Alpha-1-B glycoprotein: Difference between revisions
m (→Protein) |
m (→Protein) |
||
| Line 151: | Line 151: | ||
== Protein == | == Protein == | ||
"Alpha-1B-glycoprotein (A1BG) is an acute phase protein, its concentration in serum increases in response to inflammatory processes. It has been detected in the urine of children with [steroid-resistant nephrotic syndrome] SRNS, and it has been shown that A1BG can be used to differentiate SRNS from [steroid-sensitive nephrotic syndrome] SSNS [93], [93], [94]."<ref name=Ciuntu>{{ cite journal | "Alpha-1B-glycoprotein (A1BG) is an [[acute phase protein]], its concentration in serum increases in response to inflammatory processes. It has been detected in the urine of children with [steroid-resistant nephrotic syndrome] SRNS, and it has been shown that A1BG can be used to differentiate SRNS from [steroid-sensitive nephrotic syndrome] SSNS [93], [93], [94]."<ref name=Ciuntu>{{ cite journal | ||
|author=Angela Ciuntu and Jana Bernic | |author=Angela Ciuntu and Jana Bernic | ||
|title=Biomarkers in children with kidney diseases, In: ''Urogenital Infections and Inflammations'' | |title=Biomarkers in children with kidney diseases, In: ''Urogenital Infections and Inflammations'' | ||
| Line 164: | Line 164: | ||
|pmid= | |pmid= | ||
|accessdate=8 March 2023 }}</ref> | |accessdate=8 March 2023 }}</ref> | ||
The acute-phase reaction characteristically involves [[fever]], acceleration of peripheral [[leukocytes]], circulating [[neutrophil]]s and their precursors.<ref name=Jain>{{cite journal | authors = Jain S, Gautam V, Naseem S | title = Acute-phase proteins: As diagnostic tool | journal = Journal of Pharmacy & Bioallied Sciences | volume = 3 | issue = 1 | pages = 118–27 | date = January 2011 | pmid = 21430962 | pmc = 3053509 | doi = 10.4103/0975-7406.76489 }}</ref> | |||
=== Properties === | === Properties === | ||
Revision as of 06:58, 9 March 2023
Associate Editor(s)-in-Chief: Henry A. Hoff
| A1BG | |||||||
|---|---|---|---|---|---|---|---|
| Identifiers | |||||||
| |||||||
| |||||||
| |||||||
| |||||||
| |||||||
| |||||||
| |||||||
| |||||||
| |||||||
| Species | Human | Mouse |
|---|---|---|
| Entrez | 1 | 117586 |
| Ensembl | ENSG00000121410 | ENSMUSG00000022347 |
| UniProt | P04217 | Q19LI2 |
| RefSeq (mRNA) | NM_130786 | NM_001081067 |
| RefSeq (protein) | NP_570602 | NP_001074536 |
| Location (UCSC) | Chr 19: 58.35 – 58.35 Mb | Chr 15: 60.9 – 60.92 Mb |
| PubMed search | [1] | [2] |
| VALUE_ERROR (nil) | |||||||
|---|---|---|---|---|---|---|---|
| Identifiers | |||||||
| Aliases | |||||||
| External IDs | GeneCards: [3] | ||||||
| Orthologs | |||||||
| Species | Human | Mouse | |||||
| Entrez |
|
| |||||
| Ensembl |
|
| |||||
| UniProt |
|
| |||||
| RefSeq (mRNA) |
|
| |||||
| RefSeq (protein) |
|
| |||||
| Location (UCSC) | n/a | n/a | |||||
| PubMed search | n/a | n/a | |||||
| Wikidata | |||||||
| |||||||
Alpha-1-B glycoprotein is a 54.3 kDa protein in humans that is encoded by the A1BG gene. [3] The protein encoded by this gene is a plasma glycoprotein of unknown function. The protein shows sequence similarity to the variable regions of some immunoglobulin supergene family member proteins.
Gene
Neighborhood
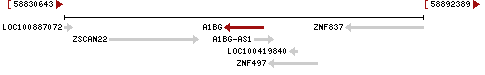
A1BG is located on the negative DNA strand of chromosome 19 from 58,858,172 – 58,864,865.[4] Additionally, A1BG is located directly adjacent to the ZSCAN22 gene (58,838,385-58,853,712) on the positive DNA strand, as well as the ZNF837 (58,878,990 - 58,892,389, complement) and ZNF497 (58865723 - 58,874,214, complement) genes on the negative strand.[4]
Transcriptions
According to one source, A1BG is transcribed from the direction of ZNF497: 3' - 58864890: CGAGCCACCCCACCGCCCTCCCTTGG+1GGCCTCATTGCTGCAGACGCTCACCCCAGACACTCACTGCACCGGAGTGAGCGCGACCATCATG : 58866601-5',[5] where the second 'G' at left of four Gs in a row is the TSS.[6] Transcription was triggered in cell cultures and the transcription start site was found using reverse transcriptase. But, the mechanism for transcription is unknown.
Controlling the transcription of A1BG may have significant immune function against snake envenomation. A1BG forms a complex that is similar to those formed between toxins from snake venom and A1BG-like plasma proteins which inhibits the toxic effect of snake venom metalloproteinases or myotoxins and protects the animal from envenomation.[7]
Expression
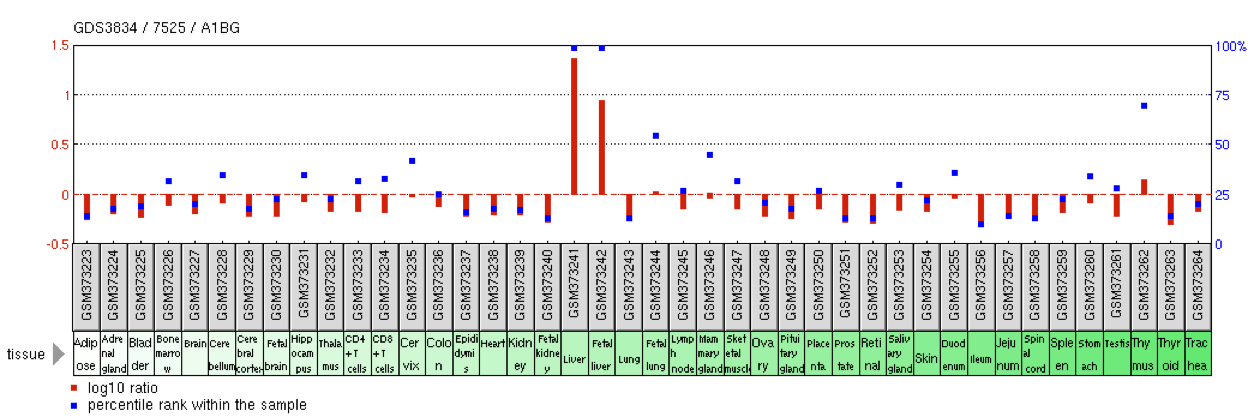
A1BG is expressed at high levels in the adult and fetal liver.[8] Additionally, the mammary gland shows roughly half as much expression as the liver.[8] Trace amounts of A1BG expression can be found in the blood, brain, lung, lymph node, ovary, testis, pancreas, and pancreas.[8] Liver tumors exhibit elevated levels of A1BG transcripts.[8]
mRNA
mRNA structure
The gene contains 20 distinct introns.[9] Transcription produces 15 different mRNAs, 10 alternatively spliced variants and 5 unspliced forms.[9] There are 4 probable alternative promoters, 4 non overlapping alternative last exons and 7 validated alternative polyadenylation sites.[9] The mRNAs appear to differ by truncation of the 5' end, truncation of the 3' end, presence or absence of 4 cassette exons, overlapping exons with different boundaries, splicing versus retention of 3 introns.[9]
Protein
"Alpha-1B-glycoprotein (A1BG) is an acute phase protein, its concentration in serum increases in response to inflammatory processes. It has been detected in the urine of children with [steroid-resistant nephrotic syndrome] SRNS, and it has been shown that A1BG can be used to differentiate SRNS from [steroid-sensitive nephrotic syndrome] SSNS [93], [93], [94]."[10]
The acute-phase reaction characteristically involves fever, acceleration of peripheral leukocytes, circulating neutrophils and their precursors.[11]
Properties
The San Diego Super Computer's Statistical Analysis of Protein (SAPS) program determined that alpha-1B glycoprotein has 495 amino acids residues, an isoelectric point of 5.47, and a molecular mass of 54.3 kDa. Additionally, it suggested that no transmembrane domains exist in alpha-1B glycoprotein.[12] According to NCBI, the amino acid sequence MLVVFLLLWGVTWGPVTEA is a signal peptide on the N-terminus of the protein that might function as an endoplasmic reticulum import signal.[12]
Post-translational modifications
The NetAcet 1.0 program calculated that the first five amino acid residues serve as an N-acetylation site.[13] The NetGlycate 1.0 program predicted that the lysines located at residue 78, 114, and 227 serve as glycation points.[14] The NetNES 1.1 program predicted the leucine at residue 47 to be a nuclear export signal.[15] The NetNGlyc 1.0 program predicted four N-glycosylation sites - two of which are highly conserved internally repeated sequences.[16][17] The NetCGlyc1.0 program predicted that none of the tryptophan residues serve as C-mannosylation sites.[18]
"The monosialylated glycan (Hexose5HexNAc4Neu5Ac1) [is] attached at ASN363 on A1BG."[19]
Protein interactions
A study by Udby et al. showed that Cysteine-rich secretory protein 3 is a ligand of alpha-1B glycoprotein in human plasma and they suggest that the A1BG-CRISP-3 complex displays a similar function in protecting the circulation from a potentially harmful effect of free CRISP-3.[20]
Protein enrichments
Alpha-1B-glycoprotein, an extracellular matrix protein, showed an enrichment in human atrial appendages.[21]
Homology
Orthologs
In addition to the table below, alpha-1B glycoprotein is also conserved in the white-cheeked crested gibbon, baboon, bolivian squirrel monkey, sheep, dog, wild boar, Chinese tree shrew, Chinese hamster, black flying fox, rabbit, guinea pig, giant panda, cow, rat, and the naked mole-rat.[22] Additionally, it is very likely that A1BG is further conserved throughout the mammalian clade.
| Genus species | Organism common name | Divergence from humans (Mya)[23] | NCBI protein accession number | Sequence identity | Protein length | Common gene name |
|---|---|---|---|---|---|---|
| Homo sapiens[24] | Humans | -- | NP_570602 | 100% | 495 | A1BG |
| Pan troglodytes[25] | Common chimp | 6.2 | XP_001146669 | 97.0% | 501 | PREDICTED: Alpha-1B-glycoprotein isoform 4 |
| Pan paniscus[26] | Bonobo | 6.3 | XP_003816677 | 97.0% | 499 | A1BG |
| Gorilla gorilla gorilla [27] | Gorilla | 8.8 | XP_004061652 | 95.0% | 275 | PREDICTED: alpha-1B-glycoprotein |
| Pongo abelii[28] | Sumatran Organutan | 15.7 | XP_002829953 | 95.0% | 495 | alpha-1B-glycoprotein isoform 1 |
| Macaca mulatta[29] | Rhesus monkey | 29.0 | XM_001101821 | 88.0% | 351 | hypothetical protein EGK_11172, partial |
| Callithrix jacchus[30] | Marmoset | 42.6 | XP_002762619 | 83.0% | 500 | A1BG |
| Mus musculus [31] | Mouse | 91.0 | NP_001074536 | 44.0% | 512 | alpha-1B-glycoprotein precursor |
| Felis catus[32] | Cat | 94.2 | XP_003997399 | 62.0% | 481 | PREDICTED: alpha-1B-glycoprotein |
| Equus caballus[33] | Horse | 97.4 | XP_001495344 | 58.0% | 568 | PREDICTED: alpha-1B-glycoprotein-like |
| Loxodonta africana [34] | African bush elephant | 104.7 | XP_003406722 | 61.0% | 520 | PREDICTED: alpha-1B-glycoprotein-like |
| Didelphis marsupialis | Southern opossum | 160.0 | AAL82794? | 0.0%? | 500? | A1BG? |
Paralogs
No paralogs have been found for alpha-1B glycoprotein.[35]
Homologous domains
An initial NCBI Blast alignment of alpha-1B glycoprotein illustrates that the protein is mainly composed of three immunoglobulin domains.[36] There is a large segment of amino acids from position 297 to 400 that is not shown to be an immunoglobulin domain. However, a NCBI BLAST alignment of just the amino acids from 297-400 does illustrate that the latter sequence is indeed a fourth immunoglobulin domain.[37] Ultimately, alpha-1B glycoprotein seems to be primarily composed of four immunoglobulin domains.
Clinical significance
Bladder cancers
"Urinary serotransferrin and haptoglobin were associated with the presence of bladder cancer, but most discriminatory was α-1B-glycoprotein (A1BG-human). This protein was detected in all Con A-captured fractions of bladder cancer patients’ samples but, more importantly, was never found to occur in non-tumor-bearing patients’ urine."[38]
"No specific function has been ascribed to A1BG to date, but an indication of a function comes from the characterization of the opossum A1BG homologue, oprin.33 Oprin is a metalloproteinase inhibitor, which in some properties, but not in sequence, resembles the tissue inhibitor of metalloproteinases (TIMPs), a family of proteins with complex roles in tumor progression and angiogenesis.34 An association of A1BG expression with cancer has been described in one previous study. In a study that utilized expressed sequence tag (EST) profiling of cDNA libraries, Yoon et al. identified A1BG as 1 of 14 genes confirmed to be highly expressed in human hepatocellular carcinoma (HCC) cell lines and liver tumor tissue specimens.35"[38]
Breast cancers
"Alpha-1B-glycoprotein is a secreted glycoprotein with some similarity to the immunoglobulin family and basically very few known functions70. [It] has been described in proteomic studies of several cancer types like breast cancer71, oral squamous carcinoma72, in the serum of non-small cell lung cancer73, and in pancreatic ductal adenocarcinoma74. Here we describe for the first time a negative correlation of alpha-1B-glycoprotein tissue expression to melanoma survival."[39]
Cervical adenocarcinoma
"The expression of high-abundance serum proteins in newly diagnosed patients with endometrial adenocarcinoma (EACa), squamous cell cervical carcinoma (SCCa) and cervical adenocarcinoma (ACCa), relative to control female subjects, was analyzed by subjecting serum samples to 2-DE followed by image analysis of the silver-stained protein profiles. The three cohorts of cancer patients demonstrated different altered expression of serum high-abundance proteins compared to negative control women. The expression of α1-antitrypsin, α1-B glycoprotein [ABG], cleaved high-molecular-weight kininogen (light chain) and antithrombin III were consistently altered in all the patients."[40]
"The upregulated expression of ABG [was] consistently observed in all the patients [relative to negative control women]."[40]
Cervical intraepithelial neoplasia
"A1BG [...] expression was elevated in several cancers including cervical intraepithelial neoplasia (28), which suggested it may be play a role in carcinogenesis."[41]
"Cervical cancer develops due to persistent infection with the oncogenic Human Papilloma Virus (HPV), which progresses into an intraepithelial lesion, then into invasive cervical cancer."[42]
"The eight highest score spots that were found to be overexpressed in the [cervical intraepithelial neoplasia, grade III] CIN III sera group were identified as α-1-B glycoprotein (A1BG), complement component 3 (C3), a pro-apolipoprotein, two apolipoproteins and three haptoglobins. Only A1BG and C3 were validated using western blot analysis, and the bands were compared between the two groups using densitometry analysis. The relative density of the bands of A1BG and C3 was found to be greater in all of the serum samples from the females with CIN III, compared with [healthy females who were Human Papilloma Virus-negative] in the control group."[42]
Chronic bronchitis
"Transforming growth factor beta-1 precursor and interferon beta precursor were up-regulated in CB, while interleukin 6, tumor necrosis factor (TNF) and a variant TNF receptor 13B were down-regulated in CB. Additionally, glycoprotein- and apolipoprotein-associated proteins including apolipoprotein A-IV and α-1-B-glycoprotein were up-regulated in CB, indicating an involvement in the pathogenesis of CB but not silicosis. By contrast, HLA-DRB1, medullasin and the proto-oncogene c-Fos were up-regulated in CB."[41]
Endometrial adenocarcinoma
"The upregulated expression of ABG [was] consistently observed in all the patients [relative to negative control women]."[40]
Hepatocellular carcinoma
"10 commonly down-regulated genes [relative to healthy cells] from the [Hepatocellular carcinoma] HCC pool [are] ALB, HP, AMBP, FGG, GC, FGB, A1BG, VTN, SERPINC1, and FGA".[43]
"Among the down-regulated genes, AMBP, GC, SERPINC1 and A1BG might be also candidates for the diagnosis and treatment of liver cancer because they are known to be immune-related proteins or plasma proteins."[43]
Non-small cell lung cancers
"Clinically, the treatment of non-small cell lung cancer (NSCLC) can be improved by the early detection and risk screening among population."[44]
"To the best of our knowledge, our study here is the first report that links the A1BG blood/tissue expression with lung cancer diseases, and we validated this linkage in a cohort of about 100 patients, which is much larger than the sample sets in above previous studies."[44]
Normal pressure hydrocephalus
The "expressed differences in cerebrospinal fluid (CSF) protein patterns [...] of normal pressure hydrocephalus (NPH) [include the] over-expression of α2HS glycoprotein, α1 antichimotrypsin and α1beta glycoprotein and the under-expression of glial fibrillary acidic protein, apolipoproteins (AIV, J and E), complement C3c, anti-thrombin, α2 antiplasmin and albumin".[45]
Oral squamous carcinomas
Pancreatic ductal adenocarcinoma
Patients who have pancreatic ductal adenocarcinoma show an overexpression of MMP-9, DJ-1 and A1BG in pancreatic juice.[46]
Papillary thyroid carcinoma
Group A consists of "papillary thyroid carcinoma patients with non-131I-avid lung metastases and [group B consists of papillary thyroid carcinoma patients with] 131I-avid lung metastases."[47]
"Of the 11 identified proteins, six were downregulated [α-1B-glycoprotein (A1BG), carboxypeptidase N-polypeptide 1, complement factor H-related 1 (CFHR1), group-specific component, inter-α (globulin) inhibitor H2 (ITIH2), and afamin] and five were upregulated [histidine-rich glycoprotein, serum amyloid P component, cp 20 kDa protein, complement component C7, and keratin, type I cytoskeletal 9 (KRT9)] in group A."[47]
"A1BG and afamin, [...] are carcinogenesis-related proteins".[47]
The "protein levels of A1BG did not differ significantly between the two groups."[47]
Rheumatoid arthritis
A1BG has been proposed as an autoantigen in rheumatoid arthritis.[48]
Rheumatoid arthritis "RA patients revealed an increase in the expression of [glial fibrillary acidic protein (GFAP)] and A1BG in the plasma as compared to osteoarthritis patients."[48]
"Presence of autoantibodies against GFAP and A1BG in the plasma samples of RA patients were confirmed by Western blot analysis after separating the recombinant pure GFAP and A1BG proteins on SDS-PAGE."[48]
Reactivity "of A1BG in RA plasma was 5 fold higher than OA plasma and 6 fold [higher] than healthy control [...].[48]
"GFAP and A1BG autoantigens are shown to be associated with RA and autoantibodies against GFAP and A1BG were isolated in RA plasma."[48]
Squamous cell cervical carcinoma
Plasma "samples from six healthy females [were] compared with six females with squamous cell carcinoma (SCC) and [...] A1BG and C3 were [identified as] overexpressed using 2D-GE analysis."[49]
"The upregulated expression of ABG [was] consistently observed in all the patients [relative to negative control women]."[40]
Steroid-resistant nephrotic syndrome
"Focal segmental glomerulosclerosis (FSGS) is a glomerular scarring disease characterized by increased extracellular matrix within the glomerular tuft [1, 2]. The sclerotic lesions occur focally and in only some segments of glomeruli, and are typically not associated with immune complex deposition [1]. It is believed that the loss of foot process of podocytes is also one of the most important characteristics of FSGS [3]. FSGS is a major cause of steroid resistant nephrotic syndrome, which could lead to end-stage renal failure [4, 5]."[50]
"A1BG (Alpha-1B-glycoprotein) is a plasma glycoprotein with homology to the immunoglobulin supergene family [49]. Implication of this protein in responsiveness to steroid drugs reported by Pyaphanee et al.[[51]] and Huang et al. [[19]]. Underrepresentation of this protein in steroid sensitive patients in our data set is thus consistent with previous findings."[50]
The alpha-1-glycoprotein is upregulated 11-fold in the urine of patients who have steroid resistant nephrotic syndrome.[51] A1BG was present in 7/19 patients with SRNS and was absent from all patients with steroid sensitive nephrotic syndrome.[51] The 13.8 kDa A1BG fragment had a high discriminatory power for steroid resistance in pediatric nephrotic syndrome, but is only present in a subset of patients.[51]
See also
- A1BG gene transcription core promoters
- A1BG gene transcription programming
- A1BG gene transcriptions
- AGC box gene transcription laboratory
- ATA box gene transcription laboratory
- C and D boxes gene transcription laboratory
- CArG box gene transcription laboratory
- CGCG box gene transcription laboratory
- CRE box gene transcription laboratory
- E2 box gene transcription laboratory
- Enhancer box gene transcription laboratory
- Factor II B recognition element gene transcription laboratory
- GA responsive complex gene transcription laboratory
- GC box gene transcription laboratory
- H box gene transcription laboratory
- HNF6 gene transcription laboratory
- HY box gene transcription laboratory
- Initiator element gene transcription laboratory
- Metal responsive element gene transcription laboratory
- STAT5 gene transcription laboratory
- TATA box gene transcription laboratory
References
- ↑ [1]
- ↑ [2]
- ↑ "Entrez Gene: Alpha-1-B glycoprotein". Retrieved 2012-11-09.
- ↑ 4.0 4.1 "A1BG alpha-1-B glycoprotein". Retrieved May 10, 2013.
- ↑ Michael David Winther, Leah Christine Knickle, Martin Haardt, Stephen John Allen, Andre Ponton, Roberto Justo De Antueno, Kenneth Jenkins, Solomon O. Nwaka, Y. Paul Goldberg (July 29, 2004). Fat Regulated Genes, Uses Thereof and Compounds for Mudulating Same. US Patent Office. Retrieved 2013-02-14.
- ↑ HGNC (February 5, 2013). A1BG alpha-1-B glycoprotein [ Homo sapiens ]. 8600 Rockville Pike, Bethesda MD, 20894 USA: National Center for Biotechnology Information, U.S. National Library of Medicine. Retrieved 2013-02-14.
- ↑ Udby L, Sørensen OE, Pass J, Johnsen AH, Behrendt N, Borregaard N, Kjeldsen L. (October 2004). "Cysteine-rich secretory protein 3 is a ligand of alpha1B-glycoprotein in human plasma". Biochemistry. 43 (40): 12877–86. doi:10.1021/bi048823e. PMID 15461460.
|access-date=requires|url=(help) - ↑ 8.0 8.1 8.2 8.3 "EST Profile - Hs.529161". UniGene. National Center for Biotechnology Information, U.S. National Library of Medicine. Retrieved 2013-05-11.
- ↑ 9.0 9.1 9.2 9.3 "AceView: A1BG". Retrieved May 11, 2013.
- ↑ Angela Ciuntu and Jana Bernic (24 November 2021). Bjerklund Johansen TE, Wagenlehner FME, Matsumoto T, Cho YH, Krieger JN, Shoskes D, Naber KG, eds. "Biomarkers in children with kidney diseases, In: Urogenital Infections and Inflammations". Berlin: German Medical Science GMS Publishing House. doi:10.5680/lhuii000060. Retrieved 8 March 2023.
- ↑ Jain S, Gautam V, Naseem S (January 2011). "Acute-phase proteins: As diagnostic tool". Journal of Pharmacy & Bioallied Sciences. 3 (1): 118–27. doi:10.4103/0975-7406.76489. PMC 3053509. PMID 21430962.
- ↑ 12.0 12.1 Brendel V, Bucher P, Nourbakhsh IR, Blaisdell BE, Karlin S (March 1992). "Methods and algorithms for statistical analysis of protein sequences". Proc. Natl. Acad. Sci. U.S.A. 89 (6): 2002–6. doi:10.1073/pnas.89.6.2002. PMC 48584. PMID 1549558.
- ↑ Kiemer L, Bendtsen JD, Blom N (April 2005). "NetAcet: prediction of N-terminal acetylation sites". Bioinformatics. 21 (7): 1269–70. doi:10.1093/bioinformatics/bti130. PMID 15539450.
- ↑ Johansen MB, Kiemer L, Brunak S (September 2006). "Analysis and prediction of mammalian protein glycation". Glycobiology. 16 (9): 844–53. doi:10.1093/glycob/cwl009. PMID 16762979.
- ↑ la Cour T, Kiemer L, Mølgaard A, Gupta R, Skriver K, Brunak S (June 2004). "Analysis and prediction of leucine-rich nuclear export signals". Protein Eng. Des. Sel. 17 (6): 527–36. doi:10.1093/protein/gzh062. PMID 15314210. Retrieved May 10, 2013.
- ↑ Gupta, R. "Prediction of N-glycosylation sites in human proteins". Retrieved May 10, 2013.
- ↑ Higgins DG, Bleasby AJ, Fuchs R (April 1992). "CLUSTAL V: improved software for multiple sequence alignment". Comput. Appl. Biosci. 8 (2): 189–91. doi:10.1093/bioinformatics/8.2.189. PMID 1591615.
- ↑ Julenius, Karin (2007). "NetCGlyc1.0: Prediction of mammalian C-mannosylation sites". Glycobiology. 17: 868–876. doi:10.1093/glycob/cwm050. PMID 17494086. Retrieved May 10, 2013.
- ↑ 19.0 19.1 Jincui Huang, Hyeyoung Lee, Angela M. Zivkovic, Jennifer T. Smilowitz, Nancy Rivera, J. Bruce German, Carlito B. Lebrilla (7 February 2014). "Glycomic Analysis of High Density Lipoprotein Shows a Highly Sialylated Particle". Journal of Proteome Research. 13 (2): 681–691. doi:10.1021/pr4012393. PMID 24417605. Retrieved 22 March 2020.
- ↑ Udby L, Sørensen OE, Pass J, Johnsen AH, Behrendt N, Borregaard N, Kjeldsen L (October 2004). "Cysteine-rich secretory protein 3 is a ligand of alpha1B-glycoprotein in human plasma". Biochemistry. 43 (40): 12877–86. doi:10.1021/bi048823e. PMID 15461460.
- ↑ Javier Barallobre-Barreiro, Shashi K. Gupta, Anna Zoccarato, Rika Kitazume-Taneike, Marika Fava, Xiaoke Yin, Tessa Werner, Marc N Hirt, Anna Zampetaki, Alessandro Viviano, Mei Chong, Marshall Bern, Antonios Kourliouros, Nieves Domenech, Peter Willeit, Ajay M Shah, Marjan Jahangiri, Liliana Schaefer, Jens W. Fischer, Renato V. Iozzo, Rosa Viner, Thomas Thum, Joerg Heineke, Antoine Kichler, Kinya Otsu and Manuel Mayr (13 September 2016). "Glycoproteomics Reveals Decorin Peptides with Anti-Myostatin Activity in Human Atrial Fibrillation". Circulation. 134 (11): 817–832. doi:10.1161/CIRCULATIONAHA.115.016423. PMID 27559042. Retrieved 31 January 2021.
- ↑ "NCBI Blast results for A1BG protein sequence". Retrieved May 11, 2013.
- ↑ "Time Tree".
- ↑ "alpha-1-B glycoprotein [Homo sapiens]". Retrieved May 11, 2013.
- ↑ "PREDICTED: alpha-1B-glycoprotein isoform 4 [Pan troglodytes]". NCBI. Retrieved May 10, 2013.
- ↑ "PREDICTED: alpha-1B-glycoprotein [Pan paniscus]". Retrieved May 11, 2013.
- ↑ "PREDICTED: alpha-1B-glycoprotein". Retrieved May 10, 2013.
- ↑ "Send to: PREDICTED: alpha-1B-glycoprotein isoform 1 [Pongo abelii]". Retrieved May 11, 2013.
- ↑ "hypothetical protein EGK_11172, partial [Macaca mulatta]". Retrieved May 11, 2013.
- ↑ "PREDICTED: alpha-1B-glycoprotein [Callithrix jacchus]". Retrieved May 11, 2013.
- ↑ "alpha-1B-glycoprotein precursor [Mus musculus]". Retrieved May 11, 2013.
- ↑ "PREDICTED: alpha-1B-glycoprotein [Felis catus]". Retrieved May 11, 2013.
- ↑ "PREDICTED: alpha-1B-glycoprotein-like". Retrieved May 11, 2013.
- ↑ "PREDICTED: alpha-1B-glycoprotein-like [Loxodonta africana]".
- ↑ "A1BG Gene". Weissman Institute of Science. Retrieved May 10, 2013.
- ↑ "NCBI conserved domain search". Retrieved May 10, 2013.
- ↑ "NCBI Blast: Protein Sequence". Retrieved May 10, 2013.
- ↑ 38.0 38.1 Paweena Kreunin, Jia Zhao, Charles Rosser, Virginia Urquidi, David M. Lubman, and Steve Goodison (July 2007). "Bladder Cancer Associated Glycoprotein Signatures Revealed by Urinary Proteomic Profiling". Journal of Proteome Research. 6 (7): 2631–2639. doi:10.1021/pr0700807. PMID 17518487. Retrieved 22 March 2020.
- ↑ Lazaro Hiram Betancourt, Krzysztof Pawłowski, Jonatan Eriksson, A. Marcell Szasz, Shamik Mitra, Indira Pla, Charlotte Welinder, Henrik Ekedahl, Per Broberg, Roger Appelqvist, Maria Yakovleva, Yutaka Sugihara, Kenichi Miharada, Christian Ingvar, Lotta Lundgren, Bo Baldetorp, Håkan Olsson, Melinda Rezeli, Elisabet Wieslander, Peter Horvatovich, Johan Malm, Göran Jönsson, and György Marko-Varga (26 March 2019). "Improved survival prognostication of node-positive malignant melanoma patients utilizing shotgun proteomics guided by histopathological characterization and genomic data". Scientific Reports. 9: 5154. doi:10.1038/s41598-019-41625-z. PMID 30914758. Retrieved 13 March 2020.
- ↑ 40.0 40.1 40.2 40.3 Puteri S. Abdul‐Rahman, Boon‐Kiong Lim, Onn H. Hashim (18 June 2007). "Expression of high-abundance proteins in sera of patients with endometrial and cervical cancers: Analysis using 2-DE with silver staining and lectin detection methods". Electrophoresis. 28 (12): 1989–96. doi:10.1002/elps.200600629. PMID 17503403. Retrieved 20 March 2020.
- ↑ 41.0 41.1 Rongming Miao, Bangmei Ding, Yingyi Zhang, Qian Xia, Yong Li, Baoli Zhu (March 2016). "Proteomic profiling differences in serum from silicosis and chronic bronchitis patients: a comparative analysis". Journal of Thoracic Disease. 8 (3): 439–50. doi:10.21037/jtd.2016.02.68. PMID 27076939. Retrieved 20 March 2020.
- ↑ 42.0 42.1 Norma Angélica Galicia Canales, Vicente Madrid Marina, Jorge Salmerón Castro, Alfredo Antúnez Jiménez, Guillermo Mendoza-Hernández, Elizabeth Langley McCarron, Margarita Bahena Roman, and Julieta Ivone Castro-Romero (August 2014). "A1BG and C3 are overexpressed in patients with cervical intraepithelial neoplasia III". Oncology Letters. 8 (2): 939–947. doi:10.3892/ol.2014.2195. PMID 25009667. Retrieved 20 March 2020.
- ↑ 43.0 43.1 Sun Young Yoon, Jeong-Min Kim, Jung-Hwa Oh, Yeo-Jin Jeon, Dong-Seok Lee, Joo Heon Kim, Jong Young Choi, Byung Min Ahn, Sangsoo Kim, Hyang-Sook Yoo, Yong Sung Kim, and Nam-Soon Kim (1 August 2006). "Gene expression profiling of human HBV- and/or HCV-associated hepatocellular carcinoma cells using expressed sequence tags". International Journal of Oncology. 29 (2): 315–327. doi:10.3892/ijo.29.2.315. PMID 16820872. Retrieved 23 March 2020.
- ↑ 44.0 44.1 Yansheng Liu, Xiaoyang Luo, Haichuan Hu, Rui Wang, Yihua Sun, Rong Zeng, and Haiquan Chen (19 December 2012). "Integrative Proteomics and Tissue Microarray Profiling Indicate the Association between Overexpressed Serum Proteins and Non-Small Cell Lung Cancer". PLoS One. 7 (12): e51748. doi:10.1371/journal.pone.0051748. PMID 23284758. Retrieved 31 January 2021.
- ↑ Antonio Scollato, Alessandro Terreni, Anna Caldini, Benedetta Salvadori, Pasquale Gallina, Simona Francese, Guido Mastrobuoni, Giuseppe Pieraccini, Gloriano Moneti, Luca Bini, Gianni Messeri & Nicola Di Lorenzo (June 2010). "CSF proteomic analysis in patients with normal pressure hydrocephalus selected for the shunt: CSF biomarkers of response to surgical treatment". Neurological Sciences. 31 (3): 283–91. doi:10.1007/s10072-009-0181-0. PMID 19936883. Retrieved 23 March 2020.
- ↑ Tian M, Cui YZ, Song GH, Zong MJ, Zhou XY, Chen Y, Han JX (2008). "Proteomic analysis identifies MMP-9, DJ-1 and A1BG as overexpressed proteins in pancreatic juice from pancreatic ductal adenocarcinoma patients". BMC Cancer. 8: 241. doi:10.1186/1471-2407-8-241. PMC 2528014. PMID 18706098.
- ↑ 47.0 47.1 47.2 47.3 Hong-Jun Song, Yan-Li Xue, Zhong-Ling Qiu, and Quan-Yong Luo (December 2013). "Comparative serum proteomic analysis identified afamin as a downregulated protein in papillary thyroid carcinoma patients with non-131I-avid lung metastases". Nuclear Medicine Communications. 34 (12): 1196–1203. doi:10.1097/MNM.0000000000000001. PMID 24089082. Retrieved 22 March 2020.
- ↑ 48.0 48.1 48.2 48.3 48.4 Sagarika Biswas, Saurabh Sharma, Ashish Saroha, D. S. Bhakuni, Rajesh Malhotra, Muzna Zahur, Michael Oellerich, Hasi R. Das, Abdul R. Asif (13 February 2013). "Identification of novel autoantigen in the synovial fluid of rheumatoid arthritis patients using an immunoproteomics approach". PLoS One. 8 (2): e56246. doi:10.1371/journal.pone.0056246. PMID 23418544. Retrieved 20 March 2020.
- ↑ Dae Hoon Jeong, Hyoung Kyu Kim, Abd-El Bary Prince, Dae Sim Lee, Young Nam Kim, Jin Han, and Ki Tae Kim (30 September 2008). "Plasma proteomic analysis of patients with squamous cell carcinoma of the uterine cervix". Journal of Gynecologic Oncology. 19 (3): 173–80. doi:10.3802/jgo.2008.19.3.173. PMID 19471570. Retrieved 20 March 2020.
- ↑ 50.0 50.1 Shiva Kalantari, Mohsen Nafar, Dorothea Rutishauser, Shiva Samavat, Mostafa Rezaei-Tavirani, Hongqian Yang & Roman A Zubarev (2 September 2014). "Predictive urinary biomarkers for steroid-resistant and steroid-sensitive focal segmental glomerulosclerosis using high resolution mass spectrometry and multivariate statistical analysis". BMC Nephrology. 15: 141. doi:10.1186/1471-2369-15-141. PMID 25182141. Retrieved 21 March 2020.
- ↑ 51.0 51.1 51.2 51.3 Piyaphanee N, Ma Q, Kremen O, Czech K, Greis K, Mitsnefes M, Devarajan P, Bennett MR (June 2011). "Discovery and initial validation of α 1-B glycoprotein fragmentation as a differential urinary biomarker in pediatric steroid-resistant nephrotic syndrome". Proteomics: Clinical Applications. 5 (5–6): 334–42. doi:10.1002/prca.201000110. PMID 21591266.
External links
- Human A1BG genome location and A1BG gene details page in the UCSC Genome Browser.