Hepatitis C: Difference between revisions
Varun Kumar (talk | contribs) No edit summary |
Varun Kumar (talk | contribs) No edit summary |
||
| Line 1: | Line 1: | ||
'''For patient information click [[{{PAGENAME}} (patient information)|here]]'''. | '''For patient information click [[{{PAGENAME}} (patient information)|here]]'''. | ||
{{Infobox_Disease | | |||
Name = Hepatitis C | | |||
Image = | | |||
Caption = | | |||
DiseasesDB = 5783 | | |||
ICD10 = {{ICD10|B|17|1|b|15}}, {{ICD10|B|18|2|b|15}} | | |||
ICD9 = {{ICD9|070.4}}, {{ICD9|070.5}} | | |||
ICDO = | | |||
OMIM = 609532 | | |||
MedlinePlus = 000284 | | |||
MeshID = D006526 | | |||
}} | |||
{{InfectiousDisease | {{InfectiousDisease | ||
|description=Hepatitis C overview | |description=Hepatitis C overview | ||
| Line 13: | Line 25: | ||
}} | }} | ||
==[[Hepatitis C screening|Screening]]== | ==[[Hepatitis C screening|Screening]]== | ||
Revision as of 20:40, 8 March 2012
For patient information click here.
| Hepatitis C | |
| ICD-10 | B17.1, B18.2 |
|---|---|
| ICD-9 | 070.4, 070.5 |
| OMIM | 609532 |
| DiseasesDB | 5783 |
| MedlinePlus | 000284 |
| MeSH | D006526 |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-In-Chief: Varun Kumar, M.B.B.S. [2]; Assistant Editor-In-Chief: Nina Axiotakis [3]{{#meta: itemprop="medicalWebPageAudiences" content="patient"}}{{#meta: itemprop="medicalWebPageSpecialities" content="cardiology"}}{{#meta: itemprop="medicalWebPageInfoTypes" content="symptoms,diagnosis,treatment,causes,prognosis,complications"}} Classification Classic::Classification Atypical::
Overview
|
Hepatitis C |
|
Diagnosis |
|
Treatment |
|
Hepatitis C On the Web |
|
American Roentgen Ray Society Images of Hepatitis C |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [4]; Associate Editor(s)-In-Chief: Yazan Daaboul, Serge Korjian
Overview
Hepatitis C virus (HCV) is a single-stranded RNA virus that causes liver damage. Initially discovered in 1989, HCV was found to be a bloodborne infection that develops into a chronic state in most cases. Although the exact pathogenesis and life cycle of HCV are not well understood, it has been demonstrated that impaired innate and adaptive immunity to acute HCV may contribute to the development of chronic infection. While the transfusion of blood and blood products along with injectable therapy were considered the most common risk factors for HCV in the past, the use of illegal intravenous drugs is currently the most important risk factor. In the absence of treatment, chronic HCV leads to liver cirrhosis several years after the initial infection, a course complicated by decompensated liver failure or hepatocellular carcinoma. Other extra-hepatic manifestations are also common. Specific patient populations should be screened for HCV first using HCV serological testing or, rarely, directly by measuring HCV RNA in patients who have had previous HCV exposure, treatment-induced clearance, or immunosuppression. The diagnosis is made when tests for anti-HCV and HCV RNA both return positive results. Measures to slow the progression of liver disease, such as vaccines against other diseases and increased awareness of the risks associated with the use of alcohol or drugs that injure the liver should be taken following diagnosis. Classically, interferon (IFN) therapy was used to treat HCV, followed by the use of ribavirin. More recently, protease inhibitors emerged as effective drugs of choice for HCV infection.
Historical Perspective
The discovery of the hepatitis C virus was made based on early findings of patients with signs and symptoms of viral hepatitis lacking positive serologies for hepatitis A or B. These patients were originally described in 1974-5 as having "non-A, non-B viral hepatitis" (NANBH). It was not until 1989 that hepatitis C virus (HCV) was truly discovered when Qui-Lim Choo and colleagues successfully isolated the first cDNA clone 5-1-1 derived from the NANBH genome. The first interferon-alpha (INF-a) to treat HCV was developed in 1986 and approved in 1991. Approximately 10-15 years later, global efforts to reduce the burden of HCV were launched; worldwide campaigns were led by the World Health Organization (WHO) and Centers for Disease Control and Prevention (CDC).
Pathophysiology
In cases of isolated acute HCV infection, the host immune system stimulates the secretion of interferon-alpha and the activation of natural killer cells, which is followed by the activation of the adaptive immune system. Chronic HCV is characterized by the impairment of these mechanisms. Eventually, chronic HCV infection leads to local inflammation and fibrogenesis, which causes hepatic injury and cirrhosis. Hepatocellular carcinoma, a known complication of chronic HCV infection, arises in cases of cirrhosis; the role of oncogenic proteins of HCV in the pathogenesis of hepatocellular carcinoma has yet to be elucidated.
Causes
The hepatitis C virus (HCV) is the causative agent of hepatitis C. It is a member of the genus Hepacivirus that belongs to the Flaviviridae family. It is an enveloped, single-stranded RNA virus. The RNA genome acts as a template for viral replication and eventually, protein biosynthesis. Humans are considered the only natural hosts for HCV. The virus is primarily transmitted by exposure to contaminated blood.
Classification
HCV can be classified based on the isolated genotype and subtype. Six major genotypes have been identified. Several new genotypes and subtypes were recently discovered.
Epidemiology and Demographics
Hepatitis C is a major health problem that affects approximately 2 to 4 million people in the United States, 5 to 10 million people in Europe, and 12 million people in India. Approximately 150,000 new cases occur annually in the United States and in Western Europe, although incidence rates are difficult to estimate with accuracy given the asymptomatic nature of early stages of the disease. While the prevalence of the disease appears to be declining, hepatitis C is still highly prevalent in specific areas of the world. Egypt is the country with the highest prevalence of HCV, HCV-associated cirrhosis, and hepatocellular carcinoma, and the prevalence tends to increase with age, suggesting ongoing new cases of HCV. Approximately one-fourth of all cases of cirrhosis and hepatocellular carcinoma are attributed to HCV worldwide. Hepatitis C affects males and females equally.
Risk Factors
Risk factors for hepatitis C include intravenous drug use (most important), multiple blood transfusions prior to 1992, therapeutic injections for hemophilia prior to 1987, and work in the healthcare field (given that exposure to contaminated blood products is the most important mode of transmission).
Other, less common risk factors include occupational exposure to blood, such as contaminated needle sticks, Hemodialysis, solid organ transplantation from infected donors, birth to infected mother (in cases of detectable maternal HCV PCR at delivery), sexual intercourse with an infected partner, sexual intercourse with multiple partners, HIV infection, and tattoos or piercings with infected needle sticks.
Screening
People living in regions highly prevalent with HCV and who have engaged in high-risk behaviors should be screened. Screening by serological testing, confirmed by nucleic acid amplification (NAT) for HCV RNA, is required. Additionally, screening for other bloodborne infections, such as HBV and HIV, is required once a diagnosis is made. The ideal frequency of testing in these patients is unclear and should be personalized according to the frequency of a patient's exposure to risk.
Differentiating Hepatitis C from other Diseases
Hepatitis C must be differentiated from other diseases that cause hepatic injury and abnormal liver function tests such as other viral hepatitides (Hepatitis A, Hepatitic B, and Hepatitis E), and non-viral etiologies (e.g., alcoholic liver disease, non-alcoholic steatohepatitis, drug-induced liver injury, autoimmune hepatitis, and hepatocellular carcinoma).
Natural History and Prognosis
The majority of individuals infected with HCV will become chronic carriers. The most common complications of HCV are hepatic, including liver cirrhosis years after the onset of infection and the consequent development of hepatocellular carcinoma. Other classical extrahepatic manifestations, such as cryoglobulinemia, lichen planus, membranoproliferative glomerulonephritis, and porphyria cutanea tarda are also complications of chronic HCV infection. Treatment is necessary for patients with chronic stable HCV infection; otherwise, prognosis is poor and progression of the disease may be fatal.
Treatment
The treatment of hepatitis C has changed dramatically over the past decade. Whereas relatively new protease inhibitors telaprevir and boceprevir were added to the regular regimen of IFN and ribavirin in 2011 to treat patients with genotype 1 HCV, newer oral agents sofosbuvir and simeprevir have demonstrated greater efficacy in viral clearance along with a better safety profile. New guidelines from the AASLD and the IDSA have endorsed the use of these oral agents (particularly sofosbuvir) as first-line agents in the treatment of chronic HCV in both relapsers and treatment-naive patients.
Historical Perspective
|
Hepatitis C |
|
Diagnosis |
|
Treatment |
|
Hepatitis C On the Web |
|
American Roentgen Ray Society Images of Hepatitis C |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [5]; Associate Editor(s)-In-Chief: Yazan Daaboul, Serge Korjian
Overview
The discovery of the hepatitis C virus was made based on early findings of patients with signs and symptoms of viral hepatitis lacking positive serologies for hepatitis A or B. These patients were originally described in 1974-5 as having "non-A, non-B viral hepatitis" (NANBH). It was not until 1989 that hepatitis C virus (HCV) was truly discovered when Qui-Lim Choo and colleagues successfully isolated the first cDNA clone 5-1-1 derived from the NANBH genome. The first interferon-alpha (INF-a) to treat HCV was developed in 1986 and approved in 1991. Approximately 10-15 years later, global efforts to reduce the burden of HCV were launched; worldwide campaigns were led by the World Health Organization (WHO) and Centers for Disease Control and Prevention (CDC).
Discovery of Non-A, Non-B Viral Hepatitis
In the early 1960s, scientists only differentiated between hepatitis A, an acute infection of short incubation period transmitted via the fecal-oral route, and hepatitis B, a potentially chronic infection of long incubation period transmitted via blood exposure. Consequently, the discovery of hepatitis C only occurred with the availability of diagnostic assays in the early 1970s.[1][2][3] In 1974-5, the works of Alfred Prince, Stephen Feinstone, and Harvey Alter led to the new discovery that was termed "non-A, non-B viral hepatitis" (NANBH) when serological markers for hepatitis A and B were both absent in patients with signs and symptoms of viral hepatitis.[1][2][4] Shortly after, studies scrutinizing the new discovery revealed that most patients with NANBH had been exposed to blood transfusions[5][6] and hepatic cirrhosis ensued in as many as 20% of these patients.[7]
With no techniques available with which to isolate the virus, the infective potential and mode of transmission of NANBH were later confirmed in 1978 when chimpanzees were infected with the virus following injection of human blood from patients confirmed to have the disease.[8][9][10] Experiments using chimpanzee models contributed to the discovery of various NANBH variants and agents, and allowed scientists to study the pathological aspects of hepatic injury in these infections.[11][12][13]
Discovery of Hepatitis C Virus
The attempt to discover the first NANBH antibodies persisted for several years. Although the virus was originally described in the early 1970s, the identification of NANBH-specific antibodies did not occur until 1985 by Yohko Shimizu and colleagues.[14] In 1989, the full isolation of a cDNA clone 5-1-1 derived from NANBH genome was finally successful. Qui-Lim Choo and colleagues were able to construct a random-primed complementary DNA (cDNA) from plasma containing an NANBH agent, showing that the virus is a positive-stranded RNA molecule composed of of 10,000 nucleotides.[15] With the isolation of the new agent, hepatitis C virus (HCV) was discovered and the term replaced NANBH for the first time in 1989.[15] In the early 1990s, HCV was then classified into 6 major genotypes and several subtypes.[16] Due to similarity with other RNA viruses, it was considered a type member of the genus Hepacivirus within the family Flavividae.[16][17][18]
Sequelae Following HCV Discovery
Following its discovery, blood tests were available by 1990 to detect the presence of HCV in blood products, to determine chronicity, and to reveal its association with such cancers as hepatocellular carcinoma and non-Hodgkin's lymphoma.[19][20][18] Worldwide efforts to prevent the transmission of HCV have been somewhat successful in reducing the global burden of HCV.[18]
Global Awareness Campaigns
In 2007, the World Health Organization (WHO) declared July 28 "World Hepatitis Day" and the Centers for Disease Control and Prevention (CDC) launched its national "Know More Hepatitis" campaign on July 28, 2011 to educate individuals born between 1945-1965 about HCV and launched its "Hepatitis Testing Day" on May 19, 2012.
Use of First Antiviral Drug for HCV
Even before the identification of the virus in 1989, the first effort to develop interferon-alpha (IFN-a) treatment against HCV was initiated in 1986 by Jay Houston Hoofnagle and colleagues.[21] The drug was finally approved by the Food and Drug Administration (FDA) for HCV treatment in 1991 at a dose of 3 million units subcutaneously three times weekly for 6 months. Ribavirin was first used to treat HCV in 1997; the drug demonstrated a decrease in aminotransferase levels and a good safety profile, despite its lack of antiviral activity as monotherapy.[22] The FDA approved the use of combination therapy of interferon alpha and ribavirin in 1998. Finally, pegylated interferon, which has a higher concentration and longer half life than interferon, was approved for use in 2001. Newer HCV protease inhibitors, such as telepravir, were developed in 2007.
References
- ↑ 1.0 1.1 Prince AM, Brotman B, Grady GF, Kuhns WJ, Hazzi C, Levine RW; et al. (1974). "Long-incubation post-transfusion hepatitis without serological evidence of exposure to hepatitis-B virus". Lancet. 2 (7875): 241–6. PMID 4136143.
- ↑ 2.0 2.1 Feinstone SM, Kapikian AZ, Purcell RH, Alter HJ, Holland PV (1975). "Transfusion-associated hepatitis not due to viral hepatitis type A or B." N Engl J Med. 292 (15): 767–70. doi:10.1056/NEJM197504102921502. PMID 163436.
- ↑ Seeff LB, Zimmerman HJ, Wright EC, Finkelstein JD, Garcia-Pont P, Greenlee HB; et al. (1977). "A randomized, double blind controlled trial of the efficacy of immune serum globulin for the prevention of post-transfusion hepatitis. A Veterans Administration cooperative study". Gastroenterology. 72 (1): 111–21. PMID 318578.
- ↑ Alter HJ, Holland PV, Morrow AG, Purcell RH, Feinstone SM, Moritsugu Y (1975). "Clinical and serological analysis of transfusion-associated hepatitis". Lancet. 2 (7940): 838–41. PMID 53329.
- ↑ Berman M, Alter HJ, Ishak KG, Purcell RH, Jones EA (1979). "The chronic sequelae of non-A, non-B hepatitis". Ann Intern Med. 91 (1): 1–6. PMID 464417.
- ↑ Aach RD, Szmuness W, Mosley JW, Hollinger FB, Kahn RA, Stevens CE; et al. (1981). "Serum alanine aminotransferase of donors in relation to the risk of non-A,non-B hepatitis in recipients: the transfusion-transmitted viruses study". N Engl J Med. 304 (17): 989–94. doi:10.1056/NEJM198104233041701. PMID 6782484.
- ↑ Hoofnagle JH, Alter HJ (1985). "Chronic non-A, non-B hepatitis". Prog Clin Biol Res. 182: 63–9. PMID 3929263.
- ↑ Alter HJ, Purcell RH, Holland PV, Popper H (1978). "Transmissible agent in non-A, non-B hepatitis". Lancet. 1 (8062): 459–63. PMID 76017.
- ↑ Tabor E, Gerety RJ, Drucker JA, Seeff LB, Hoofnagle JH, Jackson DR; et al. (1978). "Transmission of non-A, non-B hepatitis from man to chimpanzee". Lancet. 1 (8062): 463–6. PMID 76018.
- ↑ Hollinger FB, Gitnick GL, Aach RD, Szmuness W, Mosley JW, Stevens CE; et al. (1978). "Non-A, non-B hepatitis transmission in chimpanzees: a project of the transfusion-transmitted viruses study group". Intervirology. 10 (1): 60–8. PMID 632054.
- ↑ Shimizu YK, Feinstone SM, Purcell RH, Alter HJ, London WT (1979). "Non-A, non-B hepatitis: ultrastructural evidence for two agents in experimentally infected chimpanzees". Science. 205 (4402): 197–200. PMID 451589.
- ↑ Hollinger FB, Mosley JW, Szmuness W, Aach RD, Peters RL, Stevens C (1980). "Transfusion-transmitted viruses study: experimental evidence for two non-A, non-B, hepatitis agents". J Infect Dis. 142 (3): 400–7. PMID 6255037.
- ↑ Trépo C, Vitvitski L, Hantz O (1981). "Non-A, Non-B hepatitis virus: identification of a core antigen-antibody system that cross reacts with hepatitis B core antigen and antibody". J Med Virol. 8 (1): 31–47. PMID 6795310.
- ↑ Shimizu YK, Oomura M, Abe K, Uno M, Yamada E, Ono Y; et al. (1985). "Production of antibody associated with non-A, non-B hepatitis in a chimpanzee lymphoblastoid cell line established by in vitro transformation with Epstein-Barr virus". Proc Natl Acad Sci U S A. 82 (7): 2138–42. PMC 397508. PMID 2984683.
- ↑ 15.0 15.1 Choo QL, Kuo G, Weiner AJ, Overby LR, Bradley DW, Houghton M (1989). "Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome". Science. 244 (4902): 359–62. PMID 2523562.
- ↑ 16.0 16.1 Simmonds P, Holmes EC, Cha TA, Chan SW, McOmish F, Irvine B; et al. (1993). "Classification of hepatitis C virus into six major genotypes and a series of subtypes by phylogenetic analysis of the NS-5 region". J Gen Virol. 74 ( Pt 11): 2391–9. PMID 8245854.
- ↑ Bukh J, Miller RH, Purcell RH (1995). "Genetic heterogeneity of hepatitis C virus: quasispecies and genotypes". Semin Liver Dis. 15 (1): 41–63. doi:10.1055/s-2007-1007262. PMID 7597443.
- ↑ 18.0 18.1 18.2 Houghton M (2009). "Discovery of the hepatitis C virus". Liver Int. 29 Suppl 1: 82–8. doi:10.1111/j.1478-3231.2008.01925.x. PMID 19207970.
- ↑ Colombo M, Kuo G, Choo QL, Donato MF, Del Ninno E, Tommasini MA; et al. (1989). "Prevalence of antibodies to hepatitis C virus in Italian patients with hepatocellular carcinoma". Lancet. 2 (8670): 1006–8. PMID 2572740.
- ↑ Craxì A, Laffi G, Zignego AL (2008). "Hepatitis C virus (HCV) infection: a systemic disease". Mol Aspects Med. 29 (1–2): 85–95. doi:10.1016/j.mam.2007.09.017. PMID 18177700.
- ↑ Hoofnagle JH, Mullen KD, Jones DB, Rustgi V, Di Bisceglie A, Peters M; et al. (1986). "Treatment of chronic non-A,non-B hepatitis with recombinant human alpha interferon. A preliminary report". N Engl J Med. 315 (25): 1575–8. doi:10.1056/NEJM198612183152503. PMID 3097544.
- ↑ Bodenheimer HC, Lindsay KL, Davis GL, Lewis JH, Thung SN, Seeff LB (1997). "Tolerance and efficacy of oral ribavirin treatment of chronic hepatitis C: a multicenter trial". Hepatology. 26 (2): 473–7. doi:10.1002/hep.510260231. PMID 9252161.
Pathophysiology
|
Hepatitis C |
|
Diagnosis |
|
Treatment |
|
Hepatitis C On the Web |
|
American Roentgen Ray Society Images of Hepatitis C |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [6]; Associate Editor(s)-In-Chief: Yazan Daaboul, Serge Korjian, Seyedmahdi Pahlavani, M.D. [7], Javaria Anwer M.D.[8]
Overview
In isolated acute HCV infection, the host immune system stimulates the secretion of interferon alpha and the activation of natural killer cells, which is followed by the activation of the adaptive immune system. Chronic HCV is characterized by the impairment of these mechanisms. Eventually, chronic HCV infection leads to local inflammation and fibrogenesis, which cause hepatic injury and cirrhosis. Hepatocellular carcinoma, a known complication of chronic HCV infection, arises in cases of cirrhosis; the role of oncogenic proteins of HCV in the pathogenesis of hepatocellular carcinoma has yet to be elucidated.
Transmission
The transmission of HCV can be defined as percutaneous, sexual, healthcare-associated, or maternal-infant in nature.
Percutaneous Transmission
- Blood and blood components transfusion
- More than 90% of seronegative recipients who are transfused with blood from HCV-antibody positive donors will acquire infection.[1]
- Contaminated shared needles among intravenous drug users
- Before 1992, at least two-thirds of new HCV infections in the United States were associated with illicit drug use; the number has since decreased significantly.[2]
- Chronic hemodialysis
- The frequency of anti-HCV in patients on hemodialysis ranges from less than 10% in the United States to 55% to 85% in Jordan, Saudi Arabia, and Iran.[3]
Sexual Transmission
- HCV RNA has been detected in semen and saliva.[4] People with multiple sexual partners and commercial sex workers have a high HCV prevalence.[5]
Health care Associated
- Nosocomial transmission has been observed under several different conditions (e.g. needle stick, organ transplant, during surgery); now, however, because of infection control protocols, nosocomial transmission of HCV is rare except in cases of breach of protocols.[6][7]
Maternal Infant Transmission
HCV Clearance and Persistence
Acute viral infection and HCV replication triggers the activation of host immune responses, first by secretion of type I interferon alpha (IFN-alpha) and activation of natural killer (NK) cells. Nonetheless, secretion of endogenous IFN does not seem to effectively inhibit HCV replication.[10][11][12]
HCV proteins play a crucial role in inhibiting IFN-alpha effectors, such as IFN regulatory factor-3 (IRF-3), double stranded RNA-dependent protein kinase (PKR), and the JAK-STAT signaling pathway.[13][14][15] More importantly, chronic carriage of HCV is associated with impaired activation of NK cells despite IFN-alpha secretion. It is believed that the cross-linking of CD81 and the envelope protein E2 of the virus is a key mechanism by which NK cells are inactivated and INF-gamma is not produced by these cells.[16]
The activation of IFN-gamma is a prerequisite for the appropriate clearance of HCV. When activation occurs normally, antibodies start to form 7-31 weeks later.[17][18][19][20] While most epitopes for antibodies have not been discovered yet, hypervariable region 1 (HVR1) of the E2 envelope glycoprotein was found to be a target for anti-HVR1 antibodies. Antibodies play a role in clearing the virus from the host. It is currently unknown whether "escape" mechanisms are present in HCV that favor persistent HCV infection despite an adequate antibody response.[17][18][19][20]
Similarly, the activation of the CD4+ and CD8+ T-cell response is required for viral clearance. This cellular response allows for the development of long-term immunity against HCV.[21] Studies also proved that delayed or inadequate activation of T-cell response is associated with persistence of infection. It is not known why T-cell response may fail in response to acute infection, but it is hypothesized that persistence might be related to viral inhibition of T-cell maturation, defective dendritic cells, and/or failure of interleukin (IL) 12 activation.[21][22][23][24][25][26]
Liver Injury and Cirrhosis, and Hepatocellular Carcinoma
HCV is directly associated with hepatic steatosis, which is fat accumulation in the liver. It seems that core proteins may play a role in regulating lipid accumulation in hepatocytes, contributing to steatosis. However, steatosis is not observed in all genotypes of HCV infection; it is classically described in genotype 3, which perhaps is the only genotype that has a direct role in the development of steatosis irrespective of alcohol consumption or metabolic elements. Apart from steatosis, HCV per se has not been shown to have damaging effects on hepatocytes. The viral burden also does not seem to be directly related to the extent of liver injury.[27][28][29][30][31][32][33]
In chronic hepatitis C infections, the local immune response leads to portal lymphoid infiltration and chronic inflammation, which give way to bridging necrosis and degenerative lobular lesions.[16] Hepatic injury is directly associated with the degree of Th1 cytokine expression. The adaptive immune system, namely the cytotoxic T-cell response, injures infected cells as well as bystander cells. Nonetheless, it has not been confirmed whether the number of cytotoxic T cells is associated with the extent of liver injury.
Chronic inflammation ultimately leads to fibrogenesis due to deposition extracellular matrix elements in hepatic parenchyma. It is unknown whether viral components are directly responsible in the particular mechanism of hepatic cirrhosis in chronic HCV infection; although cirrhosis is definitely worsened in HCV patients who are also exposed to other risk factors, such as alcohol, obesity, and HIV.[16]
Hepatocellular Carcinoma
Hepatocellular carcinoma (HCC) occurs following chronic HCV infection complicated by liver cirrhosis. The precise role of HCV components in the development of HCC is poorly understood. Pinpointing which viral protein is directly related to carcinogenesis has been difficult, but studies have shown that NS3, NS4B, and NS5A all have oncogenic properties.[34][35][36][37][38]
Histology
Click on the arrow to view the pathologic findings in viral hepatitis: {{#ev:youtube|_hXvbpSxFZw}}
Mechanisms involved in extra-hepatic manifestations
- Cryoglobulinemic vasculitis: Chronic antigen stimulation reduces the threshold for activation and proliferation of B-lymphocyte and induces Bcl-2 activation and t(14;18) translocation. It results in decreased apoptosis. As a result, CD21−CD27+ cells produce antibodies against the Fc portion of IgG, forming immune complexes that precipitate in small blood vessels.[39]
- B-cell lymphoma: A continuous HCV antigen stimulation and permanent genetic damage caused by viral proteins cause clonal proliferation of CD21−CD27+. It also down-regulates tumor-suppressive signals (such as, microRNA-26b). Oncogenic signals are further enhanced and additional tumor suppressor genes such as Bcl-6, p53, and β-catenin undergo mutation. Hence, the reduced levels of caspase 3, 7, and 9 reduce their sensitivity to Fas-induced apoptosis.[39]
- Cardiovascular disease: Local vascular damage is caused by an increased expression of adhesion molecules on endothelial surface. Smooth cells in the media proliferate and apoptosis is inhibited, with local macrophages producing proinflammatory cytokines and free radicals. These processes result in accelerated atherosclerosis, procoagulant effects, and lead to major cardiovascular events.[39]
- Chronic kidney disease: Direct HCV cytopathic effect, chronic inflammation from atherosclerosis and insulin resistance, endothelial and mesangial inflammation, and podocyte and tubular injury caise CKD. Cryoprecipitates deposit at glomeruli also manifested as type I membranoproliferative glomerulonephritis.[39]
- Type 2 diabetes: Caused by both hepatic and peripheral insulin resistance. In the liver, HCV leads to PI3K-AKT insulin-signaling pathway reduction via insulin receptor substrate 1 inhibition and impaired Glut2–mediated hepatic glucose intake. In the extrahepatic tissue, insulin resistance is a consequence of soluble endocrine mediators released by hepatocytes. Up-regulation of TNF, G6P, and resistin, with an imbalance in the adipocytokine profile, increases gluconeogenesis in these sites.[39]
References
- ↑ Vrielink H, van der Poel CL, Reesink HW, Zaaijer HL, Scholten E, Kremer LC, Cuypers HT, Lelie PN, van Oers MH (1995). "Look-back study of infectivity of anti-HCV ELISA-positive blood components". Lancet. 345 (8942): 95–6. PMID 7815889.
- ↑ Alter MJ (1997). "Epidemiology of hepatitis C". Hepatology. 26 (3 Suppl 1): 62S–65S. doi:10.1002/hep.510260711. PMID 9305666.
- ↑ Jadoul M, Barril G (2012). "Hepatitis C in hemodialysis: epidemiology and prevention of hepatitis C virus transmission". Contrib Nephrol. 176: 35–41. doi:10.1159/000333761. PMID 22310779.
- ↑ Liou TC, Chang TT, Young KC, Lin XZ, Lin CY, Wu HL (1992). "Detection of HCV RNA in saliva, urine, seminal fluid, and ascites". J. Med. Virol. 37 (3): 197–202. PMID 1331308.
- ↑ van Doornum GJ, Hooykaas C, Cuypers MT, van der Linden MM, Coutinho RA (1991). "Prevalence of hepatitis C virus infections among heterosexuals with multiple partners". J. Med. Virol. 35 (1): 22–7. PMID 1940879.
- ↑ Martínez-Bauer E, Forns X, Armelles M, Planas R, Solà R, Vergara M, Fàbregas S, Vega R, Salmerón J, Diago M, Sánchez-Tapias JM, Bruguera M (2008). "Hospital admission is a relevant source of hepatitis C virus acquisition in Spain". J. Hepatol. 48 (1): 20–7. doi:10.1016/j.jhep.2007.07.031. PMID 17998149.
- ↑ Alter MJ (2008). "Healthcare should not be a vehicle for transmission of hepatitis C virus". J. Hepatol. 48 (1): 2–4. doi:10.1016/j.jhep.2007.10.007. PMID 18023493.
- ↑ Ohto H, Terazawa S, Sasaki N, Sasaki N, Hino K, Ishiwata C, Kako M, Ujiie N, Endo C, Matsui A (1994). "Transmission of hepatitis C virus from mothers to infants. The Vertical Transmission of Hepatitis C Virus Collaborative Study Group". N. Engl. J. Med. 330 (11): 744–50. doi:10.1056/NEJM199403173301103. PMID 8107740.
- ↑ Zanetti AR, Tanzi E, Paccagnini S, Principi N, Pizzocolo G, Caccamo ML, D'Amico E, Cambiè G, Vecchi L (1995). "Mother-to-infant transmission of hepatitis C virus. Lombardy Study Group on Vertical HCV Transmission". Lancet. 345 (8945): 289–91. PMID 7530793.
- ↑ Thimme R, Oldach D, Chang KM, Steiger C, Ray SC, Chisari FV (2001). "Determinants of viral clearance and persistence during acute hepatitis C virus infection". J Exp Med. 194 (10): 1395–406. PMC 2193681. PMID 11714747.
- ↑ Thimme R, Bukh J, Spangenberg HC, Wieland S, Pemberton J, Steiger C; et al. (2002). "Viral and immunological determinants of hepatitis C virus clearance, persistence, and disease". Proc Natl Acad Sci U S A. 99 (24): 15661–8. doi:10.1073/pnas.202608299. PMC 137773. PMID 12441397.
- ↑ Su AI, Pezacki JP, Wodicka L, Brideau AD, Supekova L, Thimme R; et al. (2002). "Genomic analysis of the host response to hepatitis C virus infection". Proc Natl Acad Sci U S A. 99 (24): 15669–74. doi:10.1073/pnas.202608199. PMC 137774. PMID 12441396.
- ↑ Katze MG, He Y, Gale M (2002). "Viruses and interferon: a fight for supremacy". Nat Rev Immunol. 2 (9): 675–87. doi:10.1038/nri888. PMID 12209136.
- ↑ Foy E, Li K, Wang C, Sumpter R, Ikeda M, Lemon SM; et al. (2003). "Regulation of interferon regulatory factor-3 by the hepatitis C virus serine protease". Science. 300 (5622): 1145–8. doi:10.1126/science.1082604. PMID 12702807.
- ↑ Blindenbacher A, Duong FH, Hunziker L, Stutvoet ST, Wang X, Terracciano L; et al. (2003). "Expression of hepatitis c virus proteins inhibits interferon alpha signaling in the liver of transgenic mice". Gastroenterology. 124 (5): 1465–75. PMID 12730885.
- ↑ 16.0 16.1 16.2 Pawlotsky JM (2004). "Pathophysiology of hepatitis C virus infection and related liver disease". Trends Microbiol. 12 (2): 96–102. doi:10.1016/j.tim.2003.12.005. PMID 15036326.
- ↑ 17.0 17.1 Farci P, Alter HJ, Wong DC, Miller RH, Govindarajan S, Engle R; et al. (1994). "Prevention of hepatitis C virus infection in chimpanzees after antibody-mediated in vitro neutralization". Proc Natl Acad Sci U S A. 91 (16): 7792–6. PMC 44488. PMID 7519785.
- ↑ 18.0 18.1 Shimizu YK, Igarashi H, Kiyohara T, Cabezon T, Farci P, Purcell RH; et al. (1996). "A hyperimmune serum against a synthetic peptide corresponding to the hypervariable region 1 of hepatitis C virus can prevent viral infection in cell cultures". Virology. 223 (2): 409–12. doi:10.1006/viro.1996.0497. PMID 8806581.
- ↑ 19.0 19.1 Bartosch B, Dubuisson J, Cosset FL (2003). "Infectious hepatitis C virus pseudo-particles containing functional E1-E2 envelope protein complexes". J Exp Med. 197 (5): 633–42. PMC 2193821. PMID 12615904.
- ↑ 20.0 20.1 Puntoriero G, Meola A, Lahm A, Zucchelli S, Ercole BB, Tafi R; et al. (1998). "Towards a solution for hepatitis C virus hypervariability: mimotopes of the hypervariable region 1 can induce antibodies cross-reacting with a large number of viral variants". EMBO J. 17 (13): 3521–33. doi:10.1093/emboj/17.13.3521. PMC 1170689. PMID 9649423.
- ↑ 21.0 21.1 Bertoletti A, Ferrari C (2003). "Kinetics of the immune response during HBV and HCV infection". Hepatology. 38 (1): 4–13. doi:10.1053/jhep.2003.50310. PMID 12829979.
- ↑ Bain C, Fatmi A, Zoulim F, Zarski JP, Trépo C, Inchauspé G (2001). "Impaired allostimulatory function of dendritic cells in chronic hepatitis C infection". Gastroenterology. 120 (2): 512–24. PMID 11159892.
- ↑ Wedemeyer H, He XS, Nascimbeni M, Davis AR, Greenberg HB, Hoofnagle JH; et al. (2002). "Impaired effector function of hepatitis C virus-specific CD8+ T cells in chronic hepatitis C virus infection". J Immunol. 169 (6): 3447–58. PMID 12218168.
- ↑ Lechner F, Wong DK, Dunbar PR, Chapman R, Chung RT, Dohrenwend P; et al. (2000). "Analysis of successful immune responses in persons infected with hepatitis C virus". J Exp Med. 191 (9): 1499–512. PMC 2213430. PMID 10790425.
- ↑ Appay V, Dunbar PR, Callan M, Klenerman P, Gillespie GM, Papagno L; et al. (2002). "Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections". Nat Med. 8 (4): 379–85. doi:10.1038/nm0402-379. PMID 11927944.
- ↑ Kittlesen DJ, Chianese-Bullock KA, Yao ZQ, Braciale TJ, Hahn YS (2000). "Interaction between complement receptor gC1qR and hepatitis C virus core protein inhibits T-lymphocyte proliferation". J Clin Invest. 106 (10): 1239–49. doi:10.1172/JCI10323. PMC 381434. PMID 11086025.
- ↑ Poynard T, Ratziu V, McHutchison J, Manns M, Goodman Z, Zeuzem S; et al. (2003). "Effect of treatment with peginterferon or interferon alfa-2b and ribavirin on steatosis in patients infected with hepatitis C." Hepatology. 38 (1): 75–85. doi:10.1053/jhep.2003.50267. PMID 12829989.
- ↑ Barba G, Harper F, Harada T, Kohara M, Goulinet S, Matsuura Y; et al. (1997). "Hepatitis C virus core protein shows a cytoplasmic localization and associates to cellular lipid storage droplets". Proc Natl Acad Sci U S A. 94 (4): 1200–5. PMC 19768. PMID 9037030.
- ↑ Rubbia-Brandt L, Quadri R, Abid K, Giostra E, Malé PJ, Mentha G; et al. (2000). "Hepatocyte steatosis is a cytopathic effect of hepatitis C virus genotype 3". J Hepatol. 33 (1): 106–15. PMID 10905593.
- ↑ Serfaty L, Andreani T, Giral P, Carbonell N, Chazouillères O, Poupon R (2001). "Hepatitis C virus induced hypobetalipoproteinemia: a possible mechanism for steatosis in chronic hepatitis C." J Hepatol. 34 (3): 428–34. PMID 11322205.
- ↑ Castéra L, Hézode C, Roudot-Thoraval F, Bastie A, Zafrani ES, Pawlotsky JM; et al. (2003). "Worsening of steatosis is an independent factor of fibrosis progression in untreated patients with chronic hepatitis C and paired liver biopsies". Gut. 52 (2): 288–92. PMC 1774979. PMID 12524415.
- ↑ Sulkowski MS, Thomas DL (2003). "Hepatitis C in the HIV-Infected Person". Ann Intern Med. 138 (3): 197–207. PMID 12558359.
- ↑ Pol S, Vallet-Pichard A, Fontaine H, Lebray P (2002). "HCV infection and hemodialysis". Semin Nephrol. 22 (4): 331–9. PMID 12118398.
- ↑ National Institutes of Health (2002). "National Institutes of Health Consensus Development Conference Statement: Management of hepatitis C: 2002--June 10-12, 2002". Hepatology. 36 (5 Suppl 1): S3–20. doi:10.1053/jhep.2002.37117. PMID 12407572.
- ↑ Ray RB, Lagging LM, Meyer K, Ray R (1996). "Hepatitis C virus core protein cooperates with ras and transforms primary rat embryo fibroblasts to tumorigenic phenotype". J Virol. 70 (7): 4438–43. PMC 190377. PMID 8676467.
- ↑ Sakamuro D, Furukawa T, Takegami T (1995). "Hepatitis C virus nonstructural protein NS3 transforms NIH 3T3 cells". J Virol. 69 (6): 3893–6. PMC 189112. PMID 7745741.
- ↑ Park JS, Yang JM, Min MK (2000). "Hepatitis C virus nonstructural protein NS4B transforms NIH3T3 cells in cooperation with the Ha-ras oncogene". Biochem Biophys Res Commun. 267 (2): 581–7. doi:10.1006/bbrc.1999.1999. PMID 10631105.
- ↑ Ghosh AK, Steele R, Meyer K, Ray R, Ray RB (1999). "Hepatitis C virus NS5A protein modulates cell cycle regulatory genes and promotes cell growth". J Gen Virol. 80 ( Pt 5): 1179–83. PMID 10355764.
- ↑ 39.0 39.1 39.2 39.3 39.4 Cacoub P, Desbois AC, Comarmond C, Saadoun D (November 2018). "Impact of sustained virological response on the extrahepatic manifestations of chronic hepatitis C: a meta-analysis". Gut. 67 (11): 2025–2034. doi:10.1136/gutjnl-2018-316234. PMID 29703790.
Epidemiology and Demographics
|
Hepatitis C |
|
Diagnosis |
|
Treatment |
|
Hepatitis C On the Web |
|
American Roentgen Ray Society Images of Hepatitis C |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [9]; Associate Editor(s)-In-Chief: Yazan Daaboul, Serge Korjian, Seyedmahdi Pahlavani, M.D. [10], Javaria Anwer M.D.[11]
Overview
Hepatitis C is a major health problem that affects approximately 2 to 4 million people in the United States, 5 to 10 million people in Europe, and 12 million people in India. Approximately 150,000 new cases occur annually in the United States and in Western Europe, although accurate incidence rates are difficult to estimate given the asymptomatic nature of the early stages of the disease. While the prevalence of the disease appears to be declining, hepatitis C is still highly prevalent in certain areas of the world. Egypt is the country with the highest prevalence of HCV, HCV-associated cirrhosis, and hepatocellular carcinoma, and the prevalence tends to increase with age, suggesting ongoing development of new cases of HCV. Approximately one-fourth of all cases of cirrhosis and hepatocellular carcinoma are attributed to HCV worldwide. Hepatitis C affects males and females equally.
Epidemiology and Demographics
Incidence and Prevalence
- According to the World Health Organization (WHO), approximately 3% of the global population are infected with chronic hepatitis C virus (HCV). More than 170 million people are infected chronically around the world. The prevalence of HCV varies among different nations; for example, 1.3% to 1.6% of the U.S. population are affected, while up to 30% of Egypt population are infected with hepatitis C virus.[1]
- Figures in individual countries also vary greatly: Approximately 2-4 million persons are infected with chronic HCV in the United States, 5-10 million in Europe, and more than 10 million in India.[2]
- Acute HCV infection follows an asymptomatic course, which makes the accurate determination of HCV incidence difficult. Additionally, many countries lack sufficient epidemiological data. Nonetheless, it is estimated that approximately 150,000 new cases are reported in the United States and Western Europe annually, whereas the incidence in Japan is as high as 350,000 new cases each year. More than 60-80% of patients with HCV infection become chronic carriers of the disease, with an overall number of chronic carriers reaching approximately 170 million patients. The trend today is marked by a progressive decrease in new HCV infections, characterized by a remarkable 80% decrease since the infection was first discovered in 1989-1990.[2]
- In 2014, a total of 2,194 cases of acute hepatitis C were reported to the CDC from 40 states.
- The overall incidence rate for 2014 was 0.7 cases per 100,000 people, an increase from 2010–2012.[3]

Prevalence of HCV rises significantly in specific populations[2]:
- Intravenous drug users: > 70% (Accounts for most modern cases of HCV infection)
- Hemophilia patients: > 70%
- Hemodialysis: 20-30%
Of note, nosocomial sources of HCV infection, such as infected blood and surgical products, have been significantly reduced due to the increased testing of products prior to utilization.[2]

- The morbidity associated wth chronic hepatitis C, mainly due to complications is estimated to be 350,000 liver-related deaths per year.[4]
Age
- The age of infected patients varies across regions. In the United States, Australia, and Western Europe, more than 65% of HCV infections are observed in patients between 30-50 years.[5] These numbers suggest that most cases of HCV in these regions occurred before 1990. On the other hand, there is an increase of HCV prevalence with age in countries such as Turkey, Spain, Italy, Japan, China, and Egypt. Most patients in these countries are older than 50 years of age.[5]
- In 2014, among all age groups, people ages 20–29 years had the highest rate (2.20 cases per 100,000 people) and people aged 0–19 and ≥60 years had the lowest rate (0.12 cases per 100,000 people) of acute hepatitis C.[3]
Gender
In 2014, rates of HCV among males and females in the United States were 0.8 and 0.7 cases per 100,000 people, respectively.[3]
Morbidity and Mortality
Approximately 27% of cases of cirrhosis and 25% of hepatocellular carcinoma (HCC) are attributed to chronic HCV infection.[5]
Geographic Distribution
HCV is a global disease. The most highly endemic region of HCV—especially genotype 4a—is Egypt, due to a history of non-hygenic medical and paramedical practices in the country.[5] As many as 25% of Egyptian blood donors are chronic carriers of HCV infection. In contrast, the United Kingdom and Scandinavia have a low prevalence of HCV compared to other regions.[5]
In some countries, HCV is prevalent in specific regions rather than the entire counrty. Such patterns are observed in Italy, China, and Japan.[5]
References
- ↑ Bryan JS, Krasne FB (1977). "Presynaptic inhibition: the mechanism of protection from habituation of the crayfish lateral giant fiber escape response". J. Physiol. (Lond.). 271 (2): 369–90. PMC 1353577. PMID 200735.
- ↑ 2.0 2.1 2.2 2.3 World Health Organization. Global Alert Response. Hepatitis C: Surveillance and control. Accessed online on July 27, 2014. http://www.who.int/csr/disease/hepatitis/whocdscsrlyo2003/en/index4.html
- ↑ 3.0 3.1 3.2 "Commentary | U.S. 2014 Surveillance Data for Viral Hepatitis | Statistics & Surveillance | Division of Viral Hepatitis | CDC". Retrieved October 5, 2016.
- ↑ Cacoub, Patrice; Longo, Dan L.; Saadoun, David (2021). "Extrahepatic Manifestations of Chronic HCV Infection". New England Journal of Medicine. 384 (11): 1038–1052. doi:10.1056/NEJMra2033539. ISSN 0028-4793.
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 Alter MJ (2007). "Epidemiology of hepatitis C virus infection". World J Gastroenterol. 13 (17): 2436–41. PMID 17552026.
Risk Factors
|
Hepatitis C |
|
Diagnosis |
|
Treatment |
|
Hepatitis C On the Web |
|
American Roentgen Ray Society Images of Hepatitis C |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [12]; Associate Editor(s)-In-Chief: Yazan Daaboul, Serge Korjian;Javaria Anwer M.D.[13]
Overview
The most potent risk factor in the development of hepatitis C is intravenous drug use. Other risk factors include occupational exposure to blood, sexual intercourse with infected individuals, multiple bloods transfusions prior to 1992, and HIV infection.
Risk Factors
Percutaneous exposure to blood is the primary mode of HCV transmission. The following are the most important risk factors for HCV infection:[1][2]:
- Individuals are majorly infected via percutaneous exposure to infected blood. Most persons with HCV were infected.
- Injecting drug use is the most important risk factors nowadays
- Transfusion of blood and blood products, especially before 1992
- Unsafe therapeutic injections, especially in hemophilia patients prior to 1987
Other, less important risk factors include:[1][2]
- Hemodialysis (Higher rates of infection are observed)
- Solid organ transplantation from infected donors
- Occupational exposure to blood, such as contaminated needle sticks
- Birth to infected mother in cases of detectable maternal HCV PCR at delivery (at the rate of 4%–5%). Breastfeeding is not associated with the transmission.
- Sexual intercourse with infected partner
- Sexual intercourse with multiple partners
- HIV infection
- Tattoo or piercing with infected needle sticks (low risk for transmission after strict infection control measures)
References
Screening
|
Hepatitis C |
|
Diagnosis |
|
Treatment |
|
Hepatitis C On the Web |
|
American Roentgen Ray Society Images of Hepatitis C |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [14]; Associate Editor(s)-In-Chief: Yazan Daaboul, Serge Korjian
Overview
People living in regions with high HCV prevalence and who have engaged in high-risk activities should be screened. Screening by serological testing, confirmed by nucleic acid amplification (NAT) for HCV RNA, is required. Additionally, screening for other bloodborne infections, such as HBV and HIV, is required once the diagnosis is made. The ideal frequency of testing in these patients is unclear and should be individualized according to the frequency of exposure to risk.
Screening
Initial Testing
Screening for HCV is performed by HCV serological testing.[1] In patients who test positive, a confirmation for chronic HCV status is required by nucleic acid amplification (NAT) or HCV RNA. HCV RNA may be directly tested in immunocompromised patients or patients who already had spontaneous or treatment-related clearance.[1]
Screening for other infections, such as HBV and HIV, is also indicated when patients are identified as HCV-positive. In some endemic areas and high-risk populations, screening for tuberculosis (TB) is also warranted.[1]
Interpretation of Screening Results
- If an anti-HCV test is negative and patients are suspected to have liver disease, a follow-up HCV antibody or HCV RNA test is recommended if HCV exposure occurs within 6 months.[1]
- If an anti-HCV test is positive but HCV RNA is negative, patients have no evidence of active infection.[1]
- If an anti-HCV test is positive and HCV RNA is positive, a diagnosis of HCV infection is made. Quantitative HCV RNA tests should be performed before initiation of antiviral therapy to document baseline levels of viremia. Additionally, HCV genotyping is recommended to guide therapy.[1]
Frequency of Screening
The ideal frequency of testing in patients is unclear and should be individualized according to frequency of exposure to risk. In patients who use intravenous drugs or HIV-positive men who have unprotected sex with men, annual screening may be considered.[2]
Summary of Screening Recommendations
American Association for the Study of Liver Disease (AASLD) - Infectious Diseases Society of America (IDSA) "Recommendations for Testing, Managing, and Treating Hepatitis C": 2014[1]
According to the AASLD - IDSA recommendations in 2014, the following patients should be screened for HCV:
- People born between 1945 and 1965
Risk Behaviors
- Injection-drug use (current or ever, including those who injected once)
- Intranasal illicit drug use
Risk Exposures
- Long-term hemodialysis (ever)
- Getting a tattoo in an unregulated setting
- Healthcare, emergency medical, and public safety workers after needle sticks, sharps, or mucosal exposures to HCV-infected blood
- Children born to HCV-infected women
- Prior recipients of transfusions or organ transplants, including persons who:
- were notified that they received blood from a donor who later tested positive for HCV infection
- received a transfusion of blood or blood components or underwent an organ transplant before July 1992
- received clotting factor concentrates produced before 1987
- were ever incarcerated
Other Medical Conditions
- HIV infection
- Unexplained chronic liver disease and chronic hepatitis including elevated alanine aminotransferase levels
World Health Organization "Guidelines for the Screening, Care, and Treatment of Persons with HCV": 2014
Generally, the World Health Organization (WHO) Guidelines for the Screening, Care, and Treatment of Persons with Hepatitis C Infection[1] published on April 2014 recommends HCV screening for all people living in regions of high HCV prevalence with positive history for risk exposure and behavior.[1]
Screening includes:[1]
- Persons who received medical or dental interventions in health-care settings where infection control practices are substandard
- Persons who received blood transfusions prior to the time when serological testing of blood donors for HCV was initiated
- Persons who received blood transfusions in countries where serological testing of blood donations for HCV is not routinely performed
- Persons who inject drugs (PWID)
- Persons who have had tattoos, body piercings, or scarification procedures are done where infection control practices are substandard
- Persons with HIV infection
- Persons who have used intranasal drugs
- Prisoners and previously incarcerated persons
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 1.9 "World Health Organization (WHO) Guidelines for the Screening, Care, and Treatment of Persons with Hepatitis C Infection" (PDF). World Health Organization. WHO. April 2014. Retrieved July 27, 2014.
- ↑ Aberg JA, Gallant JE, Ghanem KG, Emmanuel P, Zingman BS, Horberg MA (2014). "Primary care guidelines for the management of persons infected with HIV: 2013 update by the HIV Medicine Association of the Infectious Diseases Society of America". Clin Infect Dis. 58 (1): 1–10. doi:10.1093/cid/cit757. PMID 24343580.
Causes
|
Hepatitis C |
|
Diagnosis |
|
Treatment |
|
Hepatitis C On the Web |
|
American Roentgen Ray Society Images of Hepatitis C |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [15]
Overview
Hepatitis C infection is caused by the hepatitis C virus.
Hepatitis C Virus
Viral Characteristics
The hepatitis C virus (HCV) is a member of the genus Hepacivirus that belongs to the Flaviviridae family. It is an enveloped, single-stranded RNA virus that measures approximately 60 nm in diameter.
Mode of Transmission
HCV is primarily transmitted by blood. Exposure to blood is observed primarily in healthcare settings, such as in blood transfusions, surgical procedures, needle injuries, and hemodialysis. Also, the role of intravenous drug use has recently emerged as a great risk for viral transmission after the relatively successful control of nosocomial HCV transmission.[1]
Life Cycle
Humans are considered the only natural hosts for HCV. The full life cycle of the virus is poorly understood due to difficulty to culture in vitro. The expression of E1-E2, two important envelope glycoprotein complexes, on the surface of HCV allows the virus to interact with host-cell molecules (glycosaminoglycans) by acting as ligands for cellular receptors, such as tetraspanin CD81, scavenger receptor class B type I (SR-BI), and mannose binding lectins DC-SIGN and L-SIGN. This interaction is believed to have a crucial role in cell recognition and cellular tropism.[2][3][4][5][6][7][8][9][10] The exact mechanism by which viral genome enters the host cell is poorly understood, but it is believed to be via receptor-mediated endocytosis. Then envelope glycoproteins utilize pH-dependent mechanisms to mediate fusion of the viral envelope using endosomal membrane.[6][7] As soon as it is released into the cytoplasm, the viral nucleocapsid uncoats by unknown mechanisms.
Template HCV RNA allows viral replication to take place and protein synthesis is thus facilitated. Cap-independent protein translation takes place when ribosomal 40S subunit binds to internal ribosome entry site (IRES).[11] IRES is a stem-loop structure that is located at the 5' untranslated region (UTR) of the virus and the initial 30-40 nucleotides of the viral core-encoding region.[11] Nonetheless, full polyprotein translation also requires the use of 80S ribosomes and the viral 3' UTR, both of which presumably play a role in regulation of the translational process.[12]
Translation is accompanied by co-translational processes and followed by post-translational processes, all of which yield a total of 10 mature proteins.[13]
The following proteins are produced:
Structural Proteins:
- Core (C) protein[12]
- Envelope 1 (E1) glycoprotein[12]
- Envelope 2 (E2) glycoprotein[12]
C, E1, and E2 are separated from the remaining 7 non-structural proteins by the activity of p7, a small membrane polypeptide that belongs to viroporin family.[12] The 3 proteins are released by the activity of signal peptidases mediated by the host cell.
Non-Structural (NS) Proteins:
- NS2: Zn-dependent proteinase[12][14]
- NS3: Serine-dependent proteinase, helicase, and NTPase[12][14]
- NS4A: Cofactor NS3 proteinase[12][14]
- NS4B: Membrane anchor[12][14]
- NS5A: Regulation of RNA polymerase activity and inhibition of antiviral activity of interferon[12][14]
- NS5B: RNA-dependent RNA polymerase[12][14]
- p7: Separation of structural from non-structural proteins and possible formation of ion channel[12][14]
Non-structural proteins NS3 to NS5B play an important role in the formation of a replication complex that includes an intracellular "membranous web", at least partially derived from host endoplasmic reticulum.[15] The replication complex is responsible for synthesis template negative-strand RNA and consequent synthesis of its positive-strand counterpart. These RNA molecules are then enclosed in new virions.
Formation of Nucleocapsid and Envelope
New HCV nucleocapsid is formed by the action of core protein C along with viral genomic positive-strand RNA.[12] The envelope of the newly formed nucleocapsid is later formed by budding action into the lumen of the endoplasmic reticulum. Nonetheless, envelope glycoproteins do not yet mature early on at this stage. When new virions are exported outside the host cell, via cellular secretory mechanisms, glycoproteins of the envelope finally mature.[12]
References
- ↑ National Institutes of Health (2002). "National Institutes of Health Consensus Development Conference Statement: Management of hepatitis C: 2002--June 10-12, 2002". Hepatology. 36 (5 Suppl 1): S3–20. doi:10.1053/jhep.2002.37117. PMID 12407572.
- ↑ Op De Beeck A, Cocquerel L, Dubuisson J (2001). "Biogenesis of hepatitis C virus envelope glycoproteins". J Gen Virol. 82 (Pt 11): 2589–95. PMID 11602769.
- ↑ Penin F, Combet C, Germanidis G, Frainais PO, Deléage G, Pawlotsky JM (2001). "Conservation of the conformation and positive charges of hepatitis C virus E2 envelope glycoprotein hypervariable region 1 points to a role in cell attachment". J Virol. 75 (12): 5703–10. doi:10.1128/JVI.75.12.5703-5710.2001. PMC 114285. PMID 11356980.
- ↑ Barth H, Schafer C, Adah MI, Zhang F, Linhardt RJ, Toyoda H; et al. (2003). "Cellular binding of hepatitis C virus envelope glycoprotein E2 requires cell surface heparan sulfate". J Biol Chem. 278 (42): 41003–12. doi:10.1074/jbc.M302267200. PMID 12867431.
- ↑ Pileri P, Uematsu Y, Campagnoli S, Galli G, Falugi F, Petracca R; et al. (1998). "Binding of hepatitis C virus to CD81". Science. 282 (5390): 938–41. PMID 9794763.
- ↑ 6.0 6.1 Bartosch B, Vitelli A, Granier C, Goujon C, Dubuisson J, Pascale S; et al. (2003). "Cell entry of hepatitis C virus requires a set of co-receptors that include the CD81 tetraspanin and the SR-B1 scavenger receptor". J Biol Chem. 278 (43): 41624–30. doi:10.1074/jbc.M305289200. PMID 12913001.
- ↑ 7.0 7.1 Hsu M, Zhang J, Flint M, Logvinoff C, Cheng-Mayer C, Rice CM; et al. (2003). "Hepatitis C virus glycoproteins mediate pH-dependent cell entry of pseudotyped retroviral particles". Proc Natl Acad Sci U S A. 100 (12): 7271–6. doi:10.1073/pnas.0832180100. PMC 165865. PMID 12761383.
- ↑ Scarselli E, Ansuini H, Cerino R, Roccasecca RM, Acali S, Filocamo G; et al. (2002). "The human scavenger receptor class B type I is a novel candidate receptor for the hepatitis C virus". EMBO J. 21 (19): 5017–25. PMC 129051. PMID 12356718.
- ↑ Lozach PY, Lortat-Jacob H, de Lacroix de Lavalette A, Staropoli I, Foung S, Amara A; et al. (2003). "DC-SIGN and L-SIGN are high affinity binding receptors for hepatitis C virus glycoprotein E2". J Biol Chem. 278 (22): 20358–66. doi:10.1074/jbc.M301284200. PMID 12609975.
- ↑ Pöhlmann S, Zhang J, Baribaud F, Chen Z, Leslie GJ, Lin G; et al. (2003). "Hepatitis C virus glycoproteins interact with DC-SIGN and DC-SIGNR". J Virol. 77 (7): 4070–80. PMC 150620. PMID 12634366.
- ↑ 11.0 11.1 Tsukiyama-Kohara K, Iizuka N, Kohara M, Nomoto A (1992). "Internal ribosome entry site within hepatitis C virus RNA". J Virol. 66 (3): 1476–83. PMC 240872. PMID 1310759.
- ↑ 12.00 12.01 12.02 12.03 12.04 12.05 12.06 12.07 12.08 12.09 12.10 12.11 12.12 12.13 Pawlotsky JM (2004). "Pathophysiology of hepatitis C virus infection and related liver disease". Trends Microbiol. 12 (2): 96–102. doi:10.1016/j.tim.2003.12.005. PMID 15036326.
- ↑ Grakoui A, Wychowski C, Lin C, Feinstone SM, Rice CM (1993). "Expression and identification of hepatitis C virus polyprotein cleavage products". J Virol. 67 (3): 1385–95. PMC 237508. PMID 7679746.
- ↑ 14.0 14.1 14.2 14.3 14.4 14.5 14.6 Penin F, Dubuisson J, Rey FA, Moradpour D, Pawlotsky JM (2004). "Structural biology of hepatitis C virus". Hepatology. 39 (1): 5–19. doi:10.1002/hep.20032. PMID 14752815.
- ↑ Egger D, Wölk B, Gosert R, Bianchi L, Blum HE, Moradpour D; et al. (2002). "Expression of hepatitis C virus proteins induces distinct membrane alterations including a candidate viral replication complex". J Virol. 76 (12): 5974–84. PMC 136238. PMID 12021330.
Differential Diagnosis

Editor-In-Chief: C. Michael Gibson, M.S., M.D. [16]; Associate Editor(s)-In-Chief: Yazan Daaboul, Serge Korjian
Overview
Hepatitis C must be differentiated from other diseases that cause hepatic injury and abnormal liver function tests such as other viral hepatitides (Hepatitis A, Hepatitis B, and Hepatitis E) and non-viral etiologies such as alcoholic liver disease, non-alcoholic steatohepatitis, drug-induced liver injury, autoimmune hepatitis, hepatocellular carcinoma, liver abscess, pancreatitis, and bowel obstruction.
Differential Diagnosis
The differential diagnosis of hepatitis C includes other etiologies of viral hepatitis and non-viral etiologies:[1]
Viral Hepatitis Differential Diagnosis
Non-Viral Hepatitis Differential Diagnosis
- Autoimmune hepatitis
- Alcoholic liver disease
- Non-alcoholic steatohepatitis (NASH)
- Drug-induced liver injury (DILI)
- Liver abscess
- Hepatocellular carcinoma
- Cholelithiasis
- Cholecystitis
- Cholangitis
- Chronic biliary disease
- Trauma
- Abdominal aneurysm
- Gastritis
- Gastroenteritis
- Peptic ulcer disease
- Bowel obstruction
- Pancreatitis
- Pancreatic cancer
- Malignant lymphoma
- Hereditary metabolic disorders (Wilson disease, alpha-1 antitrypsin deficiency)
Differential diagnosis of jaundice as one pf symptoms of hepatitic C are: [2][3][4][5][6]
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Differential diagnosis of cirrhosis if happened in hepatitis C based on altered hepatic function are:
| Condition | Differentiating signs and symptoms | Differentiating Tests |
|---|---|---|
| Cirrhosis |
|
Ultrasound findings in cirrhosis are as follows:[7][8][9][10][11][12][13][14]
Abdominal MRI may also be helpful in the diagnosis of portal hypertension. Findings on MRI suggestive of cirrhosis with portal hypertension include:[15][16][17][18]
Transient elastography and the Acoustic Radiation Force Impulse (ARFI) technique are well-established methods for the staging of fibrosis in various liver diseases: [19][20][21][22][23][24][25][26][27][28][29]
|
| Constrictive pericarditis |
|
|
| Budd-Chiari Syndrome |
|
|
| Splenic vein thrombosis | Signs and symptoms of:
|
|
| Portal vein thrombosis |
|
|
| Schistosomiasis |
|
|
| Sarcoidosis |
|
|
| Inferior vena cava obstruction |
|
|
| Nodular regenerative hyperplasia | None |
|
| Idiopathic portal hypertension (hepatoportal sclerosis) | None |
|
| Vitamin A intoxication, arsenic, and vinyl chloride toxicity | None |
|
References
- ↑ Giannini EG, Testa R, Savarino V (2005). "Liver enzyme alteration: a guide for clinicians". CMAJ. 172 (3): 367–79. doi:10.1503/cmaj.1040752. PMC 545762. PMID 15684121.
- ↑ Fargo MV, Grogan SP, Saguil A (2017). "Evaluation of Jaundice in Adults". Am Fam Physician. 95 (3): 164–168. PMID 28145671.
- ↑ Leevy CB, Koneru B, Klein KM (1997). "Recurrent familial prolonged intrahepatic cholestasis of pregnancy associated with chronic liver disease". Gastroenterology. 113 (3): 966–72. PMID 9287990.
- ↑ Hov JR, Boberg KM, Karlsen TH (2008). "Autoantibodies in primary sclerosing cholangitis". World J. Gastroenterol. 14 (24): 3781–91. PMC 2721433. PMID 18609700.
- ↑ Bond LR, Hatty SR, Horn ME, Dick M, Meire HB, Bellingham AJ (1987). "Gall stones in sickle cell disease in the United Kingdom". Br Med J (Clin Res Ed). 295 (6592): 234–6. PMC 1247079. PMID 3115390.
- ↑ Malakouti M, Kataria A, Ali SK, Schenker S (2017). "Elevated Liver Enzymes in Asymptomatic Patients - What Should I Do?". J Clin Transl Hepatol. 5 (4): 394–403. doi:10.14218/JCTH.2017.00027. PMC 5719197. PMID 29226106.
- ↑ Udell JA, Wang CS, Tinmouth J, FitzGerald JM, Ayas NT, Simel DL, Schulzer M, Mak E, Yoshida EM (2012). "Does this patient with liver disease have cirrhosis?". JAMA. 307 (8): 832–42. doi:10.1001/jama.2012.186. PMID 22357834.
- ↑ Becker CD, Scheidegger J, Marincek B (1986). "Hepatic vein occlusion: morphologic features on computed tomography and ultrasonography". Gastrointest Radiol. 11 (4): 305–11. PMID 3533689.
- ↑ Di Lelio A, Cestari C, Lomazzi A, Beretta L (1989). "Cirrhosis: diagnosis with sonographic study of the liver surface". Radiology. 172 (2): 389–92. doi:10.1148/radiology.172.2.2526349. PMID 2526349.
- ↑ Sanford NL, Walsh P, Matis C, Baddeley H, Powell LW (1985). "Is ultrasonography useful in the assessment of diffuse parenchymal liver disease?". Gastroenterology. 89 (1): 186–91. PMID 3891495.
- ↑ Giorgio A, Amoroso P, Lettieri G, Fico P, de Stefano G, Finelli L, Scala V, Tarantino L, Pierri P, Pesce G (1986). "Cirrhosis: value of caudate to right lobe ratio in diagnosis with US". Radiology. 161 (2): 443–5. doi:10.1148/radiology.161.2.3532188. PMID 3532188.
- ↑ Simonovský V (1999). "The diagnosis of cirrhosis by high resolution ultrasound of the liver surface". Br J Radiol. 72 (853): 29–34. doi:10.1259/bjr.72.853.10341686. PMID 10341686.
- ↑ Trinchet JC, Chaffaut C, Bourcier V, Degos F, Henrion J, Fontaine H, Roulot D, Mallat A, Hillaire S, Cales P, Ollivier I, Vinel JP, Mathurin P, Bronowicki JP, Vilgrain V, N'Kontchou G, Beaugrand M, Chevret S (2011). "Ultrasonographic surveillance of hepatocellular carcinoma in cirrhosis: a randomized trial comparing 3- and 6-month periodicities". Hepatology. 54 (6): 1987–97. doi:10.1002/hep.24545. PMID 22144108.
- ↑ "EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma". J. Hepatol. 56 (4): 908–43. 2012. doi:10.1016/j.jhep.2011.12.001. PMID 22424438.
- ↑ Procopet, Bogdan; Berzigotti, Annalisa (2017). "Diagnosis of cirrhosis and portal hypertension: imaging, non-invasive markers of fibrosis and liver biopsy". Gastroenterology Report. 5 (2): 79–89. doi:10.1093/gastro/gox012. ISSN 2052-0034.
- ↑ Aagaard, J; Jensen, LI; Sorensen, TI; Christensen, U; Burcharth, F (1982). "Recanalized umbilical vein in portal hypertension". American Journal of Roentgenology. 139 (6): 1107–1110. doi:10.2214/ajr.139.6.1107. ISSN 0361-803X.
- ↑ Cho, K C; Patel, Y D; Wachsberg, R H; Seeff, J (1995). "Varices in portal hypertension: evaluation with CT". RadioGraphics. 15 (3): 609–622. doi:10.1148/radiographics.15.3.7624566. ISSN 0271-5333.
- ↑ Bandali, Murad Feroz; Mirakhur, Anirudh; Lee, Edward Wolfgang; Ferris, Mollie Clarke; Sadler, David James; Gray, Robin Ritchie; Wong, Jason Kam (2017). "Portal hypertension: Imaging of portosystemic collateral pathways and associated image-guided therapy". World Journal of Gastroenterology. 23 (10): 1735. doi:10.3748/wjg.v23.i10.1735. ISSN 1007-9327.
- ↑ Castera L, Pinzani M (2010). "Biopsy and non-invasive methods for the diagnosis of liver fibrosis: does it take two to tango?". Gut. 59 (7): 861–6. doi:10.1136/gut.2010.214650. PMID 20581229.
- ↑ Friedrich-Rust M, Nierhoff J, Lupsor M, Sporea I, Fierbinteanu-Braticevici C, Strobel D, Takahashi H, Yoneda M, Suda T, Zeuzem S, Herrmann E (2012). "Performance of Acoustic Radiation Force Impulse imaging for the staging of liver fibrosis: a pooled meta-analysis". J. Viral Hepat. 19 (2): e212–9. doi:10.1111/j.1365-2893.2011.01537.x. PMID 22239521.
- ↑ Friedrich-Rust M, Ong MF, Martens S, Sarrazin C, Bojunga J, Zeuzem S, Herrmann E (2008). "Performance of transient elastography for the staging of liver fibrosis: a meta-analysis". Gastroenterology. 134 (4): 960–74. doi:10.1053/j.gastro.2008.01.034. PMID 18395077.
- ↑ Ziol M, Handra-Luca A, Kettaneh A, Christidis C, Mal F, Kazemi F, de Lédinghen V, Marcellin P, Dhumeaux D, Trinchet JC, Beaugrand M (2005). "Noninvasive assessment of liver fibrosis by measurement of stiffness in patients with chronic hepatitis C". Hepatology. 41 (1): 48–54. doi:10.1002/hep.20506. PMID 15690481.
- ↑ Sandrin L, Fourquet B, Hasquenoph JM, Yon S, Fournier C, Mal F, Christidis C, Ziol M, Poulet B, Kazemi F, Beaugrand M, Palau R (2003). "Transient elastography: a new noninvasive method for assessment of hepatic fibrosis". Ultrasound Med Biol. 29 (12): 1705–13. PMID 14698338.
- ↑ Bamber J, Cosgrove D, Dietrich CF, Fromageau J, Bojunga J, Calliada F, Cantisani V, Correas JM, D'Onofrio M, Drakonaki EE, Fink M, Friedrich-Rust M, Gilja OH, Havre RF, Jenssen C, Klauser AS, Ohlinger R, Saftoiu A, Schaefer F, Sporea I, Piscaglia F (2013). "EFSUMB guidelines and recommendations on the clinical use of ultrasound elastography. Part 1: Basic principles and technology". Ultraschall Med. 34 (2): 169–84. doi:10.1055/s-0033-1335205. PMID 23558397.
- ↑ "EASL-ALEH Clinical Practice Guidelines: Non-invasive tests for evaluation of liver disease severity and prognosis". J. Hepatol. 63 (1): 237–64. 2015. doi:10.1016/j.jhep.2015.04.006. PMID 25911335.
- ↑ Castera L, Bedossa P (2011). "How to assess liver fibrosis in chronic hepatitis C: serum markers or transient elastography vs. liver biopsy?". Liver Int. 31 Suppl 1: 13–7. doi:10.1111/j.1478-3231.2010.02380.x. PMID 21205132.
- ↑ Chou R, Wasson N (2013). "Blood tests to diagnose fibrosis or cirrhosis in patients with chronic hepatitis C virus infection: a systematic review". Ann. Intern. Med. 158 (11): 807–20. doi:10.7326/0003-4819-158-11-201306040-00005. PMID 23732714.
- ↑ Khallafi H, Qureshi K (2015). "Imaging Based Methods of Liver Fibrosis Assessment in Viral Hepatitis: A Practical Approach". Interdiscip Perspect Infect Dis. 2015: 809289. doi:10.1155/2015/809289. PMC 4686715. PMID 26779260.
- ↑ Singh S, Fujii LL, Murad MH, Wang Z, Asrani SK, Ehman RL, Kamath PS, Talwalkar JA (2013). "Liver stiffness is associated with risk of decompensation, liver cancer, and death in patients with chronic liver diseases: a systematic review and meta-analysis". Clin. Gastroenterol. Hepatol. 11 (12): 1573–84.e1–2, quiz e88–9. doi:10.1016/j.cgh.2013.07.034. PMC 3900882. PMID 23954643.
- ↑ Foucher J, Chanteloup E, Vergniol J; et al. (2006). "Diagnosis of cirrhosis by transient elastography (FibroScan): a prospective study". Gut. 55 (3): 403–8. doi:10.1136/gut.2005.069153. PMID 16020491.
- ↑ Xie L, Chen X, Guo Q, Dong Y, Guang Y, Zhang X (2012). "Real-time elastography for diagnosis of liver fibrosis in chronic hepatitis B". Journal of Ultrasound in Medicine : Official Journal of the American Institute of Ultrasound in Medicine. 31 (7): 1053–60. PMID 22733854.
Natural History, Complications & Prognosis
Natural History
|
Hepatitis C |
|
Diagnosis |
|
Treatment |
|
Hepatitis C On the Web |
|
American Roentgen Ray Society Images of Hepatitis C |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [17]; Associate Editor(s)-In-Chief: Yazan Daaboul, Serge Korjian, Javaria Anwer M.D.[18]
Overview
The majority of individuals infected with HCV will become chronic carriers. The primary and most severe complications of HCV are hepatic, namely liver cirrhosis years after the onset of infection and the consequent development of hepatocellular carcinoma. Other classical extra-hepatic manifestations (e.g., cryoglobulinemia, lichen planus, membranoproliferative glomerulonephritis, and porphyria cutanea tarda) are also complications of chronic HCV infection. Treatment is necessary for patients with chronic stable HCV infection; otherwise, the prognosis is poor and progression of the disease is potentially fatal.
Natural History
Upon exposure, HCV causes an acute phase that is asymptomatic in approximately 80% of cases. Acute HCV persists for less than 6 months. In 15-45% of cases, HCV is an isolated acute infection with almost no chronic sequelae, even in the absence of treatment. Patients exposed to HCV will subsequently develop anti-HCV antibodies but will demonstrate undetectable viral levels and negative HCV RNA.
In the majority of cases, however, HCV persists beyond 6 months and individuals become chronic carriers of HCV. Chronic HCV occurs in approximately 55-85% of patients. These patients will have positive anti-HCV antibodies and positive nucleic acid test (NAT) for HCV RNA, demonstrating the persistence of HCV and the inability of the body to appropriately clear the infection.[1][2]
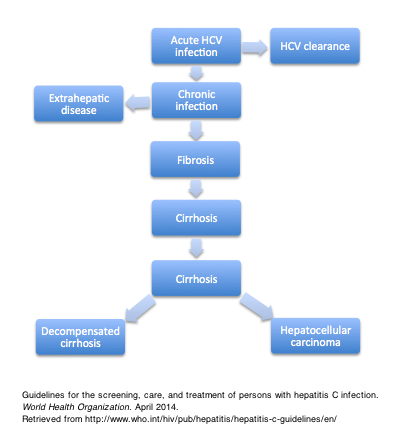
Complications
Hepatic Manifestations
A major complication of chronic HCV infection is cirrhosis, which ultimately leads to a decompensated state or transforms into a malignant hepatocellular carcinoma (HCC).[3] Not all patients develop cirrhosis at the same rate; exposure to other risk factors of cirrhosis, such as alcohol, HBV, or HIV infection, or immunocompromised status may hasten fibrosis of the liver and the development of HCC.[4]
Chronic HCV often eventually progresses to cause cirrhosis of the liver due to persistence of tissue inflammation and necrosis, along with fibrogenesis and deposition of components in the extracellular matrix. In the absence of treatment, 15-30% of patients with chronic HCV develop cirrhosis within 20 years.[3]
Similarly, hepatocellular carcinoma (HCC) is a known complication of chronic HCV infection. Patients with HCV develop HCC when the liver reaches its cirrhotic stage. The annual risk of developing hepatocellular carcinoma in a patient with cirrhosis is approximately 2-4%.
Extrahepatic complications of HCV infection
Extrahepatic complications of HCV infection include:[5][3]
- Lymphopriliferative disorders
- Benign:Cryoglobulinemia: Divided into Type I, and mixed types; Type II, and III cryoglobulinemia. Mixed cryoglobulinemic vasculitis is usually mildly symptomatic, which include palpable purpura, arthralgia, and fatigue. Peripheral nervous system involvement demonstrating as asymmetric, painful paresthesia subsequently becoming symmetric is not uncommon. Motor deficit affecting legs in the later stages of the diseases is also seen. Rarely, the disease may progress to a life-threatening widespread vasculitis with involvement of the CNS, heart, or gastrointestinal tract.[6]
- Malignant: Hodgkin's lymphoma and non-Hodgkin's lymphoma[6]
- Membranoproliferative glomerulonephritis[6]
- Autoimmune thyroiditis
- Sjogren syndrome
- Insulin resistance and type-2 diabetes mellitus
- Porphyria cutanea tarda
- Lichen Planus
- Seronegative arthritis
- Cognitive dysfunction
- Depression
- Autoimmune hemolytic anemia (AIHA)[6]
- immune thrombocytopenia[6]
- Monoclonal gammopathy of undetermined significance (MGUS)[6]
Prognosis
Acute HCV Infection[7]
- Spontaneous clearance of HCV RNA: 15-45%
- Progression to chronic infection: 55-85%
- Fulminant hepatitis: Rare
Chronic HCV Infection[7]
- Cirrhosis after 20 years: 10-20%
- Decompensated cirrhosis: 50% survival rate at 5 years
- Hepatocellular carcinoma: 1-4% per year
References
- ↑ Thomson EC, Fleming VM, Main J, Klenerman P, Weber J, Eliahoo J; et al. (2011). "Predicting spontaneous clearance of acute hepatitis C virus in a large cohort of HIV-1-infected men". Gut. 60 (6): 837–45. doi:10.1136/gut.2010.217166. PMC 3095479. PMID 21139063.
- ↑ Gerlach JT, Diepolder HM, Zachoval R, Gruener NH, Jung MC, Ulsenheimer A; et al. (2003). "Acute hepatitis C: high rate of both spontaneous and treatment-induced viral clearance". Gastroenterology. 125 (1): 80–8. PMID 12851873.
- ↑ 3.0 3.1 3.2 "Guidelines for the screening, care, and treatment of persons with HCV infection" (PDF). WHO. WHO. April 2014. Retrieved July 27, 2014.
- ↑ Freeman AJ, Law MG, Kaldor JM, Dore GJ (2003). "Predicting progression to cirrhosis in chronic hepatitis C virus infection". J Viral Hepat. 10 (4): 285–93. PMID 12823595.
- ↑ Fletcher NF, McKeating JA (2012). "Hepatitis C virus and the brain". J Viral Hepat. 19 (5): 301–6. doi:10.1111/j.1365-2893.2012.01591.x. PMID 22497808.
- ↑ 6.0 6.1 6.2 6.3 6.4 6.5 Cacoub, Patrice; Longo, Dan L.; Saadoun, David (2021). "Extrahepatic Manifestations of Chronic HCV Infection". New England Journal of Medicine. 384 (11): 1038–1052. doi:10.1056/NEJMra2033539. ISSN 0028-4793.
- ↑ 7.0 7.1 Chen SL, Morgan TR (2006). "The natural history of hepatitis C virus (HCV) infection". Int J Med Sci. 3 (2): 47–52. PMC 1415841. PMID 16614742.
Diagnosis
{{#ask:Used To Diagnose::Hepatitis C |?Sort Order |format=list |headers=hide |link=none |sep= | |template=MedicalTestQuery |sort=Sort Order }}
Treatment
{{#ask:Used To Treat::Hepatitis C |?Sort Order |format=list |headers=hide |link=none |sep= | |template=MedicalTreatmentQuery |sort=Sort Order }} {{#ask:Prevents::Hepatitis C |?Sort Order |intro= | |format=list |headers=hide |link=none |sep= | |template=MedicalTreatmentQuery2 |sort=Sort Order }}
Screening
Causes
Diagnosis
History & Symptoms | Physical Examination | Lab Tests | Electrocardiogram | Chest X Ray | CT | MRI | Ultrasound | Other Imaging Findings | Other Diagnostic Studies
Treatment
Medical Therapy | Surgery | Primary Prevention | Secondary Prevention
Special Population
Children | HIV Co-infection | Kidney Disease | Treatment of African Americans | Cirrhosis Patients | Liver Transplantation Patients | Pregnancy
Resources
- CDC's Hepatitis C Fact Sheet
- CDC's Hepatitis C Frequently Asked Questions
- Hepatitis C Fact Sheet
- Hepatitis C Fact Sheet
References
Template:Viral diseases Template:Gastroenterology Template:WH Template:WS