Ligand (biochemistry)
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
In biochemistry, a ligand (latin ligare = to bind) is a molecule that is able to bind to and form a complex with a biomolecule to serve a biological purpose. In a narrower sense, it is an effector molecule binding to a site on a target protein, by intermolecular forces such as ionic bonds, hydrogen bonds and Van der Waals forces. The docking (association) is usually reversible (dissociation). Actual irreversible covalent binding between a ligand and its target molecule is rare in biological systems. As opposed to the meaning in metalorganic and inorganic chemistry, it is irrelevant, whether or not the ligand actually binds at a metal site, as it is the case in hemoglobin. Ligand binding to receptors alters the chemical conformation, i.e. the three dimensional shape of the receptor protein. The conformational state of a receptor protein determines the functional state of a receptor. The tendency or strength of binding is called affinity. Ligands include substrates, inhibitors, activators, and neurotransmitters. Radioligands are radioisotope labeled compounds and used in vivo as tracers in PET studies and for in vitro binding studies.
Receptor/Ligand binding affinity
The interaction of most ligands with their binding sites can be characterized in terms of a binding affinity. In general, high affinity ligand binding results from greater intermolecular force between the ligand and its receptor while low affinity ligand binding involves less intermolecular force between the ligand and its receptor. In general, high affinity binding involves a longer residence time for the ligand at its receptor binding site than is the case for low affinity binding. High affinity binding of ligands to receptors is often physiologically important when some of the binding energy can be used to cause a conformational change in the receptor, resulting in altered behavior of an associated ion channel or enzyme.
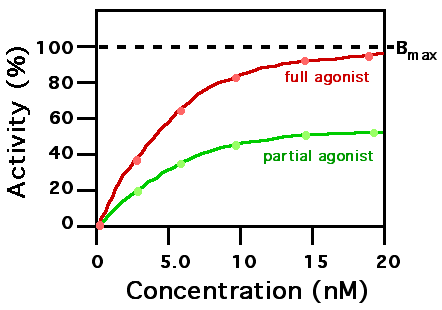
A ligand that can bind to a receptor, alter the function of the receptor and trigger a physiological response is called an agonist for that receptor. Agonist binding to a receptor can be characterized both in terms of how much physiological response can be triggered and the concentration of the agonist that is required to produce the physiological response. High affinity ligand binding implies that a relatively low concentration of a ligand is adequate to maximally occupy a ligand binding site and trigger a physiological response. Low affinity binding implies that a relatively high concentration of a ligand is required before the binding site is maximally occupied and the maximum physiological response to the ligand is achieved. In the example shown to the right, two different ligands bind to the same receptor binding site. Only one of the agonists shown can maximally stimulate the receptor and thus can be defined as a "full agonist". An agonist that can only partially activate the physiological response is called a "partial agonist". Ligands that bind to a receptor but fail to activate the physiological response are receptor "antagonists". In this example, the concentration at which the full agonist (red curve) can half-maximally activate the receptor is about 5 x 10-9 Molar (nM = nanomolar).
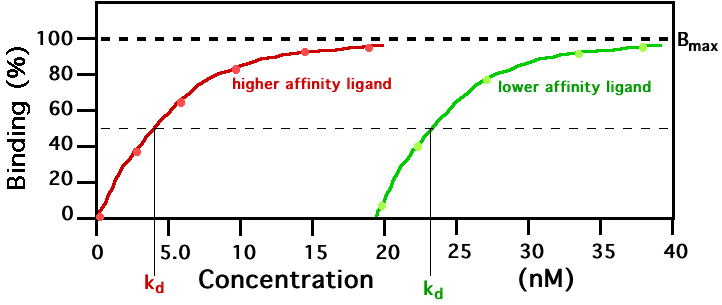
In the example shown to the left, ligand binding curves are shown for two ligands with different binding affinities. Ligand binding is often characterized in terms of the concentration of ligand at which half of the receptor binding sites are occupied (kd). The ligand illustrated by the red curve has a higher binding affinity and smaller kd than the ligand illustrated by the green curve. If these two ligands were present at the same time, more of the higher affinity ligand would be bound to the available receptor binding sites. This is how carbon monoxide can compete with oxygen in binding to hemoglobin, resulting in carbon monoxide poisoning.
See also
References
- Ligand binding to hormone receptors in Endocrinology: An Integrated Approach by Stephen Nussey and Saffron Whitehead (2001) Published by BIOS Scientific Publishers Ltd. ISBN 1-85996-252-1.
- Molecular Recognition Processes in Molecular Biology of the Cell 3rd edition (1994) by Bruce Alberts, Dennis Bray, Julian Lewis, Martin Raff, Keith Roberts and James D. Watson. See Figure 3-9, Equilibrium ligand binding.
External Links
- BindingDB, a public database of measured protein-ligand binding affinities.