Vandetanib
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Kiran Singh, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Vandetanib is an tyrosine kinase inhibitor that is FDA approved for the treatment of symptomatic or progressive medullary thyroid cancer. Common adverse reactions include diarrhea, nausea, fatigue, rash, headache, abdominal pain, dyspepsia, hypocalcemia, cough, depression.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
Medullary Thyroid Cancer (MTC)
- Vandetanib is indicated for the treatment of symptomatic or progressive medullary thyroid cancer in patients with unresectable locally advanced or metastatic disease.
- Use of vandetanib in patients with indolent, asymptomatic or slowly progressing disease should be carefully considered because of the treatment related risks of vandetanib.
Dosage
- The recommended daily dose is 300 mg of vandetanib taken orally. Vandetanib treatment should be continued until patients are no longer benefiting from treatment or an unacceptable toxicity occurs.
- Vandetanib may be taken with or without food.
- If a patient misses a dose, the missed dose should not be taken if it is less than 12 hours before the next dose.
For Patients who have Difficulty Swallowing Solids
- Vandetanib tablets should not be crushed. If vandetanib tablets cannot be taken whole, the tablets can be dispersed in a glass containing 2 ounces of non-carbonated water and stirred for approximately 10 minutes until the tablet is dispersed (will not completely dissolve). No other liquids should be used. The dispersion should be swallowed immediately. To ensure the full dose is received, any residues in the glass should be mixed again with an additional 4 ounces of non-carbonated water and swallowed.
- The dispersion can also be administered through nasogastric or gastrostomy tubes.
- Direct contact of crushed tablets with the skin or mucous membranes should be avoided. If such contact occurs, wash thoroughly. Avoid exposure to crushed tablets.
Dosage Adjustment
- In the event of corrected QT interval, Fridericia (QTcF) greater than 500 ms, interrupt dosing until QTcF returns to less than 450 ms, then resume at a reduced dose.
- For CTCAE (Common Terminology Criteria for Adverse Events) grade 3 or greater toxicity, interrupt dosing until toxicity resolves or improves to CTCAE grade 1, and then resume at a reduced dose.
- Because of the 19-day half-life, adverse reactions including a prolonged QT interval may not resolve quickly. Monitor appropriately.
- The 300-mg daily dose can be reduced to 200 mg (two 100-mg tablets) and then to 100 mg for CTCAE grade 3 or greater toxicities.
Elderly
- No adjustment in starting dose is required for patients over 65 years of age. There are limited data for patients over the age of 75.
Concomitant Strong CYP3A4 Inducers
- Avoid the concomitant use of strong CYP3A4 inducers (e.g., dexamethasone, phenytoin, carbamazepine, rifampin, rifabutin, rifapentine, phenobarbital). Patients should also avoid taking St. John’s Wort.
Patients With Renal Impairment
- The starting dose should be reduced to 200 mg in patients with moderate (creatinine clearance ≥30 to <50 mL/min) and severe (creatinine clearance <30 mL/min) renal impairment.
Patients with Hepatic Impairment
- Single dose pharmacokinetic data from volunteers with hepatic impairment receiving 800 mg suggest that there were no differences in pharmacokinetics compared to patients with normal hepatic function. There are limited data in patients with liver impairment (serum bilirubin greater than 1.5 times the upper limit of normal). Vandetanib is not recommended for use in patients with moderate (Child-Pugh B) and severe (Child-Pugh C) hepatic impairment, as safety and efficacy have not been established.
DOSAGE FORMS & STRENGTHS
- Vandetanib 100-mg tablets are white, round, biconvex, film-coated, and intagliated with ‘Z 100‘ on one side and plain on the reverse side.
- Vandetanib 300-mg tablets are white, oval, biconvex, film-coated, and intagliated with ‘Z 300’ on one side and plain on the reverse side.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Vandetanib in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Vandetanib in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Vandetanib FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Vandetanib in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Vandetanib in pediatric patients.
Contraindications
- Do not use in patients with congenital long QT syndrome.
Warnings
Prolongation and Torsades de Pointes
- Vandetanib can prolong the QT interval in a concentration-dependent manner. Torsades de pointes, ventricular tachycardia and sudden deaths have been reported in patients administered vandetanib.
- Vandetanib treatment should not be started in patients whose QTcF interval is greater than 450 ms. Vandetanib should not be given to patients who have a history of torsades de pointes, congenital long QT syndrome, bradyarrhythmias or uncompensated heart failure. Vandetanib has not been studied in patients with ventricular arrhythmias or recent myocardial infarction. Vandetanib exposure is increased in patients with impaired renal function. The starting dose should be reduced to 200 mg in patients with moderate to severe renal impairment and QT interval should be monitored closely.
- An ECG and levels of serum potassium, calcium, magnesium and TSH should be obtained at baseline, at 2-4 weeks and 8-12 weeks after starting treatment with vandetanib and every 3 months thereafter. Electrolytes and ECGs may require more frequent monitoring in case of diarrhea. Following any dose reduction for QT prolongation, or any dose interruptions greater than 2 weeks, QT assessments should be conducted as described above. Serum potassium levels should be maintained at 4 mEq/L or higher (within normal range) and serum magnesium and serum calcium should be kept within normal range to reduce the risk of electrocardiogram QT prolongation.
- Avoid using vandetanib with drugs known to prolong the electrocardiogram QT interval. If such drugs are given to patients already receiving vandetanib and no alternative therapy exists, ECG monitoring of the QT interval should be performed more frequently.
- Patients who develop a QTcF greater than 500 ms should stop taking vandetanib until QTcF returns to less than 450 ms. Dosing of vandetanib can be resumed at a reduced dose.
Skin Reactions and Stevens-Johnson Syndrome
- Severe skin reactions (including Stevens-Johnson syndrome), some leading to death, have been reported with vandetanib. Treatment of severe skin reactions has included systemic corticosteroids and permanent discontinuation of vandetanib. Mild to moderate skin reactions may manifest as rash, acne, dry skin, dermatitis, pruritis and other skin reactions (including photosensitivity reactions and palmar-plantar erythrodysesthesia syndrome). Mild to moderate skin reactions have been treated with topical and systemic corticosteroids, oral antihistamines, and topical and systemic antibiotics. If CTCAE grade 3 or greater skin reactions occur, vandetanib treatment should be stopped until improved. Upon improvement, consideration should be given to continuing treatment at a reduced dose or permanent discontinuation of vandetanib.
- Photosensitivity reactions are increased with vandetanib. Patients should be advised to wear sunscreen and protective clothing when exposed to the sun. Due to the long half-life of vandetanib, protective clothing and sunscreen should continue for 4 months after discontinuation of treatment.
Interstitial Lung Disease
- Interstitial Lung Disease (ILD) or pneumonitis has been observed with vandetanib and deaths have been reported. Consider a diagnosis of ILD in patients presenting with non-specific respiratory signs and symptoms such as hypoxia, pleural effusion, cough, or dyspnea, and in whom infectious, neoplastic, and other causes have been excluded by means of appropriate investigations. Advise patients to report promptly any new or worsening respiratory symptoms.
- Patients who develop radiological changes suggestive of ILD and have few or no symptoms may continue vandetanib therapy with close monitoring at the discretion of the treating physician.
- If symptoms are moderate, consider interrupting therapy until symptoms improve. The use of corticosteroids and antibiotics may be indicated.
- For cases where symptoms of ILD are severe, discontinue vandetanib therapy and the use of corticosteroids and antibiotics may be indicated until clinical symptoms resolve. Even upon resolution of severe ILD, permanent discontinuation of vandetanib should be considered.
Ischemic Cerebrovascular Events
- Ischemic cerebrovascular events have been observed with vandetanib and some cases have been fatal. In the randomized medullary thyroid cancer (MTC) study, ischemic cerebrovascular events were observed more frequently with vandetanib compared to placebo (1.3% compared to 0%) and no deaths were reported. The safety of resumption of vandetanib therapy after resolution of an ischemic cerebrovascular event has not been studied. Discontinue vandetanib in patients who experience a severe ischemic cerebrovascular event.
Hemorrhage
- Serious hemorrhagic events, which in some cases were fatal, have been observed with vandetanib. There were no fatal bleeding events in the randomized MTC study. Three patients died of fatal bleeding events while on vandetanib therapy in clinical studies. Do not administer vandetanib to patients with recent history of hemoptysis of ≥ 1/2 teaspoon of red blood. Discontinue vandetanib in patients with severe hemorrhage.
Heart Failure
- Heart failure has been observed with vandetanib and some cases have been fatal. Discontinuation of vandetanib may be necessary in patients with heart failure. Heart failure may not be reversible upon stopping vandetanib. Monitor for signs and symptoms of heart failure.
Diarrhea
- Diarrhea was observed in patients who received vandetanib. Routine anti-diarrheal agents are recommended. Diarrhea may cause electrolyte imbalances. Since QT prolongation is seen with vandetanib, serum electrolytes and ECGs should be carefully monitored in patients with diarrhea.If severe diarrhea develops, vandetanib treatment should be stopped until diarrhea improves. Upon improvement, treatment with vandetanib should be resumed at a reduced dose.
Hypothyroidism
- In the randomized MTC study where 90% of the patients enrolled had prior thyroidectomy, increases in the dose of the thyroid replacement therapy were required in 49% of the patients randomized to vandetanib compared to 17% of the patients randomized to placebo. Thyroid-stimulating hormone (TSH) should be obtained at baseline, at 2 to 4 weeks and 8 to 12 weeks after starting treatment with vandetanib and every 3 months thereafter. If signs or symptoms of hypothyroidism occur, thyroid hormone levels should be examined and thyroid replacement therapy should be adjusted accordingly.
Hypertension
- Hypertension, including hypertensive crisis, has been observed with vandetanib. All patients should be monitored for hypertension and it should be controlled as appropriate. Dose reduction or interruption may be necessary. If high blood pressure cannot be controlled, vandetanib should not be restarted.
Reversible posterior leukoencephalopathy syndrome
- Reversible posterior leukoencephalopathy syndrome (RPLS), a syndrome of subcortical vasogenic edema diagnosed by an MRI of the brain, has been observed with vandetanib. This syndrome should be considered in any patient presenting with seizures, headache, visual disturbances, confusion or altered mental function. In clinical studies, three of four patients who developed RPLS while taking vandetanib, including one pediatric patient, also had hypertension. Discontinuation of vandetanib treatment in patients with RPLS should be considered.
Drug Interactions
- The administration of vandetanib with agents that are strong CYP3A4 inducers should be avoided.
- The administration of vandetanib with anti-arrhythmic drugs (including, but not limited to amiodarone, disopyramide, procainamide, sotalol, dofetilide) and other drugs that may prolong the QT interval (including but not limited to cloroquine, clarithromycin, dolasetron, granisetron, haloperidol, methadone, moxifloxacin, and pimozide) should be avoided.
Renal Impairment
- Vandetanib exposure is increased in patients with impaired renal function. The starting dose should be reduced to 200 mg in patients with moderate to severe renal impairment and QT interval should be monitored closely. There is no information available for patients with end-stage renal disease requiring dialysis.
Hepatic Impairment
- Vandetanib is not recommended for use in patients with moderate and severe hepatic impairment, as safety and efficacy have not been established.
Use in Pregnancy
- Vandetanib can cause fetal harm when administered to a pregnant woman. There are no adequate and well-controlled studies in pregnant women using vandetanib. In nonclinical studies in rats, vandetanib was embryotoxic, fetotoxic, and teratogenic, at exposures equivalent to or lower than those expected at the recommended human dose of 300 mg/day. As expected from its pharmacological actions, vandetanib has shown significant effects on all stages of female reproduction in rats.
- If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the fetus. Women of childbearing potential should be advised to avoid becoming pregnant during treatment with vandetanib. Women should be advised that they must use effective contraception to prevent pregnancy during treatment and for at least four months following the last dose of vandetanib.
Vandetanib REMS (Risk Evaluation and Mitigation Strategy) Program
- Because of the risk of QT prolongation, torsades de pointes, and sudden death, vandetanib is available only through a restricted distribution program called Vandetanib REMS Program. Only prescribers and pharmacies certified with the program are able to prescribe and dispense vandetanib.
- An overview of the requirements for prescribers and pharmacies is included below.
- To be certified, prescribers must review the educational materials, agree to comply with the REMS requirements and enroll in the program.
- To be certified, pharmacies that dispense vandetanib must enroll in the program, train their pharmacy staff to verify that each prescription is written by a certified prescriber before dispensing to a patient, and agree to comply with the REMS requirements.
- To learn about the specific REMS requirements and to enroll in the Vandetanib REMS Program call 1-800-236-9933 or visit www.vandetanibrems.com.
Adverse Reactions
Clinical Trials Experience
- The most commonly reported adverse drug reactions (>20%) have been diarrhea, rash, acne, nausea, hypertension, headache, fatigue, decreased appetite, and abdominal pain. The most common laboratory abnormalities (>20%) were decreased calcium, increased ALT, and decreased glucose.
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Clinical Studies Experience
- Patients with unresectable locally advanced or metastatic medullary thyroid cancer were treated with vandetanib 300 mg (n=231) or Placebo (n= 99). Patients with investigator-determined progression or patients who continued treatment after the data cut-off could receive open label vandetanib. The following adverse reactions have been reported

- Adverse reactions resulting in death in patients receiving vandetanib (N=5) were respiratory failure, respiratory arrest, aspiration pneumonia, cardiac failure with arrhythmia, and sepsis. Adverse reactions resulting in death in patients receiving placebo were gastrointestinal hemorrhage (1%) and gastroenteritis (1%). In addition there was one sudden death and one death from cardiopulmonary arrest, in patients receiving vandetanib after data cut-off. Causes of discontinuation in vandetanib-treated patients in >1 patient included asthenia, fatigue, rash, arthralgia, diarrhea, hypertension, prolonged QT interval, increase in creatinine and pyrexia. Serious adverse events in vandetanib-treated patients in >2% of patients included diarrhea, pneumonia, and hypertension. Clinically important uncommon adverse drug reactions in patients who received vandetanib versus patients who received placebo included pancreatitis (0.4% vs. 0%) and heart failure (0.9% vs. 0%). In the integrated summary of safety database, the most common cause of death in patients who received vandetanib was pneumonia.
- The incidence of Grade 1-2 bleeding events was 14% in patients receiving vandetanib compared with 7% on placebo in the randomized portion of the medullary thyroid cancer (MTC) study. The incidence was similar in the 300 mg monotherapy safety program with a 13% incidence.
- Blurred vision was more common in patients who received vandetanib versus patients who received placebo for medullary thyroid cancer (9% vs. 1%, respectively). Scheduled slit lamp examinations have revealed corneal opacities (vortex keratopathies) in treated patients, which can lead to halos and decreased visual acuity. It is unknown if this will improve after discontinuation. Ophthalmologic examination, including slit lamp, is recommended in patients who report visual changes. If a patient has blurred vision, do not drive or operate machinery.
- Table 2 provides the frequency and severity of laboratory abnormalities reported for patients with medullary thyroid cancer receiving randomized treatment with vandetanib or placebo.
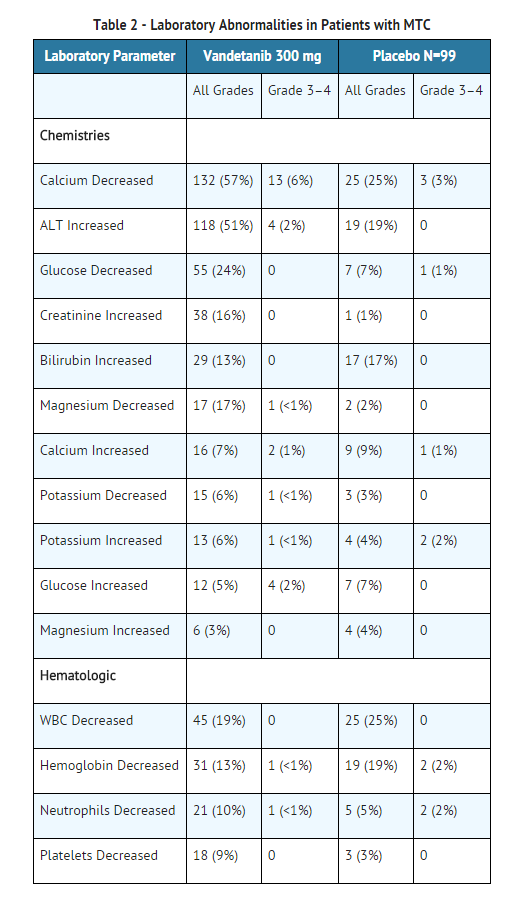
- Alanine aminotransferase elevations occurred in 51% of patients on vandetanib in the randomized medullary thyroid cancer (MTC) study. Grade 3-4 ALT elevations were seen in 2% of patients and no patients had a concomitant increase in bilirubin. Elevations in ALT have resulted in temporary discontinuation of vandetanib. However, 16 of 22 patients with a grade 2 elevation in ALT continued 300 mg vandetanib. Seven patients who continued vandetanib had a normal ALT within 6 months. In the protocol, ALT was monitored every 3 months and more frequently as indicated.
Postmarketing Experience
There is limited information regarding Vandetanib Postmarketing Experience in the drug label.
Drug Interactions
CYP3A4 Inducers
- Drugs that are CYP3A4 inducers can alter vandetanib plasma concentrations. The concomitant use of known strong CYP3A4 inducers should be avoided while receiving vandetanib therapy. St. John’s Wort may decrease vandetanib exposure unpredictably and should be avoided.
CYP3A4 Inhibitors
- In healthy subjects, no clinically significant interaction was shown between vandetanib and the potent CYP3A4 inhibitor, itraconazole.
Drugs that Prolong the QT Interval
- The administration of vandetanib with agents that may prolong the QT interval should be avoided.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): Pregnancy Category D.
- Vandetanib can cause fetal harm when administered to a pregnant woman. There are no adequate and well-controlled studies of vandetanib in pregnant women. Vandetanib is embryotoxic, fetotoxic, and teratogenic to rats, at exposures equivalent to or lower than those expected at the recommended human dose of 300 mg/day. When vandetanib was administered to female rats prior to mating and through the first week of pregnancy, there were increases in pre-implantation loss and post-implantation loss resulting in a significant reduction in the number of live embryos. This dose administered to rats during organogenesis, caused an increase in post-implantation loss including embryofetal death. Vandetanib caused total litter loss when administered at a dose of 25 mg/kg/day during organogenesis until expected parturition. When administered during organogenesis, vandetanib doses of 1, 10 and 25 mg/kg/day (approximately 0.03, 0.4, and 1.0 times respectively, the Cmax in patients with cancer at the recommended human dose) caused malformations of the heart vessels and delayed ossification of the skull, vertebrae and sternum, indicating delayed fetal development. A no effect level for these malformations was not identified in this study. In a rat pre- and post-natal development study, at doses producing maternal toxicity (1 and 10 mg/kg/day) during gestation and/or lactation, vandetanib, decreased pup survival, and/or reduced post-natal pup growth. Reduced post-natal pup growth was associated with a delay in physical development.
- If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the fetus. Women of childbearing potential should be advised to avoid pregnancy while taking vandetanib and for at least four months following the last dose of vandetanib.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Vandetanib in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Vandetanib during labor and delivery.
Nursing Mothers
- In nonclinical studies, vandetanib was excreted in rat milk and found in plasma of pups following dosing to lactating rats. Vandetanib transfer in breast milk resulted in relatively constant exposure in pups due to the long half-life of the drug. It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from vandetanib, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
- Safety and efficacy of vandetanib in pediatric patients have not been established.
Geriatic Use
- In total, 18% of medullary thyroid cancer patients treated with vandetanib were age 65 years or older, and 3% were 75 years and older. No overall differences in safety and efficacy were observed between elderly and younger patients. No adjustment in starting dose is required for patients over 65 years of age. There are limited data for patients over the age of 75 years.
Gender
There is no FDA guidance on the use of Vandetanib with respect to specific gender populations.
Race
There is no FDA guidance on the use of Vandetanib with respect to specific racial populations.
Renal Impairment
- The pharmacokinetics of vandetanib were evaluated after a single dose of 800 mg in subjects with mild (n = 6), moderate (n = 8), and severe (n = 6) renal impairment and normal (n = 10) renal function. Subjects with mild renal impairment had comparable mean AUC and clearance values to those with normal renal function. In subjects with moderate and severe renal impairment, the average AUC of vandetanib increased by 39% and 41%, respectively, compared to patients with normal renal function.
- The starting dose should be reduced to 200 mg in patients with moderate and severe renal impairment.
Hepatic Impairment
- The pharmacokinetics of vandetanib were evaluated after a single dose of 800 mg in subjects with mild (n = 8), moderate (n = 7), and severe (n = 6) hepatic impairment and normal hepatic function (n = 5). Subjects with mild (Child-Pugh class A), moderate (Child-Pugh class B), and severe (Child-Pugh class C) hepatic impairment had comparable mean AUC and clearance values to those with normal hepatic function.
- There are limited data in patients with liver impairment (serum bilirubin greater than 1.5 times the upper limit of normal). Vandetanib is not recommended for use in patients with moderate and severe hepatic impairment, as safety and efficacy have not been established.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Vandetanib in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Vandetanib in patients who are immunocompromised.
Administration and Monitoring
Administration
- ORAL
Monitoring
There is limited information regarding Vandetanib Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Vandetanib and IV administrations.
Overdosage
- There is no specific treatment in the event of overdose with vandetanib and possible symptoms of overdose have not been established. Because of the 19-day half-life, adverse reactions may not resolve quickly. In phase 1 clinical trials, a limited number of patients were treated with daily doses of up to 600 mg and healthy volunteers with daily doses up to 1200 mg. An increase in the frequency and severity of some adverse reactions, like rash, diarrhea and hypertension, was observed at multiple doses at and above 300 mg in healthy volunteer studies and in patients. In addition the possibility of QTc prolongation and Torsades de pointes should be considered.
- Adverse reactions associated with overdose are to be treated symptomatically; in particular, severe diarrhea must be managed appropriately. In the event of an overdose, further doses of vandetanib must be interrupted, and appropriate measures taken to assure that an adverse event has not occurred, i.e., ECG within 24 hours to determine QTc prolongation.
Pharmacology
Mechanism of Action
- Vandetanib is a tyrosine kinase inhibitor. In vitro studies have shown that vandetanib inhibits the activity of tyrosine kinases including members of the epidermal growth factor receptor (EGFR) family, vascular endothelial cell growth factor (VEGF) receptors, rearranged during transfection (RET), protein tyrosine kinase 6 (BRK), TIE2, members of the EPH receptors kinase family, and members of the Src family of tyrosine kinases. Vandetanib inhibits endothelial cell migration, proliferation, survival and new blood vessel formation in in vitro models of angiogenesis. Vandetanib inhibits EGFR-dependent cell survival in vitro. In addition, vandetanib inhibits epidermal growth factor (EGF)-stimulated receptor tyrosine kinase phosphorylation in tumor cells and endothelial cells and VEGF-stimulated tyrosine kinase phosphorylation in endothelial cells.
- In vivo vandetanib administration reduced tumor cell-induced angiogenesis, tumor vessel permeability, and inhibited tumor growth and metastasis in mouse models of cancer.
- There is no evidence of a relationship between RET mutations and efficacy with vandetanib.
Structure
- Vandetanib tablets for daily oral administration are available in two dosage strengths, 100 mg and 300 mg, containing 100 mg and 300 mg of vandetanib, respectively. The tablet cores contain the following inactive ingredients: Tablet core: calcium hydrogen phosphate dihydrate, microcrystalline cellulose, crospovidone, povidone, and magnesium stearate. The tablet film-coat contains the following inactive ingredients: hypromellose 2910, macrogol 300, and titanium dioxide E171.
- Vandetanib is chemically described as N-(4-bromo-2-fluorophenyl)-6-methoxy-7-[(1-methylpiperidin-4-yl)] methoxy]quinazolin-4-amine.
- The structural and molecular formulas are:
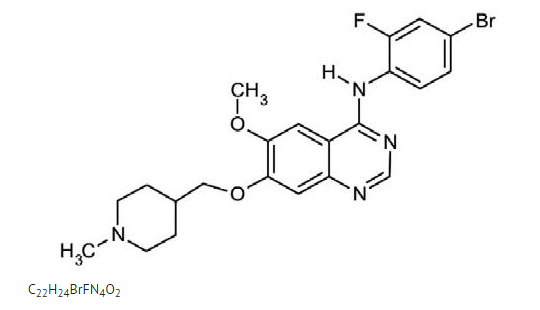
Vandetanib has a molecular weight of 475.36. Vandetanib exhibits pH-dependent solubility, with increased solubility at lower pH. Vandetanib is practically insoluble in water with a value of 0.008 mg/mL at 25°C (77°F ).
Pharmacodynamics
There is limited information regarding Vandetanib Pharmacodynamics in the drug label.
Pharmacokinetics
- A population pharmacokinetic analysis of vandetanib was conducted in 231 patients with MTC following oral administration of 300 mg daily doses. The pharmacokinetics of vandetanib at the 300 mg dose in MTC patients are characterized by a mean clearance of approximately 13.2 L/h, a mean volume of distribution of approximately 7450 L, and a median plasma half-life of 19 days.
Absorption
- Following oral administration of vandetanib, absorption is slow with peak plasma concentrations typically achieved at a median of 6 hours, range 4-10 hours, after dosing. Vandetanib accumulates approximately 8-fold on multiple dosing with steady state achieved from approximately 3 months.
- Exposure to vandetanib is unaffected by food.
Distribution
- Vandetanib binds to human serum albumin and α1-acid-glycoprotein with in vitro protein binding being approximately 90%. In ex vivo plasma samples from colorectal cancer patients at steady state exposure after 300 mg once daily, the mean percentage protein binding was 93.7% (range 92.2 to 95.7%).
Metabolism
- Following oral dosing of 14C-vandetanib, unchanged vandentanib and metabolites vandetanib N-oxide and N-desmethyl vandetanib were detected in plasma, urine and feces. A glucuronide conjugate was seen as a minor metabolite in excreta only. N-desmethyl-vandetanib is primarily produced by CYP3A4 and vandetanib-N-oxide by flavin–containing monooxygenase enzymes FMO1 and FMO3. N-desmethyl-vandetanib and vandetanib-N-oxide circulate at concentrations of approximately 7-17.1% and 1.4-2.2%, respectively, of those of vandetanib.
Excretion
- Within a 21-day collection period after a single dose of 14C-vandetanib, approximately 69% was recovered with 44% in feces and 25% in urine. Excretion of the dose was slow and further excretion beyond 21 days would be expected based on the plasma half-life.
- Vandetanib was not a substrate of hOCT2 expressed in HEK293 cells. Vandetanib inhibits the uptake of the selective OCT2 marker substrate 14C-creatinine by HEK-OCT2 cells, with a mean IC50 of approximately 2.1 μg/mL. This is higher than vandetanib plasma concentrations (approximately 0.81 μg/mL) observed after multiple dosing at 300 mg. Inhibition of renal excretion of creatinine by vandetanib provides an explanation for increases in plasma creatinine seen in human subjects receiving vandetanib.
Special Populations
Effects of Age and Gender
- In a population pharmacokinetic evaluation in cancer patients, no relationship was apparent between oral clearance and patient age or gender.
Ethnicity
- Based on a cross-study comparison in a limited number of patients, Japanese (N=3) and Chinese (N=7) patients had on average exposures that were higher than Caucasian (N=7) patients receiving the same dose.
Pediatric
- The pharmacokinetics of vandetanib have not been evaluated in pediatric patients.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- Carcinogenicity studies have not been conducted with vandetanib.
- Vandetanib was not mutagenic in vitro in the bacterial reverse mutation (Ames) assay and was not clastogenic in both the in vitro cytogenetic assay using human lymphocytes or in the in vivo rat micronucleus assay.
- Based on nonclinical findings, male and female fertility may be impaired by treatment with vandetanib. In a fertility study in male rats, vandetanib had no effect on copulation or fertility rate when undosed females were mated with males administered 1, 5, or 20 mg/kg/day of vandetanib (approximately 0.03, 0.22, or 0.40 times, respectively, the AUC in patients with cancer at the recommended human dose of 300 mg/day). There was a slight decrease in the number of live embryos at 20 mg/kg/day and an increase in preimplantation loss at >5 mg/kg/day. In a female fertility study, there was a trend towards increased estrus cycle irregularity, a slight reduction in pregnancy incidence and an increase in implantation loss. In a repeat-dose toxicity study in rats, there was a decrease in the number of corpora lutea in the ovaries of rats administered 75 mg/kg/day vandetanib (approximately 1.8 times the AUC in patients with cancer at the recommended human dose) for 1 month.
Animal Pharmacology and/or Toxicology
- In an animal model of wound-healing, mice dosed with vandetanib had reduced skin-breaking strength compared with controls. This suggests that vandetanib slows but does not prevent wound healing. The appropriate interval between discontinuation of vandetanib and subsequent elective surgery required to avoid the risks of impaired wound healing has not been determined.
- Nodular masses were observed in a 6-month toxicology study in rats during treatment with ≥5 mg/kg/day vandetanib (approximately 0.22 or 0.40 times, respectively, the AUC in patients with cancer at the recommended human dose of 300 mg/day). Masses were palpable during clinical assessments as early as week 13, were observed in multiple organs, and were associated with hemorrhagic or inflammatory findings.
Clinical Studies
- A double-blind, placebo-controlled study randomized patients with unresectable locally advanced or metastatic medullary thyroid cancer to vandetanib 300 mg (n=231) versus Placebo (n=100).
- The primary objective was demonstration of improvement in progression-free survival (PFS) with vandetanib compared to placebo. Other endpoints included evaluation of overall survival and overall objective response rate (ORR). Centralized, independent blinded review of the imaging data was used in the assessment of PFS and ORR. Upon objective disease progression based on the investigator’s assessment, patients were discontinued from blinded study treatment and given the option to receive open-label vandetanib. Nineteen percent (44/231) of the patients initially randomized to vandetanib opted to receive open-label vandetanib after disease progression, and 58% (58/100) of the patients initially randomized to placebo opted to receive open-label vandetanib after disease progression.
- The result of the PFS analysis, based on the central review RECIST assessment, showed a statistically significant improvement in PFS for patients randomized to vandetanib (Hazard Ratio (HR) = 0.35; 95% Confidence Interval (CI) = 0.24-0.53; p<0.0001). Analyses in the subgroups of patients who were symptomatic or had progressed within 6 months prior to their enrollment showed similar PFS results (HR = 0.31 95% CI: 0.19, 0.53 for symptomatic patients; HR = 0.41 95% CI: 0.25, 0.66 for patients who had progressed within 6 months prior to enrollment).
- At the time of the primary analysis of PFS, 15% of the patients had died and there was no significant difference in overall survival between the two treatment groups. The overall objective response rate (ORR) for patients randomized to vandetanib was 44% compared to 1% for patients randomized to placebo. All objective responses were partial responses.


How Supplied
- 100 mg Tablets Available in bottles containing 30 tablets (NDC 0310–7810–30).
- 300 mg Tablets Available in bottles containing 30 tablets (NDC 0310–7830–30).
Storage
- Vandetanib tablets should be stored at 25°C (77°F); excursions permitted to 15oC – 30oC (59oF – 86oF) [See USP controlled room temperature].
Images
Drug Images
{{#ask: Page Name::Vandetanib |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel



{{#ask: Label Page::Vandetanib |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
QT Interval
- Vandetanib can prolong the QT interval in a concentration-dependent manner. Torsades de pointes, ventricular tachycardia and sudden death have been reported in patients administered vandetanib. Patients should be advised that their electrolytes and the electrical activity of their heartbeat (via an ECG) should be monitored regularly during treatment with vandetanib.
Rash
- Patients taking vandetanib should be told they may be more susceptible to sunburn and to use appropriate sun protection (e.g., sunscreen and/or clothing) while taking vandetanib and for at least 4 months after drug discontinuation. Patients should consult their physician promptly if they develop a skin rash.
Interstitial lung disease
- Patients should be told to contact their physician promptly if they develop sudden onset or worsening of breathlessness, persistent cough or fever.
Diarrhea
- Patients should be informed that they may experience diarrhea while taking vandetanib. Patients should also be advised to use standard anti-diarrheal medications and to seek medical attention if their diarrhea becomes persistent or severe. Patients with diarrhea should contact their physician to have their electrolytes monitored.
Reversible Posterior Leukoencephalopathy Syndrome
- Patients should be told to contact their physician promptly if they experience seizures, headaches, visual disturbances, confusion or difficulty thinking.
Pregnancy and Nursing
- Patients of childbearing potential must be told to use effective contraception during therapy and for at least four months following their last dose of vandetanib.
- Breast-feeding mothers are advised to discontinue nursing while receiving vandetanib therapy.
Drug Handling
- Vandetanib tablets should not be crushed. Direct contact of crushed tablets with the skin or mucous membranes should be avoided.
Precautions with Alcohol
Alcohol-Vandetanib interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- VANDETANIB ®[1]
Look-Alike Drug Names
There is limited information regarding Vandetanib Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.