Sandbox:Aditya
Typhus fever
- Typhus refers to a group of zoonotic diseases caused by bacteria that are spread to humans by fleas, lice, and chiggers.
- Typhus fevers include scrub typhus, murine typhus, and epidemic typhus.
- The most common symptoms are fever, headaches, and sometimes rash.
Historical perspective
- In 1083, Typhus was first identified as a disease in Spain.
- In 1489, during the Spanish siege of Moorish Granada, the first reliable description of the disease was made.
- In 1546, Fracastoro extensively described the disease and distinguished it from plague in his book Contagione.
- In 1676, Von Zavorziz wrote a book on typhus called The Infection of Military Camps.
- In 1739, Huxham stated typhus and typhoid as two different entities, later in the same year Boissier de Sauvages confirmed this and called it exanthematic typhus.
- In 1829, Louis, French clinician clearly differentiated Typhus Fever from Typhoid Fever.
- In 1836, Gerhard(United States) clearly distinguished the two diseases from each other based on pathologic findings.
- In 1909, Charles Nicolle for the first time described the role of lice bite in transmission of typhus. In 1928, he was awarded the Nobel Prize for his discovery.
- In 1916, Weil and Felix reported the isolation of a Proteus that was agglutinated by the sera of patients with typhus, which was the basis for the first serological test for the disease.
- In 1916, DaRocha-Lima isolated and identified Rickettsia prowazeki.
- In 1926, Maxcy described the various forms of typhus.
- In 1938, Starzyk demonstrated that patients are infected by the feces and not the bite of the louse.
- In 1922, Wolbach described the human histopathology of R prowazekii infection.[1]
- In 1938, Cox was successful in growing cell cultures of R prowazekii in embryonated eggs.[2]
- In 1940, Cox and Bell prepared an Epidemic Typhus vaccine based upon the use of tissue culture.
- In 1943–1944, during World war II DDT (a pesticide) was employed to control lice and typhus.
- In 1998, Andersson et al, sequenced the entire genome after much study of the fundamental mechanisms of R prowazekii's intracellular life and its effects on host cells.[3]
Pathophysiology
Typhus fever is a zoonotic disease, Humans could be infected by bites from ticks, lice, inhalation of the bacteria, and direct contact of bacteria with skin wounds or mucous membranes. Following transmission, white blood cells phagocyte the pathogen and transports it via hematologic or lymphatic route to different organs, specially to those of the reticuloendothelial system. The pathophysiology of typhus fever can be described in the following steps.
Transmission
- Rickettsial pathogens are harboured by parasites such as fleas, lice, mites, and ticks.
- Organisms are transmitted by the bites from these parasites or by the inoculation of infectious fluids or feces from the parasites into the skin.
class="wikitable"
| Disease | Etiological agent | Vector |
|---|---|---|
| Epidemic typhus | Rickettsia prowazekii | Human body louse |
| Murine typhus | Rickettsia typhi | Infected fleas |
| Scrub typhus | Orientia tsutsugamushi | Larval mites |
Dissemination
- Scratching a louse-bite site allows the rickettsia-laden excrement to be inoculated into the bite wound.
- Following transmission, rickettsia are ingested by macrophages and polymorphonuclear cells. On ingestion, they replicate intracellularly inside the lysed cells and disseminate systemically.
Incubation
Incubation period of Typhus fever varies from one to two weeks.
Pathogenesis
- On transmission, Rickettsia is actively phagocytosed by the endothelial cells of the small venous, arterial, and capillary vessels.
- It is followed by systemic hematogenous spread resulting in multiple localizing vasculitis. The major pathology is caused by vasculitis and its complications.
- This process of inflammatory response (aggregation of leukocytes, macrophages, and platelets) along with occlusion of small blood vessels results in formation of nodules.
- Occlusion of supplying blood vessels also causes gangrene of the distal portions of the extremities, nose, ear lobes, and genitalia.
- This vasculitic process also results destruction of the endothelial cells and leakage of the blood leading to volume depletion and subsequently leading to decreased tissue perfusion and, possibly, organ failure.
- Endothelial damage lead to activation of clotting system
Natural history
Without treatment, fever may last 2 weeks, followed by a prolonged recovery time and a significantly greater chance of developing complications. Delay in treatment may result in advanced disease, including neurologic manifestations such as confusion, seizures, or coma, and widespread vasculitis (damage to the endothelial cells that line blood vessels).
Complications
- Hearing loss
- Myocarditis
- Vasculitis
- Aseptic meningitis
Prognosis
Prognosis depends on age and immunization status of the individual. With prompt, appropriate treatment, most individuals recover completely.The fatality rate for epidemic typhus varies from 1% to 20%.
History
- History of travel to endemic areas
- History of tick bite
Symptoms
| Typhus fever | Rash |
|---|---|
| Scrub typhus | About 25–50% of scrub typhus patients develop a rash. The rash is usually macular or maculopapular. Typically, it will begin on the abdomen of an infected individual and then spread to the extremities. Petechiae are uncommon |
| Murine Typhus | The rash typically occurs at the end of the first week of the illness and lasts 1–4 days. It generally starts as a maculopapular eruption on the trunk and spreads peripherally, sparing the palms of the hands and soles of the feet. Rash may vary among individuals, or may be absent altogether and should not be relied upon for diagnosis. |
| Epidemic Typhus | The rash usually begins a couple of days after the onset of symptoms. It typically begins as a maculopapular eruption on the trunk of the body and spreads to the extremities, usually sparing the palms of hands and soles of feet. When the disease is severe, petechiae may develop. The rash may be variable among individuals and stage of infection, or may be absent altogether and should not be relied upon for diagnosis |
About 25–50% of scrub typhus patients develop a rash. The rash is usually macular or maculopapular. Typically, it will begin on the abdomen of an infected individual and then spread to the extremities. Petechiae are uncommon. Murine Typhus The rash typically occurs at the end of the first week of the illness and lasts 1–4 days. It generally starts as a maculopapular eruption on the trunk and spreads peripherally, sparing the palms of the hands and soles of the feet. Rash may vary among individuals, or may be absent altogether and should not be relied upon for diagnosis. Epidemic Typhus The rash usually begins a couple of days after the onset of symptoms. It typically begins as a maculopapular eruption on the trunk of the body and spreads to the extremities, usually sparing the palms of hands and soles of feet. When the disease is severe, petechiae may develop. The rash may be variable among individuals and stage of infection, or may be absent altogether and should not be relied upon for diagnosis. Most common symptoms
- Fever
- Headache
- Malaise
- Maculopapular, vesicular, or petechial rash
- Eschar
- Nausea and vomiting.
Less common symptoms
- Abdominal pain
- Cough
- Prostration
- Confusion
- Photophobia
- Diarrhea
Lab diagnosis
- Laboratory studies are not particularly helpful in confirming a diagnosis of typhus.
- They assess the degree of severity of the illness and in help in excluding other diseases.
- The diagnosis of typhus is clinically suggested when the appropriate historical elements are elicited from a patient who presents with the characteristic symptoms and signs.
- Antibiotic therapy should begin promptly when the diagnosis is suspected; thereafter, appropriate laboratory studies can be serially performed as needed.
- Diagnosis may be confirmed using laboratory tests; however, more than one week may pass before patients mount a demonstrable immune response that can be measured serologically.
- Typhus is a vasculitic process, any organ may be affected, and multiorgan system dysfunction or failure may occur if the illness is not diagnosed and treated in the early stages.
- Renal - Azotemia/proteinuria
- Hematologic
- Leukopenia (common in the early stages of disease)
- WBC count normal/mildly elevated later
- Thrombocytopenia
- Hepatic - Mild transaminase elevations
- Metabolic - Hypoalbuminemia/electrolyte abnormalities (particularly hyponatremia)
- Indirect immunofluorescence assay (IFA) or enzyme immunoassay (EIA) testing can be used to evaluate for a rise in the immunoglobulin M (IgM) antibody titer, which indicates an acute primary disease.
- Brill-Zinsser disease can be confirmed in a patient with a history of primary epidemic typhus who has recurrent symptoms and signs of typhus and a rise in the immunoglobulin G (IgG) antibody titer, which indicates a secondary immune response.
- IFA and EIA tests can be used to confirm a diagnosis of typhus, but they do not identify the various rickettsial species.
- Polymerase chain reaction (PCR) amplification of rickettsial DNA of serum or skin biopsy specimens can be used for diagnosing typhus. [9]
- The complement fixation (CF) test is a serological test that can be used to demonstrate which specific rickettsial organism is causing disease by detection of specific antibodies.
Histologic Findings
- Rickettsia may be observed in tissue sections using Giemsa or Gimenez staining techniques.
Xray chest
- No imaging studies are specifically indicated to aid in diagnosing typhus.
- Imaging studies are indicated only on a case-by-case basis to evaluate potential complications or as needed.
- Chest radiography may be a complementary tool to evaluate the clinical course of scrub typhus.
- Chest radiographic examinations should be obtained during the first week after the onset of illness.
Differential
| Disease | Findings |
|---|---|
| Ebola | Presents with fever, chills vomiting, diarrhea, generalized pain or malaise, and sometimes internal and external bleeding, that follow an incubation period of 2-21 days. |
| Typhoid fever | Presents with fever, headache, rash, gastrointestinal symptoms, with lymphadenopathy, relative bradycardia, cough and leucopenia and sometimes sore throat. Blood and stool culture can confirm the presence of the causative bacteria. |
| Malaria | Presents with acute fever, headache and sometimes diarrhea (children). A blood smears must be examined for malaria parasites. The presence of parasites does not exclude a concurrent viral infection. An antimalarial should be prescribed as an empiric therapy. |
| Lassa fever | Disease onset is usually gradual, with fever, sore throat, cough, pharyngitis, and facial edema in the later stages. Inflammation and exudation of the pharynx and conjunctiva are common. |
| Yellow fever and other Flaviviridae | Present with hemorrhagic complications. Epidemiological investigation may reveal a pattern of disease transmission by an insect vector. Virus isolation and serological investigation serves to distinguish these viruses. Confirmed history of previous yellow fever vaccination will rule out yellow fever. |
| Shigellosis & other bacterial enteric infections | Presents with diarrhea, possibly bloody, accompanied by fever, nausea, and sometimes toxemia, vomiting, cramps, and tenesmus. Stools contain blood and mucous in a typical case. A search for possible sites of bacterial infection, together with cultures and blood smears, should be made. Presence of leucocytosis distinguishes bacterial infections from viral infections. |
| Others | Leptospirosis, viral hepatitis, rheumatic fever, and mononucleosis can produce signs and symptoms that may be confused with Ebola in the early stages of infection. |
| Diseases | Clinical features | Diagnosis | |||||
|---|---|---|---|---|---|---|---|
| Fever | Rash | Diarrhea | Cough | Specific | |||
| Ebola | |||||||
| Typhoid fever | |||||||
| Malaria | |||||||
| Lassa fever | |||||||
| Yellow fever and
other Flaviviridae |
|||||||
| Shigellosis &
other bacterial enteric infections |
|||||||
Labs
Serologic assays are the most frequently used methods for confirming cases of scrub typhus. The indirect immunofluorescence assay (IFA) is generally considered the reference standard, but is usually not available in developing countries where this disease is endemic. Other serological tests include ELISA and indirect immunuoperoxidase (IIP) assays. Weil-Felix OX-K agglutination assays may be used in some international settings but lack sensitivity and specificity and are not generally used in the United States. These assays can detect either IgG or IgM antibodies. Diagnosis is typically confirmed by documenting a four-fold rise in antibody titer between acute and convalescent samples. Acute specimens are taken during the first week of illness and convalescent samples are taken 2–4 weeks later. IgG antibodies are considered more accurate than IgM, but detectable levels of IgG antibody generally do not appear until 7–10 days after the onset of illness.
Because antibody titers may persist in some individuals for years after the original exposure, only demonstration of recent changes in titers between paired specimens can be considered reliable confirmation of an acute scrub typhus infection. The most rapid and specific diagnostic assays for scrub typhus rely on molecular methods like polymerase chain reaction (PCR), which can detect DNA in a whole blood, eschar swab, or tissue sample. Immunostaining procedures can also be performed on formalin-fixed tissue samples. Since scrub typhus is not common in the United States, confirmatory tests are not typically available at state and local health departments; nonetheless, IFA, culture, and PCR assays can all be performed at the CDC through submission from state health departments.
Murine typhus
Rickettsia typhi can be detected via indirect immunofluorescence antibody (IFA) assay, immunohistochemistry (IHC), polymerase chain reaction (PCR) assays using blood, plasma, or tissue samples, or culture isolation. PCR is most sensitive on samples taken during the first week of illness, but prior to the start of doxycycline.
Serologic tests (typically using IFA) are the most common means of confirming murine typhus and can be used to detect either IgG or IgM antibodies. Diagnosis is usually confirmed by demonstrating a four-fold rise in antibody titer between acute and convalescent samples. Acute specimens are taken during the first week of illness and convalescent samples are taken 2–4 weeks later. IgG antibodies are considered more accurate than IgM. Detectable levels of IgG antibody generally do not appear until 7–10 days after the onset of illness.
Because antibody titers may persist in some individuals for years after the original exposure, only demonstration of recent changes in titers between paired specimens can be considered reliable serological confirmation of an acute murine typhus infection. R. typhi antigens frequently cross-react with those of R. prowazekii and R. felis, and less often with R. rickettsii. When possible, species-specific assays for R. typhi, R. prowazekii, R. felis, and R. rickettsii should be run in parallel. IHC can be used to detect infection with typhus group Rickettsia (including R. prowazekii and R. typhi) in formalin-fixed tissue samples. PCR of whole blood or tissue can distinguish between infection with R. typhi and R. prowazekii although the sensitivity of these assays varies considerably based on the sample type, timing of sample collection, and the severity of disease.
Epidimic
Rickettsia prowazekii can be detected via indirect immunofluorescence antibody (IFA) assay, immunohistochemistry (IHC), polymerase chain reaction (PCR) assay of blood, plasma, or tissue samples, or culture isolation. Serologic tests are the most common means of confirmation and can be used to detect either IgG or IgM antibodies. Diagnosis is typically confirmed by documenting a four-fold rise in antibody titer between acute and convalescent samples. Acute specimens are taken during the first week of illness and convalescent samples are taken 2–4 weeks later. Detectable levels of IgG or IgM antibodies generally do not appear until 7–10 days after the onset of illness.
Because IgG antibody titers may persist in some individuals for years after the original exposure, only demonstration of recent changes in titers between paired specimens can be considered reliable serological confirmation of an acute epidemic typhus infection. R. prowazekii antigens may cross react with those of R. typhi, and occasionally with R. rickettsii. When possible, species-specific serological assays for R. prowazekii, R. typhi, and R. rickettsii should be run in parallel. Persons with Brill-Zinsser disease generally show a rise in IgG but not IgM antibodies to R. prowazekii. IHC can be used to detect infection with typhus group Rickettsia (including R. prowazekii and R. typhi) in formalin-fixed tissue samples. PCR of whole blood or tissue can distinguish between infection with R. typhi and R. prowazekii although the sensitivity of these assays vary considerably based on the sample type, timing of sample collection, and the severity of disease. Since epidemic typhus is not common in the United States, testing is not typically available at state and local health departments. IFA, culture, and PCR can all be performed at the CDC, through submission from state health departments.
Epidemiology and Demographics
All age groups are at risk for rickettsial infections during travel to endemic areas. Both short and long-term travelers are at risk for infection. Transmission is increased during outdoor activities in the spring and summer months when ticks and fleas are most active. However, infection can occur throughout the year. Because of the 5- to 14-day incubation period for most rickettsial diseases, tourists may not necessarily experience symptoms during their trip, and onset may coincide with their return home or develop within a week after returning. Although the most commonly diagnosed rickettsial diseases in travelers are usually in the spotted fever or typhus groups, travelers may acquire a wide range of rickettsioses, including emerging and newly recognized species. Tickborne spotted fever rickettsioses are the most frequently reported travel-associated rickettsial infections. Game hunting and traveling to southern Africa from November through April are risk factors for African tick-bite fever, and this consistently remains the most commonly reported rickettsial infection acquired during travel. Mediterranean spotted fever infections are less commonly reported but occur over an even larger region, including (but not limited to) much of Europe, Africa, India, and the Middle East. Rocky Mountain spotted fever (also known as Brazilian spotted fever, as well as other local names) is reported throughout much of the Western Hemisphere, including Canada, the United States, Mexico, and various countries in Central and South America. Contact with dogs (in both rural and urban settings) and outdoor activities such as hiking, hunting, fishing, and camping increase the risk of infection.
Scrub typhus, which is transmitted by mites encountered in high grass and brush, is endemic in northern Japan, Southeast Asia, the western Pacific Islands, eastern Australia, China, maritime areas and several parts of south-central Russia, India, and Sri Lanka. More than 1 million cases occur annually. Most travel-acquired cases of scrub typhus occur during visits to rural areas in endemic countries for activities such as camping, hiking, or rafting, but urban cases have also been described.
R. typhi and R. felis, which are transmitted by fleas, are widely distributed, especially throughout the tropics and subtropics and in port cities and coastal regions with rodents. Humans exposed to flea-infested cats, dogs, and peridomestic animals while traveling in endemic regions, or who enter or sleep in areas infested with rodents, are at most risk for fleaborne rickettsioses. Murine typhus has been reported among travelers returning from Asia, Africa, and the Mediterranean Basin and has also been reported from Hawaii, California, and Texas in the United States.
R. akari, the causative agent of rickettsialpox, is transmitted by house-mouse mites, and circulates in mainly urban centers in Ukraine, South Africa, Korea, the Balkan states, and the United States. Outbreaks of rickettsialpox most often occur after contact with infected rodents and their mites, especially during natural die-offs or exterminations of infected rodents that cause the mites to seek out new hosts, including humans. The agent may spill over and occasionally be found in other wild rodent populations.
Epidemic typhus is rarely reported among tourists but can occur in communities and refugee populations where body lice are prevalent. Outbreaks often occur during the colder months when infested clothing is not laundered. Travelers at most risk for epidemic typhus include those who may work with or visit areas with large homeless populations, impoverished areas, refugee camps, and regions that have recently experienced war or natural disasters. Active foci of epidemic typhus are known in the Andes regions of South America and some parts of Africa (including but not limited to Burundi, Ethiopia, and Rwanda). Louseborne epidemic typhus does not regularly occur in the United States, but a zoonotic reservoir occurs in the southern flying squirrel, and sporadic sylvatic epidemic typhus cases are reported. Tick-associated reservoirs of R. prowazekii have been described in Ethiopia, Mexico, and Brazil, but documented human cases are rare.
Ehrlichiosis and anaplasmosis are tickborne infections most commonly reported in the United States. A variety of species are implicated in infection, but E. chaffeensis and A. phagocytophilum are most common. Infections with various Ehrlichia and Anaplasma spp. have also been reported in Europe, Asia, and South America. Neoehrlichia mikurensis is a tickborne pathogen that occurs in Europe and Asia. Sennetsu fever, caused by Neorickettsia sennetsu, occurs in Japan, Malaysia, and possibly other parts of Asia. This disease can be contracted from eating raw infected fish.
Physical examination
Vitals
- Fever
- Relative bradycardia with the fever.
- Tachypnea and cough
Skin
- Rash
The macular, maculopapular, or petechial rash initially occurs on the trunk and axilla and spreads to involve the rest of the body except for the face, palms, and soles. Rash may be petechial in patients with epidemic or murine typhus.
- Eschar
This is found in the scrub form of typhus and is essential in confirming a clinical diagnosis. It occurs in up to 60% of cases. Eschar occurs at the site of the arthropod bite. It starts as a painless papule, and the lesion becomes indurated and enlarged. The center of the lesion becomes necrotic and develops into a black scab. Other features
Lymph nodes
Regional lymphadenopathy Lymph nodes are often tender and enlarged. Generalized lymphadenopathy
Abdomnen
- Hepatomegaly
- Splenomegaly
HEENT
Conjunctival suffusion occurs in scrub typhus.
Medical Therapy
Pharmacotherapy
Typhus, louse-borne
- Louse born typhus, Rickettsia prowazekii (epidemic typhus, sylvatic typhus and Brill–Zinsser typhus [4]
- Pathogen-directed antimicrobial therapy
- In adults
- Preferred regimen (1): Doxycycline 200 mg PO for 5 days or 2-3 days after defervescence
- Preferred regimen (2): Doxycycline 100-200 mg PO single dose in outbreak situation
- Alternative regimen: Chloramphenicol 60 to 75 mg/kg/day PO in four divided doses
- In children
- Preferred regimen (1): Doxycycline 100-200 mg PO single dose
- In pregnant women
- Preferred regimen: Doxycycline 100-200 mg PO single dose
Typhus, murine
- Murine typhus,Rickettsia typhi (flea-borne infection) [4]
- Pathogen-directed antimicrobial therapy
- 1. Adults
- Preferred regimen : Doxycycline 100 mg PO bid continued for 3 days after the symptoms have resolved, Doxycycline 100-200 mg, PO single dose
- Alternative regimen (1): Oxacillin 2-12 g/24 hr IV q4-6h IV (maximum dose: 12 g/24)
- Alternative regimen (2): Chloramphenicol 60 to 75 mg/kg/day PO in qid
- 2. Children
- Preferred regimen: Doxycycline 100-200 mg, PO for 3-7 days
- Alternative regimen: Chloramphenicol 50-75 mg/kg/24 hr IV/PO q 6-8 hr
- 3. Pregnant women
- Preferred regimen: Doxycycline 100-200 mg, PO single dose ( late trimester)
- Alternative regimen (1): Erythromycin Base: 333 mg PO tid or estolate/stearate/ base: 250-500 mg PO qid
- Alternative regimen (2): Chloramphenicol 50 mg/kg/24 hr IV/PO q6h (maximum dose: 4 g/24 hr) (early trimester: first and second trimesters)
Typhus, scrub
- Scrub typhus, Orientia tsutsugamushi (previously called Rickettsia tsutsugamushi- mite-borne infectious disease) [5]
- Pathogen-directed antimicrobial therapy
- Preferred regimen (1): Doxycycline 100 mg PO/IV q12h for 3 days
- Preferred regimen (2): Chloramphenicol 500 mg PO/IV q6h
- Alternative regimen: Azithromycin 500 mg PO day 1 followed by 250 mg for 4 days
Prevention
Primary Prevention
Avoid areas where you might encounter rat fleas or lice. Good sanitation and public health measures reduce the rat population. Measures to get rid of lice when an infection has been found include:
- Bathing
- Boiling clothes or avoiding infested clothing for at least 5 days (lice will die without feeding on blood)
- Using insecticides (10% DDT, 1% malathion, or 1% permethrin)
Vaccine
The first major step in the development of the vaccine was Charles Nicolle's 1909 discovery that lice were the vectors for epidemic typhus. This made it possible to isolate the bacteria causing the disease and develop a vaccine; he was awarded the 1928 Nobel Prize in Physiology or Medicine for this work. Nicolle attempted a vaccine but was not successful in making one that worked on a large enough scale.[6]
Henrique da Rocha Lima in 1916 then proved that the bacteria Rickettsia prowazekii was the agent responsible for typhus; he named bacteria after H. T. Ricketts and Stanislaus von Prowazek, two zoologists who died investigating a typhus epidemic in a prison camp in 1915. Once these crucial facts were recognized, Rudolf Weigl in 1930 was able to fashion a practical and effective vaccine production method by grinding up the guts of infected lice that had been drinking blood. It was, however, very dangerous to produce, and carried a high likelihood of infection to those who were working on it.
A safer mass-production-ready method using egg yolks was developed by Herald R. Cox in 1938.[7] This vaccine was used heavily by 1943.
Colonic abscess
A colonic abscess develops as a complication of diverticulitis. A colonic abscess is a localized collection of pus within the wall of the colon that may cause swelling and destroy tissue. If the abscess is small and remains within the wall of the colon, it may clear up with antibiotics alone. If the abscess is large > 5cms, or unresponsive to medical treatment, it must be drained using a catheter facilitated by sonography or x-ray.
Causes
Colon abscess is a rare entity and arises as a compliecation of diseases such as IBD, colorectal cancer, diverticulosis or diverticulitis. Natural gut flora which includes gram negative and anaerobic bacteria play a major role in the development of colonic abscess.[8]
Most common causes
Less common causes
Pathophysiology
- The primary process is thought to be an erosion of the diverticular wall by increased intraluminal pressure or inspissated food particles.
- Inflammation and focal necrosis ensue, resulting in the abscess formation.
Gross Pathology
- The serosal surface of the colon looks pale with rough edges and yellowish exudate along with hyperemia
Microscopic findings
- A focally necrotic debris is seen in the mucosal wall.
- Intravascular fibrin is seen in medium-sized blood vessels.
- Clusters of neutrophils are seen on the serosal aspect.
Risk factors
Risk factors in the development of colonic abscess include same as that of diverticular diseases of the colon, such as advanced age, chronic constipation, connective tissue diseases (such as Marfan syndrome or Ehlers-Danlos syndrome), low dietary fiber intake, high intake of fat and red meat, and obesity.
Screening
Screening for colonic abscess is not recommended in the general population.
Epidemiology and Demographics
Prevalance
- The prevalence of diverticulosis is age-dependent.
- The prevalence increases from fewer than 20% at age 40 to approximately 60% by age 60.[9][10]
Incidence
- Incidence rates among age groups 18 to 44 is 0.151 to 0.251 per 1000 population.
- Incidence rates among age groups 45 to 64 years of age is 0.659 to 0.777 per 1000 population.
Gender
- At young age (<50 years), males are more commonly affected with diverticulosis than females.
- At older age, women are more frequently affected with diverticulosis than males.[11]
Race
- There is a slight racial predilection to the development of diverticulosis.
- Caucasian individuals are at higher risk of developing diverticulosis compared with Asian and non-African Black individuals.[12]
Natural history
If left untreated colonic abscess will rupture through the wall, and this may eventually lead to death if peritonitis develops.
Complications
- Peritonitis
- Septicemia
- Hemorrhage
- Death
Prognosis
- Majority of the patients with colonic abscess recover quickly with drain and IV antibiotics, but complications can occur if treatment is delayed or if peritonitis occurs.[3][4]
- It usually takes between 10 and 28 days to recover completely.
- Typical abscess responds quickly to antibiotics and percutaneous drain and resolves spontaneously.
History and symptoms
The most common symptom of colonic abscess is left lower quadrant abdominal pain along with fever and chills. The most common sign is tenderness around the left side of the lower abdomen. Nausea, vomiting, chills, cramping, diarrhea and constipation may occur as well. The severity of symptoms depends on the extent of the infection.
Differentiating Colonic abscess from other diseases
| Diseases | Clinical features | Diagnosis | Associated findings | |||||
|---|---|---|---|---|---|---|---|---|
| Symptoms | Signs | Laboratory fingdings | Radiological findings | |||||
| Fever | Abdominal pain | Nausea
vomiting |
Diarrhea | |||||
| Crohn's disease | + |
LLQ continuous localized pain |
+ |
Bloody |
[ASCA]) are found in Crohn disease |
Transmural ulcerations are seen on colonoscopy |
| |
| Gastroenteritis
(Bacterial and viral) |
+ |
Diffuse crampy intermittent abdominal pain |
+ |
Bloody or watery |
|
No specific findings |
| |
| Primary peritonitis | + |
Abrupt diffuse abdominal pain |
+ |
Bloody/watery |
Abdominal distension, rebound tenderness |
Peritoneal fluid shows >500/microliter count and >25% polymorphonuclear leukocytosis. |
||
| Pelvic inflammatory disease | + |
Bilateral lower quadrant pain |
+ | - |
|
|
Transvaginal ultrasonographic scanning or magnetic resonance imaging (MRI) shows thickened fluid-filled tubes with or without free pelvic fluid or tubo-ovarian abscess (TOA). |
Laparoscopy helps in confirmation of the diagnosis |
| Ruptured ectopic pregnancy | + |
Diffuse abdominal pain |
+ | - |
Ultrasound reveals presence of mass in fallopian tubes. |
| ||
Laboratory findings
Hematologic parameters suggestive of infection like, leukocytosis, anemia, abnormal platelet counts, and abnormal liver function frequently are present in patients with colonic abscess, although patients who are debilitated or elderly often fail to mount reactive leukocytosis or fever. Blood cultures indicating persistent polymicrobial bacteremia strongly implicate the presence of an abscess.
CT Abdomen
- Colonic and paracolic inflammation in the presence of underlying diverticula (diverticula are identified on CT scans as outpouchings of the colonic wall).
- Symmetric thickening of the colonic of approximately 4-5 mm is common.
- Enhancement of the colonic wall is commonly noted. This usually has inner and outer high-attenuation layers, with a thick middle layer of low attenuation.
- Free diverticular perforation results in the extravasation of air and fluid into the pelvis and peritoneal cavity.
- Air in the bladder in the presence of a nearby segment of diverticulitis is suggestive of a colovesical fistula.
Medical therapy
Antibiotics should be started immediately once the diagnosis of abscess is made. Preoperative antibiotics have been associated with lower rates of wound and intra-abdominal infections.[8] [13]
- 1 Emperic therapy:
- 1.1 Single agent:
- Preferred regimen (1): Imipenem-Cilastatin 500 mg IV q6h OR 1 g q8h
- Preferred regimen (2): Meropenem 1 g IV q8h
- Preferred regimen (3): Doripenem 500 mg IV q8h
- Preferred regimen (4): Piperacillin-tazobactam 3.375 g IV q6h
- 1.2 Combination:
- Preferred regimen (1): Cefepime 2 g q8–12 h AND Metronidazole 500 mg IV q8–12 h or 1500 mg q24h
- Preferred regimen (2): Ceftazidime 2 g q8h AND Metronidazole 500 mg IV q8–12 h or 1500 mg q24h
- Preferred regimen (3): Ciprofloxacin 400 mg q12h AND Metronidazole 500 mg IV q8–12 h or 1500 mg q24h
- Preferred regimen (4): Levofloxacin 750 mg q24h AND Metronidazole 500 mg IV q8–12 h or 1500 mg q24h
- Note: Antimicrobial therapy of established infection should be limited to 4–7 days, unless it is difficult to achieve adequate source control. Longer durations of therapy have not been associated with improved outcome.
Surgery
Percutaneous drainage can be performed under ultrasound or CT guidance, using either the Seldinger or trocar technique. Ultrasound is limited if the abscess is small, obscured by other structures, or if precise placement is required because of nearby vessels or organs. In these cases, CT is the optimal imaging modality. When an abscess is deep in the pelvis, depending on the specific location of the fluid collection, access may be obtained via transgluteal, transvaginal, or transrectal approaches. If the fluid collection is sterile, a transgluteal approach is preferred because it allows for sterile technique. Depending on the location of abscess, patient is placed in prone or supine position on the CT table. Localization scan using CT allows in selecting a safe window of access into the collection. A coaxial micropuncture introducer set is advanced into the abscess under CT guidance. An Amplatz guidewire is advanced through the sheath and coiled within the abscess. After serial dilatation of the tract with a dilator, an pigtail drain is advanced over the guidewire and deployed. {{#ev:youtube|f5KvsjHaOnI}}
Prevention
Dietary fiber and a vegetarian diet may reduce the incidence of symptomatic diverticular disease by decreasing intestinal inflammation and altering the intestinal microbiota.[14]. Vigorous physical activity appears to reduce the risk of diverticulitis and diverticular bleeding.[15].
Acute kidney injury in cancer patients
Types of Acute Kidney Injury in Patients with Hematologic Cancers
- Tumor infiltration of the kidneys
- Obstructive nephropathy related to retroperitoneal lymphadenopathy
- Lysozymuria (CMML or AML) with direct tubular injury
- Hemophagocytic lymphohistiocytosis with acute interstitial disease
- Vascular occlusion associated with DIC and hyperleukocytosis (rare)
- Hypercalcemia with hemodynamic acute kidney injury and acute nephrocalcinosis
- Glomerular diseases (minimal change disease, focal segmental glomerulosclerosis, membranoproliferative glomerulonephritis, membranous
nephropathy, amyloidosis, immuno-tactoid glomerulonephritis, fibrillary glomerulonephritis, crescentic glomerulonephritis)
- Nephrotoxicity (including thrombotic microangiopathy, acute tubular injury, tubulointerstitial nephritis, and glomerular disease)
- Tumor lysis syndrome with acute uric acid nephropathy (may occur spontaneously)
- Intratubular obstruction from medications (e.g., methotrexate)
Other types of injuries
- Volume depletion
- Sepsis and septic shock
- Nephrotoxicity of radiocontrast agents
- Nephrotoxicity of common medications, such as NSAIDs, ACE inhibitors,
- ARBs, and antibiotics
Cancers and association
- MM
- RCC
Types of Haemophilus influenzae Infections
H. influenzae, including Hib, can cause many different kinds of infections. These infections can range from mild ear infections to severe diseases, like bloodstream infections. When the bacteria invade parts of the body that are normally free from germs, like spinal fluid or blood, this is known as "invasive disease." Invasive disease is usually severe and can sometimes result in death.
The most common types of invasive disease caused by H. influenzae are:
- Pneumonia
- Bacteremia
- Meningitis (infection of the covering of the brain and spinal cord)
- Epiglottitis (swelling of the windpipe that can cause breathing trouble)
- Cellulitis (skin infection)
- Infectious arthritis (inflammation of the joint)
H. influenzae can also be a common cause of ear infections in children and bronchitis in adults.
Causes
- Haemophilus influenzae disease is caused by the bacterium Haemophilus influenzae.
- There are six identifiable types of H. influenzae (a through f) and other non-identifiable types (called nontypeable). The one that people are most familiar with is H. influenzae type b, or Hib.
- These bacteria live in the nose and throat, and usually cause no harm. However, the bacteria can sometimes move to other parts of the body and cause infection. Some of these infections are considered “invasive” and can be very serious and sometimes even deadly.
Incubation period
The incubation period (time between exposure and first symptoms) of H. influenzae disease is not certain, but could be as short as a few days. .
Natural history
- Between 3% to 6% of Hib cases in children are fatal; up to 20% of patients who survive Hib meningitis have permanent hearing loss or other long-term neurological sequelae.
- Patients ≥65 years of age with invasive H. influenzae disease (Hib, non-b, and nontypeable) have higher case-fatality ratios than children and young adults.
Epidemiology
Incidence
- Before vaccine became available in 1988, the annual attack rate of invasive Hib disease was estimated at 64-129 cases per 100,000 children younger than 5 years.
- The estimated annual incidence of Hib infection is 0.04 cases per 100,000 general population.
- The estimated annual incidence of non-Hib infection is 0.36 cases per 100,000 general population.
Gender
- Hib disease has no sexual predilection
Age
- Hib infections are rare in patients older than 6 years.
- Hib infections are most common in children aged 6 months to 6 years.
Risk factors
- Unimmunized children younger than 4 years of age, as well as household contacts and daycare classmates of a person with Hib disease are at increased risk of Hib disease.
- Close contacts of patients with non-b or nontypeable H. influenzae has not been identified.
- Patients with sickle cell disease, asplenia, HIV, and certain immunoglobulin and complement component deficiencies, as well as recipients of hematopoietic stem cell transplant and chemotherapy or radiation therapy for malignant neoplasms are at increased risk for invasive H. influenzae disease.
- Immigrants
Complications
- Complications depend on the type of invasive infection caused the bacteria.
- Meningitis can result in hearing loss.
- Bacteremia (blood infection) can result in loss of limb(s).
- Invasive H. influenzae infections can sometimes result in death. Even with antibiotic treatment, about 3 to 6 out of every 100 children with meningitis caused by Hib die from the disease.
- When H. influenzae cause a non-invasive infection, like bronchitis or an ear infection, complications are rare and typically not severe. If appropriate, antibiotics can be given to prevent complications
Medical therapy
A number of medical treatments are utilized with the goal of putting and keeping the disease in remission. These include 5-aminosalicylic acid (5-ASA) formulations (Pentasa capsules, Asacol tablets, Lialda tablets, Rowasa retention enemas), steroid medications, immunomodulators (such as azathioprine, mercaptopurine (6-MP), and methotrexate), and newer biological medications, such as infliximab (Remicade) and adalimumab (Humira).[16]Also in January 2008 the U.S. Food and Drug Administration approved a new biological medication known as natalizumab (Tysabri) for both induction of remission and maintenance of remission in moderate and severe Crohn's Disease. Treatment is only needed for people exhibiting symptoms. The therapeutic approach to Crohn's disease is sequential: to treat acute disease and then to maintain remission. Treatment initially involves the use of medications to treat any infection and to reduce inflammation. This usually involves the use of aminosalicylate anti-inflammatory drugs and corticosteroids, and may include antibiotics.
Once remission is induced, the goal of treatment becomes maintaining remission and avoiding flares. Because of side-effects, the prolonged use of corticosteroids must be avoided. Although some people are able to maintain remission with aminosalicylates alone, many require immunosuppressive drugs. On 14 January 2008 the U.S. Food and Drug Administration approved natalizumab (Tysabri) for both induction of remission and maintenance of remission in Crohns. Natalizumab is humanized monoclonal antibody (MAb), and the first alpha-4 antagonist in a new class of agents called selective adhesion-molecule (SAM) inhibitors. Alpha-4 integrin is required for leukocytes to adhere to the walls of blood vessels and migrate into the gut; natalizumab prevents leukocytes from doing that. Natalizumab was previously approved for multiple sclerosis. However, because it suppresses the immune system, natalizumab has been linked to a very rare adverse effect that is usually fatal if undetected. Leukocytes also protect the body from viruses, and 2 patients on natalizumab, who were also receiving other immuno-suppressive drugs (Avonex and Immuran), died of a rare brain infection, progressive multifocal leukoencephalopathy. Because of this danger, patients must be in a special monitoring program, and natalizumab is given as a mono-therapy.[17] As of late December 2007, more than 21,000 MS patients were receiving natalizumab mono-therapy without a single incidence of PML occurring.[18]
Surgery may be required for complications such as obstructions, fistulas and/or abscesses, or if the disease does not respond to drugs within a reasonable time. For patients with an obstruction due to a stricture, two options for treatment are strictureplasty and resection of that portion of bowel. According to a retrospective review at the Cleveland Clinic, there is no statistical significance between strictureplasty alone versus strictureplasty and resection specifically in cases of duodenal involvement. In these cases, re-operation rates were 31% and 27%, respectively, indicating that strictureplasty is a safe and effective treatment for selected patients with duodenal involvement.[19]
Recent studies using Helminthic therapy or hookworms to treat Crohn's Disease and other (non-viral) auto-immune diseases seem to yield promising results.[20][21][22]
Pathophysiology of Sepsis
- Sepsis is defined as a collection of physiologic responses by the immune system in response to an infectious agent.
- The clinical course of sepsis depends on the type and resistance of the infectious organism, the site and size of the infecting insult, and the genetically determined or acquired properties of the host's immune system.
- The pathogenesis of sepsis can be discussed as follows
Immune system activation
- The immune system is activated by pathogen entry which is facilitated by contamination of tissue either by surgery or infection, foreign body insertion (catheters) and in an immunocompromised state.
- Products of activation include
- Bacterial cell wall products such as lipopolysaccharide
- Binding to host receptors, including Toll-like receptors (TLRs).
- Toll-like receptors are found in leukocytes and macrophages, and endothelial cells.
- These have specificity for different bacterial, fungal, or viral products.
- TLRs are associated with a predisposition to shock with gram-negative organisms.
- Activation of the innate immune system results in a complex series of cellular and humoral responses.
Immune repsonse
- Immune response includes the release of pro-inflammatory cytokines, such as tumor necrosis factor (TNF)-alpha and interleukins 1 and 6 along with reactive oxygen species, nitric oxide (NO), proteases, and pore-forming molecules, which bring about activation of immune cells and bacterial killing.
- Nitrous oxide is responsible for vasodilatation and increased capillary permeability and has been implicated in sepsis-induced mitochondrial dysfunction.
- The complement system also gets activated which mediates activation of leukocytes, attracting them to the site of infection (phagocytes, cytotoxic T lymphocytes),
- Complement system also helps as a mediator for antigen-presenting cells and B lymphocytes
- This response by complement system helps the B lymphocyte to produce memory cells in case of future infection and is responsible for the increased production and chemotaxis of more T helper cells.
The endothelium and coagulation system
- The vascular endothelium plays a major role in the host's defense to an invading organism, but also in the development of sepsis.
- Activated endothelium not only allows the adhesion and migration of stimulated immune cells but becomes porous to large molecules such as proteins, resulting in the tissue edema.
- Alterations in the coagulation systems include an increase in procoagulant factors, such as plasminogen activator inhibitor type I and tissue factor, and reduced circulating levels of natural anticoagulants, including antithrombin III and activated protein C (APC), which also carry anti-inflammatory and modulatory roles.
Inflammation and organ dysfunction
- Through vasodilatation (causing reduced systemic vascular resistance) and increased capillary permeability (causing extravasation of plasma), sepsis results in relative and absolute reductions in circulating volume.
- A number of factors combine to produce multiple organ dysfunctions.
- Relative and absolute hypovolemia are compounded by reduced left ventricular contractility to produce hypotension.
- Initially, through an increased heart rate, cardiac output increases to compensate and maintain perfusion pressures, but as this compensatory mechanism becomes exhausted, hypoperfusion and shock may result.
- Impaired tissue oxygen delivery is exacerbated by pericapillary edema.
- It makes oxygen to diffuse a greater distance to reach target cells.
- There is a reduction of capillary diameter due to mural edema and the procoagulant state results in capillary microthrombus formation.
Additional contributing factors
- Disordered blood flow through capillary beds, resulting from a combination of shunting of blood through collateral channels and an increase in blood viscosity secondary to loss of red cell flexibility.
- As a result, organs become hypoxic, even with increase blood flow.
- These abnormalities leads to lactic acidosis, cellular dysfunction, and multiorgan failure.
- Cellular energy levels fall as metabolic activity begins to exceed production.
- However, cell death appears to be uncommon in sepsis, implying that cells shut down as part of the systemic response.
- This could explain why relatively few histologic changes are found at autopsy, and the eventual rapid resolution of severe symptoms, such as complete anuria and hypotension, once the systemic inflammation resolves.
H influenza infection
Overview
H.influenzae is a type of bacteria that can cause infections in people of all ages ranging from mild, such as an ear infection, to severe, such as a bloodstream infection.
Causes
- Haemophilus influenza disease is caused by the bacterium Haemophilus influenza.
- There are six identifiable types of H.influenza (a through f) and other non-identifiable types (called nontypeable).
- The most common type of H.influeza that is most familiar is H. influenza type b, or Hib.
- These bacteria are a normal commensal of throat and nose. However, the bacteria can sometimes move to other parts of the body and cause infection.
- Some of these infections are considered “invasive” and can be very serious.
Classification
H. influenzae, including Hib, can cause many different kinds of infections. These infections can range from mild ear infections to severe diseases, like bloodstream infections. When the bacteria invade parts of the body that are normally free from germs, like spinal fluid or blood, this is known as "invasive disease." Invasive disease is usually severe and can sometimes result in death.
The most common types of invasive disease caused by H.influenza are:
- Pneumonia
- Bacteremia
- Meningitis (infection of the covering of the brain and spinal cord)
- Epiglottitis (swelling of the windpipe that can cause breathing trouble)
- Cellulitis (skin infection)
- Infectious arthritis (inflammation of the joint)
The most common types of Non-invasive disease caused by H.influenza are:
- Otitis media
- Conjuctivitis
| H influenza infection | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Infection due to capsulated H influenza | Infection due to non-capsulated H influenza | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Meningitis | Cellulitis | Epiglottitis | Pneumonia | Pericarditis | Septic arthritis | otitis media | Conjunctivitis | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Pathophysiology
Transmission
- Transmission is by direct contact or by inhalation of respiratory tract droplets.
- Neonates can acquire the infection by aspiration of amniotic fluid or contact with genital tract secretions containing the bacteria.
Incubation period
The incubation period (time between exposure and first symptoms) of H. influenzae disease is not certain, but could be as short as a few days.
Seeding
- A larger bacterial load or the presence of a concomitant viral infection can potentiate the infection.
- The colonizing bacteria invade the mucosa and enter the bloodstream.
- The spread of bacteria by direct extension to the eustachian tubes causes otitis media.
- Spread to the sinuses leads to sinusitis.
- Spread down the respiratory tract results in bronchitis and pneumonia.
- Eustachian tube dysfunction, antecedent viral upper respiratory tract infection (URTI), foreign bodies, and mucosal irritants, including smoking, can promote infection.
- In patients with underlying chronic obstructive pulmonary disease (COPD) or cystic fibrosis (CF), NTHi frequently colonizes the lower respiratory tract and can exacerbate the disease.
Pathogenesis
- The capsule of H influenza plays a key role in the pathogenesis of the all the capsulated H influenza infections.
- The antiphagocytic nature of the Hib capsule makes it resistant to natural host phagocytic defense mechanisms and facilitating bacterial proliferation.
- After proliferation, the bacterial load disseminates to various sites, including meninges, subcutaneous tissue, joints, pleura, pericardiam, and lungs triggering an inflammatory response and subsequently activating the complement system.
- Capsulated H influenza can penetrate the normal epithelium and are therefore responsible for invasive infections.
- Non-capsulated are non-invasive but can still induce the inflammatory response similar to that of capsulated organisms
- The Hib conjugate vaccine induces protection by inducing antibodies against the PRP capsule.
- The Hib conjugate vaccine does not provide protection against Non-typable H influenza strains. Since the widespread use of the Hib conjugate vaccine, Non-typable H influenza strains has become more of a pathogen
Toxoplasmosis
Transmission
Transmission may occur through:
- Ingestion of raw or partly cooked meat, especially pork, lamb, or venison containing Toxoplasma cysts. Infection prevalence in countries where undercooked meat is traditionally eaten, such as France, has been related to this transmission method. Oocysts may also be ingested during hand-to-mouth contact after handling undercooked meat, or from using knives, utensils, or cutting boards contaminated by raw meat.[23]
- Ingestion of contaminated cat faeces. This can occur through hand-to-mouth contact following gardening, cleaning a cat's litter box, contact with children's sandpits, or touching anything that has come into contact with cat faeces.
- Drinking water contaminated with Toxoplasma.
- Transplacental infection in utero.
- Receiving an infected organ transplant or blood transfusion, although this is extremely rare.[23]
- Accidental inoculation of tachyzoites
Transplacental Transmission
- infection in 1st trimester - incidence of transplacental infection is low (15%) but disease in neonate is most severe.
- Infection in 3rd trimester - incidence of transplacental infection is high (65%) but infant is usually asymptomatic at birth.
The cyst form of the parasite is extremely hardy, capable of surviving exposure to freezing down to −12 degrees Celsius, moderate temperatures and chemical disinfectants such as bleach, and can survive in the environment for over a year. It is, however, susceptible to high temperatures—above 66 degrees Celsius, and is thus killed by thorough cooking, and would be killed by 24 hours in a typical domestic freezer.[24]
Cats excrete the pathogen in their faeces for a number of weeks after contracting the disease, generally by eating an infected rodent. Even then, cat faeces are not generally contagious for the first day or two after excretion, after which the cyst 'ripens' and becomes potentially pathogenic. Studies have shown that only about 2% of cats are shedding oocysts at any one time, and that oocyst shedding does not recur even after repeated exposure to the parasite. Although the pathogen has been detected on the fur of cats, it has not been found in an infectious form, and direct infection from handling cats is generally believed to be very rare.
Pathophysiology
Life cycle of Toxoplasma gondii
- T gondii has 2 distinct life cycles.
- The sexual cycle occurs only in cats, the definitive host.
- The asexual cycle occurs in other mammals (including humans) and various strains of birds.
- It consists of 2 forms: tachyzoites (the rapidly dividing form observed in the acute phase of infection) and bradyzoites (the slowly growing form observed in tissue cysts).
- A cat becomes infected with T gondii by eating contaminated raw meat, wild birds, or mice.
- The organism’s sexual cycle then begins in the cat’s gastrointestinal (GI) tract. Macrogametocytes and microgametocytes develop from ingested bradyzoites and fuse to form zygotes.
- The zygotes then become encapsulated within a rigid wall and are shed as oocysts.
- The zygote sporulates and divides to form sporozoites within the oocyst.
- Sporozoites become infectious 24 hours or more after the cat sheds the oocyst via feces.
- During a primary infection, the cat can excrete millions of oocysts daily for 1-3 weeks.
- The oocysts are very strong and may remain infectious for more than one year in warm humid environments.
- T gondii oocysts, tachyzoites, and bradyzoites can cause infection in humans.
Transmission
- Infection can occur by ingestion of oocysts following the handling of contaminated soil or cat litter or through the consumption of contaminated water or food sources (eg, unwashed garden vegetables).
- Transmission of tachyzoites to the fetus can occur via the placenta following primary maternal infection.
- Rarely, infection by tachyzoites occurs from ingestion of unpasteurized milk or by direct entry into the bloodstream through a blood transfusion or laboratory accident.
- Transmission can also occur via ingestion of tissue cysts (bradyzoites) in undercooked or uncooked meat or through transplantation of an organ that contains tissue cysts. (Slaughterhouse workers and butchers may be at increased risk of infection.)
Seeding
- T gondii oocysts are ingested in material contaminated by feces from infected cats.
- When T gondii is ingested, bradyzoites are released from cysts or sporozoites are released from oocysts, and the organisms enter gastrointestinal cells. *Host cell receptors consisting of laminin, lectin, and SAG1 are involved in T gondii tachyzoite attachment and penetration.
- Tachyzoites multiply, rupture cells, and infect contiguous cells.
- The ability of T gondii to actively penetrate host cells results in formation of a parasitophorous vacuole that is derived from the plasma membrane, which is entirely distinct from a normal phagocytic or endocytic compartment.
- Following apical attachment, the parasite rapidly enters the host cell in a process that is significantly faster than phagocytosis.
- The vacuole is formed primarily by invagination of the host cell plasma membrane, which is pulled over the parasite through the concerted action of the actin-myosin cytoskeleton of the parasite.
- During invasion, the host cell is essentially passive and no change is detected in membrane ruffling, the actin cytoskeleton, or phosphorylation of host cell proteins.
Dissemination
- They are transported via the lymphatics and are disseminated hematogenously throughout the tissues.
Immune response
- Tachyzoites proliferate, producing necrotic foci surrounded by a cellular reaction.
- Upon the development of a normal immune response, tachyzoites disappear from tissues.
- In immunodeficient individuals and in some apparently immunologically healthy patients, the acute infection progresses, resulting in potentially lethal consequences such as pneumonitis, myocarditis, and necrotizing encephalitis.
Changes in T-lymphocyte levels
- Alterations in subpopulations of T lymphocytes are profound and prolonged during acute acquired T gondii infection.
- These have been correlated with disease syndromes but not with disease outcome.
- Some patients with prolonged fever and malaise have lymphocytosis, increased suppressor T-cell counts, and a decreased helper-to-suppressor T-cell ratio.
- In some patients with lymphadenopathy, helper-cell counts are diminished for more than 6 months after infection onset.
- Ratios of T-cell subpopulations may also be abnormal in asymptomatic patients.
- Some patients with disseminated toxoplasmosis have a very marked reduction in T cells and a marked depression in the ratio of helper to suppressor T lymphocytes.
- Depletion of inducer T lymphocytes in patients with AIDS may contribute to the severe manifestations of toxoplasmosis observed in these patients.
Retinochoroiditis
- Retinochoroiditis usually results from reactivation of congenital infection, although cases have been recorded that were part of acute infection.
- There are 5 hypotheses related to the inflammatory process of ocular toxoplasmosis, as follows
- Infection and inflammatory response after spontaneous cyst rupture
- Parasitic toxic mediators released from T gondii
- Lytic effect of inflammatory mediators
- Delayed-type hypersensitivity reaction to antigens of T gondii
- Cell-mediated immunity against retinal antigens
- When the organism reaches the eye through the bloodstream, depending on the host's immune status, a clinical or subclinical focus of infection begins in the retina.
- As the host's immune system responds and the tachyzoites convert themselves into bradyzoites, the cyst forms.
- The cyst is extremely resistant to the host's defenses, and a chronic, latent infection ensues.
- If a subclinical infection is present, no funduscopic changes are observed.
- The cyst remains in the normal-appearing retina. Whenever the host's immune function declines for any reason, the cyst wall may rupture, releasing organisms into the retina, and the inflammatory process restarts.
- If an active clinical lesion is present, healing occurs as a retinochoroidal scar.
- The cyst often remains inactive within or adjacent to the scar.
Strongyloidiasis
Strongyloidiasis is a disease caused by a nematode, or a roundworm, in the genus Strongyloides. Though there are over 40 species within this genus that can infect birds, reptiles, amphibians, livestock and other primates, Strongyloides stercoralis is the primary species that accounts for human disease. The larvae are small; the longest reach about 1.5mm in length -- the size of a mustard seed or a large grain of sand.
Classification
Acute strongyloidiasis
Chronic strongyloidiasis
Hyperinfection syndrome and disseminated strongyloidiasis
Pathogenesis
Strongyloides is classified as a soil-transmitted helminth. This means that the primary mode of infection is through contact with soil that is contaminated with free-living larvae. When the larvae come in contact with skin, they are able to penetrate it and migrate through the body, eventually finding their way to the small intestine where they burrow and lay their eggs. Unlike other soil-transmitted helminths such as hookworm and whipworm whose eggs do not hatch until they are in the environment, the eggs of Strongyloides hatch into larvae in the intestine. Most of these larvae will be excreted in the stool, but some of the larvae may molt and immediately re-infect the host either by burrowing into the intestinal wall, or by penetrating the perianal skin. This characteristic of Strongyloides is termed auto-infection. The significance of auto-infection is that unless treated for Strongyloides, persons may remain infected throughout their lifetime.
Transmission
- Contact with soil and auto-infection, there have been rare cases of person-to-person transmission in:
- Organ transplantation
- Institutions for the development nfeclly disabled
- Long-term care facilities
- Daycare centers
Incubation period
Most people do not know when their exposure occurred. For those who do, a local rash can occur immediately. The cough usually occurs several days later. Abdominal symptoms typically occur approximately 2 weeks later, and larvae can be found in the stool about 3 to 4 weeks later.
Life cycle
- The Strongyloides life cycle is more complex than that of most nematodes with its alternation between free-living and parasitic cycles, and its potential for autoinfection and multiplication within the host.
- Two types of cycles exist:
Free-living cycle:
- The rhabditiform larvae passed in the stool can either become infective filariform larvae (direct development) or free living adult males and females
- These adult forms mate and produce eggs from which rhabditiform larvae hatch, eventually become infective filariform larvae.
- The filariform larvae penetrate the human host skin to initiate the parasitic cycle.
Parasitic cycle:
- Filariform larvae in contaminated soil penetrate the human skin and by various, often random routes, migrate into the small intestine.
- Historically it was believed that the larvae migrate via the bloodstream to the lungs, where they are eventually coughed up and swallowed.
- However, there is also evidence that larvae can migrate directly to the intestine via connective tissues.
- In the small intestine they molt twice and become adult female worms.
- The females live threaded in the epithelium of the small intestine and by parthenogenesis produce eggs which yield rhabditiform larvae.
- The rhabditiform larvae can either be passed in the stool or can cause autoinfection.
- In autoinfection, the rhabditiform larvae become infective filariform larvae, which can penetrate either the intestinal mucosa (internal autoinfection) or the skin of the perianal area (external autoinfection); in either case, the filariform larvae may disseminate throughout the body.
- To date, occurrence of autoinfection in humans with helminthic infections is recognized only in Strongyloides stercoralis and Capillaria philippinensis infections.
- In the case of Strongyloides, autoinfection may explain the possibility of persistent infections for many years in persons who have not been in an endemic area and of hyperinfections in immunosuppressed individuals.

Microscopic findings
{{#ev youtube|TSwN602mcn4&t=57s}}
Signs and Symptoms
The majority of people infected with Strongyloides are without symptoms.The symptomatic spectrum of Strongyloides ranges from subclinical in acute and chronic infection to severe and fatal in hyperinfection syndrome and disseminated strongyloidiasis, which have case-fatality rates that approach 90%. In either case, patients’ symptoms are a result of the parasite’s larval form migrating through various organs of the body. Those who do develop symptoms tend to have non-specific, or generalized complaints. Some people develop abdominal pain, bloating, heartburn, intermittent episodes of diarrhea and constipation, a dry cough, and rashes. Rarely people will develop arthritis, kidney problems, and heart conditions. Strongyloidiasis can be severe and life-threatening in persons who:
- People who use oral or intravenous steroids -- such as those with asthma or chronic obstructive pulmonary disease (COPD) exacerbations, lupus, gout, or in persons using steroids for immunosuppression or symptomatic relief
- HTLV-1 infection
- Have hematologic malignancies such as leukemia or lymphoma
- Transplant recipients.
Acute strongyloidiasis
- The initial sign of acute strongyloidiasis, if noticed at all, is a localized pruritic, erythematous rash at the site of skin penetration.
- Patients may then develop tracheal irritation and a dry cough as the larvae migrate from the lungs up through the trachea.
- After the larvae are swallowed into the gastrointestinal tract, patients may experience diarrhea, constipation, abdominal pain, and anorexia.
Chronic strongyloidiasis
- Chronic strongyloidiasis is generally asymptomatic, but in patients with clinical disease gastrointestinal and cutaneous manifestations are the most common. *Of the gastrointestinal complaints, epigastric pain, postprandial fullness, heartburn, and brief episodes of intermittent diarrhea and constipation are the most frequent.
- Less commonly, patients may present with fecal occult blood, or massive colonic and gastric hemorrhage.
- Presentations resembling inflammatory bowel disease, specifically ulcerative colitis, are rare. Also rare, but documented, are endoscopic exams revealing pathology similar to pseudopolyposis.
- Cutaneous symptoms include chronic urticaria and the pathognomonic larva currens- a recurrent serpiginous maculopapular or urticarial rash along the buttocks, perineum, and thighs due to repeated auto-infection.
- It has been described as advancing as rapidly as 10cm/hr.
- Rarely, patients with chronic strongyloidiasis have complained of arthritis, cardiac arrhythmias, and signs and symptoms consistent with chronic malabsorption, duodenal obstruction, nephrotic syndrome, and recurrent asthma.
- Up to 75% of people with chronic strongyloidiasis have mild peripheral eosinophilia or elevated IgE levels.
Hyperinfection syndrome and disseminated strongyloidiasis
- Hyperinfection syndrome and disseminated strongyloidiasis are most frequently associated with subclinical infection in patients receiving high-dose corticosteroids for the treatment of asthma or chronic obstructive pulmonary disease (COPD) exacerbations.
- Subsequent impaired host immunity leads to accelerated autoinfection and an overwhelming number of migrating larvae.
- Whereas in chronic strongyloidiasis and in hyperinfection syndrome the larvae are limited to the GI tract and the lungs, in disseminated strongyloidiasis the larvae invade numerous organs.
- Left untreated, the mortality rates of hyperinfection syndrome and disseminated strongyloidiasis can approach 90%.
The following are signs and symptoms that can be seen with hyperinfection syndrome and disseminated strongyloidiasis:
Gastrointestinal manifestations
- Abdominal pain, nausea, vomiting, diarrhea, ileus, bowel edema, intestinal obstruction, mucosal ulceration, massive hemorrhage, and subsequent peritonitis or bacterial sepsis
Pulmonary manifestations and findings
- Cough, wheezing, dyspnea, hoarseness, pneumonitis, hemoptysis, respiratory failure, diffuse interstitial infiltrates or consolidation on chest radiographs
Neurologic findings
- Aseptic or gram-negative meningitis
- Larvae have been reported in the CSF, meningeal vessels, dura, epidural, subdural, and subarachnoid spaces
Systemic signs and symptoms
- Peripheral edema and ascites secondary to hypoalbuminemia from protein losing enteropathy
- Recurrent gram negative bacteremia/sepsis from larvae carrying bacteria that penetrate mucosal walls
- Syndrome of inappropriate secretion of anti-diuretic hormone (SIADH)
- Peripheral eosinophilia is frequently absent
Cutaneous manifestations
- Recurrent maculopapular or urticarial rash most commonly found along the buttocks, perineum, and thighs due to repeated auto-infection, but can be found anywhere on the skin
- Larva currens - pathognomonic serpiginous or urticarial rash that advances as rapidly as 10cm/hr.
Physical examination
Overview
The physical examination findings in ascariasis vary and it is usually dependent on the worm burden and the involved organ.
Physical Examination
General appearance
Most patients generally appear well with minimal or no symptoms on physical examination.
Vital signs
A low-grade fever may occur in some patients with ascariasis.A high grade fever may be seen when there are complications such as acute cholangitis, hepatic abscess, etc.
HEENT
An icteric sclera due to obstructive jaundice from biliary ascariasis may be seen.
Chest
- Dyspnea
- Dry rales
- Wheezing resulting from bronchospasms
Abdomen
- Abdominal tenderness- Abdominal tenderness can be secondary to intestinal obstruction, appendicitis, biliary colic, acute cholangitis, acute cholecystitis, hepatic abscess, etc
- Abdominal distension
- Signs of acute bowel obstruction
Skin
The patients with ascariasis can present with urticaria.
Natural history, Complications and Prognosis
Natural history
If left untreated, the subclinical strongyloidiasis can disseminate and transform into hyper infection syndrome with a mortality rate of 90%.
Complications
- Disseminated strongyloidiasis
- Eosinophilic pneumonia
- Malnutrition due to problems absorbing nutrients from the gastrointestinal tract (malabsorption)
Prgonosis
With good treatment, people should make a full recovery and the parasites should be removed. Sometimes treatment needs to be repeated. Infections that are severe or widespread often have a poor outcome, especially in people with a weakened immune system.
Laboratory findings
- The gold standard for the diagnosis of Strongyloides is serial stool examination.
- However, traditional stool examinations are insensitive and require up to seven stool exams to reach a sensitivity of 100%.
- Specialized stool exams include Baermann concentration, Horadi-Mori filter paper culture, quantitative acetate concentration technique, and nutrient agar plate cultures.
- Duodenal aspirate is more sensitive than stool examination, and duodenal biopsy may reveal parasites in the gastric crypts, in the duodenal glands, or eosinophilic infiltration in the lamina propria.
- Frequently, larvae can be seen by a simple wet-mount in fluid from a bronchoalveolar lavage (BAL).
- Many of the serologic tests that are available are quite sensitive, but cross-react with other filarial parasites, schistosomes, and Ascaris lumbricoides, decreasing the specificity of the tests.
- Furthermore, it can be difficult to distinguish between active cases and historical cases as traditional antibodies can persist for some time.
- More sensitive and specific serologic tests using recombinant antigens have been, and are being developed, and are available at specific laboratories.
- An additional advantage to these serologic tests is that there is typically a significant drop in titer by 6 months after parasite eradication, which may make it possible to use these tests as a "test of cure."raph
Epidimeology and Demographics
Geographic distrubution
- Strongyloides is known to exist on all continents except for Antarctica, but it is most common in the tropics, subtropics, and in warm temperate regions.
Incidence and Prevalance
- The global prevalence of Strongyloides is unknown, but experts estimate that there are between 30–100 million infected persons worldwide.
- In the United States, a series of small studies in select populations have shown that between 600-1000 per 100,000 persons sampled were infected.
- Studies in immigrant populations have shown a much higher percentage of infected persons ranging from 460-1000 per 100.000 persons.
Risk Factors
- Strongyloides is found more frequently in the socioeconomically disadvantaged, in institutionalized populations, and in rural areas. It is often associated with agricultural activities.
- The most common way of becoming infected with Strongyloides is by contacting soil that is contaminated with Strongyloides larvae. Therefore, activities that increase contact with the soil increase the risk of becoming infected, such as:
- Walking with bare feet
- Contact with human waste or sewage
- Occupations that increase contact with contaminated soil such as farming and coal mining.
- Association with Strongyloides and infection with Human T-Cell Lymphotropic Virus-1 (HTLV-1).
differentiating strongyloidosis
| Differentiating Ascaris lumbricoides infection from other Nematode infections[25][26] | ||||||
|---|---|---|---|---|---|---|
| Nematode | Transmission | Direct Person-Person Transmission | Duration of Infection | Pulmonary Manifestation | Location of Adult worm(s) | Treatment |
| Ascaris lumbricoides | Ingestion of infective ova | No | 1-2 years |
|
Free in the lumen of the small bowel
(primarily jejunum) |
|
| Trichuris trichiura
(whipworm) |
Ingestion of infective ova | No | 1-3 years | No pulmonary migration, therefore, no pulmonary manifestation | Anchored in the superficial mucosa of cecum and colon | |
| Hookworm (Necator americanus and Ancylostoma duodenale) | Skin penetration by filariform larvae | No |
|
|
Attached to the mucosa of mid-upper portion of the small bowel | |
| Strongyloides stercoralis | Filariform larvae penetrates skin or bowel mucosa | Yes | Lifetime of the host |
|
Embedded in the mucosa of the duodenum, jejunum | |
| Enterobius vermicularis (pinworm) | Ingestion of infective ova | Yes | 1 month | Extraintestinal migration is very rare[27] | Free in the lumen of cecum, appendix, adjacent colon | |
Historical Perspective
- In 1876, Louis Alexis Normand, a French physician discovered strongyloides for the first time.
- Later in the same year, Professor Arthur Réné Jean Baptiste Bavay at the French Conseil Supérieur de Santé, gave a detailed description of the worm.
- In 1883, Karl Georg Friedrich Rudolf Leuckart German parasitologist discovered the alternation of generations involving parasitic and free-living phases. *The discovery that infection occurred through the skin was made by a Belgian physician, Paul Van Durme, whose studies were based on the work of Looss.
- In 1940, detailed studies on disseminated infections of strongyloides in immunosuppressed patients were described.
Treatment
Acute and chronic strongyloidiasis
- First line therapy: Ivermectin, in a single dose, 200 µg/kg orally for 1-2 days
- Alternative Albendazole: 400 mg orally two times a day for 7 days.
In patients with positive stool examination for Strongyloides and persistent symptoms, follow-up stool exams should be performed 2-4 weeks after treatment to confirm clearance of infection. If recrudescence of larvae is observed, retreatment is indicated.
Hyperinfection syndrome/Disseminated strongyloidiasis
If possible, immunosuppressive therapy should be stopped or reduced, and:
Ivermectin, 200 µg/kg per day orally until stool and/or sputum exams are negative for 2 weeks.
For patients unable to tolerate oral therapy, such as those with ileus, obstruction, or known or suspected malabsorption, published case reports have demonstrated efficacy with rectal administration.
If oral and/or rectal administrations are not possible, there have been instances where Investigational New Drug (IND) exemptions for the veterinary subcutaneous formulation of ivermectin have been granted by the FDA.
Sepsis Differential
FibromyalgiaHistorical perspective
ClassificationDSM 5 divides fibromyalgia into four groups based on the differences in psychological and autonomic nervous system profiles among affected individuals
Risk FactorsThe possible risk factors for fibromyalgia include:
DifferentialIt is a clinical diagnosis and all other conditions that present with muscular pain and stiffness all over the body such as polymyalgia rheumatica, myofascial pain syndrome (MPS), chronic fatigue syndrome, myositis, malingering Epidemiology and DemographicsIncidence and Prevalance
Gender
Age20-50 year age group is more commonly affected RaceFibromyalgia has no racial predilication. Natural HistoryIf left untreated, chronic pain could cause permanent changes in how the body perceives pain. ComplicationsComplications that can develop as a result of Fibromyalgia are
PrognosisFibromyalgia is a long-term disorder. Various factors play a key role in the outcomes
Even with appropriate treatment, symptoms of fibromyalgia improve other times, the pain may get worse and continue for months or years. DiagnosisThe most widely accepted set of diagnostic criteria for fibromyalgia was elaborated in 2010 by the Multicenter Criteria Committee of the the American College of Rheumatology. CriteriaA patient satisfies diagnostic criteria for fibromyalgia if the following 3 conditions are met:
Ascertainment1) WPI: note the number areas in which the patient has had pain over the last week. In how many areas has the patient had pain?
2) SS scale score:
For the each of the 3 symptoms above, indicate the level of severity over the past week using the following scale:
Considering somatic symptoms in general, indicate whether the patient has:
The SS scale score is the sum of the severity of the 3 symptoms (fatigue, waking unrefreshed, cognitive symptoms) plus the extent (severity) of somatic symptoms in general. The final score is between 0 and 12. History and symtpomsThe defining symptoms of fibromyalgia are chronic, widespread pain and tenderness to light touch. Other symptoms include
Laboratory findingsFibromyalgia is a diagnosed clinically by careful history and examination. Blood and urine tests are usually normal. However, tests may be done to rule out other conditions that may have similar symptoms. Medical therapySingle agent therapy
Combination Thereapy
AND
AND
PathophysiologyThe cause of fibromyalgia is unknown. In fact it is not be due to a singular factor at all, but r due to a multiplicity of causes. Fibromyalgia can, but most often does not, start as a result of some trauma such as a traffic accident, major surgery, or disease. Some evidence shows that Lyme Disease may be a trigger of fibromyalgia symptoms.[31] Another study suggests that more than one clinical entity may be involved, ranging from a mild, idiopathic inflammatory process to clinical depression[32] GeneticsBy using self-reported "Chronic Widespread Pain" (CWP) as a surrogate marker for fibromyalgia, the Swedish Twin Registry found that a modest genetic contribution may exist:[33][34]
Stress
Dopamine abnormality
Serotonin
Sleep disturbance
Human growth hormone
Deposition disease
Other hypotheses
Associated Conditions
Fibromyalgia Differential
Physical examinationGeneral appearance
Musculoskeletal
 Neurological
Extremites
However, a steady interest in the disorder on the part of academic researchers as well as pharmaceutical interests has led to improvements in its treatment, which ranges from symptomatic prescription medication to alternative and complementary medicine. The European League Against Rheumatism (EULAR) issued the first guidelines for the treatment of fibromyalgia syndrome (FMS) and published them in the September 17th On-line First issue of the Annals of the Rheumatic Diseases. Many medications are used to treat specific symptoms of fibromyalgia, such as muscle pain and insomnia. 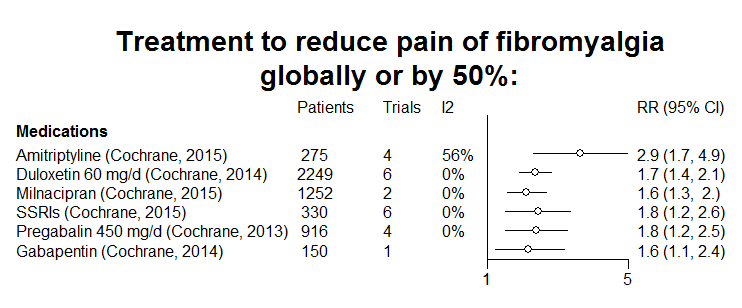 Pain ReliefA number of pain relievers have been prescribed for fibromyalgia. This includes NSAID medications over the counter, COX-2 inhibitors, and tramadol in prescription form for more advanced cases. Recently, pregabalin (marketed as Lyrica) has been given FDA approval for the treatment of diagnosed Fibromyalgia. Muscle RelaxantsMuscle relaxants, such as cyclobenzaprine (Flexeril) or tizanidine (Zanaflex), may be used to treat the muscle pain associated with the disorder. Tricyclic antidepressants (TCAs)Traditionally, low doses of sedating antidepressants (e.g. amitriptyline and trazodone) have been used to reduce the sleep disturbances that are associated with fibromyalgia and are believed by some practitioners to alleviate the symptoms of the disorder. Because depression often accompanies chronic illness, these antidepressants may provide additional benefits to patients suffering from depression. Amitriptyline is often favoured as it can also have the effect of providing relief from neuralgenic or neuropathic pain. It is to be noted that Fibromyalgia is not considered a depressive disorder; antidepressants are used for their sedating effect to aid in sleep. Selective serotonin reuptake inhibitors (SSRIs)Research data consistently contradict the utility of agents with specificity as serotonin reuptake inhibitors for the treatment of core symptoms of fibromyalgia. [72][73][74] Moreover, SSRIs are known to aggravate many of the comorbidities that commonly affect patients with fibromyalgia including restless legs syndrome and sleep bruxism[75][76][77]. Anti-seizure drugsAnti-seizure drugs are also sometimes used, such as gabapentin[78] and pregabalin (Lyrica). Pregabalin, originally used for the nerve pain suffered by diabetics, has been approved by the American Food and Drug Administration for treatment of fibromyalgia. A randomized controlled trial of pregabalin 450 mg/day found that a number needed to treat of 6 patients for one patient to have 50% reduction in pain.[79] Dopamine agonistsDopamine agonists (e.g. pramipexole (Mirapex) and ropinirole (ReQuip)) have been studied for use in the treatment of fibromyalgia with good results. [80] A trial of transdermal rotigotine is currently on going [81]. Combination therapyA controlled clinical trial of amitriptyline and fluoxetine demonstrated utility when used in combination.[82] Cannabis and cannabinoidsFibromyalgia patients frequently self-report using cannabis therapeutically to treat symptoms of the disorder.[83] Writing in the July 2006 issue of the journal Current Medical Research and Opinion, investigators at Germany's University of Heidelberg evaluated the analgesic effects of oral THC (∆9-tetrahydrocannabinol) in nine patients with fibromyalgia over a 3-month period. Subjects in the trial were administered daily doses of 2.5 to 15 mg of THC, but received no other pain medication during the trial. Among those participants who completed the trial, all reported a significant reduction in daily recorded pain and electronically induced pain.[84] Previous clinical and preclinical trials have shown that both naturally occurring and endogenous cannabinoids hold analgesic qualities,[85] particularly in the treatment of cancer pain and neuropathic pain,[86][87] both of which are poorly treated by conventional opioids. As a result, some experts have suggested that cannabinoid agonists would be applicable for the treatment of chronic pain conditions unresponsive to opioid analgesics such as fibromyalgia, and they propose that the disorder may be associated with an underlying clinical deficiency of the endocannabinoid system.[88] Topical RemediesUsers of Epsom Salts in the gel form (Magnesium Sulfate), have reported significant and lasting relief from pain associated with fibromyalgia. Epsom Salts have long been touted for its ability to reduce pain and swelling. Injection TherapyInterventional therapy can ease pain by blocking nerve conduction between specific areas of the body and the brain. Approaches range from injections of local anesthetics, steroids, proliferative agents (Prolotherapy) into affected soft tissues, joints, or nerve roots to more complex nerve blocks. Chronic use of steroid injections may lead to increased functional impairment. Physical treatmentsStudies have found exercise improves fitness and sleep and may reduce pain and fatigue in some people with fibromyalgia.[89] Many patients find temporary relief by applying heat to painful areas. Those with access to physical therapy, massage, or acupuncture may find them beneficial.[90] Most patients find exercise, even low intensity exercise to be extremely helpful.[91] Osteopathic manipulative therapy can also temporarily relieve pain due to fibromyalgia.[92] Transcutaneous electrical nerve stimulation (TENS) is administered by a battery-powered device that sends mild electric pulses along nerve fibers to block pain signals to the brain. Small electrodes placed on the skin at or near the site of pain generate nerve impulses that block incoming pain signals from the peripheral nerves. TENS may also help stimulate the brain’s production of endorphins (chemicals that have pain-relieving properties). A holistic approach—including managing diet, sleep, stress, activity, and pain—is used by many patients. Dietary supplements, massage, chiropractic care, managing blood sugar levels, and avoiding known triggers when possible means living as well as it is in the patient's power to do. Dietary treatmentIn a 2001 review of four case studies, symptom alleviation was found by minimizing consumption of monosodium glutamate.[93] References
|
|}