Sofosbuvir
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Alonso Alvarado, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Sofosbuvir is an antiviral that is FDA approved for the treatment of chronic hepatitis C (CHC) infection. Common adverse reactions include diarrhea, anemia, headache, insomnia, fatigue.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
The recommended dose of sofosbuvir is one 400 mg tablet, taken orally, once daily with or without food.
Sofosbuvir should be used in combination with ribavirin or in combination with pegylated interferon and ribavirin for the treatment of CHC in adults. The recommended regimen and treatment duration for sofosbuvir combination therapy is provided in the table below.
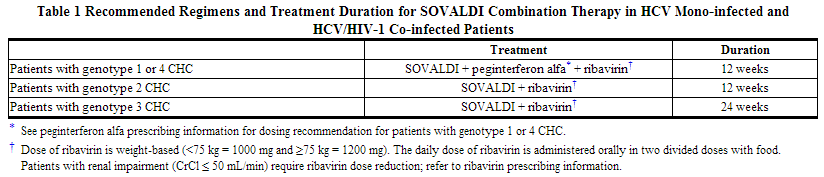
SOVALDI in combination with ribavirin for 24 weeks can be considered as a therapeutic option for CHC patients with genotype 1 infection who are ineligible to receive an interferon-based regimen [See Use in Specific Populations (8.8) and Clinical Studies (14.4)]. Treatment decision should be guided by an assessment of the potential benefits and risks for the individual patient.
Patients with Hepatocellular Carcinoma Awaiting Liver Transplantation
Sofosbuvir in combination with ribavirin is recommended for up to 48 weeks or until the time of liver transplantation, whichever occurs first, to prevent post-transplant HCV reinfection [See Use in Specific Populations (8.9)].
Dose Modification
Dose reduction of sofosbuvir is not recommended.
Genotype 1 and 4
If a patient has a serious adverse reaction potentially related to peginterferon alfa and/or ribavirin, the peginterferon alfa and/or ribavirin dose should be reduced or discontinued. Refer to the peginterferon alfa and ribavirin prescribing information for additional information about how to reduce and/or discontinue the peginterferon alfa and/or ribavirin dose.
Genotype 2 and 3
If a patient has a serious adverse reaction potentially related to ribavirin, the ribavirin dose should be modified or discontinued, if appropriate, until the adverse reaction abates or decreases in severity. Table 2 provides guidelines for dose modifications and discontinuation based on the patient's hemoglobin concentration and cardiac status.

Discontinuation of Dosing
If the other agents used in combination with sofosbuvir are permanently discontinued, sofosbuvir should also be discontinued.
Severe Renal Impairment and End Stage Renal Disease
No dose recommendation can be given for patients with severe renal impairment (estimated Glomerular Filtration Rate (eGFR) <30 mL/min/1.73m2) or with end stage renal disease (ESRD) due to higher exposures (up to 20-fold) of the predominant sofosbuvir metabolite [See Use in Specific Populations (8.6) and Clinical Pharmacology (12.3)].
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Sofosbuvir in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Sofosbuvir in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Sofosbuvir FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Sofosbuvir in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Sofosbuvir in pediatric patients.
Contraindications
- When sofosbuvir is used in combination with ribavirin or peginterferon alfa/ribavirin, the contraindications applicable to those agents are applicable to combination therapies. Refer to the prescribing information of peginterferon alfa and ribavirin for a list of their contraindications.
- Sofosbuvir combination treatment with ribavirin or peginterferon alfa/ribavirin is contraindicated in women who are pregnant or may become pregnant and men whose female partners are pregnant because of the risks for birth defects and fetal death associated with ribavirin.
Warnings
Pregnancy: Use with Ribavirin or Peginterferon Alfa/Ribavirin
Ribavirin may cause birth defects and/or death of the exposed fetus and animal studies have shown that interferons have abortifacient effects. Extreme care must be taken to avoid pregnancy in female patients and in female partners of male patients. Ribavirin therapy should not be started unless a report of a negative pregnancy test has been obtained immediately prior to initiation of therapy.
When sofosbuvir is used in combination with ribavirin or peginterferon alfa/ribavirin, women of childbearing potential and their male partners must use two forms of effective contraception during treatment and for at least 6 months after treatment has concluded. Routine monthly pregnancy tests must be performed during this time. There are no data on the effectiveness of systemic hormonal contraceptives in women taking sofosbuvir, therefore, two non-hormonal methods of contraception should be used during treatment with sofosbuvir and concomitant ribavirin. Refer also to the prescribing information for ribavirin.
Use with Potent P-gp Inducers
Drugs that are potent P-gp inducers in the intestine (e.g., rifampin, St. John's wort) may significantly decrease sofosbuvir plasma concentrations and may lead to a reduced therapeutic effect of sofosbuvir. Rifampin and St. John's wort should not be used with sofosbuvir.
Adverse Reactions
Clinical Trials Experience
Sofosbuvir should be administered with ribavirin or peginterferon alfa/ribavirin. Refer to the prescribing information of peginterferon alfa and ribavirin for a description of adverse reactions associated with their use.
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety assessment of sofosbuvir is based on pooled Phase 3 clinical trial data (both controlled and uncontrolled) including 650 subjects who received sofosbuvir + ribavirin (RBV) combination therapy for 12 weeks, 98 subjects who received sofosbuvir + ribavirin combination therapy for 16 weeks, 250 subjects who received sofosbuvir + ribavirin combination therapy for 24 weeks, 327 subjects who received sofosbuvir + peginterferon (Peg-IFN) alfa + ribavirin combination therapy for 12 weeks, 243 subjects who received peginterferon alfa + ribavirin for 24 weeks and 71 subjects who received placebo (PBO) for 12 weeks.
The proportion of subjects who permanently discontinued treatment due to adverse events was 4% for subjects receiving placebo, 1% for subjects receiving sofosbuvir + ribavirin for 12 weeks, <1% for subjects receiving sofosbuvir + ribavirin for 24 weeks, 11% for subjects receiving peginterferon alfa + ribavirin for 24 weeks and 2% for subjects receiving sofosbuvir + peginterferon alfa + ribavirin for 12 weeks.
Treatment-emergent adverse events observed in ≥15% of subjects in clinical trials are provided in the table below. A side-by-side tabulation is to simplify presentation; direct comparison across trials should not be made due to differing trial designs.
The most common adverse events (≥ 20%) for sofosbuvir + ribavirin combination therapy were fatigue and headache. The most common adverse events (≥ 20%) for sofosbuvir + peginterferon alfa + ribavirin combination therapy were fatigue, headache, nausea, insomnia and anemia.

With the exception of anemia and neutropenia, the majority of events presented in Table 3 occurred at severity of grade 1 in sofosbuvir-containing regimens.
Less Common Adverse Reactions Reported in Clinical Trials (<1%)
The following ADRs occurred in <1% of subjects receiving sofosbuvir in a combination regimen in any one trial. These events have been included because of their seriousness or assessment of potential causal relationship.
- Hematologic Effects: Pancytopenia (particularly in subjects receiving concomitant pegylated interferon).
- Psychiatric Disorders: Severe depression (particularly in subjects with pre-existing history of psychiatric illness), including suicidal ideation and suicide.
Laboratory Abnormalities
Changes in selected hematological parameters are described in the table below. A side-by-side tabulation is to simplify presentation; direct comparison across trials should not be made due to differing trial designs.

Bilirubin Elevations
Total bilirubin elevation of more than 2.5×ULN was observed in none of the subjects in the sofosbuvir + peginterferon alfa + ribavirin 12 weeks group and in 1%, 3% and 3% of subjects in the peginterferon alfa + ribavirin 24 weeks, sofosbuvir + ribavirin 12 weeks and sofosbuvir + ribavirin 24 weeks groups, respectively. Bilirubin levels peaked during the first 1 to 2 weeks of treatment and subsequently decreased and returned to baseline levels by post-treatment Week 4. These bilirubin elevations were not associated with transaminase elevations.
Creatine Kinase Elevations
Creatine kinase was assessed in the FISSION and NEUTRINO trials. Isolated, asymptomatic creatine kinase elevation of greater than or equal to 10×ULN was observed in <1%, 1% and 2% of subjects in the peginterferon alfa + ribavirin 24 weeks, sofosbuvir + peginterferon alfa + ribavirin 12 weeks and sofosbuvir + ribavirin 12 weeks groups, respectively.
Lipase Elevations
Isolated, asymptomatic lipase elevation of greater than 3×ULN was observed in <1%, 2%, 2%, and 2% of subjects in the SOVALDI + peginterferon alfa + ribavirin 12 weeks, SOVALDI + ribavirin 12 weeks, SOVALDI + ribavirin 24 weeks and peginterferon alfa + ribavirin 24 weeks groups, respectively.
Postmarketing Experience
There is limited information regarding adverse reactions in postmarketing experience of Sofosbuvir provided by the label.
Drug Interactions
Potential for Drug Interactions
After oral administration, sofosbuvir is rapidly converted to the predominant circulating metabolite GS-331007 that accounts for greater than 90% of drug related material systemic exposure, while the parent sofosbuvir accounts for approximately 4% of drug related material. In clinical pharmacology studies, both sofosbuvir and GS-331007 were monitored for purposes of pharmacokinetic analyses.
Sofosbuvir is a substrate of drug transporter P-gp and breast cancer resistance protein (BCRP) while GS-331007 is not. Drugs that are potent P-gp inducers in the intestine (e.g., rifampin or St. John's wort) may decrease sofosbuvir plasma concentration leading to reduced therapeutic effect of sofosbuvir and thus should not be used with sofosbuvir. Coadministration of sofosbuvir with drugs that inhibit P-gp and/or BCRP may increase sofosbuvir plasma concentration without increasing GS-331007 plasma concentration; accordingly, sofosbuvir may be coadministered with P-gp and/or BCRP inhibitors. Sofosbuvir and GS-331007 are not inhibitors of P-gp and BCRP and thus are not expected to increase exposures of drugs that are substrates of these transporters.
The intracellular metabolic activation pathway of sofosbuvir is mediated by generally low affinity and high capacity hydrolase and nucleotide phosphorylation pathways that are unlikely to be affected by concomitant drugs.
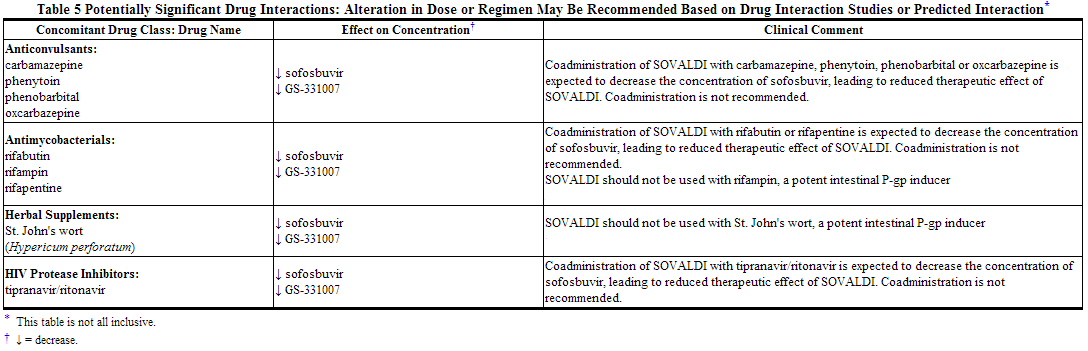
Potentially Significant Drug Interactions
Drug interaction information for sofosbuvir with potential concomitant drugs is summarized in the table below. The drug interactions described are based on potential drug interactions that may occur with sofosbuvir. The table is not all-inclusive.
Drugs without Clinically Significant Interactions with Sofosbuvir
In addition to the drugs included in the table above, the interaction between sofosbuvir and the following drugs was evaluated in clinical trials and no dose adjustment is needed for either drug: cyclosporine, darunavir/ritonavir, efavirenz, emtricitabine, methadone, raltegravir, rilpivirine,tacrolimus, or tenofovir disoproxil fumarate.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): B There are no adequate and well-controlled studies with sofosbuvir in pregnant women.
Animal Data
- No effects on fetal development have been observed in rats and rabbits at the highest doses tested. In the rat and rabbit, AUC exposure to the predominant circulating metabolite GS-331007 increased over the course of gestation from approximately 5- to 10-fold and 12- to 28-fold the exposure in humans at the recommended clinical dose, respectively.
Pregnancy Category X: Use with Ribavirin or Peginterferon Alfa/Ribavirin
- Extreme caution must be taken to avoid pregnancy in female patients and female partners of male patients while taking this combination. Women of childbearing potential and their male partners should not receive ribavirin unless they are using two forms of effective contraception during treatment with ribavirin and for 6 months after treatment has concluded. There are no data on the effectiveness of systemic hormonal contraceptives in women taking sofosbuvir. Therefore, two effective non-hormonal methods of contraception should be used during treatment with sofosbuvir and concomitant ribavirin.
- In case of exposure during pregnancy, a Ribavirin Pregnancy Registry has been established to monitor maternal-fetal outcomes of pregnancies in female patients and female partners of male patients exposed to ribavirin during treatment and for 6 months following cessation of treatment. Healthcare providers and patients are encouraged to report such cases by calling Ribavirin Pregnancy Registry at 1-800-593-2214. For patients who are HCV/HIV-1 co-infected and taking concomitant antiretrovirals, an Antiretroviral Pregnancy Registry is also available at 1-800-258-4263.
Animal Data
- Significant teratogenic and/or embryocidal effects have been demonstrated in all animal species exposed to ribavirin; and therefore ribavirin is contraindicated in women who are pregnant and in the male partners of women who are pregnant. Interferons have abortifacient effects in animals and should be assumed to have abortifacient potential in humans.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Sofosbuvir in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Sofosbuvir during labor and delivery.
Nursing Mothers
It is not known whether sofosbuvir and its metabolites are present in human breast milk. The predominant circulating metabolite GS-331007 was the primary component observed in the milk of lactating rats, without effect on nursing pups. Because of the potential for adverse reactions from the drug in nursing infants, a decision must be made whether to discontinue nursing or discontinue treatment with ribavirin-containing regimens, taking into account the importance of the therapy to the mother. See also the prescribing information for ribavirin.
Pediatric Use
Safety and effectiveness of sofosbuvir in children less than 18 years of age have not been established.
Geriatic Use
Sofosbuvir was administered to 90 subjects aged 65 and over. The response rates observed for subjects over 65 years of age were similar to that of younger subjects across treatment groups. No dose adjustment of sofosbuvir is warranted in geriatric patients
Gender
There is no FDA guidance on the use of Sofosbuvir with respect to specific gender populations.
Race
There is no FDA guidance on the use of Sofosbuvir with respect to specific racial populations.
Renal Impairment
No dose adjustment of sofosbuvir is required for patients with mild or moderate renal impairment. The safety and efficacy of sofosbuvir have not been established in patients with severe renal impairment (eGFR <30 mL/min/1.73m2) or end stage renal disease (ESRD) requiring hemodialysis. No dose recommendation can be given for patients with severe renal impairment or ESRD. Refer also to ribavirin and peginterferon alfa prescribing information for patients with CrCl <50 mL/min.
Hepatic Impairment
No dose adjustment of sofosbuvir is required for patients with mild, moderate or severe hepatic impairment (Child-Pugh Class A, B or C). Safety and efficacy of sofosbuvir have not been established in patients with decompensated cirrhosis. See peginterferon alfa prescribing information for contraindication in hepatic decompensation.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Sofosbuvir in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Sofosbuvir in patients who are immunocompromised.
Others
Patients with HCV/HIV-1 Co-infection
The safety and efficacy of sofosbuvir was assessed in 223 HCV/HIV-1 co-infected subjects. The safety profile in HCV/HIV-1 co-infected subjects was similar to that observed in HCV mono-infected subjects. Elevated total bilirubin (grade 3 or 4) was observed in 30/32 (94%) subjects receiving atazanavir as part of the antiretroviral regimen. None of the subjects had concomitant transaminase increases. Among subjects not taking atazanavir, grade 3 or 4 elevated total bilirubin was observed in 2 (1.5%) subjects, similar to the rate observed with HCV mono-infected subjects receiving sofosbuvir + ribavirin in Phase 3 trials.
Patients with Hepatocellular Carcinoma Awaiting Liver Transplantation
Sofosbuvir was studied in HCV-infected subjects with hepatocellular carcinoma prior to undergoing liver transplantation in an open-label clinical trial evaluating the safety and efficacy of sofosbuvir and ribavirin administered pre-transplant to prevent post-transplant HCV reinfection. The primary endpoint of the trial was post-transplant virologic response (pTVR) defined as HCV RNA < lower limit of quantification (LLOQ) at 12 weeks post-transplant. HCV-infected subjects, regardless of genotype, with hepatocellular carcinoma (HCC) meeting the MILAN criteria (defined as the presence of a tumor 5 cm or less in diameter in patients with single hepatocellular carcinomas and no more than three tumor nodules, each 3 cm or less in diameter in patients with multiple tumors and no extrahepatic manifestations of the cancer or evidence of vascular invasion of tumor) received 400 mg sofosbuvir and weight-based 1000–1200 mg ribavirin daily for 24–48 weeks or until the time of liver transplantation, whichever occurred first. An interim analysis was conducted on 61 subjects who received sofosbuvir and ribavirin; 45 subjects had HCV genotype 1; 44 subjects had a baseline CPT score less than 7 and all subjects had a baseline unadjusted MELD score ≤14. Of these 61 subjects, 41 subjects underwent liver transplantation following up to 48 weeks of treatment with sofosbuvir and ribavirin; 37 had HCV RNA < LLOQ at the time of transplantation. Of the 37 subjects, the post-transplant virologic response (pTVR) rate is 64% (23/36) in the 36 evaluable subjects who have reached the 12 week post-transplant time point. The safety profile of sofosbuvir and ribavirin in HCV-infected subjects prior to liver transplantation was comparable to that observed in subjects treated with sofosbuvir and ribavirin in Phase 3 clinical trials.
Post-Liver Transplant Patients
The safety and efficacy of sofosbuvir have not been established in post-liver transplant patients.
CHC Patients with Genotype 5 or 6 HCV Infection
Available data on subjects with genotype 5 or 6 HCV infection are insufficient for dosing recommendations.
Administration and Monitoring
Administration
Oral
Monitoring
In case of exposure during pregnancy, a Ribavirin Pregnancy Registry has been established to monitor maternal-fetal outcomes of pregnancies in female patients and female partners of male patients exposed to ribavirin during treatment and for 6 months following cessation of treatment. Healthcare providers and patients are encouraged to report such cases by calling Ribavirin Pregnancy Registry at 1-800-593-2214. For patients who are HCV/HIV-1 co-infected and taking concomitant antiretrovirals, an Antiretroviral Pregnancy Registry is also available at 1-800-258-4263.
IV Compatibility
There is limited information regarding the compatibility of Sofosbuvir and IV administrations.
Overdosage
The highest documented dose of sofosbuvir was a single supratherapeutic dose of sofosbuvir 1200 mg administered to 59 healthy subjects. In that trial, there were no untoward effects observed at this dose level, and adverse events were similar in frequency and severity to those reported in the placebo and sofosbuvir 400 mg treatment groups. The effects of higher doses are not known.
No specific antidote is available for overdose with sofosbuvir. If overdose occurs the patient must be monitored for evidence of toxicity. Treatment of overdose with sofosbuvir consists of general supportive measures including monitoring of vital signs as well as observation of the clinical status of the patient. A 4-hour hemodialysis session removed 18% of the administered dose.
Pharmacology
Mechanism of Action
Sofosbuvir is a direct-acting antiviral agent against the hepatitis C virus.
Structure
sofosbuvir is a nucleotide analog inhibitor of HCV NS5B polymerase.
The IUPAC name for sofosbuvir is (S)-Isopropyl 2-((S)-(((2R,3R,4R,5R)-5-(2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)-4-fluoro-3-hydroxy-4-methyltetrahydrofuran-2-yl)methoxy)-(phenoxy)phosphorylamino)propanoate. It has a molecular formula of C22H29FN3O9P and a molecular weight of 529.45. It has the following structural formula:

Sofosbuvir is a white to off-white crystalline solid with a solubility of ≥ 2 mg/mL across the pH range of 2–7.7 at 37 °C and is slightly soluble in water.
Sofosbuvir tablets are for oral administration. Each tablet contains 400 mg of sofosbuvir. The tablets include the following inactive ingredients: colloidal silicon dioxide, croscarmellose sodium, magnesium stearate, mannitol, and microcrystalline cellulose. The tablets are film-coated with a coating material containing the following inactive ingredients: polyethylene glycol, polyvinyl alcohol, talc, titanium dioxide, and yellow iron oxide.
Pharmacodynamics
Effect on Electrocardiogram
The effect of sofosbuvir 400 and 1200 mg on QTc interval was evaluated in a randomized, single-dose, placebo-, and active-controlled (moxifloxacin 400 mg) four period crossover thorough QT trial in 59 healthy subjects. At a dose three times the maximum recommended dose, sofosbuvir does not prolong QTc to any clinically relevant extent.
Pharmacokinetics
Absorption
The pharmacokinetic properties of sofosbuvir and the predominant circulating metabolite GS-331007 have been evaluated in healthy adult subjects and in subjects with chronic hepatitis C. Following oral administration of sofosbuvir was absorbed with a peak plasma concentration observed at ~0.5–2 hour post-dose, regardless of dose level. Peak plasma concentration of GS-331007 was observed between 2 to 4 hours post-dose. Based on population pharmacokinetic analysis in subjects with genotype 1 to 6 HCV infection who were coadministered ribavirin (with or without pegylated interferon), geometric mean steady state sofosbuvir (N=838) and GS-331007 (N=1695) AUC0–24 were 828 ng∙hr/mL and 6790 ng∙hr/mL, respectively. Relative to healthy subjects administered sofosbuvir alone (N = 272), the sofosbuvir AUC0–24 was 39% higher and GS-331007 AUC0–24 was 39% lower, respectively, in HCV-infected subjects. Sofosbuvir and GS-331007 AUCs are near dose proportional over the dose range of 200 mg to 1200 mg.
Effect of Food
Relative to fasting conditions, the administration of a single dose of sofosbuvir with a standardized high fat meal did not substantially affect the sofosbuvir Cmax or AUC0–inf. The exposure of GS-331007 was not altered in the presence of a high-fat meal. Therefore, sofosbuvir can be administered without regard to food.
Distribution
Sofosbuvir is approximately 61–65% bound to human plasma proteins and the binding is independent of drug concentration over the range of 1 µg/mL to 20 µg/mL. Protein binding of GS-331007 was minimal in human plasma. After a single 400 mg dose of [14C]-sofosbuvir in healthy subjects, the blood to plasma ratio of 14C-radioactivity was approximately 0.7.
Metabolism
Sofosbuvir is extensively metabolized in the liver to form the pharmacologically active nucleoside analog triphosphate GS-461203. The metabolic activation pathway involves sequential hydrolysis of the carboxyl ester moiety catalyzed by human cathepsin A (CatA) or carboxylesterase 1 (CES1) and phosphoramidate cleavage by histidine triad nucleotide-binding protein 1 (HINT1) followed by phosphorylation by the pyrimidine nucleotide biosynthesis pathway. Dephosphorylation results in the formation of nucleoside metabolite GS-331007 that cannot be efficiently rephosphorylated and lacks anti-HCV activity in vitro. After a single 400 mg oral dose of [14C]-sofosbuvir, sofosbuvir and GS-331007 accounted for approximately 4% and >90% of drug related material (sum of molecular weight-adjusted AUC of sofosbuvir and its metabolites) systemic exposure, respectively.
Elimination
Following a single 400 mg oral dose of [14C]-sofosbuvir, mean total recovery of the dose was greater than 92%, consisting of approximately 80%, 14%, and 2.5% recovered in urine, feces, and expired air, respectively. The majority of the sofosbuvir dose recovered in urine was GS-331007 (78%) while 3.5% was recovered as sofosbuvir. These data indicate that renal clearance is the major elimination pathway for GS-331007. The median terminal half-lives of sofosbuvir and GS-331007 were 0.4 and 27 hours, respectively.
Specific Populations
Race
Population pharmacokinetics analysis in HCV-infected subjects indicated that race had no clinically relevant effect on the exposure of sofosbuvir and GS-331007.
Gender
No clinically relevant pharmacokinetic differences have been observed between men and women for sofosbuvir and GS-331007.
Pediatric Patients
The pharmacokinetics of sofosbuvir in pediatric patients have not been established.
Geriatric Patients
Population pharmacokinetic analysis in HCV-infected subjects showed that within the age range (19 to 75 years) analyzed, age did not have a clinically relevant effect on the exposure to sofosbuvir and GS-331007.
Patients with Renal Impairment
The pharmacokinetics of sofosbuvir were studied in HCV negative subjects with mild (eGFR ≥ 50 and < 80 mL/min/1.73m2), moderate (eGFR ≥30 and <50 mL/min/1.73m2), severe renal impairment (eGFR <30 mL/min/1.73m2) and subjects with end stage renal disease (ESRD) requiring hemodialysis following a single 400 mg dose of sofosbuvir. Relative to subjects with normal renal function (eGFR >80 mL/min/1.73m2), the sofosbuvir AUC0–inf was 61%, 107% and 171% higher in mild, moderate and severe renal impairment, while the GS-331007 AUC0–inf was 55%, 88% and 451% higher, respectively. In subjects with ESRD, relative to subjects with normal renal function, sofosbuvir and GS-331007 AUC0–inf was 28% and 1280% higher when sofosbuvir was dosed 1 hour before hemodialysis compared with 60% and 2070% higher when sofosbuvir was dosed 1 hour after hemodialysis, respectively. A 4 hour hemodialysis session removed approximately 18% of administered dose. No dose adjustment is required for patients with mild or moderate renal impairment. The safety and efficacy of sofosbuvir have not been established in patients with severe renal impairment or ESRD. No dose recommendation can be given for patients with severe renal impairment or ESRD.
Patients with Hepatic Impairment
The pharmacokinetics of sofosbuvir were studied following 7-day dosing of 400 mg sofosbuvir in HCV-infected subjects with moderate and severe hepatic impairment (Child-Pugh Class B and C). Relative to subjects with normal hepatic function, the sofosbuvir AUC0–24 were 126% and 143% higher in moderate and severe hepatic impairment, while the GS-331007 AUC0–24 were 18% and 9% higher, respectively. Population pharmacokinetics analysis in HCV-infected subjects indicated that cirrhosis had no clinically relevant effect on the exposure of sofosbuvir and GS-331007. No dose adjustment of sofosbuvir is recommended for patients with mild, moderate and severe hepatic impairment.
Assessment of Drug Interactions
The effects of coadministered drugs on the exposure of sofosbuvir and GS-331007 are shown in Table 6. The effects of sofosbuvir on the exposure of coadministered drugs are shown in Table 7.

No effect on the pharmacokinetic parameters of sofosbuvir and GS-331007 was observed with raltegravir.

No effect on the pharmacokinetic parameters of the following coadministered drugs was observed with sofosbuvir: cyclosporine, darunavir/ritonavir, efavirenz, emtricitabine, methadone or rilpivirine.
Microbiology
Mechanism of Action
Sofosbuvir is an inhibitor of the HCV NS5B RNA-dependent RNA polymerase, which is essential for viral replication. Sofosbuvir is a nucleotide prodrug that undergoes intracellular metabolism to form the pharmacologically active uridine analog triphosphate (GS-461203), which can be incorporated into HCV RNA by the NS5B polymerase and acts as a chain terminator. In a biochemical assay, GS-461203 inhibited the polymerase activity of the recombinant NS5B from HCV genotype 1b, 2a, 3a and 4a with IC50 values ranging from 0.7 to 2.6 µM. GS-461203 is not an inhibitor of human DNA and RNA polymerases nor an inhibitor of mitochondrial RNA polymerase.
Antiviral Activity
In HCV replicon assays, the EC50 values of sofosbuvir against full-length replicons from genotype 1a, 1b, 2a, 3a and 4a, and chimeric 1b replicons encoding NS5B from genotype 2b, 5a or 6a ranged from 0.014 to 0.11 µM. The median EC50 value of sofosbuvir against chimeric replicons encoding NS5B sequences from clinical isolates was 0.062 µM for genotype 1a (range 0.029–0.128 µM; N=67), 0.102 µM for genotype 1b (range 0.045–0.170 µM; N=29), 0.029 µM for genotype 2 (range 0.014–0.081 µM; N=15) and 0.081 µM for genotype 3a (range 0.024–0.181 µM; N=106). In infectious virus assays, the EC50 values of sofosbuvir against genotype 1a and 2a were 0.03 and 0.02 µM, respectively. The presence of 40% human serum had no effect on the anti-HCV activity of sofosbuvir. Evaluation of sofosbuvir in combination with interferon alpha or ribavirin showed no antagonistic effect in reducing HCV RNA levels in replicon cells.
Resistance
In Cell Culture
HCV replicons with reduced susceptibility to sofosbuvir have been selected in cell culture for multiple genotypes including 1b, 2a, 2b, 3a, 4a, 5a and 6a. Reduced susceptibility to sofosbuvir was associated with the primary NS5B substitution S282T in all replicon genotypes examined. An M289L substitution developed along with the S282T substitution in genotype 2a, 5 and 6 replicons. Site-directed mutagenesis of the S282T substitution in replicons of 8 genotypes conferred 2- to 18-fold reduced susceptibility to sofosbuvir and reduced the replication viral capacity by 89% to 99% compared to the corresponding wild-type. In biochemical assays, recombinant NS5B polymerase from genotypes 1b, 2a, 3a and 4a expressing the S282T substitution showed reduced susceptibility to GS-461203 compared to respective wild-types.
In Clinical Trials
In a pooled analysis of 982 subjects who received sofosbuvir in Phase 3 trials, 224 subjects had post-baseline NS5B genotypic data from next generation nucleotide sequencing (assay cutoff of 1%).
Treatment-emergent substitutions L159F (n= 6) and V321A (n= 5) were detected in post-baseline samples from GT3a-infected subjects across the Phase 3 trials. No detectable shift in the phenotypic susceptibility to sofosbuvir of subject isolates with L159F or V321A substitutions was seen. The sofosbuvir-associated resistance substitution S282T was not detected at baseline or in the failure isolates from Phase 3 trials. However, an S282T substitution was detected in one genotype 2b subject who relapsed at Week 4 post-treatment after 12 weeks of sofosbuvir monotherapy in the Phase 2 trial P7977-0523 [ELECTRON]. The isolate from this subject displayed a mean 13.5-fold reduced susceptibility to sofosbuvir. For this subject, the S282T substitution was no longer detectable at Week 12 post-treatment by next generation sequencing with an assay cut off of 1%.
In the trial done in subjects with hepatocellular carcinoma awaiting liver transplantation where subjects received up to 48 weeks of sofosbuvir and ribavirin, the L159F substitution emerged in multiple subjects with GT1a or GT2b HCV who experienced virologic failure (breakthrough and relapse). Furthermore, the presence of substitutions L159F and/or C316N at baseline was associated with sofosbuvir breakthrough and relapse post-transplant in multiple subjects infected with GT1b HCV. In addition, S282R and L320F substitutions were detected on-treatment by next generation sequencing in a subject infected with GT1a HCV with a partial treatment response.
The clinical significance of these substitutions is not known.
Cross Resistance
HCV replicons expressing the sofosbuvir-associated resistance substitution S282T were susceptible to NS5A inhibitors and ribavirin. HCV replicons expressing the ribavirin-associated substitutions T390I and F415Y were susceptible to sofosbuvir. Sofosbuvir was active against HCV replicons with NS3/4A protease inhibitor, NS5B non-nucleoside inhibitor and NS5A inhibitor resistant variants.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis and Mutagenesis
Use with Ribavirin and/or Peginterferon alfa: Ribavirin was shown to be genotoxic in several in vitro and in vivo assays. Ribavirin was not oncogenic in a 6-month p53+/- transgenic mouse study or a 2-year carcinogenicity study in rats. See the prescribing information for ribavirin. Carcinogenicity studies of sofosbuvir in mice and rats are ongoing.
Sofosbuvir was not genotoxic in a battery of in vitro or in vivo assays, including bacterial mutagenicity, chromosome aberration using human peripheral blood lymphocytes and in vivo mouse micronucleus assays.
Impairment of Fertility
Use with Ribavirin and/or Peginterferon alfa: In fertility studies in male animals, ribavirin induced reversible testicular toxicity, while peginterferon alfa may impair fertility in females. Refer to prescribing information for ribavirin and peginterferon alfa for additional information.
Sofosbuvir had no effects on embryo-fetal viability or on fertility when evaluated in rats. At the highest dose tested, AUC exposure to the predominant circulating metabolite GS-331007 was approximately 8-fold the exposure in humans at the recommended clinical dose.
Animal Toxicology and/or Pharmacology
Heart degeneration and inflammation were observed in rats following GS-9851 (a stereoisomeric mixture containing approximately 50% sofosbuvir) doses of 2000 mg/kg/day for up to 5 days. At this dose, AUC exposure to the predominant metabolite GS-331007 is approximately 29-fold higher than human exposure at the recommended clinical dose. No heart degeneration or inflammation was observed in rats following sofosbuvir doses of up to 500 mg/kg/day for 6 months at a GS-331007 AUC exposure approximately 9-fold higher than human exposure at the recommended clinical dose. In dogs and mice, heart degeneration and inflammation were not observed following sofosbuvir doses of up to 500 and 1000 mg/kg/day for 9 and 3 months, respectively, the highest doses tested. At these doses, GS-331007 AUC exposures are approximately 27- and 41-fold higher, respectively, than human exposure at the recommended clinical dose.
Clinical Studies
Description of Clinical Trials
The safety and efficacy of sofosbuvir was evaluated in five Phase 3 trials in a total of 1724 HCV mono-infected subjects with genotypes 1 to 6 chronic hepatitis C (CHC) and one Phase 3 trial in 223 HCV/HIV-1 co-infected subjects with genotype 1, 2 or 3 CHC. Among the five trials in HCV mono-infected subjects, one was conducted in treatment-naïve subjects with genotype 1, 4, 5 or 6 CHC in combination with peginterferon alfa 2a and ribavirin and the other four were conducted in subjects with genotype 2 or 3 CHC in combination with ribavirin, including one in treatment-naïve subjects, one in interferon intolerant, ineligible or unwilling subjects, one in subjects previously treated with an interferon-based regimen, and one in all subjects irrespective of prior treatment history or ability to take interferon. The trial in HCV/HIV-1 co-infected subjects was conducted in combination with [ribavirin]] in treatment-naïve subjects with genotype 1 CHC and all subjects with genotype 2 or 3 CHC irrespective of prior treatment history or ability to take interferon. Subjects in these trials had compensated liver disease including cirrhosis. Sofosbuvir was administered at a dose of 400 mg once daily. The ribavirin (RBV) dose was weight-based at 1000–1200 mg daily administered in two divided doses when used in combination with sofosbuvir, and the peginterferon alfa 2a dose, where applicable, was 180 micrograms per week. Treatment duration was fixed in each trial and was not guided by subjects' HCV RNA levels (no response guided algorithm). Plasma HCV RNA values were measured during the clinical trials using the COBAS TaqMan HCV test (version 2.0), for use with the High Pure System. The assay had a lower limit of quantification (LLOQ) of 25 IU per mL. Sustained virologic response (SVR) was the primary endpoint which was defined as HCV RNA less than LLOQ at 12 weeks after the end of treatment.
Clinical Trials in Subjects with Genotype 1 or 4 CHC
Treatment-Naïve Adults — NEUTRINO (Study 110)
NEUTRINO was an open-label, single-arm trial that evaluated 12 weeks of treatment with sofosbuvir in combination with peginterferon alfa 2a and ribavirin in treatment-naïve subjects with genotype 1, 4, 5 or 6 HCV infection compared to pre-specified historical control.
Treated subjects (N=327) had a median age of 54 years (range: 19 to 70); 64% of the subjects were male; 79% were White, 17% were Black; 14% were Hispanic or Latino; mean body mass index was 29 kg/m2 (range: 18 to 56 kg/m2); 78% had baseline HCV RNA greater than 6 log10 IU per mL; 17% had cirrhosis; 89% had HCV genotype 1; 9% had HCV genotype 4 and 2% had HCV genotype 5 or 6. Table 8 presents the response rates for the treatment group of sofosbuvir + peginterferon alfa + ribavirin.

Response rates for selected subgroups are presented in Table 9.

SVR rates were 98% (93/95) in subjects with baseline IL28B C/C allele and 87% (202/232) in subjects with baseline IL28B non-C/C alleles.
It is estimated that the response rate in patients who previously failed pegylated interferon and ribavirin therapy will approximate the observed response rate in NEUTRINO subjects with multiple baseline factors traditionally associated with a lower response to interferon-based treatment (Table 9). The SVR rate in the NEUTRINO trial in genotype 1 subjects with IL28B non-C/C alleles, HCV RNA >800,000 IU/mL and Metavir F3/F4 fibrosis was 71% (37/52).
Clinical Trials in Subjects with Genotype 2 or 3 CHC
Treatment-Naïve Adults — FISSION (Study 1231)
FISSION was a randomized, open-label, active-controlled trial that evaluated 12 weeks of treatment with sofosbuvir and ribavirin compared to 24 weeks of treatment with peginterferon alfa 2a and ribavirin in treatment-naïve subjects with genotype 2 and 3 HCV. The ribavirin doses used in the sofosbuvir + ribavirin and peginterferon alfa 2a + ribavirin arms were weight-based 1000–1200 mg per day and 800 mg per day regardless of weight, respectively. Subjects were randomized in a 1:1 ratio and stratified by cirrhosis (presence vs. absence), HCV genotype (2 vs. 3) and baseline HCV RNA level (<6 log10IU/mL vs. ≥6 log10IU/mL). Subjects with genotype 2 or 3 HCV were enrolled in an approximately 1:3 ratio.
Treated subjects (N=499) had a median age of 50 years (range: 19 to 77); 66% of the subjects were male; 87% were White, 3% were Black; 14% were Hispanic or Latino; mean body mass index was 28 kg/m2 (range: 17 to 52 kg/m2); 57% had baseline HCV RNA levels greater than 6 log10 IU per mL; 20% had cirrhosis; 72% had HCV genotype 3. Table 10 presents the response rates for the treatment groups of sofosbuvir + ribavirin and peginterferon alfa + ribavirin.

Response rates for subjects with cirrhosis at baseline are presented in Table 11 by genotype.

Interferon Intolerant, Ineligible or Unwilling Adults — POSITRON (Study 107)
POSITRON was a randomized, double-blinded, placebo-controlled trial that evaluated 12 weeks of treatment with sofosbuvir and ribavirin (N=207) compared to placebo (N=71) in subjects who are interferon intolerant, ineligible or unwilling. Subjects were randomized in 3:1 ratio and stratified by cirrhosis (presence vs. absence).
Treated subjects (N=278) had a median age of 54 years (range: 21 to 75); 54% of the subjects were male; 91% were White, 5% were Black; 11% were Hispanic or Latino; mean body mass index was 28 kg/m2 (range: 18 to 53 kg/m2); 70% had baseline HCV RNA levels greater than 6 log10 IU per mL; 16% had cirrhosis; 49% had HCV genotype 3. The proportions of subjects who were interferon intolerant, ineligible, or unwilling were 9%, 44%, and 47%, respectively. Most subjects had no prior HCV treatment (81%). Table 12 presents the response rates for the treatment groups of sofosbuvir + ribavirin and placebo.

Table 13 presents the subgroup analysis by genotype for cirrhosis and interferon classification.

Previously Treated Adults — FUSION (Study 108)
FUSION was a randomized, double-blinded trial that evaluated 12 or 16 weeks of treatment with sofosbuvir and ribavirin in subjects who did not achieve SVR with prior interferon-based treatment (relapsers and nonresponders). Subjects were randomized in a 1:1 ratio and stratified by cirrhosis (presence vs. absence) and HCV genotype (2 vs. 3).
Treated subjects (N=201) had a median age of 56 years (range: 24 to 70); 70% of the subjects were male; 87% were White; 3% were Black; 9% were Hispanic or Latino; mean body mass index was 29 kg/m2 (range: 19 to 44 kg/m2); 73% had baseline HCV RNA levels greater than 6log10 IU per mL; 34% had cirrhosis; 63% had HCV genotype 3; 75% were prior relapsers. Table 14 presents the response rates for the treatment groups of sofosbuvir + ribavirin for 12 weeks and 16 weeks.
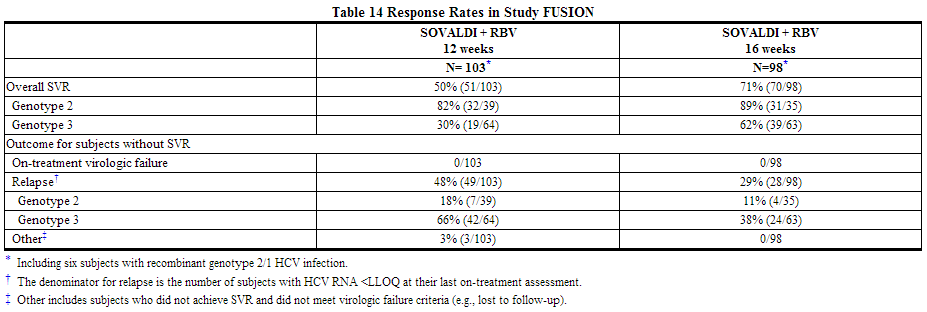
Table 15 presents the subgroup analysis by genotype for cirrhosis and response to prior HCV treatment.

Treatment-Naïve and Previously Treated Adults — VALENCE (Study 133)
The VALENCE trial evaluated sofosbuvir in combination with weight-based ribavirin for the treatment of genotype 2 or 3 HCV infection in treatment-naïve subjects or subjects who did not achieve SVR with prior interferon-based treatment, including subjects with compensated cirrhosis. The original trial design was a 4 to 1 randomization to sofosbuvir + ribavirin for 12 weeks or placebo. Based on emerging data, this trial was unblinded and all genotype 2 HCV-infected subjects continued the original planned treatment and received sofosbuvir + ribavirin for 12 weeks, and duration of treatment with sofosbuvir + ribavirin in genotype 3 HCV-infected subjects was extended to 24 weeks. Eleven genotype 3 subjects had already completed sofosbuvir + ribavirin for 12 weeks at the time of the amendment.
Treated subjects (N=419) had a median age of 51 years (range: 19 to 74); 60% of the subjects were male; mean body mass index was 26 kg/m2 (range: 17 to 44 kg/m2); the mean baseline HCV RNA level was 6.4 log10 IU per mL; 78% had HCV genotype 3; 58% of the subjects were treatment-experienced and 65% of those subjects experienced relapse/breakthrough to prior HCV treatment.
Table 16 presents the response rates for the treatment groups of sofosbuvir + ribavirin for 12 weeks and 24 weeks.

Table 17 presents the subgroup analysis by genotype for cirrhosis and prior HCV treatment experience.

Clinical Trials in Subjects Co-infected with HCV and HIV-1
Sofosbuvir was studied in an open-label clinical trial (Study PHOTON-1) evaluating the safety and efficacy of 12 or 24 weeks of treatment with sofosbuvir and ribavirin in subjects with genotype 1, 2 or 3 chronic hepatitis C co-infected with HIV-1. Genotype 2 and 3 subjects were either HCV treatment-naïve or experienced, whereas genotype 1 subjects were all treatment-naïve. Subjects received 400 mg sofosbuvir and weight-based ribavirin (1000 mg for subjects weighing <75 kg or 1200 mg for subjects weighing ≥75kg) daily for 12 or 24 weeks based on genotype and prior treatment history. Subjects were either not on antiretroviral therapy with a CD4+ cell count >500 cells/mm3 or had virologically suppressed HIV-1 with a CD4+ cell count >200 cells/mm3. Efficacy data 12 weeks post treatment are available for 210 subjects.

In subjects with HCV genotype 1 infection, the SVR rate was 82% (74/90) in subjects with genotype 1a infection and 54% (13/24) in subjects with genotype 1b infection, with relapse accounting for the majority of treatment failures. SVR rates in subjects with HCV genotype 1 infection were 80% (24/30) in subjects with baseline IL28B C/C allele and 75% (62/83) in subjects with baseline IL28B non-C/C alleles.
In the 223 CHC subjects with HIV-1 co-infection, the percentage of CD4+ cells did not change during treatment. Median CD4+ cell count decreases of 85 cells/mm3 and 84 cells/mm3 were observed at the end of treatment with sofosbuvir + ribavirin for 12 or 24 weeks, respectively. HIV-1 rebound during sofosbuvir + ribavirin treatment occurred in 2 subjects (0.9%) on antiretroviral therapy.
How Supplied
- Sofosbuvir tablets are yellow, capsule-shaped, film-coated tablets containing 400 mg sofosbuvir debossed with "GSI" on one side and "7977" on the other side.
- Each bottle contains 28 tablets (NDC 61958-1501-1), a silica gel desiccant, polyester coil and is closed with a child-resistant closure.
Storage
- Store at room temperature below 30 °C (86 °F).
Images
Drug Images
{{#ask: Page Name::Sofosbuvir |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Sofosbuvir |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Pregnancy
Ribavirin must not be used by women who are pregnant or by men whose female partners are pregnant. Ribavirin therapy should not be initiated until a report of a negative pregnancy test has been obtained immediately before starting therapy. When sofosbuvir is used in combination with peginterferon alfa/ribavirin or ribavirin, patients must be advised of the teratogenic/embryocidal risks of ribavirin and should be advised that extreme care must be taken to avoid pregnancy in female patients and in female partners of male patients both during treatment and for 6 months after the completion of treatment.
Women of childbearing potential and their male partners must use at least two forms of effective contraception during treatment and for 6 months after the treatment has been stopped; routine monthly pregnancy tests must be performed during this time. There is no data on the effectiveness of systemic hormonal contraceptives in women taking sofosbuvir; therefore, two alternative non-hormonal methods of contraception should be used.
Patients should be advised to notify their health care provider immediately in the event of a pregnancy. There is a Ribavirin Pregnancy Registry established to monitor maternal and fetal outcomes of pregnant women exposed to ribavirin. Patients should be encouraged to register by calling 1-800-593-2214. For patients who are HCV/HIV-1 co-infected and taking concomitant antiretrovirals, an Antiretroviral Pregnancy Registry is also available at 1-800-258-4263.
Hepatitis C Virus Transmission
Patients should be informed that the effect of treatment of hepatitis C infection on transmission is not known, and that appropriate precautions to prevent transmission of the hepatitis C virus during treatment or in the event of treatment failure should be taken.
Administration
Patients should be advised that the recommended regimen for patients with genotype 1 or 4 HCV infection is sofosbuvir administered in combination with peginterferon alfa and ribavirin and the recommended regimen for patients with genotype 2 or 3 HCV infection is sofosbuvir administered in combination with ribavirin. If peginterferon alfa and/or ribavirin are permanently discontinued, sofosbuvir should also be discontinued.
Patients should be advised that the dose of sofosbuvir must not be reduced and it should be taken on a regular dosing schedule with or without food. If a patient did not take the sofosbuvir at the regular time, it could be taken later in the day. However, no more than 400 mg of sofosbuvir should be taken on any calendar day. The patient should resume the regular dosing schedule on the next day.
Patient Information
Read this Patient Information before you start taking sofosbuvir and each time you get a refill. There may be new information. This information does not take the place of talking with your healthcare provider about your medical condition or your treatment.
Sofosbuvir is used in combination with other antiviral medicines. When taking sofosbuvir with ribavirin or in combination with peginterferon alfa and ribavirin you should also read those Medication Guides.
The information in this Patient Information Leaflet talks about sofosbuvir when it is used with ribavirin and in combination with peginterferon alfa and ribavirin.
What is the most important information I should know about SOVALDI?
Sofosbuvir , in combination with ribavirin or peginterferon alfa and ribavirin, may cause birth defects or death of your unborn baby. If you are pregnant or your sexual partner is pregnant or plans to become pregnant, do not take these medicines. You or your sexual partner should not become pregnant while taking sofosbuvir with ribavirin or in combination with peginterferon alfa and ribavirin, and for 6 months after treatment is over.
- Females and males must use 2 effective forms of birth control during treatment and for the 6 months after treatment with sofosbuvir and ribavirin or in combination with peginterferon alfa and ribavirin. Talk to your healthcare provider about forms of birth control that may be used during this time.
- Females must have a negative pregnancy test before starting treatment with sofosbuvir and ribavirin or in combination with peginterferon alfa and ribavirin, every month while being treated, and for 6 months after your treatment ends.
- If you or your female sexual partner becomes pregnant while taking or within 6 months after you stop taking sofosbuvir and ribavirin, or sofosbuvir in combination with peginterferon alfa and ribavirin, tell your healthcare provider right away. You or your healthcare provider should contact the Ribavirin Pregnancy Registry by calling 1-800-593-2214. The Ribavirin Pregnancy Registry collects information about what happens to mothers and their babies if the mother takes ribavirin while she is pregnant. If you are also infected with HIV and taking medicines to treat your HIV infection, an Antiretroviral Pregnancy Registry is also available at 1-800-258-4263.
You should not take Sofosbuvir alone
Sofosbuvir should be used together with ribavirin or in combination with peginterferon alfa and ribavirin to treat chronic hepatitis C infection.
What is Sofosbuvir ?
- Sofosbuvir is a prescription medicine used with other antiviral medicines to treat chronic (lasting a long time) hepatitis C infection in adults.
- Sofosbuvir should not be taken alone.
It is not known if sofosbuvir is safe and effective in children under 18 years of age.
Who should not take Sofosbuvir ?
See "What is the most important information I should know about Sofosbuvir ?"
What should I tell my healthcare provider before taking Sofosbuvir?
Before taking Sofosbuvir, tell your healthcare provider if you:
- Have liver problems other than hepatitis C infection
- Have had a liver transplant
- Have severe kidney problems or you are on dialysis
- Have HIV
- Have any other medical condition
- Are breastfeeding or plan to breastfeed. It is not known if sofosbuvir passes into your breast milk. You and your healthcare provider should decide if you will take sofosbuvir or breastfeed. You should not do both.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Other medicines may affect how sofosbuvir works
Especially tell your healthcare provider if you take any of the following medicines:
- Carbamazepine (Carbatrol®, Epitol®, Equetro®, Tegretol®)
- Oxcarbazepine (Trileptal®, Oxtellar XR™)
- Phenytoin (Dilantin®, Phenytek®)
- Phenobarbital (Luminal®)
- Rifabutin (Mycobutin®)
- Rifampin (Rifadin®, Rifamate®, Rifater®, Rimactane®)
- Rifapentine (Priftin®)
- St. John's wort (Hypericum perforatum) or a product that contains St. John's wort
- Tipranavir (Aptivus®)
Know the medicines you take. Keep a list of your medicines and show it to your healthcare provider and pharmacist when you get a new medicine.
How should I take sofosbuvir ?
- Take sofosbuvir exactly as your healthcare provider tells you to take it. Do not change your dose unless your healthcare provider tells you to.
- Do not stop taking sofosbuvir without first talking with your healthcare provider. If you think there is a reason to stop taking sofosbuvir, talk to your healthcare provider before doing so.
- Take sofosbuvir 1 time each day with or without food.
- If you miss a dose of sofosbuvir, take the missed dose as soon as you remember the same day. Do not take more than 1 tablet (400 mg) of sofosbuvir in a day. Take your next dose of sofosbuvir at your regular time the next day.
- If you take too much sofosbuvir, call your healthcare provider or go to the nearest hospital emergency room right away.
What are the possible side effects of sofosbuvir?
See "What is the most important information I should know about sofosbuvir?" The most common side effects of sofosbuvir when used in combination with ribavirin include:
- Tiredness
- Headache
The most common side effects of sofosbuvir when used in combination with peginterferon alfa and ribavirin include:
Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of sofosbuvir. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store sofosbuvir?
- Store sofosbuvir at room temperature below 86°F (30°C).
- Keep sofosbuvir in its original container.
- Do not use sofosbuvir if the seal over the bottle opening is broken or missing.
- Keep sofosbuvir and all medicines out of the reach of children.
General information about the safe and effective use of sofosbuvir
It is not known if treatment with sofosbuvir will prevent you from infecting another person with the hepatitis C virus during treatment. Talk with your healthcare provider about ways to prevent spreading the hepatitis C virus.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use sofosbuvir for a condition for which it was not prescribed. Do not give sofosbuvir to other people, even if they have the same symptoms you have. It may harm them.
If you would like more information about sofosbuvir, talk with your healthcare provider. You can ask your healthcare provider or pharmacist for information about sofosbuvir that is written for health professionals.
For more information, call 1-800-445-3235 or go to www.SOVALDI.com.
What are the ingredients in sofosbuvir?
- Active ingredient: sofosbuvir
- Inactive ingredients: colloidal silicon dioxide, croscarmellose sodium, magnesium stearate, mannitol, and microcrystalline cellulose.
- The tablet film-coat contains: polyethylene glycol, polyvinyl alcohol, talc, titanium dioxide, and yellow iron oxide.
This Patient Information has been approved by the U.S. Food and Drug Administration.
Precautions with Alcohol
Alcohol-Sofosbuvir interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
Sovaldi
Look-Alike Drug Names
There is limited information regarding Sofosbuvir Look-Alike Drug Names in the drug label.
Drug Shortage Status
Drug Shortage
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Label Page=Sofosbuvir |Label Name=SofosbuvirPackage.png
}}