHON mtDNA mutation may be precipitated by vitamin B-12 deficiency. Therefore, known carriers should take care to have an adequate dietary intake of vitamin B-12 and malabsorption syndromes like those occurring in familial pernicious anaemia or after gastric surgery should be excluded."
Normal requirement, and in pregnancy and breastfeeding
The Dietary Reference Intake for an adult ranges from 2 to 3 µg (micrograms). The recommended optimal daily intake (ODI) is 10 to 15 µg.
Vitamin B-12 is believed to be safe when used orally in amounts that do not exceed the recommended dietary allowance (RDA). The RDA for vitamin B-12 in pregnant women is 2.6 µg per day and 2.8 µg during lactation periods. There is insufficient reliable information available about the safety of consuming greater amounts of Vitamin B-12 during pregnancy.
Other medical uses
Hydroxycobalamin, or hydoxocobalamin, also known as Vitamin B-12a, is used in Europe both for vitamin B-12 deficiency and as a treatment for cyanide poisoning, sometimes with a large amount (5-10 g) given intravenously, and sometimes in combination with sodium thiosulfate.[40] The mechanism of action is straightforward: the hydroxycobalamin hydroxide ligand is displaced by the toxic cyanide ion, and the resulting harmless B-12 complex is excreted in urine. In the United States, the FDA has approved in 2006 the use of hydroxocobalamin for acute treatment of cyanide poisoning.
Interactions
Interactions with drugs
- Alcohol (ethanol): Excessive alcohol intake lasting longer than two weeks can decrease vitamin B-12 absorption from the gastrointestinal tract, leading to Korsakoff's Syndrome.
- Aminosalicylic acid (para-aminosalicylic acid, PAS, Paser): Aminosalicylic acid can reduce oral vitamin B-12 absorption, possibly by as much as 55%, as part of a general malabsorption syndrome. Megaloblastic changes, and occasional cases of symptomatic anemia have occurred, usually after doses of 8 to 12 grams/day for several months. Vitamin B-12 levels should be monitored in people taking aminosalicylic acid for more than one month.
- Antibiotics: An increased bacterial load can bind significant amounts of vitamin B-12 in the gut, preventing its absorption. In people with bacterial overgrowth of the small bowel, antibiotics such as metronidazole (Flagyl®) can actually improve vitamin B-12 status. The effects of most antibiotics on gastrointestinal bacteria are unlikely to have clinically significant effects on vitamin B-12 levels.
- Hormonal contraception: The data regarding the effects of oral contraceptives on vitamin B-12 serum levels are conflicting. Some studies have found reduced serum levels in oral contraceptive users, but others have found no effect despite use of oral contraceptives for up to 6 months. When oral contraceptive use is stopped, normalization of vitamin B-12 levels usually occurs. Lower vitamin B-12 serum levels seen with oral contraceptives probably are not clinically significant.
- Chloramphenicol (Chloromycetin®): Limited case reports suggest that chloramphenicol can delay or interrupt the reticulocyte response to supplemental vitamin B-12 in some patients. Blood counts should be monitored closely if this combination cannot be avoided.
- Cobalt irradiation: Cobalt irradiation of the small bowel can decrease gastrointestinal (GI) absorption of vitamin B-12.
- Colchicine: Colchicine in doses of 1.9 to 3.9mg/day can disrupt normal intestinal mucosal function, leading to malabsorption of several nutrients, including vitamin B-12. Lower doses do not seem to have a significant effect on vitamin B-12 absorption after 3 years of colchicine therapy. The significance of this interaction is unclear. Vitamin B-12 levels should be monitored in people taking large doses of colchicine for prolonged periods.
- Colestipol (Colestid®), Cholestyramine (Questran®): These resins used for sequestering bile acids in order to decrease cholesterol, can decrease gastrointestinal (GI) absorption of vitamin B-12. It is unlikely that this interaction will deplete body stores of vitamin B-12 unless there are other factors contributing to deficiency. In a group of children treated with cholestyramine for up to 2.5 years there was not any change in serum vitamin B-12 levels. Routine supplements are not necessary.
- H2-receptor antagonists: include cimetidine (Tagamet®), famotidine (Pepcid®), nizatidine (Axid®), and ranitidine (Zantac®). Reduced secretion of gastric acid and pepsin produced by H2 blockers can reduce absorption of protein-bound (dietary) vitamin B-12, but not of supplemental vitamin B-12. Gastric acid is needed to release vitamin B-12 from protein for absorption. Clinically significant vitamin B-12 deficiency and megaloblastic anemia are unlikely, unless H2 blocker therapy is prolonged (2 years or more), or the person's diet is poor. It is also more likely if the person is rendered achlorhydric (with complete absence of gastric acid secretion), which occurs more frequently with proton pump inhibitors than H2 blockers. Vitamin B-12 levels should be monitored in people taking high doses of H2 blockers for prolonged periods.
- Metformin (Glucophage®): Metformin may reduce serum folic acid and vitamin B-12 levels. These changes can lead to hyperhomocysteinemia, adding to the risk of cardiovascular disease in people with diabetes. There are also rare reports of megaloblastic anemia in people who have taken metformin for 5 years or more. Reduced serum levels of vitamin B-12 occur in up to 30% of people taking metformin chronically.[41][42] However, clinically significant deficiency is not likely to develop if dietary intake of vitamin B-12 is adequate. Deficiency can be corrected with vitamin B-12 supplements even if metformin is continued. The metformin-induced malabsorption of vitamin B-12 is reversible by oral calcium supplementation.[43] The general clinical significance of metformin upon B-12 levels is as yet unknown.[44]
- Neomycin: Absorption of vitamin B-12 can be reduced by neomycin, but prolonged use of large doses is needed to induce pernicious anemia. Supplements are not usually needed with normal doses.
- Nicotine: Nicotine can reduce serum vitamin B-12 levels. The need for vitamin B-12 supplementation in smokers has not been adequately studied.
- Nitrous oxide: Nitrous oxide inactivates the cobalamin form of vitamin B-12 by oxidation. Symptoms of vitamin B-12 deficiency, including sensory neuropathy, myelopathy, and encephalopathy, can occur within days or weeks of exposure to nitrous oxide anesthesia in people with subclinical vitamin B-12 deficiency. Symptoms are treated with high doses of vitamin B-12, but recovery can be slow and incomplete. People with normal vitamin B-12 levels have sufficient vitamin B-12 stores to make the effects of nitrous oxide insignificant, unless exposure is repeated and prolonged (such as recreational use). Vitamin B-12 levels should be checked in people with risk factors for vitamin B-12 deficiency prior to using nitrous oxide anesthesia. Chronic nitrous oxide B-12 poisoning (usually from use of nitrous oxide as a recreational drug), however, may result in B-12 functional deficiency even with normal measured blood levels of B-12 [45]
- Phenytoin (Dilantin®), phenobarbital, primidone (Mysoline®): These anticonvulsants have been associated with reduced vitamin B-12 absorption, and reduced serum and cerebrospinal fluid levels in some patients. This may contribute to the megaloblastic anemia, primarily caused by folate deficiency, associated with these drugs. It's also suggested that reduced vitamin B-12 levels may contribute to the neuropsychiatric side effects of these drugs. Patients should be encouraged to maintain adequate dietary vitamin B-12 intake. Folate and vitamin B-12 status should be checked if symptoms of anemia develop.
- Proton pump inhibitors (PPIs): The PPIs include omeprazole (Prilosec®, Losec®), lansoprazole (Prevacid®), rabeprazole (Aciphex®), pantoprazole (Protonix®, Pantoloc®), and esomeprazole (Nexium®). The reduced secretion of gastric acid and pepsin produced by PPIs can reduce absorption of protein-bound (dietary) vitamin B-12, but not supplemental vitamin B-12. Gastric acid is needed to release vitamin B-12 from protein for absorption. Reduced vitamin B-12 levels may be more common with PPIs than with H2-blockers, because they are more likely to produce achlorhydria (complete absence of gastric acid secretion). However, clinically significant vitamin B-12 deficiency is unlikely, unless PPI therapy is prolonged (2 years or more) or dietary vitamin intake is low. Vitamin B-12 levels should be monitored in people taking high doses of PPIs for prolonged periods.
- Zidovudine (AZT, Combivir®, Retrovir®): Reduced serum vitamin B-12 levels may occur when zidovudine therapy is started. This adds to other factors that cause low vitamin B-12 levels in people with HIV, and might contribute to the hematological toxicity associated with zidovudine. However, data suggests vitamin B-12 supplements are not helpful for people taking zidovudine.
Interactions with herbs and dietary supplements
- Folic acid: Folic acid, particularly in large doses, can mask vitamin B-12 deficiency by completely correcting hematological abnormalities. In vitamin B-12 deficiency, folic acid can produce complete resolution of the characteristic megaloblastic anemia, while allowing potentially irreversible neurological damage (from continued inactivity of methylmalonyl mutase) to progress. Thus, vitamin B-12 status should be determined before folic acid is given as monotherapy.
- Potassium: Potassium supplements can reduce absorption of vitamin B-12 in some people. This effect has been reported with potassium chloride and, to a lesser extent, with potassium citrate. Potassium might contribute to vitamin B-12 deficiency in some people with other risk factors, but routine supplements are not necessary.[46]
External links
- Template:Pauling
- fact sheet at NIH
- Vitamin B-12. Medline Plus (National Library of Medicine). Part of it was used for this article (US Government public domain), specially for drug and other interactions.
- Vitamin B12 Deficiency
- Vitamin B-12 deficiency article in American Family Physician journal
- Vitamin B-12: Vital Nutrient for Good Health at the Weston A. Price Foundation
- Cyanocobalamin at the US National Library of Medicine Medical Subject Headings (MeSH)
References
- ↑ "Tobacco amblyopia". grande.nal.usda.gov. Retrieved 2008-03-26.
- ↑ Accessed Dec. 3, 2007
- ↑ http://www.ajcn.org/cgi/reprint/48/3/852.pdf Accessed Dec 3., 2007 See especially discussion on activated charcoal column purification for the origin of this compound.
- ↑ Bioorganometallics: Biomolecules, Labeling, Medicine; Jaouen, G., Ed. Wiley-VCH: Weinheim, 2006.3-527-30990-X.
- ↑ G. Loeffler (2005). Basiswissen Biochemie. p. 606. Unknown parameter
|isdn=ignored (help) - ↑ {{cite journal | author= Woodward,RB | title= The Total Synthesis of Vitamin Template:Ssub
- ↑ Khan,AG and Easwaran,SV (2003). "Woodward's Synthesis of Vitamin B-12". Resonance. 8: 8–16.
- ↑ Eschenmoser, A. and Wintner, C. (1976). "Natural Product Synthesis and Vitamin B-Template:Ssub". Science. 196: 1410–20.
- ↑ Riether, D. and Mulzer, J. (2003). "Total Synthesis of Cobyric Acid: Historical Development and Recent Synthetic Innovations". Eur. J. Org. Chem. (1): 30–45.
- ↑ J.H. Martens, H. Barg, M.J. Warren and D. Jahn (2002). "Microbial production of vitamin B-12". Applied Microbiology and Biotechnology. 58: 275–285.
- ↑ Donald and Judith Voet (1995). Biochemistry (2nd ed.). John Wiley & Sons Ltd. p. 675. ISBN 0-471-58651-X.
- ↑ http://www.dizziness-and-balance.com/disorders/central/B-12.html
- ↑ Banerjee RV, Matthews RG (1990). "Cobalamin-dependent methionine synthase" (PDF). FASEB J. 4 (5): 1450–9. PMID 2407589.
- ↑ Wickramasinghe SN (1995). "Morphology, biology and biochemistry of cobalamin- and folate-deficient bone marrow cells". Baillieres Clin Haematol. 8: 441–459. PMID 8534956.
- ↑ Case 87: Subacute Combined Degeneration - Naidich and Ho 237 (1): 101 - Radiology
- ↑ http://www.emedicine.com/med/topic1799.htm Accessed Dec. 21, 2007
- ↑ www.mayoclinicproceedings.com
- ↑ Dietary Supplement Fact Sheet: Vitamin B12
- ↑ Clinical Neurology by Anatol Dowżenko (ISBN 83-200-1197-3), page 451, translated to English:
- ↑ Sailer, Christian, Wasner, Susanne. Differential Diagnosis Pocket. Hermosa Beach, CA: Borm Bruckmeir Publishing LLC, 2002:77 ISBN 1591032016
- ↑ Kahan, Scott, Smith, Ellen G. In A Page: Signs and Symptoms. Malden, Massachusetts: Blackwell Publishing, 2004:68 ISBN 140510368X
- ↑ Doscherholmen A et al. (1975). "Proc Soc Exp Biol Med, Sep;149(4):987-90;".
- ↑ Wakayama EJ, Dillwith JW, Howard RW, Blomquist GJ (1984). "Vitamin B-12 levels in selected insects". Insect Biochemistry. 14: 175–179.
- ↑ 24.0 24.1 Walsh, Stephen, RD. "Vegan Society B-12 factsheet". Vegan Society. Retrieved 2008-01-17.
- ↑ Norris, Jack, RD. "B-12 in Tempeh, Seaweeds, Organic Produce, and Other Plant Foods". VeganHealth.org. Retrieved 2008-01-17.
- ↑ Donaldson, MS. "Metabolic vitamin B-12 status on a mostly raw vegan diet with follow-up using tablets, nutritional yeast, or probiotic supplements". Ann Nutr Metab. 2000;44:229-234.
- ↑ Reed Mangels, Ph.D., R.D. "Vitamin B-12 in the Vegan Diet". Vegetarian Resource Group. Retrieved 2008-01-17. External link in
|publisher=(help) - ↑ "Don't Vegetarians Have Trouble Getting Enough Vitamin B-12?". Physicians Committee for Responsible Medicine. Retrieved 2008-01-17.
- ↑ Sharabi A, Cohen E, Sulkes J, Garty M. Replacement therapy for vitamin B-12 deficiency: comparison between the sublingual and oral route. Br J Clin Pharmacol. 2003 Dec;56(6):635-8. PMID 14616423.
- ↑ Andersson HC, Shapira E (1998). "Biochemical and clinical response to hydroxocobalamin versus cyanocobalamin treatment in patients with methylmalonic acidemia and homocystinuria (cblC)". J. Pediatr. 132 (1): 121–4. PMID 9470012.
- ↑ Roze E, Gervais D, Demeret S; et al. (2003). "Neuropsychiatric disturbances in presumed late-onset cobalamin C disease". Arch. Neurol. 60 (10): 1457–62. doi:10.1001/archneur.60.10.1457. PMID 14568819.
- ↑ Thauvin-Robinet C, Roze E, Couvreur G; et al. (2008). "The adolescent and adult form of cobalamin C disease: clinical and molecular spectrum". J. Neurol. Neurosurg. Psychiatr. doi:10.1136/jnnp.2007.133025. PMID 18245139.
- ↑ Heil SG, Hogeveen M, Kluijtmans LA; et al. (2007). "Marfanoid features in a child with combined methylmalonic aciduria and homocystinuria (CblC type)". J. Inherit. Metab. Dis. 30 (5): 811. doi:10.1007/s10545-007-0546-6. PMID 17768669.
- ↑ Tsai AC, Morel CF, Scharer G; et al. (2007). "Late-onset combined homocystinuria and methylmalonic aciduria (cblC) and neuropsychiatric disturbance". Am. J. Med. Genet. A. 143 (20): 2430–4. doi:10.1002/ajmg.a.31932. PMID 17853453.
- ↑ Bolaman Z, Kadikoylu G, Yukselen V, Yavasoglu I, Barutca S, Senturk T (2003). "Oral versus intramuscular cobalamin treatment in megaloblastic anemia: a single-center, prospective, randomized, open-label study". Clin Ther. 25 (12): 3124–34. PMID 14749150.
- ↑ Lane LA, Rojas-Fernandez C (2002). "Treatment of vitamin b(12)-deficiency anemia: oral versus parenteral therapy". Ann Pharmacother. 36 (7–8): 1268–72. PMID 12086562.
- ↑ Butler CC, Vidal-Alaball J, Cannings-John R; et al. (2006). "Oral vitamin B12 versus intramuscular vitamin B12 for vitamin B12 deficiency: a systematic review of randomized controlled trials". Fam Pract. 23 (3): 279–85. doi:10.1093/fampra/cml008. PMID 16585128.
- ↑ Park S, Johnson MA (2006). "What is an adequate dose of oral vitamin B12 in older people with poor vitamin B12 status?". Nutr. Rev. 64 (8): 373–8. PMID 16958314.
- ↑ Pott JW, Wong KH (2006). "Leber's hereditary optic neuropathy and vitamin B12 deficiency". Graefes Arch. Clin. Exp. Ophthalmol. 244 (10): 1357–9. doi:10.1007/s00417-006-0269-7. PMID 16523300.
- ↑ Hall AH, Rumack BH. Hydroxycobalamin/sodium thiosulfate as a cyanide antidote. J Emerg Med. 1987;5(2):115-21. PMID 3295013.
- ↑ Andrès E, Noel E, Goichot B (2002). "Metformin-associated vitamin B-12 deficiency". Arch Intern Med. 162 (19): 2251–2. PMID 12390080.
- ↑ Gilligan M (2002). "Metformin and vitamin B-12 deficiency". Arch Intern Med. 162 (4): 484–5. PMID 11863489.
- ↑ Bauman WA, Shaw S, Jayatilleke E, Spungen AM, Herbert V. Increased intake of calcium reverses vitamin B-12 malabsorption induced by metformin. Diabetes Care. 2000 Sep;23(9):1227-31. PMID 10977010.
- ↑ Samantha Copp (2005-12-01). "What effect does metformin have on vitamin B-12 levels?". UK Medicines Information, NHS - Full report (DOC). External link in
|publisher=(help) - ↑ [1] Accessed Dec. 21, 2007]
- ↑ Palva IP, Salokannel, SJ, Timonen T, et al: Drug induced malabsorption of vitamin B-12 - IV - malabsorption and deficiency of B-12 during treatment with slow-release potassium chloride, Acta Med Scand, 1972, 191(4):355-7
ar:فيتامين بي12 ca:Cianocobalamina cs:Vitamín B12 de:Cobalamine eo:Kobalamino ko:바이타민 B12 hr:Vitamin B-12 it:Cobalamina he:ויטמין B12 lb:Cobalamin lt:Kobalaminas ms:Vitamin B-12 nl:Cobalamine no:Kobalamin sk:Kobalamín sr:Витамин Б12 sh:Vitamin B-12 fi:B-12-vitamiini sv:Kobalamin th:วิตามินบี12
- Pages with script errors
- Pages with reference errors
- Pages with citations using unsupported parameters
- CS1 maint: Multiple names: authors list
- CS1 errors: external links
- CS1 maint: Explicit use of et al.
- Pages using duplicate arguments in template calls
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Articles containing unverified chemical infoboxes
- B vitamins
- Organometallic chemistry
- Cofactors