Ixabepilone
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Alberto Plate [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING: TOXICITY IN HEPATIC IMPAIRMENT
See full prescribing information for complete Boxed Warning.
Condition Name:
IXEMPRA in combination with capecitabine is contraindicated in patients with AST or ALT >2.5 x ULN or bilirubin >1 x ULN due to increased risk of toxicity and neutropenia-related death
|
Overview
Ixabepilone is an antineoplastic agent, epothilone and mitotic inhibitor that is FDA approved for the treatment of patients with metastatic or locally advanced breast cancer resistant to treatment with an anthracycline and a taxane, or whose cancer is taxane resistant and for whom further anthracycline therapy is contraindicated.
IXEMPRA is indicated as monotherapy for the treatment of metastatic or locally advanced breast cancer in patients whose tumors are resistant or refractory to anthracyclines, taxanes, and capecitabine. There is a Black Box Warning for this drug as shown here. Common adverse reactions include peripheral sensory neuropathy, fatigue/asthenia, myalgia/arthralgia, alopecia, nausea, vomiting, stomatitis/mucositis, diarrhea, and musculoskeletal pain.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
There is limited information regarding Ixabepilone FDA-Labeled Indications and Dosage (Adult) in the drug label.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Ixabepilone in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Ixabepilone in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Ixabepilone FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Ixabepilone in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Ixabepilone in pediatric patients.
Contraindications
- IXEMPRA is contraindicated in patients with a history of a severe (CTC grade 3/4) hypersensitivity reaction to agents containing Cremophor® EL or its derivatives (eg, polyoxyethylated castor oil).
- IXEMPRA is contraindicated in patients who have a neutrophil count <1500 cells/mm3 or a platelet count <100,000 cells/mm3.
- IXEMPRA in combination with capecitabine is contraindicated in patients with AST or ALT >2.5 x ULN or bilirubin >1 x ULN.
Warnings
|
WARNING: TOXICITY IN HEPATIC IMPAIRMENT
See full prescribing information for complete Boxed Warning.
Condition Name:
IXEMPRA in combination with capecitabine is contraindicated in patients with AST or ALT >2.5 x ULN or bilirubin >1 x ULN due to increased risk of toxicity and neutropenia-related death
|
Peripheral Neuropathy
- Peripheral neuropathy was common (see Table 3). Patients treated with IXEMPRA should be monitored for symptoms of neuropathy, such as burning sensation, hyperesthesia, hypoesthesia, paresthesia, discomfort, or neuropathic pain. Neuropathy occurred early during treatment; ~75% of new onset or worsening neuropathy occurred during the first 3 cycles. Patients experiencing new or worsening symptoms may require a reduction or delay in the dose of IXEMPRA. In clinical studies, peripheral neuropathy was managed through dose reductions, dose delays, and treatment discontinuation. Neuropathy was the most frequent cause of treatment discontinuation due to drug toxicity. In Studies 046 and 081, 80% and 87%, respectively, of patients with peripheral neuropathy who received IXEMPRA had improvement or no worsening of their neuropathy following dose reduction. For patients with grade 3/4 neuropathy in Studies 046 and 081, 76% and 79%, respectively, had documented improvement to baseline or grade 1, twelve weeks after onset.

- A pooled analysis of 1540 cancer patients treated with IXEMPRA indicated that patients with diabetes mellitus or preexisting peripheral neuropathy may be at increased risk of severe neuropathy. Prior therapy with neurotoxic chemotherapy agents did not predict the development of neuropathy. Patients with moderate to severe neuropathy (grade 2 or greater) were excluded from studies with IXEMPRA. Caution should be used when treating patients with diabetes mellitus or preexisting peripheral neuropathy.
Myelosuppression
- Myelosuppression is dose-dependent and primarily manifested as neutropenia. In clinical studies, grade 4 neutropenia (<500 cells/mm3) occurred in 36% of patients treated with IXEMPRA in combination with capecitabine and 23% of patients treated with IXEMPRA monotherapy. Febrile neutropenia and infection with neutropenia were reported in 5% and 6% of patients treated with IXEMPRA in combination with capecitabine, respectively, and 3% and 5% of patients treated with IXEMPRA as monotherapy, respectively. Neutropenia-related death occurred in 1.9% of 414 patients with normal hepatic function or mild hepatic impairment treated with IXEMPRA in combination with capecitabine. The rate of neutropenia-related deaths was higher (29%, 5 out of 17) in patients with AST or ALT >2.5 x ULN or bilirubin >1.5 x ULN. Neutropenia-related death occurred in 0.4% of 240 patients treated with IXEMPRA as monotherapy. No neutropenia-related deaths were reported in 24 patients with AST or ALT >2.5 x ULN or bilirubin >1.5 x ULN treated with IXEMPRA monotherapy. IXEMPRA must not be administered to patients with a neutrophil count <1500 cells/mm3. To monitor for myelosuppression, frequent peripheral blood cell counts are recommended for all patients receiving IXEMPRA. Patients who experience severe neutropenia or thrombocytopenia should have their dose reduced.
Hepatic Impairment
- Patients with baseline AST or ALT >2.5 x ULN or bilirubin >1.5 x ULN experienced greater toxicity than patients with baseline AST or ALT ≤2.5 x ULN or bilirubin ≤1.5 x ULN when treated with IXEMPRA at 40 mg/m2 in combination with capecitabine or as monotherapy in breast cancer studies. In combination with capecitabine, the overall frequency of grade 3/4 adverse reactions, febrile neutropenia, serious adverse reactions, and toxicity-related deaths was greater. With monotherapy, grade 4 neutropenia, febrile neutropenia, and serious adverse reactions were more frequent. The safety and pharmacokinetics of IXEMPRA as monotherapy were evaluated in a dose escalation study in 56 patients with varying degrees of hepatic impairment. Exposure was increased in patients with elevated AST or bilirubin.
- IXEMPRA in combination with capecitabine is contraindicated in patients with AST or ALT >2.5 x ULN or bilirubin >1 x ULN due to increased risk of toxicity- and neutropenia-related death. Patients who are treated with IXEMPRA as monotherapy should receive a reduced dose depending on the degree of hepatic impairment. Use in patients with AST or ALT >10 x ULN or bilirubin >3 x ULN is not recommended. Limited data are available for patients with AST or ALT >5 x ULN. Caution should be used when treating these patients.
Hypersensitivity Reactions
- Patients with a history of a severe hypersensitivity reaction to agents containing Cremophor® EL or its derivatives (eg, polyoxyethylated castor oil) should not be treated with IXEMPRA. All patients should be premedicated with an H1 and an H2 antagonist approximately 1 hour before IXEMPRA infusion and be observed for hypersensitivity reactions (eg, flushing, rash, dyspnea, and bronchospasm). In case of severe hypersensitivity reactions, infusion of IXEMPRA should be stopped and aggressive supportive treatment (eg, epinephrine, corticosteroids) started. Of the 1323 patients treated with IXEMPRA in clinical studies, 9 patients (1%) had experienced severe hypersensitivity reactions (including anaphylaxis). Three of the 9 patients were able to be retreated. Patients who experience a hypersensitivity reaction in one cycle of IXEMPRA must be premedicated in subsequent cycles with a corticosteroid in addition to the H1 and H2 antagonists, and extension of the infusion time should be considered.
Cardiac Adverse Reactions
- The frequency of cardiac adverse reactions (myocardial ischemia and ventricular dysfunction) was higher in the IXEMPRA in combination with capecitabine (1.9%) than in the capecitabine alone (0.3%) treatment group. Supraventricular arrhythmias were observed in the combination arm (0.5%) and not in the capecitabine alone arm. Caution should be exercised in patients with a history of cardiac disease. Discontinuation of IXEMPRA should be considered in patients who develop cardiac ischemia or impaired cardiac function.
Potential for Cognitive Impairment from Excipients
Since IXEMPRA contains dehydrated alcohol USP, consideration should be given to the possibility of central nervous system and other effects of alcohol.
Adverse Reactions
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, the adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in other clinical trials and may not reflect the rates observed in clinical practice.
- Unless otherwise specified, assessment of adverse reactions is based on one randomized study (Study 046) and one single-arm study (Study 081). In Study 046, 369 patients with metastatic breast cancer were treated with IXEMPRA 40 mg/m2 administered intravenously over 3 hours every 21 days, combined with capecitabine 1000 mg/m2 twice daily for 2 weeks followed by a 1-week rest period. Patients treated with capecitabine as monotherapy (n=368) in this study received 1250 mg/m2 twice daily for 2 weeks every 21 days. In Study 081, 126 patients with metastatic or locally advanced breast cancer were treated with IXEMPRA 40 mg/m2 administered intravenously over 3 hours every 3 weeks.
- The most common adverse reactions (≥20%) reported by patients receiving IXEMPRA were peripheral sensory neuropathy, fatigue/asthenia, myalgia/arthralgia, alopecia, nausea, vomiting, stomatitis/mucositis, diarrhea, and musculoskeletal pain. The following additional reactions occurred in ≥20% in combination treatment: palmar-plantar erythrodysesthesia (hand-foot) syndrome, anorexia, abdominal pain, nail disorder, and constipation. The most common hematologic abnormalities (>40%) include neutropenia, leukopenia, anemia, and thrombocytopenia.
- Table 4 presents nonhematologic adverse reactions reported in 5% or more of patients. Hematologic abnormalities are presented separately in Table 5.

The following serious adverse reactions were also reported in 1323 patients treated with IXEMPRA as monotherapy or in combination with other therapies in Phase 2 and 3 studies.
- Infections and Infestations: sepsis, pneumonia, infection, neutropenic infection, urinary tract infection, bacterial infection, enterocolitis, laryngitis, lower respiratory tract infection.
- Blood and Lymphatic System Disorders: coagulopathy, lymphopenia
- Metabolism and Nutrition Disorders: hyponatremia, metabolic acidosis, hypokalemia, hypovolemia.
- Nervous System Disorders: cognitive disorder, syncope, cerebral hemorrhage, abnormal coordination, lethargy.
- Cardiac Disorders: myocardial infarction, supraventricular arrhythmia, left ventricular dysfunction, angina pectoris, atrial flutter, cardiomyopathy, myocardial ischemia.
- Vascular Disorders: hypotension, thrombosis, embolism, hemorrhage, hypovolemic shock, vasculitis.
- Respiratory, Thoracic, and Mediastinal Disorders: pneumonitis, hypoxia, respiratory failure, acute pulmonary edema, dysphonia, pharyngolaryngeal pain.
- Gastrointestinal Disorders: ileus, colitis, impaired gastric emptying, esophagitis, dysphagia, gastritis, gastrointestinal hemorrhage.
- Hepatobiliary Disorders: acute hepatic failure, jaundice.
- Skin and Subcutaneous Tissue Disorders: erythema multiforme
- Musculoskeletal, Connective Tissue, and Bone Disorders: muscular weakness, muscle spasms, trismus.
- Renal and Urinary Disorders: nephrolithiasis, renal failure.
- General Disorders and Administration Site Conditions: chills
- Investigations: increased transaminases, increased blood alkaline phosphatase, increased gamma-glutamyltransferase.
Postmarketing Experience
- Radiation recall has been reported during postmarketing use of IXEMPRA. Because this reaction was reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate the frequency or establish a causal relationship to drug exposure.
Drug Interactions
Effect of Other Drugs on Ixabepilone
Drugs That May Increase Ixabepilone Plasma Concentrations
- CYP3A4 Inhibitors: Coadministration of ixabepilone with ketoconazole, a potent CYP3A4 inhibitor, increased ixabepilone AUC by 79% compared to ixabepilone treatment alone. If alternative treatment cannot be administered, a dose adjustment should be considered. The effect of mild or moderate inhibitors (eg, erythromycin, fluconazole, or verapamil) on exposure to ixabepilone has not been studied. Therefore, caution should be used when administering mild or moderate CYP3A4 inhibitors during treatment with IXEMPRA, and alternative therapeutic agents that do not inhibit CYP3A4 should be considered. Patients receiving CYP3A4 inhibitors during treatment with IXEMPRA should be monitored closely for acute toxicities (eg, frequent monitoring of peripheral blood counts between cycles of IXEMPRA).
Drugs That May Decrease Ixabepilone Plasma Concentrations
- CYP3A4 Inducers: IXEMPRA is a CYP3A4 substrate. Coadministration of IXEMPRA with rifampin, a potent CYP3A4 inducer, decreased ixabepilone AUC by 43% compared to IXEMPRA treatment alone. Other strong CYP3A4 inducers (eg, dexamethasone, phenytoin, carbamazepine, rifabutin, and phenobarbital) may also decrease ixabepilone concentrations leading to subtherapeutic levels. Therefore, therapeutic agents with low enzyme induction potential should be considered for coadministration with IXEMPRA. St. John’s Wort may decrease ixabepilone plasma concentrations unpredictably and should be avoided. If patients must be coadministered a strong CYP3A4 inducer, a gradual dose adjustment may be considered.
Effect of Ixabepilone on Other Drugs
- Ixabepilone does not inhibit CYP enzymes at relevant clinical concentrations and is not expected to alter the plasma concentrations of other drugs.
Capecitabine
- In patients with cancer who received ixabepilone (40 mg/m2) in combination with capecitabine (1000 mg/m2), ixabepilone Cmax decreased by 19%, capecitabine Cmax decreased by 27%, and 5-fluorouracil AUC increased by 14%, as compared to ixabepilone or capecitabine administered separately. The interaction is not clinically significant given that the combination treatment is supported by efficacy data.
Use in Specific Populations
Pregnancy
- IXEMPRA may cause fetal harm when administered to pregnant women. There are no adequate and well-controlled studies with IXEMPRA in pregnant women. Women should be advised not to become pregnant when taking IXEMPRA. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the fetus.
- Ixabepilone was studied for effects on embryo-fetal development in pregnant rats and rabbits given IV doses of 0.02, 0.08, and 0.3 mg/kg/day and 0.01, 0.03, 0.11, and 0.3 mg/kg/day, respectively. There were no teratogenic effects. In rats, an increase in resorptions and post-implantation loss and a decrease in the number of live fetuses and fetal weight was observed at the maternally toxic dose of 0.3 mg/kg/day (approximately one-tenth the human clinical exposure based on AUC). Abnormalities included a reduced ossification of caudal vertebrae, sternebrae, and metacarpals. In rabbits, ixabepilone caused maternal toxicity (death) and embryo-fetal toxicity (resorptions) at 0.3 mg/kg/day (approximately one-tenth the human clinical dose based on body surface area). No fetuses were available at this dose for evaluation.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Ixabepilone in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Ixabepilone during labor and delivery.
Nursing Mothers
- It is not known whether ixabepilone is excreted into human milk. Following intravenous administration of radiolabeled ixabepilone to rats on days 7 to 9 postpartum, concentrations of radioactivity in milk were comparable with those in plasma and declined in parallel with the plasma concentrations. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from ixabepilone, a decision must be made whether to discontinue nursing or to discontinue IXEMPRA taking into account the importance of the drug to the mother.
Pediatric Use
- The effectiveness of IXEMPRA in pediatric patients has not been established. IXEMPRA was evaluated in one Phase 1 and one Phase 2 trial. The pediatric patients had a safety profile consistent with that seen in adults, and no new safety signals were identified.
- In the Phase 1 open-label, dose-finding trial, the safety of IXEMPRA was evaluated in 19 pediatric patients with advanced or refractory solid tumors and 2 with acute leukemias. IXEMPRA was administered as a one-hour IV infusion daily for the first five days of a 21-day cycle at one of 5 dose levels, ranging from 3 to 10 mg/m2. Among the 21 patients, 12 ranged in age from 2 to 12 years and 9 ranged from 13 to 18 years. The maximum tolerated dose was 8 mg/m2 IV daily for 5 days every 21 days. No significant activity was observed. The pharmacokinetics of ixabepilone were characterized by population pharmacokinetic analysis of data for 16 patients from this trial, who were aged 2 to 18 years (median 12 years). The pharmacokinetic parameters of ixabepilone in these pediatric patients were compared to the corresponding parameters of 130 adult patients enrolled in clinical trials using the same dosing schedule. The median BSA normalized clearance of ixabepilone in pediatric patients (17 L/h/m2) was similar to that in adult patients (20 L/h/m2).
- In the Phase 2 trial of 59 patients with advanced or refractory solid tumors, 28 ranged in age from 3 to 12 years and 19 ranged in age from 13 to 18 years. Twelve additional patients over the age of 18 were treated in this trial. IXEMPRA was administered at a dose of 8 mg/m2 IV daily for 5 days every 21 days. This trial was terminated early due to lack of efficacy.
Geriatic Use
- Clinical studies of IXEMPRA did not include sufficient numbers of subjects aged sixty-five and over to determine whether they respond differently from younger subjects.
- Forty-five of 431 patients treated with IXEMPRA in combination with capecitabine were ≥65 years of age and 3 patients were ≥75. Overall, the incidence of grade 3/4 adverse reactions was higher in patients ≥65 years of age versus those <65 years of age (82% versus 68%) including grade 3/4 stomatitis (9% versus 1%), diarrhea (9% versus 6%), palmar-plantar erythrodysesthesia syndrome (27% versus 20%), peripheral neuropathy (24% versus 22%), febrile neutropenia (9% versus 3%), fatigue (16% versus 12%), and asthenia (11% versus 6%). Toxicity-related deaths occurred in 2 (4.7%) of 43 patients ≥65 years with normal baseline hepatic function or mild impairment.
- Thirty-two of 240 breast cancer patients treated with IXEMPRA as monotherapy were ≥65 years of age and 6 patients were ≥75. No overall differences in safety were observed in these patients compared to those <65 years of age.
Gender
There is no FDA guidance on the use of Ixabepilone with respect to specific gender populations.
Race
There is no FDA guidance on the use of Ixabepilone with respect to specific racial populations.
Renal Impairment
- Ixabepilone is minimally excreted via the kidney. No controlled pharmacokinetic studies were conducted with IXEMPRA in patients with renal impairment. IXEMPRA in combination with capecitabine has not been evaluated in patients with calculated creatinine clearance of <50 mL/min. IXEMPRA as monotherapy has not been evaluated in patients with creatinine >1.5 times ULN. In a population pharmacokinetic analysis of IXEMPRA as monotherapy, there was no meaningful effect of mild and moderate renal insufficiency (CrCL >30 mL/min) on the pharmacokinetics of ixabepilone.
Hepatic Impairment
- IXEMPRA was evaluated in 56 patients with mild to severe hepatic impairment defined by bilirubin levels and AST levels. Compared to patients with normal hepatic function (n=17), the area under the curve (AUC0-infinity) of ixabepilone increased by:
- 22% in patients with a) bilirubin >1 – 1.5 x ULN or b) AST >ULN but bilirubin <1.5 x ULN;
- 30% in patients with bilirubin >1.5 – 3 x ULN and any AST level; and
- 81% in patients with bilirubin >3 x ULN and any AST level.
- Doses of 10 and 20 mg/m2 as monotherapy were tolerated in 17 patients with severe hepatic impairment (bilirubin >3 x ULN).
- IXEMPRA in combination with capecitabine must not be given to patients with AST or ALT >2.5 x ULN or bilirubin >1 x ULN [see Boxed Warning, Contraindications (4), and Warnings and Precautions (5.3)]. Dose reduction is recommended when administering IXEMPRA as monotherapy to patients with hepatic impairment [see Dosage and Administration (2.3)]. Because there is a need for dosage adjustment based upon hepatic function, assessment of hepatic function is recommended before initiation of IXEMPRA and periodically thereafter.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Ixabepilone in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Ixabepilone in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Ixabepilone Administration in the drug label.
Monitoring
There is limited information regarding Ixabepilone Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Ixabepilone and IV administrations.
Overdosage
- Experience with overdose of IXEMPRA is limited to isolated cases. The adverse reactions reported in these cases included peripheral neuropathy, fatigue, musculoskeletal pain/myalgia, and gastrointestinal symptoms (nausea, anorexia, diarrhea, abdominal pain, stomatitis). The highest dose mistakenly received was 100 mg/m2 (total dose 185 mg).
- There is no known antidote for overdosage of IXEMPRA. In case of overdosage, the patient should be closely monitored and supportive treatment should be administered. Management of overdose should include supportive medical interventions to treat the presenting clinical manifestations.
Pharmacology
 | |
| Clinical data | |
|---|---|
| Trade names | Ixempra |
| Synonyms | Azaepothilone B |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a608042 |
| [[Regulation of therapeutic goods |Template:Engvar data]] |
|
| Pregnancy category |
|
| Routes of administration | Intravenous infusion |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | N/A |
| Protein binding | 67 to 77% |
| Metabolism | Extensive, hepatic, CYP3A4-mediated |
| Elimination half-life | 52 hours |
| Excretion | Fecal (mostly) and renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C27H42N2O5S |
| Molar mass | 506.698 g/mol |
| 3D model (JSmol) | |
| |
| |
| | |
Mechanism of Action
- Ixabepilone is a semi-synthetic analog of epothilone B. Ixabepilone binds directly to β-tubulin subunits on microtubules, leading to suppression of microtubule dynamics. Ixabepilone suppresses the dynamic instability of αβ−II and αβ−III microtubules. Ixabepilone possesses low in vitro susceptibility to multiple tumor resistance mechanisms including efflux transporters, such as MRP-1 and P-glycoprotein (P-gp). Ixabepilone blocks cells in the mitotic phase of the cell division cycle, leading to cell death.
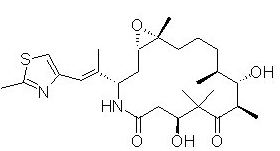
Structure
- The chemical name for ixabepilone is (1S,3S,7S,10R,11S,12S,16R)-7,11-dihydroxy-8,8,10,12,16-pentamethyl-3-(1E)-1-methyl-2-(2-methyl-4-thiazolyl)ethenyl-17-oxa-4-azabicyclo14.1.0 heptadecane-5,9-dione, and it has a molecular weight of 506.7. Ixabepilone has the following structural formula:
[[file::Ixabepilone Structure.png|none|300px]]
Pharmacodynamics
- In cancer patients, ixabepilone has a plasma concentration-dependent effect on tubulin dynamics in peripheral blood mononuclear cells that is observed as the formation of microtubule bundles. Ixabepilone has antitumor activity in vivo against multiple human tumor xenografts, including drug-resistant types that overexpress P-gp, MRP-1, and βIII tubulin isoforms, or harbor tubulin mutations. Ixabepilone is active in xenografts that are resistant to multiple agents including taxanes, anthracyclines, and vinca alkaloids. Ixabepilone demonstrated synergistic antitumor activity in combination with capecitabine in vivo. In addition to direct antitumor activity, ixabepilone has antiangiogenic activity.
Pharmacokinetics
Absorption
- Following administration of a single 40 mg/m2 dose of IXEMPRA in patients with cancer, the mean Cmax was 252 ng/mL (coefficient of variation, CV 56%) and the mean AUC was 2143 ng•hr/mL (CV 48%). Typically, Cmax occurred at the end of the 3-hour infusion. In cancer patients, the pharmacokinetics of ixabepilone were linear at doses of 15 to 57 mg/m2.
Distribution
- The mean volume of distribution of 40 mg/m2 ixabepilone at steady-state was in excess of 1000 L. In vitro, the binding of ixabepilone to human serum proteins ranged from 67 to 77%, and the blood-to-plasma concentration ratios in human blood ranged from 0.65 to 0.85 over a concentration range of 50 to 5000 ng/mL.
Metabolism
- Ixabepilone is extensively metabolized in the liver. In vitro studies indicated that the main route of oxidative metabolism of ixabepilone is via CYP3A4. More than 30 metabolites of ixabepilone are excreted into human urine and feces. No single metabolite accounted for more than 6% of the administered dose. The biotransformation products generated from ixabepilone by human liver microsomes were not active when tested for in vitro cytotoxicity against a human tumor cell line.
- In vitro studies using human liver microsomes indicate that clinically relevant concentrations of ixabepilone do not inhibit CYP3A4, CYP1A2, CYP2A6, CYP2B6, CYP2C8, CYP2C9, CYP2C19, or CYP2D6. Ixabepilone does not induce the activity or the corresponding mRNA levels of CYP1A2, CYP2B6, CYP2C9, or CYP3A4 in cultured human hepatocytes at clinically relevant concentrations. Therefore, it is unlikely that ixabepilone will affect the plasma levels of drugs that are substrates of CYP enzymes.
Elimination
- Ixabepilone is eliminated primarily as metabolized drug. After an intravenous 14[C]-ixabepilone dose to patients, approximately 86% of the dose was eliminated within 7 days in feces (65% of the dose) and in urine (21% of the dose). Unchanged ixabepilone accounted for approximately 1.6% and 5.6% of the dose in feces and urine, respectively. Ixabepilone has a terminal elimination half-life of approximately 52 hours. No accumulation in plasma is expected for ixabepilone administered every 3 weeks
Nonclinical Toxicology
There is limited information regarding Ixabepilone Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Ixabepilone Clinical Studies in the drug label.
How Supplied
There is limited information regarding Ixabepilone How Supplied in the drug label.
Storage
There is limited information regarding Ixabepilone Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Ixabepilone |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Ixabepilone |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Ixabepilone Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Ixabepilone interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Ixabepilone Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Ixabepilone Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
| File:Ixabepilone.svg | |
| Clinical data | |
|---|---|
| Synonyms | Azaepothilone B |
| Pregnancy category |
|
| Routes of administration | Intravenous infusion |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | N/A |
| Protein binding | 67 to 77% |
| Metabolism | Extensive, hepatic, CYP3A4-mediated |
| Elimination half-life | 52 hours |
| Excretion | Fecal (mostly) and renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C27H42N2O5S |
| Molar mass | 506.698 g/mol |
| 3D model (JSmol) | |
| |
|
WikiDoc Resources for Ixabepilone |
|
Articles |
|---|
|
Most recent articles on Ixabepilone Most cited articles on Ixabepilone |
|
Media |
|
Powerpoint slides on Ixabepilone |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Ixabepilone at Clinical Trials.gov Clinical Trials on Ixabepilone at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Ixabepilone
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Ixabepilone Discussion groups on Ixabepilone Patient Handouts on Ixabepilone Directions to Hospitals Treating Ixabepilone Risk calculators and risk factors for Ixabepilone
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Ixabepilone |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Overview
Ixabepilone (INN; also known as azaepothilone B, codenamed BMS-247550) is an epothilone B analog developed by Bristol-Myers Squibb as a cancer drug.
On October 16, 2007, the U.S. Food and Drug Administration approved ixabepilone for the treatment of aggressive metastatic or locally advanced breast cancer no longer responding to currently available chemotherapies. ([3]). Ixabepilone is administered through injection, and will be marketed under the trade name Ixempra.
- Pages with script errors
- Template:drugs.com link with non-standard subpage
- Articles with changed CASNo identifier
- Articles with changed EBI identifier
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Infobox drug
- Drugboxes which contain changes to verified fields
- Pages with broken file links
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Articles containing unverified chemical infoboxes
- Chemotherapeutic agents