Long QT Syndrome overview
| https://https://www.youtube.com/watch?v=oFUZeNCox8Q%7C350}} |
|
Long QT Syndrome Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Long QT Syndrome overview On the Web |
|
American Roentgen Ray Society Images of Long QT Syndrome overview |
|
Risk calculators and risk factors for Long QT Syndrome overview |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [2]
Overview
The long QT syndrome (LQTS) is a heart condition associated with prolongation of polarization (recovery) following depolarization (excitation) of the cardiac ventricles. It is associated with syncope (fainting) and sudden death due to ventricular arrhythmias. Arrhythmias in individuals with LQTS are often associated with exercise or excitement. LQTS is also associated with torsade de pointes, a the rare ventricular arrhythmia which can deteriorate into ventricular fibrillation and ultimately death.
Individuals with LQTS have a prolongation of the QT interval on the EKG. The Q wave on the EKG corresponds to ventricular depolarization while the T wave corresponds to ventricular repolarization. The QT interval is measured from the beginning of the Q wave to the end of the T wave. While many individuals with LQTS have persistent prolongation of the QT interval, some individuals do not always show QT prolongation; in these individuals, the QT interval may prolong with the administration of certain medications.
Classification
There are multiple genetic mutations that account for LQTS, but LQT1, LQT2, and LQT3 account for approximately 75% of all LQTS cases and approximately 89% genotype-positive LQTS cases. [1][2]
LQT1
LQT1 is the most common type of long QT syndrome, making up about 40-55% of all cases. This variant will sometimes come to the attention of the cardiologist following a cardiac event during exercise like swimming.[3] The LQT1 gene is KCNQ1 located on chromosome 11p15.5. KCNQ1 codes for the voltage-gated potassium channel KvLQT1 that is highly expressed in the heart. It is believed that the product of the KCNQ1 gene produces an alpha subunit that interacts with other proteins (particularly the minK beta subunit) to create the IKs ion channel, which is responsible for the delayed potassium rectifier current of the cardiac action potential.
Mutations to the KCNQ1 gene can be inherited in an autosomal dominant or an autosomal recessive pattern within the same family. In the autosomal recessive mutation of this gene, homozygous mutations in KVLQT1 leads to severe prolongation of the QT interval (due to near-complete loss of the IKs ion channel), and is associated with increased risk of ventricular arrhythmias and congenital deafness. This variant of LQT1 is known as the Jervell and Lange-Nielsen syndrome.
Most individuals with LQT1 show paradoxical prolongation of the QT interval with infusion of epinephrine. This can also unmark latent carriers of the LQT1 gene.
Many missense mutations of the LQT1 gene have been identified. These are often associated with a high risk percentage of symptomatic carriers and sudden death.
LQT2
The LQT2 type is the second most common gene location that is affected in long QT syndrome, making up about 35-45% of all cases. This variant will sometimes come to the attention of the cardiologist as a result of a cardiac event during the post partum period or after being triggered by an alarm clock.[3] This form of long QT syndrome most likely involves mutations of the human ether-a-go-go related gene (HERG) that is found on chromosome 7q36.1. The HERG gene (also known as KCNH2) codes for part of the rapid component of the potassium rectifying current (IKr). The IKr current is mainly responsible for the termination of the cardiac action potential, and therefore the length of the QT interval. The normally functioning HERG gene allows protection against early after depolarizations (EADs).
Most drugs that cause long QT syndrome do so by blocking the IKr current via the HERG gene. These include erythromycin, terfenadine, andketoconazole. The HERG channel is very sensitive to unintended drug binding due to two aromatic amino acids, the tyrosine at position 652 and the phenylalanine at position 656. These amino acid residues are poised so drug binding to them will block the channel from conducting current. Other potassium channels do not have these residues in these positions and are therefore not as prone to blockage.
LQT3
The LQT3 type of long QT syndrome accounts for 5-10% of cases, and cardiac events can occur during sleep. This variant involves a mutation of the gene that encodes the alpha subunit of the Na+ ion channel. This gene is located on chromosome 3p21-24, and is known as SCN5A (also hH1 and NaV1.5). The mutations involved in LQT3 slow the inactivation of the Na+ channel, resulting in prolongation of the Na+ influx during depolarization. Paradoxically, the mutant sodium channels inactivate more quickly, and may open repetitively during the action potential.
A large number of mutations have been characterized as leading to or predisposing LQT3. Calcium has been suggested as a regulator of SCN5A, and the effects of calcium on SCN5A may begin to explain the mechanism by which some these mutations cause LQT3. Furthermore mutations in SCN5A can cause Brugada syndrome, cardiac conduction disease, and dilated cardiomyopathy. Although rare, some affected individuals can have combinations of these diseases.
Pathophysiology
The pathophysiology of Long QT syndrome involves an inhereted or congenital abnormality in the cardiac ion channels leading to prolongation of the action potential and early after depolarization. There is also an imbalance in the sympathetic innervation of heart.
Associated syndromes
A number of syndromes are associated with LQTS.
Jervell and Lange-Nielsen syndrome
The Jervell and Lange-Nielsen syndrome (JLNS) is an autosomal recessive form of LQTS with associated congenital deafness. It is caused specifically by mutation of the KCNE1 and KCNQ1 genes.
In untreated individuals with JLNS, about 50 percent die by the age of 15 years due to ventricular arrhythmias.
Romano-Ward syndrome
Romano-Ward syndrome is an autosomal dominant form of LQTS that is not associated with deafness. It occurs more frequently than Jervell and Lange-Nielsen syndrome.
Differentiating Long QT Syndrome from Other Disorders
There are multiple causes of QT prolongation that are distinct from Long QT syndrome such as drugs (anti-arrhythmic drugs, anti-psychotic drugs), electrolyte disturbances (hyperkalaemia, hypocalcaemia, hypoglycaemia, hypokalaemia, and hypomagnesemia), neurologic events such as subarachnoid hemorrhage, and anorexia nervosa.
Epidemiology and Demographics
The prevalence of LQTS is approximately 50/100,000 individuals (i.e. between 1:1100 and 1:3,000).[4]
Risk Stratification
The genetic variant (LQT1-8), the gender and the QT interval are associated with the risk of a cardiac event. A history of prior events (syncope, fainting spells, seizures, sudden death) and a family history of cardiac events are associated with an increased risk of subsequent cardiac events.
Risk Factors for Torsade de Pointes and Sudden Cardiac Death
- Persons with a history of repeated fainting episodes or syncope. If there are more than 2 fainting spells in under 2 years, the risk of an aborted SCD or SCD is increased 18 fold.[5]
- Repeated blackouts or fainting spells in the context of the following are due to a malignant arrhythmia until proven otherwise:
- Exertion
- Loud startling noise
- Postpartum syncope
- Seizures
- A prior history of cardiac arrest.
- Family members of persons with repeated fainting, accidents, seizures or a history of cardiac arrest.
- Persons who are on certain medications that are known to cause a prolonged QT interval on electrocardiogram.
- Persons who are first degree relatives of people with know long QT syndrome.
- Persons who suffer from anorexia nervosa, or who have low levels of magnesium, calcium or potassium in their blood
Natural History
About half the patients with long QT syndrome will have an arrhythmia that degenerates into Torsade de Pointes that may terminate spontaneously or may end in sudden cardiac death.
Complications
Long QT syndrome can result in fatal heart arrhythmias and death. Certain medications can increase the risk of fatal arrhythmias and death in persons with long QT syndrome.
Prognosis
People who are treated with lifestyle modifications and medications live longer than those who are not. For people who are not treated, half of them, mostly those with the inherited form of long QT syndrome, will die within 10 years.
The Jervell and Lange-Nielsen syndrome (JLNS) is an autosomal recessive form of LQTS with associated congenital deafness. It is caused specifically by mutation of the KCNE1 and KCNQ1 genes. In untreated individuals with JLNS, about 50 percent die by the age of 15 years due to ventricular arrhythmias.
Diagnosis
History
You should inquire about the following as part of the thistory:
- Persons with a history of repeated fainting episodes or syncope. If there are more than 2 fainting spells in under 2 years, the risk of an aborted SCD or SCD is increased 18 fold.[6]
- Repeated blackouts or fainting spells in the context of the following are due to a malignant arrhythmia until proven otherwise:
- Exertion
- Loud startling noise
- Postpartum syncope
- Seizures
- A prior history of cardiac arrest.
- Family members of persons with repeated fainting, accidents, seizures or a history of cardiac arrest.
- Persons who are on certain medications that are known to cause a prolonged QT interval on electrocardiogram.
- Persons who are first degree relatives of people with know long QT syndrome.
Symptoms
- Fainting - fainting or syncope is the most common symptom in persons with long QT syndrome. The fainting can occur spontaneously without warning, or in response to certain stressors such as emotional stress, exercise, excitement or loud noises. Often when people are about to faint, they may experience lightheadedness, heart palpitations, blurred vision or weakness.
- Seizures - if the heart continues to beat abnormally, the brain can become deprived of oxygen, which can then cause seizures.
- Sudden death - in some circumstances a fatal arrhytmia that is not quickly intervened on, may cause sudden death.
Electrocardiography
The diagnosis of LQTS is difficult in so far as 2.5% of the healthy population have a prolonged QT interval, and 10% of LQTS patients have a normal QT interval (known as concealed LQTs). The presence of LQTs in the absence of QT prolongation (concealed LQTs) underscores the importance of genetic testing in the diagnosis of LQTs. It should be noted that the QT interval is often overestimated in the presence of a u wave.
How to Measure the QT
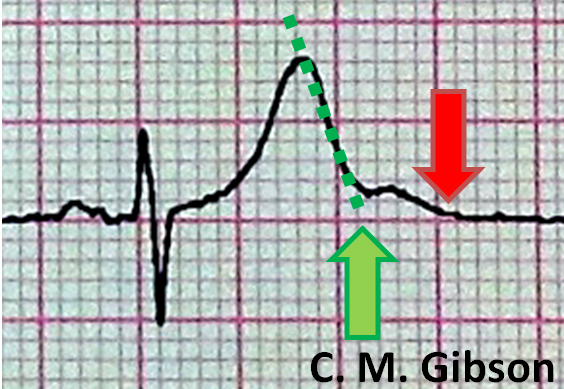
The QT interval is often measured incorrectly. It is measured incorrectly by 33% of EP physicians and 75% of general cardiologists. It is measured incorrectly by <5% of Long QT syndrome experts who deal with this on a frequent basis. [7] The presence of a U wave can often lead to a false diagnosis of QT prolongation. In order to avoid this, the "teach the tangent" or "avoid the tail" rule is applied. In this method, a line is drawn on top of the downslope of the T wave as shown below with the dotted green line. The QT interval is measured where this green dotted line intersects with the isoelectric line as shown by the large green arrow. The red arrow is an incorrect assessment of the QT interval at the end of the U wave. Using the red arrow would lead to a misdiagnosis of QT prolongation.
The LQTS Diagnostic Score
A commonly used criterion to diagnose LQTS is the LQTS "diagnostic score". Its based on several criteria giving points to each. With 4 or more points the probability is high for LQTS, and with 1 or less point the probability is low. Two or 3 points indicates intermediate probability.
- QTc (Defined as QT interval / square root of RR interval)
- >= 480 msec - 3 points
- 460-470 msec - 2 points
- 450 msec and male gender - 1 point
- Torsades de Pointes ventricular tachycardia - 2 points
- T wave alternans - 1 point
- Notched T wave in at least 3 leads - 1 point
- Low heart rate for age (children) - 0.5 points
- Syncope (one cannot receive points both for syncope and Torsades de pointes)
- With stress - 2 points
- Without stress - 1 point
- Congenital deafness - 0.5 points
- Family history (the same family member cannot be counted for LQTS and sudden death)
- Other family members with definite LQTS - 1 point
- Sudden death in immediate family (members before the age 30) - 0.5 points
Electrocardiographic Examples of the Long QT Variants
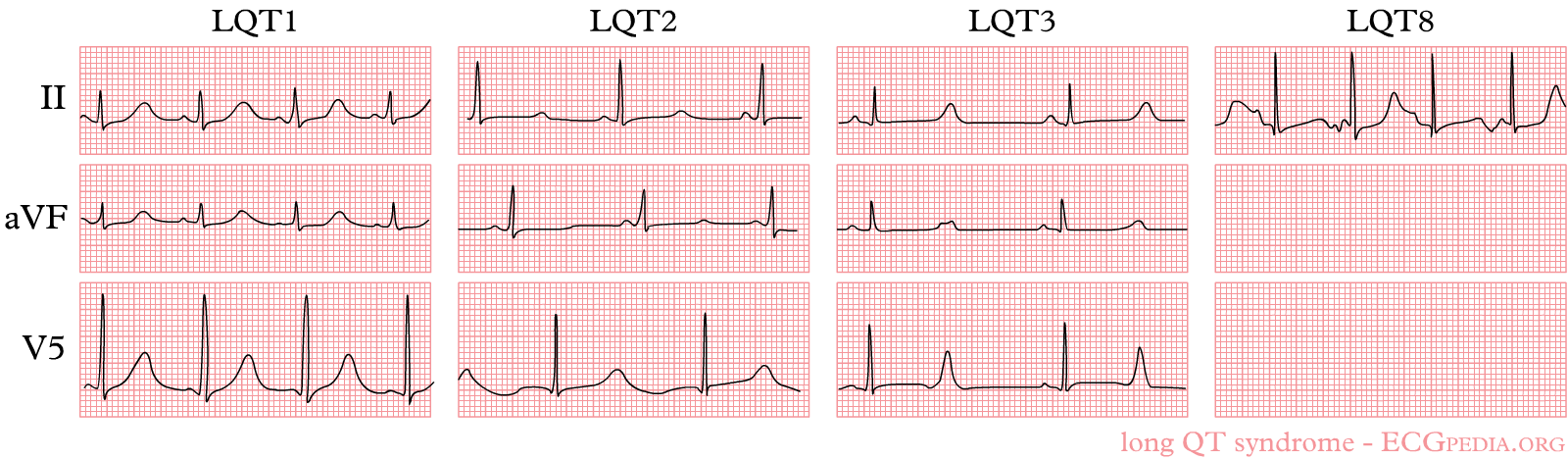
Shown below are examples of ECGs demonstrating QT prolongation in Long QT syndrome.
- LQT 1 shows 'early onset' broad based, slowly generated T waves
- LQT 2 shows small late T waves. Sometimes these T waves will be notched or double humped in lead V4.
- LQT 3 shows a flat and prolonged ST segment with a 'late onset' T wave that is normal in configuration.
Genetic Testing
Either compressive testing for all variants of LQTs or for the LQTs 1-3 variants is recommended in any patient in whom there is a strong clinical suspicion based on the family history, symptoms, resting EKG, provoked findings on an exercise treadmill test or during catecholamine infusion. Genetic studies remain to be the gold standard in the diagnosis of long QT syndrome.
Other Diagnostic Studies
The diagnosis of long QT syndrome can be difficult when abnormalities on electrocardiogram are borderline or intermittent. In cases where the history and symptoms are suggestive of long QT syndrome, but few or no abnormalities are seen on electrocardiogram, further testing can be done to unmask long QT syndrome with the use of exercise treadmill testing, and catecholamine provocation testing.
Non-exercise Catecholamine Stress Testing
During this type of test, an EKG is performed while the patient is given an infusion of epinephrine. The epinephrine challenge is a useful test to establish electrocardiographic diagnosis in silent LQT1 mutation carriers, thus allowing implementation of prophylactic measures aimed at reducing sudden cardiac death. This test can unmask what is known as concealed long QT syndrome, which shows a normal QT interval on EKG at rest. This test can show a prolonged QT interval in persons who have a history of fainting spells in response to intense exercise or emotional upset, and therefore point to the diagnosis of long QT syndrome.
Exercise Treadmill Testing
Similar to catecholamine provocation testing, an exercise treadmill test with EKG, can unmmask concealed long QT syndrome. This is also used as a provocation test for persons who have a pertinent history suggestive of long QT syndrome, or a family history of long QT syndrome, but do not have any abnormalities on resting EKG. Studies have shown not only the usefulness of exercise stress testing in the diagnosis of long QT syndrome, but also it's utility in determining the subtype of long QT syndrome that the patient has. Krahn et al. showed that the hysteresis of the QT segment observed during exercise may be helpful in the diagnosis of long QT syndrome. They also showed that differences in the QT interval that were greater than 21ms between 1 min into recovery and early exercise may be indicative of long QT syndrome [8] . A study done by Takenaka et al. demonstrated that the QTc in LQT1 patients was significantly prolonged during exercise, whereas in LQT2 patients, exercise did not cause any significant changes in the QTc, however did show a prominent notch on the descending limb of the T wave. They also showed that there is a steeper QT/R-R slope in LQT2 patients than in LQT1 patients during exercise [9].
Treatment
Beta-blockers are first line treatment in LQTs along with electrolyte repletion, and avoidance of triggers (drugs, supplements, loud noises). LQTs is one of the few diseases where genetic testing actually can provide important guidance such as who to put a AICD (defibrillator) in for primary prevention. [10] Left stellectomy is not a cure, but is second line therapy to reduce the risk of sudden cardiac death and is indicated if the patient does not tolerate beta blockers or breaks through beta blockers, as well as in young patients under the age of 12 where beta blockers are not deemed protective enough and where the morbidity of an AICD seems excessive. Patients with Long QT syndrome should undergo secondary prevention with AICD implantation for secondary prevention if they sustain an aborted cardiac arrest or sudden cardiac death.
ACC/AHA/ESC 2006 Guidelines for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death (DO NOT EDIT) [11]
Recommendations for Long QT Syndrome
| Class I |
| "1. Lifestyle modification is recommended for patients with an LQTS diagnosis (clinical and/or molecular). (Level of Evidence: B)" |
| "2. Beta blockers are recommended for patients with an LQTS clinical diagnosis (i.e., in the presence of prolonged QT interval). (Level of Evidence: B)" |
| "3. Implantation of an ICD along with use of beta blockers is recommended for LQTS patients with previous cardiac arrest and who have reasonable expectation of survival with a good functional status for more than 1 y. (Level of Evidence: A)" |
| Class IIa |
| "1. Beta blockers can be effective to reduce SCD in patients with a molecular LQTS analysis and normal QT interval. (Level of Evidence: B)" |
| "2. Implantation of an ICD with continued use of beta blockers can be effective to reduce SCD in LQTS patients experiencing syncope and/or VT while receiving beta blockers and who have reasonable expectation of survival with a good functional status for more than 1 y. (Level of Evidence: B)" |
| Class IIb |
| "1. Left cardiac sympathetic neural denervation may be considered for LQTS patients with syncope, torsades de pointes, or cardiac arrest while receiving beta blockers. (Level of Evidence: B)" |
| "2. Implantation of an ICD with the use of beta blockers may be considered for prophylaxis of SCD for patients in categories possibly associated with higher risk of cardiac arrest such as LQT2 and LQT3 and who have reasonable expectation of survival with a good functional status for more than 1 y. (Level of Evidence: B)" |
Recommendations for Drug-Induced Long QT Syndrome
| Class I |
| "1. In patients with drug-induced LQTS, removal of the offending agent is indicated. (Level of Evidence: A)" |
| Class IIa |
| "1. Management with intravenous magnesium sulfate is reasonable for patients who take QT-prolonging drugs and present with few episodes of torsades de pointes in which the QT remains long. (Level of Evidence: B)" |
| "2. Atrial or ventricular pacing or isoproterenol is reasonable for patients taking QT-prolonging drugs who present with recurrent torsades de pointes. (Level of Evidence: B)" |
| Class IIb |
| "1. Potassium ion repletion to 4.5 to 5 mmol/L may be reasonable for patients who take QT-prolonging drugs and present with few episodes of torsades de pointes in whom the QT remains long. (Level of Evidence: C)" |
Primary Prevention of Arrhythmias and Sudden Death
Withdrawal of Drugs and Supplements
Certain medications should be avoided in persons with long QT syndrome, to avoid worsening the condition. These medications include certain appetite suppressants, decongestants, and antibiotics such as erythromycin. Illicit drugs such as cocaine and amphetamines can be even more dangerous in persons with long QT syndrome.
List of Medications to be Avoided in Congenital Long QT Syndrome
- Albuterol
- Alfuzosin
- Amantadine
- Amiodarone
- Amisulpride
- Amitriptyline
- Amphetamine
- Arsenic trioxide
- Artenimol + piperaquine
- Astemizole
- Atazanavir
- Atomoxetine
- Azithromycin
- Bepridil
- Chloral hydrate
- Chloroquine
- Chlorpromazine
- Ciprofloxacin
- Cisapride
- Citalopram
- Clarithromycin
- Clomipramine
- Clozapine
- Cocaine
- Desipramine
- Dexmethylphenidate
- Diphenhydramine
- Diphenhydramine
- Disopyramide
- Dobutamine
- Dofetilide
- Dolasetron
- Domperidone
- Dopamine
- Doxepin
- Dronedarone
- Droperidol
- Ephedrine
- Epinephrine
- Eribulin
- Erythromycin
- Escitalopram
- Famotidine
- Fenfluramine
- Fingolimod
- Flecainide
- Fluconazole
- Fluoxetine
- Foscarnet
- Fosphenytoin
- Galantamine
- Gatifloxacin
- Gemifloxacin
- Granisetron
- Halofantrine
- Haloperidol
- Ibutilide
- Iloperidone
- Imipramine
- Indapamide
- Isoproterenol
- Isradipine
- Itraconazole
- Ketoconazole
- Lapatinib
- Levalbuterol
- Levofloxacin
- Levomethadyl
- Lisdexamfetamine
- Lithium
- Mesoridazine
- Metaproterenol
- Methadone
- Methylphenidate
- Midodrine
- Mirtazapine
- Moexipril / HCTZ
- Moxifloxacin
- Nicardipine
- Nilotinib
- Norepinephrine
- Nortriptyline
- Octreotide
- Ofloxacin
- Ondansetron
- Oxytocin
- Paliperidone
- Paroxetine
- Pentamidine
- Perflutren lipid microspheres
- Phentermine
- Phenylephrine
- Phenylpropanolamine
- Pimozide
- Probucol
- Procainamide
- Protriptyline
- Pseudoephedrine
- Quetiapine
- Quinidine
- Ranolazine
- Ritodrine
- Ritonavir
- Roxithromycin
- Salmeterol
- Sertindole
- Sertraline
- Sevoflurane
- Sibutramine
- Solifenacin
- Sotalol
- Sparfloxacin
- Sunitinib
- Tacrolimus
- Tamoxifen
- Telithromycin
- Terbutaline
- Terfenadine
- Thioridazine
- Tizanidine
- Tolterodine
- Trazodone
- Trimethoprim-Sulfamethoxazole
- Trimipramine
- Vandetanib
- Vardenafil
- Venlafaxine
- Voriconazole
- Ziprasidone
Correct Electrolyte Disturbances
Illness that cause hypokalemia due to vomiting and diarrhea can aggravate long QT syndrome. Medications that can lower the levels of potassium in the blood should also be avoided.
Postassium Administration
The use of potassium supplementation is experimental and is not evidence based. The hypothesis is that ff the potassium content in the blood rises, the action potential shortens and it is for this reason that increasing potassium concentration may minimize the occurrence of arrhythmias. It should work best in LQT2 since the HERG channel is especially sensible to potassium concentration, but potassium supplementation is experimental and not evidence based.
Beta Blockers
Beta blockers are first line therapy in the treatment of Long QT syndrome.
Arrhythmia suppression involves the use of medications or surgical procedures that attack the underlying cause of the arrhythmias associated with LQTS. Since the cause of arrhythmias in LQTS is after depolarizations, and these after depolarizations are increased in states of adrenergic stimulation, steps can be taken to blunt adrenergic stimulation in these individuals. beta receptor blocking agents decrease the risk of stress or catecholamine induced arrhythmias. Nadolol and propranolol are recommended, and caution should be used with atenolol.
Nadolol
Nadolol at a dose of 1.0 to 1.5 mg/kg/day or 50 mg/m2/day QD or BID is the dose
Propranolol
3-4 mg/kg/day BID for the long acting form and TID for the liquid. Often preferred in LQT3.
Mexiletine
Mexiletine is a sodium channel blocker. In LQT3 the problem is that the sodium channel does not close properly. Mexiletine closes these channels and is believed to be potentially of use when other therapies fail. It should be especially effective in LQT3 but there is limited evidence to support this recommendation.
AICD Implantation
Genotype and QT interval duration are independent predictors of recurrence of life-threatening events during beta-blockers therapy. Specifically the presence of QTc >500ms and LQT2 and LQT3 genotype are associated with the highest incidence of recurrence. In these patients primary prevention with ICD (Implantable Cardioverster Defibrilator) implantaion can be considered.[12]
An AICD should be implanted if:
- The QTc is > 550 ms and if it is not LQT1
- LQT2 in women and the QTc is > 500 ms, with or without symptoms
- In infants with 2:1 AV block (controversial)
- In JLNS (LQTS with deafness) given its malignant propensity (controversial)
Sympathetic Denervation
Videoscopic Left Cardiac Sympathetic Denervation Surgery (left stellectomy) is not a cure, but reduces the risk of sudden cardiac death and is indicated if:
- The patient does not tolerate beta blockers or breaks through beta blockers
- The patient faints while on beta blockers
- There is a history of VF terminating AICD shocks
- In young patients under the age of 12 where beta blockers are not deemed protective enough and where the morbidity of an AICD seems excessive.
Secondary Prevention
Patients with Long QT syndrome should undergo secondary prevention with AICD implantation if they sustain an aborted cardiac arrest or sudden cardiac death.
References
- ↑ Sherman J, Tester DJ, Ackerman MJ (2005). "Targeted mutational analysis of ankyrin-B in 541 consecutive, unrelated patients referred for long QT syndrome genetic testing and 200 healthy subjects". Heart Rhythm : the Official Journal of the Heart Rhythm Society. 2 (11): 1218–23. doi:10.1016/j.hrthm.2005.07.026. PMID 16253912. Unknown parameter
|month=ignored (help) - ↑ Webster G, Berul CI (2008). "Congenital long-QT syndromes: a clinical and genetic update from infancy through adulthood". Trends in Cardiovascular Medicine. 18 (6): 216–24. doi:10.1016/j.tcm.2008.11.002. PMID 19185812. Unknown parameter
|month=ignored (help) - ↑ 3.0 3.1 Tester DJ, Will ML, Haglund CM, Ackerman MJ (2005). "Compendium of cardiac channel mutations in 541 consecutive unrelated patients referred for long QT syndrome genetic testing". Heart Rhythm : the Official Journal of the Heart Rhythm Society. 2 (5): 507–17. doi:10.1016/j.hrthm.2005.01.020. PMID 15840476. Unknown parameter
|month=ignored (help) - ↑ Booker PD, Whyte SD, Ladusans EJ (2003). "Long QT syndrome and anaesthesia". British Journal of Anaesthesia. 90 (3): 349–66. PMID 12594150. Unknown parameter
|month=ignored (help) - ↑ Hobbs et al. JAMA 296:1249-1254, 2006.
- ↑ Hobbs et al. JAMA 296:1249-1254, 2006.
- ↑ Heart Rhythm 2005;2:569-574
- ↑ Krahn AD, Klein GJ, Yee R (1997). "Hysteresis of the RT interval with exercise: a new marker for the long-QT syndrome?". Circulation. 96 (5): 1551–6. PMID 9315546.
- ↑ Takenaka K, Ai T, Shimizu W, Kobori A, Ninomiya T, Otani H; et al. (2003). "Exercise stress test amplifies genotype-phenotype correlation in the LQT1 and LQT2 forms of the long-QT syndrome". Circulation. 107 (6): 838–44. PMID 12591753.
- ↑ Compton SJ, Lux RL, Ramsey MR, Strelich KR, Sanguinetti MC, Green LS, Keating MT, Mason JW. Genetically defined therapy of inherited long-QT syndrome. Correction of abnormal repolarization by potassium. Circulation. 1996 Sep 1;94(5):1018-22. PMID 8790040
- ↑ Zipes DP, Camm AJ, Borggrefe M, Buxton AE, Chaitman B, Fromer M; et al. (2006). "ACC/AHA/ESC 2006 Guidelines for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death: a report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (writing committee to develop Guidelines for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society". Circulation. 114 (10): e385–484. doi:10.1161/CIRCULATIONAHA.106.178233. PMID 16935995.
- ↑ Priori SG, Napolitano C, Schwartz PJ, Grillo M, Bloise R, Ronchetti E, Moncalvo C, Tulipani C, Veia A, Bottelli G, Nastoli J. Association of long QT syndrome loci and cardiac events among patients treated with beta-blockers. JAMA. 2004 Sep 15;292(11):1341-4.15367556