Axitinib
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Aparna Vuppala, M.B.B.S. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Axitinib is an tyrosine kinase inhibitor that is FDA approved for the treatment of advanced renal cell carcinoma (RCC) after failure of one prior systemic therapy. Common adverse reactions include diarrhea, hypertension, fatigue, decreased appetite, nausea, dysphonia, palmar-plantar erythrodysesthesia (hand-foot syndrome, weight decreased, vomiting, asthenia, and constipation.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Renal cell carcinoma (RCC)
- Axitinib is indicated for the treatment of advanced renal cell carcinoma (RCC) after failure of one prior systemic therapy.
Recommended Dosing
- The recommended starting oral dose of Axitinib is 5 mg twice daily. Administer Axitinib doses approximately 12 hours apart with or without food. Axitinib should be swallowed whole with a glass of water.
- If the patient vomits or misses a dose, an additional dose should not be taken. The next prescribed dose should be taken at the usual time.
Dose Modification Guidelines
- Dose increase or reduction is recommended based on individual safety and tolerability.
- Over the course of treatment, patients who tolerate Axitinib for at least two consecutive weeks with no adverse reactions >Grade 2 (according to the Common Toxicity Criteria for Adverse Events [CTCAE]), are normotensive, and are not receiving anti-hypertension medication, may have their dose increased. When a dose increase from 5 mg twice daily is recommended, the Axitinib dose may be increased to 7 mg twice daily, and further to 10 mg twice daily using the same criteria.
- Over the course of treatment, management of some adverse drug reactions may require temporary interruption or permanent discontinuation and/or dose reduction of Axitinib therapy . If dose reduction from 5 mg twice daily is required, the recommended dose is 3 mg twice daily. If additional dose reduction is required, the recommended dose is 2 mg twice daily.
- Strong CYP3A4/5 Inhibitors: The concomitant use of Strong CYP3A4/5 Inhibitors should be avoided (e.g., ketoconazole, itraconazole, clarithromycin, atazanavir, indinavir, nefazodone, nelfinavir, ritonavir, saquinavir, telithromycin, and voriconazole). Selection of an alternate concomitant medication with no or minimal CYP3A4/5 Inhibition is recommended. *Although Axitinib dose adjustment has not been studied in patients receiving strong CYP3A4/5 inhibitors, if a strong CYP3A4/5 inhibitor must be co-administered, a dose decrease of Axitinib by approximately half is recommended, as this dose reduction is predicted to adjust the axitinib area under the plasma concentration vs time curve (AUC) to the range observed without inhibitors. The subsequent doses can be increased or decreased based on individual safety and tolerability. If co-administration of the strong inhibitor is discontinued, the Axitinib dose should be returned (after 3 – 5 half-lives of the inhibitor) to that used prior to initiation of the strong CYP3A4/5 inhibitor .
- Hepatic Impairment: No starting dose adjustment is required when administering Axitinib to patients with mild hepatic impairment (Child-Pugh class A). Based on the pharmacokinetic data, the Axitinib starting dose should be reduced by approximately half in patients with baseline moderate hepatic impairment (Child-Pugh class B). The subsequent doses can be increased or decreased based on individual safety and tolerability. Axitinib has not been studied in patients with severe hepatic impairment (Child-Pugh class C)
Off-Label Use and Dosage (Adult)
Non–Guideline-Supported Use
Metastatic renal cell carcinoma, First-line therapy
- Metastatic renal cell carcinoma, First-line therapy: initial, 5 mg ORALLY twice daily; increases at 2-week intervals to 7 mg ORALLY twice daily, then 10 mg ORALLY twice daily was used in a clinical trial
- [1]
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
The safety and efficacy of Axitinib in pediatric patients have not been studied.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
The safety and efficacy of Axitinib in pediatric patients have not been studied.
Non–Guideline-Supported Use
The safety and efficacy of Axitinib in pediatric patients have not been studied.
Contraindications
- None
Warnings
Hypertension and Hypertensive Crisis
- In a controlled clinical study with Axitinib for the treatment of patients with RCC, hypertension was reported in 145/359 patients (40%) receiving Axitinib and 103/355 patients (29%) receiving sorafenib. Grade 3/4 hypertension was observed in 56/359 patients (16%) receiving Axitinib and 39/355 patients (11%) receiving sorafenib. Hypertensive crisis was reported in 2/359 patients (<1%) receiving Axitinib and none of the patients receiving sorafenib. The median onset time for hypertension (systolic blood pressure >150 mmHg or diastolic blood pressure >100 mmHg) was within the first month of the start of Axitinib treatment and blood pressure increases have been observed as early as 4 days after starting Axitinib. Hypertension was managed with standard antihypertensive therapy. Discontinuation of Axitinib treatment due to hypertension occurred in 1/359 patients (<1%) receiving Axitinib and none of the patients receiving sorafenib.
- Blood pressure should be well-controlled prior to initiating Axitinib. Patients should be monitored for hypertension and treated as needed with standard anti-hypertensive therapy. In the case of persistent hypertension despite use of anti-hypertensive medications, reduce the Axitinib dose. Discontinue Axitinib if hypertension is severe and persistent despite anti-hypertensive therapy and dose reduction of Axitinib, and discontinuation should be considered if there is evidence of hypertensive crisis. If Axitinib is interrupted, patients receiving antihypertensive medications should be monitored for hypotension .
Arterial Thromboembolic Events
- In clinical trials, arterial thromboembolic events have been reported, including deaths. In a controlled clinical study with Axitinib for the treatment of patients with RCC, Grade 3/4 arterial thromboembolic events were reported in 4/359 patients (1%) receiving Axitinib and 4/355 patients (1%) receiving sorafenib. Fatal cerebrovascular accident was reported in 1/359 patients (<1%) receiving Axitinib and none of the patients receiving sorafenib.
- In clinical trials with Axitinib, arterial thromboembolic events (including transient ischemic attack, cerebrovascular accident, myocardial infarction, and retinal artery occlusion) were reported in 17/715 patients (2%), with two deaths secondary to cerebrovascular accident.
- Use Axitinib with caution in patients who are at risk for, or who have a history of, these events. Axitinib has not been studied in patients who had an arterial thromboembolic event within the previous 12 months.
Venous Thromboembolic Events
- In clinical trials, venous thromboembolic events have been reported, including deaths. In a controlled clinical study with Axitinib for the treatment of patients with RCC, venous thromboembolic events were reported in 11/359 patients (3%) receiving Axitinib and 2/355 patients (1%) receiving sorafenib. Grade 3/4 venous thromboembolic events were reported in 9/359 patients (3%) receiving Axitinib (including pulmonary embolism, deep vein thrombosis, retinal vein occlusion and retinal vein thrombosis) and 2/355 patients (1%) receiving sorafenib. Fatal pulmonary embolism was reported in 1/359 patients (<1%) receiving Axitinib and none of the patients receiving sorafenib. In clinical trials with Axitinib, venous thromboembolic events were reported in 22/715 patients (3%), with two deaths secondary to pulmonary embolism.
- Use Axitinib with caution in patients who are at risk for, or who have a history of, these events. Axitinib has not been studied in patients who had a venous thromboembolic event within the previous 6 months.
Hemorrhage
- In a controlled clinical study with Axitinib for the treatment of patients with RCC, hemorrhagic events were reported in 58/359 patients (16%) receiving Axitinib and 64/355 patients (18%) receiving sorafenib. Grade 3/4 hemorrhagic events were reported in 5/359 (1%) patients receiving Axitinib (including cerebral hemorrhage, hematuria, hemoptysis, lower gastrointestinal hemorrhage, and melena) and 11/355 (3%) patients receiving sorafenib. Fatal hemorrhage was reported in 1/359 patients (<1%) receiving Axitinib (gastric hemorrhage) and 3/355 patients (1%) receiving sorafenib.
- Axitinib has not been studied in patients who have evidence of untreated brain metastasis or recent active gastrointestinal bleeding and should not be used in those patients. If any bleeding requires medical intervention, temporarily interrupt the Axitinib dose.
Cardiac Failure
- In a controlled clinical study with Axitinib for the treatment of patients with RCC, cardiac failure was reported in 6/359 patients (2%) receiving Axitinib and 3/355 patients (1%) receiving sorafenib. Grade 3/4 cardiac failure was observed in 2/359 patients (1%) receiving Axitinib and 1/355 patients (<1%) receiving sorafenib. *Fatal cardiac failure was reported in 2/359 patients (1%) receiving Axitinib and 1/355 patients (<1%) receiving sorafenib. Monitor for signs or symptoms of cardiac failure throughout treatment with Axitinib. Management of cardiac failure may require permanent discontinuation of Axitinib.
Gastrointestinal Perforation and Fistula Formation
- In a controlled clinical study with Axitinib for the treatment of patients with RCC, gastrointestinal perforation was reported in 1/359 patients (<1%) receiving Axitinib and none of the patients receiving sorafenib. In clinical trials with Axitinib, gastrointestinal perforation was reported in 5/715 patients (1%), including one death. In addition to cases of gastrointestinal perforation, fistulas were reported in 4/715 patients (1%).
- Monitor for symptoms of gastrointestinal perforation or fistula periodically throughout treatment with Axitinib.
Thyroid Dysfunction
- In a controlled clinical study with Axitinib for the treatment of patients with RCC, hypothyroidism was reported in 69/359 patients (19%) receiving Axitinib and 29/355 patients (8%) receiving sorafenib. Hyperthyroidism was reported in 4/359 patients (1%) receiving Axitinib and 4/355 patients (1%) receiving sorafenib. In patients who had thyroid stimulating hormone (TSH) <5 μU/mL before treatment, elevations of TSH to ≥10 μU/mL occurred in 79/245 patients (32%) receiving Axitinib and 25/232 patients (11%) receiving sorafenib.
- Monitor thyroid function before initiation of, and periodically throughout, treatment with Axitinib. Treat hypothyroidism and hyperthyroidism according to standard medical practice to maintain euthyroid state.
Wound Healing Complications
- No formal studies of the effect of Axitinib on wound healing have been conducted.
- Stop treatment with Axitinib at least 24 hours prior to scheduled surgery. The decision to resume Axitinib therapy after surgery should be based on clinical judgment of adequate wound healing.
Reversible Posterior Leukoencephalopathy Syndrome
- In a controlled clinical study with Axitinib for the treatment of patients with RCC, reversible posterior leukoencephalopathy syndrome (RPLS) was reported in 1/359 patients (<1%) receiving Axitinib and none of the patients receiving sorafenib . There were two additional reports of RPLS in other clinical trials with Axitinib.
- RPLS is a neurological disorder which can present with headache, seizure, lethargy, confusion, blindness and other visual and neurologic disturbances. Mild to severe hypertension may be present. Magnetic resonance imaging is necessary to confirm the diagnosis of RPLS. Discontinue Axitinib in patients developing RPLS. *The safety of reinitiating Axitinib therapy in patients previously experiencing RPLS is not known.
Proteinuria
- In a controlled clinical study with Axitinib for the treatment of patients with RCC, proteinuria was reported in 39/359 patients (11%) receiving Axitinib and 26/355 patients (7%) receiving sorafenib. Grade 3 proteinuria was reported in 11/359 patients (3%) receiving Axitinib and 6/355 patients (2%) receiving sorafenib.
- Monitoring for proteinuria before initiation of, and periodically throughout, treatment with Axitinib is recommended. For patients who develop moderate to severe proteinuria, reduce the dose or temporarily interrupt Axitinib treatment.
Elevation of Liver Enzymes
- In a controlled clinical study with Axitinib for the treatment of patients with RCC, alanine aminotransferase (ALT) elevations of all grades occurred in 22% of patients on both arms, with Grade 3/4 events in <1% of patients on the Axitinib arm and 2% of patients on the sorafenib arm.
- Monitor ALT, aspartate aminotransferase (AST) and bilirubin before initiation of and periodically throughout treatment with Axitinib.
Hepatic Impairment
- The systemic exposure to axitinib was higher in subjects with moderate hepatic impairment (Child-Pugh class B) compared to subjects with normal hepatic function. A dose decrease is recommended when administering Axitinib to patients with moderate hepatic impairment (Child-Pugh class B). Axitinib has not been studied in patients with severe hepatic impairment (Child-Pugh class C) .
Adverse Reactions
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
- The safety of Axitinib has been evaluated in 715 patients in monotherapy studies, which included 537 patients with advanced RCC. The data described reflect exposure to Axitinib in 359 patients with advanced RCC who participated in a randomized clinical study versus sorafenib.
- The following risks, including appropriate action to be taken, are discussed in greater detail in other sections of the label. hypertension, arterial thromboembolic events, venous thromboembolic events, hemorrhage, cardiac failure, gastrointestinal perforation and fistula formation, thyroid dysfunction, wound healing complications, RPLS, proteinuria, elevation of liver enzymes, hepatic impairment and fetal development.
Clinical Trials Experience
- The median duration of treatment was 6.4 months (range 0.03 to 22.0) for patients who received Axitinib and 5.0 months (range 0.03 to 20.1) for patients who received sorafenib. Dose modifications or temporary delay of treatment due to an adverse reaction occurred in 199/359 patients (55%) receiving Axitinib and 220/355 patients (62%) receiving sorafenib. Permanent discontinuation due to an adverse reaction occurred in 34/359 patients (9%) receiving Axitinib and 46/355 patients (13%) receiving sorafenib.
- The most common (≥20%) adverse reactions observed following treatment with Axitinib were diarrhea, hypertension, fatigue, decreased appetite, nausea, dysphonia, palmar-plantar erythrodysesthesia (hand-foot) syndrome, weight decreased, vomiting, asthenia, and constipation. Table 1 presents adverse reactions reported in ≥10% patients who received Axitinib or sorafenib.

- Selected adverse reactions (all grades) that were reported in <10% of patients treated with Axitinib included dizziness (9%), upper abdominal pain (8%), myalgia (7%), dehydration (6%), epistaxis (6%), anemia (4%), hemorrhoids (4%), hematuria (3%), tinnitus (3%), lipase increased (3%), glossodynia (3%), pulmonary embolism (2%), rectal hemorrhage (2%), hemoptysis (2%), deep vein thrombosis (1%), retinal-vein occlusion/thrombosis (1%), polycythemia (1%), and transient ischemic attack (1%).
- Table 2 presents the most common laboratory abnormalities reported in ≥10% patients who received Axitinib or sorafenib.

- Selected laboratory abnormalities (all grades) that were reported in <10% of patients treated with Axitinib included hemoglobin increased (above the upper limit of normal) (9% for Axitinib versus 1% for sorafenib) and hypercalcemia (6% for Axitinib versus 2% for sorafenib).
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Axitinib in the drug label.
Drug Interactions
- Co-administration of rifampin, a strong inducer of CYP3A4/5, reduced the plasma exposure of axitinib in healthy volunteers. Co-administration of Axitinib with strong CYP3A4/5 inducers (e.g., rifampin, dexamethasone, phenytoin, carbamazepine, rifabutin, rifapentin, phenobarbital, and St. John's wort) should be avoided. Selection of concomitant medication with no or minimal CYP3A4/5 induction potential is recommended . Moderate CYP3A4/5 inducers (e.g., bosentan, efavirenz, etravirine, modafinil, and nafcillin) may also reduce the plasma exposure of axitinib and should be avoided if possible.
Use in Specific Populations
Pregnancy
- Axitinib can cause fetal harm when administered to a pregnant woman based on its mechanism of action. There are no adequate and well-controlled studies in pregnant women using Axitinib. In developmental toxicity studies in mice, axitinib was teratogenic, embryotoxic and fetotoxic at maternal exposures that were lower than human exposures at the recommended clinical dose.
- Women of childbearing potential should be advised to avoid becoming pregnant while receiving Axitinib. If this drug is used during pregnancy, or if a patient becomes pregnant while receiving this drug, the patient should be apprised of the potential hazard to the fetus.
- There are no adequate and well-controlled studies with Axitinib in pregnant women. Axitinib can cause fetal harm when administered to a pregnant woman based on its mechanism of action. Axitinib was teratogenic, embryotoxic and fetotoxic in mice at exposures lower than human exposures at the recommended starting dose. If this drug is used during pregnancy, or if the patient becomes pregnant while receiving this drug, the patient should be apprised of the potential hazard to the fetus.
- Oral axitinib administered twice daily to female mice prior to mating and through the first week of pregnancy caused an increase in post-implantation loss at all doses tested (≥15 mg/kg/dose, approximately 10 times the systemic exposure (AUC) in patients at the recommended starting dose). In an embryo-fetal developmental toxicity study, pregnant mice received oral doses of 0.15, 0.5 and 1.5 mg/kg/dose axitinib twice daily during the period of organogenesis. Embryo-fetal toxicities observed in the absence of maternal toxicity included malformation (cleft palate) at 1.5 mg/kg/dose (approximately 0.5 times the AUC in patients at the recommended starting dose) and variation in skeletal ossification at ≥0.5 mg/kg/dose (approximately 0.15 times the AUC in patients at the recommended starting dose).
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Axitinib in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Axitinib during labor and delivery.
Nursing Mothers
- It is not known whether axitinib is excreted in human milk. *Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from Axitinib, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
- The safety and efficacy of Axitinib in pediatric patients have not been studied.
- Toxicities in bone and teeth were observed in immature mice and dogs administered oral axitinib twice daily for 1 month or longer. Effects in bone consisted of thickened growth plates in mice and dogs at ≥15 mg/kg/dose (approximately 6 and 15 times, respectively, the systemic exposure (AUC) in patients at the recommended starting dose). Abnormalities in growing incisor teeth (including dental caries, malocclusions and broken and/or missing teeth) were observed in mice administered oral axitinib twice daily at ≥5 mg/kg/dose (approximately 1.5 times the AUC in patients at the recommended starting dose). Other toxicities of potential concern to pediatric patients have not been evaluated in juvenile animals.
Geriatic Use
- In a controlled clinical study with Axitinib for the treatment of patients with RCC, 123/359 patients (34%) treated with Axitinib were ≥65 years of age. Although greater sensitivity in some older individuals cannot be ruled out, no overall differences were observed in the safety and effectiveness of Axitinib between patients who were ≥65 years of age and younger.
- No dosage adjustment is required in elderly patients
Gender
There is no FDA guidance on the use of Axitinib with respect to specific gender populations.
Race
There is no FDA guidance on the use of Axitinib with respect to specific racial populations.
Renal Impairment
- No dedicated renal impairment trial for axitinib has been conducted. Based on the population pharmacokinetic analyses, no significant difference in axitinib clearance was observed in patients with pre-existing mild to severe renal impairment (15 mL/min ≤creatinine clearance CLcr <89 mL/min) . No starting dose adjustment is needed for patients with pre-existing mild to severe renal impairment. Caution should be used in patients with end-stage renal disease (CLcr <15 mL/min).
Hepatic Impairment
- In a dedicated hepatic impairment trial, compared to subjects with normal hepatic function, systemic exposure following a single dose of Axitinib was similar in subjects with baseline mild hepatic impairment (Child-Pugh class A) and higher in subjects with baseline moderate hepatic impairment (Child-Pugh class B
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Axitinib in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Axitinib in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
Monitoring
- Advise patients that hypertension may develop during Axitinib treatment and that blood pressure should be monitored regularly during treatment
IV Compatibility
There is limited information regarding IV Compatibility of Axitinib in the drug label.
Overdosage
- There is no specific treatment for Axitinib overdose.
- In a controlled clinical study with Axitinib for the treatment of patients with RCC, 1 patient inadvertently received a dose of 20 mg twice daily for 4 days and experienced dizziness (Grade 1).
- In a clinical dose finding study with Axitinib, subjects who received starting doses of 10 mg twice daily or 20 mg twice daily experienced adverse reactions which included hypertension, seizures associated with hypertension, and fatal hemoptysis.
- In cases of suspected overdose, Axitinib should be withheld and supportive care instituted.
Pharmacology
There is limited information regarding Axitinib Pharmacology in the drug label.
Mechanism of Action
- Axitinib has been shown to inhibit receptor tyrosine kinases including vascular endothelial growth factor receptors (VEGFR)-1, VEGFR-2, and VEGFR-3 at therapeutic plasma concentrations. These receptors are implicated in pathologic angiogenesis, tumor growth, and cancer progression. VEGF-mediated endothelial cell proliferation and survival were inhibited by axitinib in vitro and in mouse models. Axitinib was shown to inhibit tumor growth and phosphorylation of VEGFR-2 in tumor xenograft mouse models.
Structure
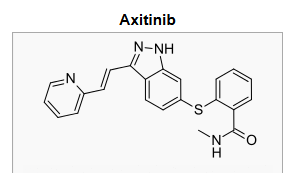
- Axitinib (axitinib) is a kinase inhibitor. Axitinib has the chemical name N-methyl-2-[3-((E)-2-pyridin-2-yl-vinyl)-1H-indazol-6-ylsulfanyl]-benzamide. The molecular formula is C22H18N4OS and the molecular weight is 386.47 Daltons. The chemical structure is:

- Axitinib is a white to light-yellow powder with a pKa of 4.8. The solubility of axitinib in aqueous media over the range pH 1.1 to pH 7.8 is in excess of 0.2 µg/mL. The partition coefficient (n-octanol/water) is 3.5.
- Axitinib is supplied as red, film-coated tablets containing either 1 mg or 5 mg of axitinib together with microcrystalline cellulose, lactose monohydrate, croscarmellose sodium, magnesium stearate, and Opadry® II red 32K15441 as inactive ingredients. The Opadry II red 32K15441 film coating contains lactose monohydrate, HPMC 2910/Hypromellose 15cP, titanium dioxide, triacetin (glycerol triacetate), and red iron oxide.
Pharmacodynamics
- The effect of a single oral dose of Axitinib (5 mg) in the absence and presence of 400 mg ketoconazole on the QTc interval was evaluated in a randomized, single-blinded, two-way crossover study in 35 healthy subjects. No large changes in mean QTc interval (i.e., >20 ms) from placebo were detected up to 3 hours post-dose. However, small increases in mean QTc interval (i.e., <10 ms) cannot be ruled out.
Pharmacokinetics
- The population pharmacokinetic analysis pooled data from 17 trials in healthy subjects and patients with cancer. A two-compartment disposition model with first-order absorption and lag-time adequately describes the axitinib concentration-time profile.
Absorption and Distribution
- Following single oral 5-mg dose administration, the median Tmax ranged from 2.5 to 4.1 hours. Based on the plasma half-life, steady state is expected within 2 to 3 days of dosing. Dosing of axitinib at 5 mg twice daily resulted in approximately 1.4-fold accumulation compared to administration of a single dose. At steady state, axitinib exhibits approximately linear pharmacokinetics within the 1-mg to 20-mg dose range. The mean absolute bioavailability of axitinib after an oral 5 mg dose is 58%.
- Compared to overnight fasting, administration of Axitinib with a moderate fat meal resulted in 10% lower AUC and a high fat, high-calorie meal resulted in 19% higher AUC. Axitinib can be administered with or without food .
- Axitinib is highly bound (>99%) to human plasma proteins with preferential binding to albumin and moderate binding to α1-acid glycoprotein. In patients with advanced RCC (n=20), at the 5 mg twice daily dose in the fed state, the geometric mean (CV%) Cmax and AUC0–24 were 27.8 (79%) ng/mL and 265 (77%) ng.h/mL, respectively. The geometric mean (CV%) clearance and apparent volume of distribution were 38 (80%) L/h and 160 (105%) L, respectively.
Metabolism and Elimination
- The plasma half life of Axitinib ranges from 2.5 to 6.1 hours. Axitinib is metabolized primarily in the liver by CYP3A4/5 and to a lesser extent by CYP1A2, CYP2C19, and UGT1A1. Following oral administration of a 5-mg radioactive dose of axitinib, approximately 41% of the radioactivity was recovered in feces and approximately 23% was recovered in urine. Unchanged axitinib, accounting for 12% of the dose, was the major component identified in feces. Unchanged axitinib was not detected in urine; the carboxylic acid and sulfoxide metabolites accounted for the majority of radioactivity in urine. In plasma, the N-glucuronide metabolite represented the predominant radioactive component (50% of circulating radioactivity) and unchanged axitinib and the sulfoxide metabolite each accounted for approximately 20% of the circulating radioactivity.
- The sulfoxide and N-glucuronide metabolites show approximately ≥400-fold less in vitro potency against VEGFR-2 compared to axitinib.
Drug-Drug Interactions
- Effects of Other Drugs on Axitinib: Axitinib is metabolized primarily in the liver by CYP3A4/5. Additionally, the aqueous solubility of axitinib is pH dependent, with higher pH resulting in lower solubility. The effects of a strong CYP3A4/5 inhibitor, a strong CYP3A4/5 inducer, and an antacid on the pharmacokinetics of axitinib are presented in Figure 1

- Effects of Axitinib on Other Drugs: In vitro studies demonstrated that axitinib has the potential to inhibit CYP1A2 and CYP2C8. However, co-administration of axitinib with paclitaxel, a CYP2C8 substrate, did not increase plasma concentrations of paclitaxel in patients.
- In vitro studies indicated that axitinib does not inhibit CYP2A6, CYP2C9, CYP2C19, CYP2D6, CYP2E1, CYP3A4/5, or UGT1A1 at therapeutic plasma concentrations. In vitro studies in human hepatocytes indicated that axitinib does not induce CYP1A1, CYP1A2, or CYP3A4/5.
- Axitinib is an inhibitor of the efflux transporter P-glycoprotein (P-gp) in vitro. However, Axitinib is not expected to inhibit P-gp at therapeutic plasma concentrations.
Pharmacokinetics in Specific Populations
- Pediatric Use: Axitinib has not been studied in patients <18 years of age.
- Hepatic Impairment: The effects of hepatic impairment on the pharmacokinetics of axitinib are presented in Figure 1 .
- Renal Impairment: Population pharmacokinetic analysis (based on pre-existing renal function) was carried out in 590 healthy volunteers and patients, including five with severe renal impairment (15 mL/min ≤CLcr <29 mL/min), 64 with moderate renal impairment (30 mL/min ≤CLcr <59 mL/min), and 139 with mild renal impairment (60 mL/min ≤CLcr <89 mL/min). Mild to severe renal impairment did not have meaningful effects on the pharmacokinetics of axitinib. Data from only one patient with end-stage renal disease are available .
- Other Intrinsic Factors: Population pharmacokinetic analyses indicate that there are no clinically relevant effects of age, gender, race, body weight, body surface area, UGT1A1 genotype, or CYP2C19 genotype on the clearance of axitinib.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- Carcinogenicity studies have not been conducted with axitinib.
- Axitinib was not mutagenic in an in vitro bacterial reverse mutation (Ames) assay and was not clastogenic in the in vitro human lymphocyte chromosome aberration assay. Axitinib was genotoxic in the in vivo mouse bone marrow micronucleus assay.
- Axitinib has the potential to impair reproductive function and fertility in humans. In repeat-dose toxicology studies, findings in the male reproductive tract were observed in the testes/epididymis (decreased organ weight, atrophy or degeneration, decreased numbers of germinal cells, hypospermia or abnormal sperm forms, reduced sperm density and count) at ≥15 mg/kg/dose administered orally twice daily in mice (approximately 7 times the systemic exposure (AUC) in patients at the recommended starting dose) and ≥1.5 mg/kg/dose administered orally twice daily in dogs (approximately 0.1 times the AUC in patients at the recommended starting dose). Findings in the female reproductive tract in mice and dogs included signs of delayed sexual maturity, reduced or absent corpora lutea, decreased uterine weights and uterine atrophy at ≥5 mg/kg/dose (approximately 1.5 or 0.3 times the AUC in patients at the recommended starting dose compared to mice and dogs, respectively).
- In a fertility study in mice, axitinib did not affect mating or fertility rate when administered orally twice daily to males at any dose tested up to 50 mg/kg/dose following at least 70 days of administration (approximately 57 times the AUC in patients at the recommended starting dose). In female mice, reduced fertility and embryonic viability were observed at all doses tested (≥15 mg/kg/dose administered orally twice daily) following at least 15 days of treatment with axitinib (approximately 10 times the AUC in patients at the recommended starting dose).
Clinical Studies
The safety and efficacy of Axitinib were evaluated in a randomized, open-label, multicenter Phase 3 study. Patients (N=723) with advanced RCC whose disease had progressed on or after treatment with 1 prior systemic therapy, including sunitinib-, bevacizumab-, temsirolimus-, or cytokine-containing regimens were randomized (1:1) to receive Axitinib (N=361) or sorafenib (N=362). Progression-free survival (PFS) was assessed by a blinded independent central review committee. Other endpoints included objective response rate (ORR) and overall survival (OS).
Of the patients enrolled in this study, 389 patients (54%) had received 1 prior sunitinib-based therapy, 251 patients (35%) had received 1 prior cytokine-based therapy (interleukin-2 or interferon-alfa), 59 patients (8%) had received 1 prior bevacizumab-based therapy, and 24 patients (3%) had received 1 prior temsirolimus-based therapy. The baseline demographic and disease characteristics were similar between the Axitinib and sorafenib groups with regard to age (median 61 years), gender (72% male), race (75% white, 21% Asian), Eastern Cooperative Oncology Group (ECOG) performance status (55% 0, 45% 1), and histology (99% clear cell).
There was a statistically significant advantage for Axitinib over sorafenib for the endpoint of PFS (see TABLE 3 and FIGURE 2). There was no statistically significant difference between the arms in OS.

How Supplied
- Axitinib tablets are supplied as follows:
1 mg tablets are red film-coated, oval tablets debossed with "Pfizer" on one side and "1 XNB" on the other; available in bottles of 180: NDC 0069-0145-01.
- 5 mg tablets are red film-coated, triangular tablets debossed with "Pfizer" on one side and "5 XNB" on the other; available in bottles of 60: NDC 0069-0151-11.
Storage
- Store at 20°C to 25°C (68°F to 77°F); excursions permitted to 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature]
Images
Drug Images
{{#ask: Page Name::Axitinib |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Axitinib |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Advise the patient to read the FDA-approved patient labeling (Patient Information)
Hypertension
- Advise patients that hypertension may develop during Axitinib treatment and that blood pressure should be monitored regularly during treatment.
Arterial/Venous Thromboembolic Events
- Advise patients that arterial and venous thromboembolic events have been observed during Axitinib treatment and to inform their doctor if they experience symptoms suggestive of thromboembolic events.
Hemorrhage
- Advise patients that Axitinib may increase the risk of bleeding and to promptly inform their doctor of any bleeding episodes .
Cardiac Failure
- Advise patients that cardiac failure may develop during Axitinib treatment and that signs or symptoms of cardiac failure should be regularly monitored for during treatment
Gastrointestinal Disorders
- Advise patients that gastrointestinal disorders such as diarrhea, nausea, vomiting, and constipation may develop during Axitinib treatment and to seek immediate medical attention if they experience persistent or severe abdominal pain because cases of gastrointestinal perforation and fistula have been reported in patients taking Axitinib.
Abnormal Thyroid Function
- Advise patients that abnormal thyroid function may develop during Axitinib treatment and to inform their doctor if symptoms of abnormal thyroid function occur.
Wound Healing Complications
- Advise patients to inform their doctor if they have an unhealed wound or if they have surgery scheduled.
Reversible Posterior Leukoencephalopathy Syndrome
- Advise patients to inform their doctor if they have worsening of neurological function consistent with RPLS (headache, seizure, lethargy, confusion, blindness and other visual and neurologic disturbances) .
Pregnancy
- Advise patients that Axitinib can cause birth defects or fetal loss and that they should not become pregnant during treatment with Axitinib. Both male and female patients should be counseled to use effective birth control during treatment with Axitinib. Female patients should also be advised against breast-feeding while receiving Axitinib .
Concomitant Medications
- Advise patients to inform their doctor of all concomitant medications, vitamins, or dietary and herbal supplements.
Precautions with Alcohol
- Alcohol-Axitinib interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Axitinib®
Look-Alike Drug Names
- A® — B®
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Hutson TE, Lesovoy V, Al-Shukri S, Stus VP, Lipatov ON, Bair AH; et al. (2013). "Axitinib versus sorafenib as first-line therapy in patients with metastatic renal-cell carcinoma: a randomised open-label phase 3 trial". Lancet Oncol. 14 (13): 1287–94. doi:10.1016/S1470-2045(13)70465-0. PMID 24206640.
- ↑ 2.0 2.1 2.2 2.3 2.4 "Axitinib (axitinib) dosing, indications, interactions, adverse effects, and more". Medscape Reference. WebMD. Retrieved 25 January 2014.
{{#subobject:
|Page Name=Axitinib |Pill Name=INLYTA_NDC_00690145.jpg |Drug Name=INLYTA |Pill Ingred=AXITINIB[AXITINIB]|+sep=; |Pill Imprint=Pfizer;1;XNB |Pill Dosage=1 mg |Pill Color=Red|+sep=; |Pill Shape=Oval |Pill Size (mm)=9 |Pill Scoring=1 |Pill Image= |Drug Author=Pfizer Laboratories Div Pfizer Inc |NDC=00690145
}}
{{#subobject:
|Page Name=Axitinib |Pill Name=INLYTA_NDC_00690151.jpg |Drug Name=INLYTA |Pill Ingred=AXITINIB[AXITINIB]|+sep=; |Pill Imprint=Pfizer;5;XNB |Pill Dosage=5 mg |Pill Color=Red|+sep=; |Pill Shape=Triangle |Pill Size (mm)=8 |Pill Scoring=1 |Pill Image= |Drug Author=Pfizer Laboratories Div Pfizer Inc |NDC=00690151
}}
{{#subobject:
|Label Page=Axitinib |Label Name=Axitinib06.png
}}
{{#subobject:
|Label Page=Axitinib |Label Name=Axitinib07.png
}}