Calcipotriol
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Sheng Shi, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Calcipotriol is a vitamin D3 analog that is FDA approved for the treatment of plaque psoriasis. Common adverse reactions include burning sensation, dermatitis, dry skin, peeling of skin, pruritus, rash and skin irritation.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Plaque Psoriasis
Dosing information
- Apply a thin layer of Calcipotriene Cream to the affected skin twice daily and rub in gently and completely. The safety and efficacy of Calcipotriene Cream have been demonstrated in patients treated for eight weeks.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
- There is limited information regarding Off-Label Guideline-Supported Use of Calcipotriol in adult patients.
Non–Guideline-Supported Use
Psoriasis vulgaris Of the body
Dosing information
- 50 mcg/g applied to the trunk and limbs once daily for up to 8 weeks.[1]
Vitiligo
Dosing information
- Applied twice daily[2]
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
- Safety and effectiveness of Calcipotriene Cream in pediatric patients have not been established
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
- There is limited information regarding Off-Label Guideline-Supported Use of Calcipotriol in pediatric patients.
Non–Guideline-Supported Use
Vitiligo
Dosing information
- 50 mcg/g was applied in the evening to depigmented skin.
Contraindications
- Calcipotriene Cream is contraindicated in those patients with a history of hypersensitivity to any of the components of the preparation. It should not be used by patients with demonstrated hypercalcemia or evidence of vitamin D toxicity. Calcipotriene Cream should not be used on the face.
Warnings
- Use of Calcipotriene Cream may cause transient irritation of both lesions and surrounding uninvolved skin. If irritation develops, Calcipotriene Cream should be discontinued.
- For external use only. Keep out of the reach of children. Always wash hands thoroughly after use.
- Reversible elevation of serum calcium has occurred with use of topical calcipotriene. If elevation in serum calcium outside the normal range should occur, discontinue treatment until normal calcium levels are restored.
Adverse Reactions
Clinical Trials Experience
- In controlled clinical trials, the most frequent adverse experiences reported for Calcipotriene (calcipotriene) Cream, 0.005% were cases of skin irritation, which occurred in approximately 10-15% of patients. Rash, pruritus, dermatitis and worsening of psoriasis were reported in 1 to 10% of patients.
Postmarketing Experience
- FDA Package Insert for Calcipotriol contains no information regarding Postmarketing.
Drug Interactions
- FDA Package Insert for Calcipotriol contains no information regarding Drug Interaction.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): C Teratogenic Effects: Pregnancy Category C
- Studies of teratogenicity were done by the oral route where bioavailability is expected to be approximately 40-60% of the administered dose. Increased rabbit maternal and fetal toxicity was noted at 12 μg/kg/day (132 μg/m2/day). Rabbits administered 36 μg/kg/day (396 μg/m2/day) resulted in fetuses with a significant increase in the incidences of pubic bones, forelimb phalanges, and incomplete bone ossification. In a rat study, oral doses of 54 μg/kg/day (318 μg/m2/day) resulted in a significantly higher incidence of skeletal abnormalities consisting primarily of enlarged fontanelles and extra ribs. The enlarged fontanelles are most likely due to calcipotriene's effect upon calcium metabolism. The maternal and fetal calculated no-effect exposures in the rat (43.2 μg/m2/day) and rabbit (17.6 μg/m2/day) studies are approximately equal to the expected human systemic exposure level (18.5 μg/m2/day) from dermal application. There are no adequate and well-controlled studies in pregnant women. Therefore, Calcipotriene Cream should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Calcipotriol in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Calcipotriol during labor and delivery.
Nursing Mothers
- There is evidence that maternal 1,25-dihydroxy vitamin D3 (calcitriol) may enter the fetal circulation, but it is not known whether it is excreted in human milk. The systemic disposition of calcipotriene is expected to be similar to that of the naturally occurring vitamin. Because many drugs are excreted in human milk, caution should be exercised when Calcipotriene Cream is administered to a nursing woman.
Pediatric Use
- Safety and effectiveness of Calcipotriene Cream in pediatric patients have not been established. Because of a higher ratio of skin surface area to body mass, pediatric patients are at greater risk than adults of systemic adverse effects when they are treated with topical medication.
Geriatic Use
- Of the total number of patients in clinical studies of calcipotriene cream, approximately 15% were 65 or older, while approximately 3% were 75 and over. There were no significant differences in adverse events for subjects over 65 years compared to those under 65 years of age. However, the greater sensitivity of older individuals cannot be ruled out.
Gender
There is no FDA guidance on the use of Calcipotriol with respect to specific gender populations.
Race
There is no FDA guidance on the use of Calcipotriol with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Calcipotriol in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Calcipotriol in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Calcipotriol in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Calcipotriol in patients who are immunocompromised.
Administration and Monitoring
Administration
- Topical
Monitoring
- FDA Package Insert for Calcipotriol contains no information regarding Drug Monitoring.
IV Compatibility
- There is limited information about the IV Compatibility.
Overdosage
- Topically applied calcipotriene can be absorbed in sufficient amounts to produce systemic effects. Elevated serum calcium has been observed with excessive use of topical calcipotriene. If elevation in serum calcium should occur, discontinue treatment until normal calcium levels are restored.
Pharmacology
Mechanism of Action
- In humans, the natural supply of vitamin D depends mainly on exposure to the ultraviolet rays of the sun for conversion of 7-dehydrocholesterol to vitamin D3 (cholecalciferol) in the skin. Calcipotriene is a synthetic analog of vitamin D3.
Structure
- Calcipotriene (calcipotriene) Cream, 0.005% contains calcipotriene monohydrate, a synthetic vitamin D3 derivative, for topical dermatological use.
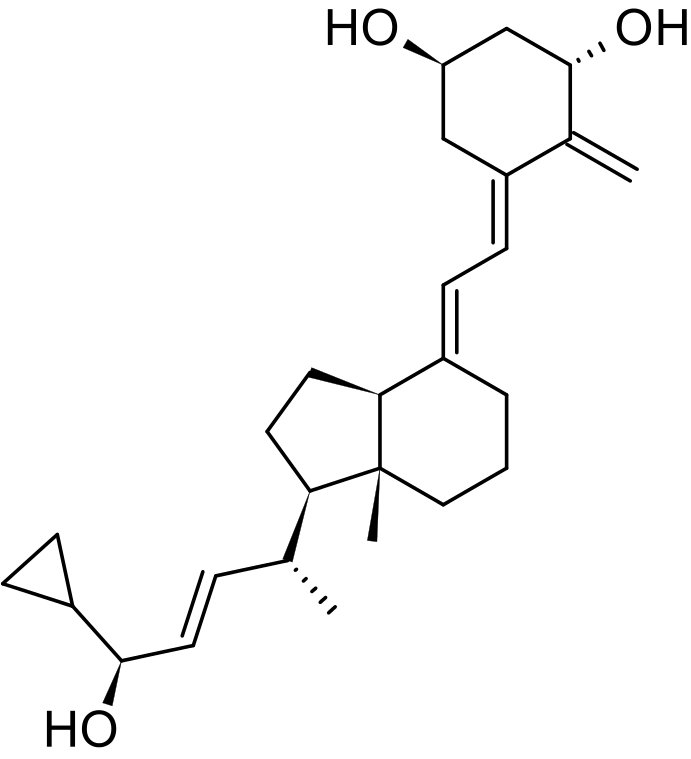
- Chemically, calcipotriene monohydrate is (5Z,7E,22E,24S)-24-cyclopropyl-9,10-secochola-5,7,10(19),22-tetraene-1α,3β,24-triol monohydrate, with the empirical formula C27H40O3•H2O, a molecular weight of 430.6, and the following structural formula:
- Calcipotriene monohydrate is a white or off-white crystalline substance. Calcipotriene Cream contains calcipotriene monohydrate equivalent to 50 μg/g anhydrous calcipotriene in a cream base of cetearyl alcohol, ceteth-20, diazolidinyl urea, dichlorobenzyl alcohol, dibasic sodium phosphate, edetate disodium, dl-alpha tocopherol, glycerin, mineral oil, petrolatum, and water.
Pharmacodynamics
- Clinical studies with radiolabelled calcipotriene ointment indicate that approximately 6% (± 3%, SD) of the applied dose of calcipotriene is absorbed systemically when the ointment is applied topically to psoriasis plaques, or 5% (± 2.6%, SD) when applied to normal skin, and much of the absorbed active is converted to inactive metabolites within 24 hours of application. Systemic absorption of the cream has not been studied.
Pharmacokinetics
- Vitamin D and its metabolites are transported in the blood, bound to specific plasma proteins. The active form of the vitamin, 1,25-dihydroxy vitamin D3 (calcitriol), is known to be recycled via the liver and excreted in the bile. Calcipotriene metabolism following systemic uptake is rapid, and occurs via a similar pathway to the natural hormone.
Nonclinical Toxicology
- When calcipotriene was applied topically to mice for up to 24 months at dosages of 3, 10 and 30 µg/kg/day (corresponding to 9, 30 and 90 µg/m2/day), no significant changes in tumor incidence were observed when compared to control. In a study in which albino hairless mice were exposed to both UVR and topically applied calcipotriene, a reduction in the time required for UVR to induce the formation of skin tumors was observed (statistically significant in males only), suggesting that calcipotriene may enhance the effect of UVR to induce skin tumors. Patients that apply Calcipotriene Cream to exposed portions of the body should avoid excessive exposure to either natural or artificial sunlight (including tanning booths, sun lamps, etc.). Physicians may wish to limit or avoid use of phototherapy in patients that use Calcipotriene Cream.
- Calcipotriene did not elicit any mutagenic effects in an Ames mutagenicity assay, a mouse lymphoma TK locus assay, a human lymphocyte chromosome aberration assay, or in a micronucleus assay conducted in mice.
- Studies in rats at doses up to 54 μg/kg/day (324 μg/m2/day) of calcipotriene indicated no impairment of fertility or general reproductive performance.
Clinical Studies
- Adequate and well-controlled trials of patients treated with Calcipotriene Cream have demonstrated improvement usually beginning after 2 weeks of therapy. This improvement continued with approximately 50% of patients showing at least marked improvement in the signs and symptoms of psoriasis after 8 weeks of therapy, but only approximately 4% showed complete clearing.
How Supplied
- Calcipotriene (calcipotriene) Cream, 0.005% is available in:
- 60 gram aluminum tubes N 50222-260-06
- 120 gram aluminum tubes N 50222-260-12
Storage
- Store at controlled room temperature 15° C - 25° C (59° F - 77° F). Do not freeze.
Images
Drug Images
{{#ask: Page Name::Calcipotriol |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Calcipotriol |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Patients using Calcipotriene Cream should receive the following information and instructions:
1. This medication is to be used only as directed by the physician. It is for external use only. Avoid contact with the face or eyes. As with any topical medication, patients should wash their hands after application. 2. This medication should not be used for any disorder other than that for which it was prescribed. 3. Patients should report to their physician any signs of adverse reactions. 4. Patients that apply Calcipotriene Cream to exposed portions of the body should avoid excessive exposure to either natural or artificial sunlight (including tanning booths, sun lamps, etc.).
Precautions with Alcohol
- Alcohol-Calcipotriol interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Dovonex
- Calcitrene
- Sorilux
Look-Alike Drug Names
- There is limited information about the look alike drug names.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Menter A, Gold LS, Bukhalo M, Grekin S, Kempers S, Boyce BM; et al. (2013). "Calcipotriene plus betamethasone dipropionate topical suspension for the treatment of mild to moderate psoriasis vulgaris on the body: a randomized, double-blind, vehicle-controlled trial". J Drugs Dermatol. 12 (1): 92–8. PMID 23377334.
- ↑ Baysal V, Yildirim M, Erel A, Kesici D (2003). "Is the combination of calcipotriol and PUVA effective in vitiligo?". J Eur Acad Dermatol Venereol. 17 (3): 299–302. PMID 12702070.
{{#subobject:
|Label Page=Calcipotriol |Label Name=Calcipotriol_label_01.jpg
}}
{{#subobject:
|Label Page=Calcipotriol |Label Name=Calcipotriol_panel_01.png
}}