Methylpentynol: Difference between revisions
m (Robot: Automated text replacement (-{{WikiDoc Cardiology Network Infobox}} +, -<references /> +{{reflist|2}}, -{{reflist}} +{{reflist|2}})) |
m (Protected "Methylpentynol": Bot: Protecting all pages from category Drug ([Edit=Allow only administrators] (indefinite) [Move=Allow only administrators] (indefinite))) |
||
| (3 intermediate revisions by one other user not shown) | |||
| Line 1: | Line 1: | ||
{{ | {{Drugbox | ||
| IUPAC_name | | Verifiedfields = changed | ||
| image | | Watchedfields = changed | ||
| verifiedrevid = 447908931 | |||
| | | IUPAC_name = 3-methylpent-1-yn-3-ol | ||
| image = Meparfynol.png | |||
<!--Clinical data--> | |||
| tradename = Oblivon | |||
| pregnancy_AU = <!-- A / B1 / B2 / B3 / C / D / X --> | |||
| pregnancy_US = <!-- A / B / C / D / X --> | |||
| pregnancy_category = | |||
| legal_AU = <!-- Unscheduled / S2 / S3 / S4 / S5 / S6 / S7 / S8 / S9 --> | |||
| legal_CA = <!-- / Schedule I, II, III, IV, V, VI, VII, VIII --> | |||
| legal_UK = <!-- GSL / P / POM / CD / Class A, B, C --> | |||
| pregnancy_AU | | legal_US = <!-- OTC / Rx-only / Schedule I, II, III, IV, V --> | ||
| pregnancy_US | | legal_status = | ||
| pregnancy_category= | | routes_of_administration = | ||
| legal_AU | |||
| legal_CA | |||
| legal_UK | |||
| legal_US | |||
| legal_status | |||
| routes_of_administration = | |||
<!--Pharmacokinetic data--> | |||
| bioavailability = | |||
| protein_bound = | |||
| metabolism = | |||
| elimination_half-life = | |||
| excretion = | |||
<!--Identifiers--> | |||
| CAS_number_Ref = {{cascite|correct|??}} | |||
| CAS_number = 77-75-8 | |||
| ATC_prefix = N05 | |||
| ATC_suffix = CM15 | |||
| PubChem = 6494 | |||
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} | |||
| DrugBank = | |||
| UNII_Ref = {{fdacite|correct|FDA}} | |||
| UNII = B017BC5B1N | |||
| ChemSpiderID_Ref = {{chemspidercite|changed|chemspider}} | |||
| ChemSpiderID = 21106516 | |||
<!--Chemical data--> | |||
| C=6 | H=10 | O=1 | |||
| molecular_weight = 98.143 g/mol | |||
| smiles = CCC(C)(C#C)O | |||
| InChI = 1/C6H10O/c1-4-6(3,7)5-2/h1,7H,5H2,2-3H3 | |||
| InChIKey = QXLPXWSKPNOQLE-UHFFFAOYAI | |||
| StdInChI_Ref = {{stdinchicite|changed|chemspider}} | |||
| StdInChI = 1S/C6H10O/c1-4-6(3,7)5-2/h1,7H,5H2,2-3H3 | |||
| StdInChIKey_Ref = {{stdinchicite|changed|chemspider}} | |||
| StdInChIKey = QXLPXWSKPNOQLE-UHFFFAOYSA-N | |||
}} | |||
__NOTOC__ | |||
{{SI}} | |||
{{CMG}} | |||
==Overview== | |||
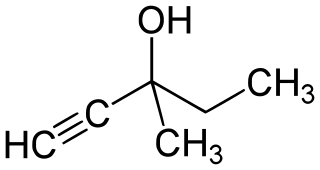
'''Methylpentynol''' ('''Methylparafynol''', '''Dormison''', '''Atemorin''') is a [[Alcohol#tertiary|tertiary]] [[hexanol]] with [[hypnotic]]/[[sedative]] and [[anticonvulsant]] effects. It was discovered by Bayer in 1913 and was used shortly thereafter for the treatment of [[insomnia]], but its use was quickly phased out in response to newer drugs with far more favorable safety profiles. | |||
To make meparfynol proper, the alcohol must be reacted with [[phosgene]] followed by ammonia to form the [[carbamate]] (see patent). Also, ethynylation of cyclohexanone is one of the preferred analogs | |||
==References== | ==References== | ||
{{reflist|2}} | {{reflist|2}} | ||
{{Hypnotics and sedatives}} | {{Hypnotics and sedatives}} | ||
[[Category:Anticonvulsants]] | |||
[[Category:Drug]] | |||
Latest revision as of 16:42, 20 August 2015
 | |
| Clinical data | |
|---|---|
| Trade names | Oblivon |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C6H10O |
| Molar mass | 98.143 g/mol |
| 3D model (JSmol) | |
| |
| |
| | |
|
WikiDoc Resources for Methylpentynol |
|
Articles |
|---|
|
Most recent articles on Methylpentynol Most cited articles on Methylpentynol |
|
Media |
|
Powerpoint slides on Methylpentynol |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Methylpentynol at Clinical Trials.gov Trial results on Methylpentynol Clinical Trials on Methylpentynol at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Methylpentynol NICE Guidance on Methylpentynol
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Methylpentynol Discussion groups on Methylpentynol Patient Handouts on Methylpentynol Directions to Hospitals Treating Methylpentynol Risk calculators and risk factors for Methylpentynol
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Methylpentynol |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Methylpentynol (Methylparafynol, Dormison, Atemorin) is a tertiary hexanol with hypnotic/sedative and anticonvulsant effects. It was discovered by Bayer in 1913 and was used shortly thereafter for the treatment of insomnia, but its use was quickly phased out in response to newer drugs with far more favorable safety profiles. To make meparfynol proper, the alcohol must be reacted with phosgene followed by ammonia to form the carbamate (see patent). Also, ethynylation of cyclohexanone is one of the preferred analogs
References
- Pages with script errors
- Articles with changed ChemSpider identifier
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Articles with changed InChI identifier
- Chemical articles with unknown parameter in Infobox drug
- Articles without EBI source
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Drugs with no legal status
- Drugboxes which contain changes to verified fields
- Drugboxes which contain changes to watched fields
- Anticonvulsants
- Drug