Arsenic trioxide
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Aparna Vuppala, M.B.B.S. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING
See full prescribing information for complete Boxed Warning.
|
Overview
Arsenic trioxide is an antineoplastic agent that is FDA approved for the treatment of induction of remission and consolidation in patients with acute promyelocytic leukemia (APL) who are refractory to, or have relapsed from, retinoid and [[anthracycline]] chemotherapy, and whose APL is characterized by the presence of the t(15;17) translocation or PML/RAR-alpha gene expression.. There is a Black Box Warning for this drug as shown here. Common adverse reactions include Most patients experienced some drug-related toxicity, most commonly leukocytosis, gastrointestinal (nausea, vomiting, diarrhea, and abdominal pain), fatigue, edema, hyperglycemia, dyspnea, cough, rash or itching, headaches, and dizziness..
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Acute promyelocytic leukemia
- Arsenic trioxide is indicated for induction of remission and consolidation in patients with acute promyelocytic leukemia (APL) who are refractory to, or have relapsed from, retinoid and anthracycline chemotherapy, and whose APL is characterized by the presence of the t(15;17) translocation or PML/RAR-alpha gene expression.
- The response rate of other acute myelogenous leukemia subtypes to Arsenic trioxide has not been examined.
Dosing Information
- Arsenic trioxide should be diluted with 100 to 250 mL 5% Dextrose Injection, USP or 0.9% Sodium Chloride Injection, USP, using proper aseptic technique, immediately after withdrawal from the ampule. The Arsenic trioxide ampule is single-use and does not contain any preservatives. Unused portions of each ampule should be discarded properly. Do not save any unused portions for later administration. Do not mix Arsenic trioxide with other medications.
- Arsenic trioxide should be administered intravenously over 1-2 hours. The infusion duration may be extended up to 4 hours if acute vasomotor reactions are observed. A central venous catheter is not required.
Dosing Regimen
- Arsenic trioxide is recommended to be given according to the following schedule:
- Induction Treatment Schedule: Arsenic trioxide should be administered intravenously at a dose of 0.15 mg/kg daily until bone marrow remission. Total induction dose should not exceed 60 doses.
- Consolidation Treatment Schedule: Consolidation treatment should begin 3 to 6 weeks after completion of induction therapy. Arsenic trioxide should be administered intravenously at a dose of 0.15 mg/kg daily for 25 doses over a period up to 5 weeks.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Arsenic trioxide in adult patients.
Non–Guideline-Supported Use
Acute promyelocytic leukemia, FAB M3
- Arsenic trioxide 10 mg IV over 2 to 3 hours per day [1]
Multiple myeloma, Relapsed or refractory
- Arsenic trioxide 0.15 mg/kg as a daily 2-hour IV infusion for 60 days.[2]
Myelodysplastic syndrome, Monotherapy in transfusion-dependent patients
- Arsenic trioxide 0.25 mg/kg/day was administered as a 1- to 2-hour IV infusion for 5 consecutive days each week for 2 weeks, repeated every 4 weeks
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
- There are limited clinical data on the pediatric use of Arsenic trioxide. Of 5 patients below the age of 18 years (age range: 5 to 16 years) treated with Arsenic trioxide, at the recommended dose of 0.15 mg/kg/day, 3 achieved a complete response.
- In an additional study, the toxicity profile observed in 13 pediatric patients with APL between the ages of 4 and 20 receiving Arsenic trioxide at 0.15 mg/kg/day was similar to that observed in adult patients .
- Safety and effectiveness in relapsed APL pediatric patients below the age of 4 years have not been studied.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Arsenic trioxide in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Arsenic trioxide in pediatric patients.
Contraindications
- Arsenic trioxide is contraindicated in patients who are hypersensitive to arsenic.
Warnings
|
WARNING
See full prescribing information for complete Boxed Warning.
|
- Arsenic trioxide should be administered under the supervision of a physician who is experienced in the management of patients with acute leukemia.
- Nine of 40 patients with APL treated with Arsenic trioxide, at a dose of 0.15 mg/kg, experienced the APL differentiation syndrome .
- Hyperleukocytosis
- Treatment with Arsenic trioxide has been associated with the development of hyperleukocytosis (≥ 10 x 10³/uL) in 20 of 40 patients. A relationship did not exist between baseline WBC counts and development of hyperleukocytosis nor baseline WBC counts and peak WBC counts. Hyperleukocytosis was not treated with additional chemotherapy. WBC counts during consolidation were not as high as during induction treatment.
- QT Prolongation
- QT/QTc prolongation should be expected during treatment with Arsenic trioxide and torsade de pointes as well as complete heart block has been reported. Over 460 ECG tracings from 40 patients with refractory or relapsed APL treated with Arsenic trioxide were evaluated for QTc prolongation. Sixteen of 40 patients (40%) had at least one ECG tracing with a QTc interval greater than 500 msec. Prolongation of the QTc was observed between 1 and 5 weeks after Arsenic trioxide infusion, and then returned towards baseline by the end of 8 weeks after Arsenic trioxide infusion. In these ECG evaluations, women did not experience more pronounced QT prolongation than men, and there was no correlation with age.
- Complete AV block
- Complete AV block has been reported with Arsenic trioxide in the published literature including a case of a patient with APL.
- Carcinogenesis
- Carcinogenicity studies have not been conducted with Arsenic trioxide by intravenous administration. The active ingredient arsenic trioxide is a human carcinogen.
Adverse Reactions
Clinical Trials Experience
- Safety information was available for 52 patients with relapsed or refractory APL who participated in clinical trials of Arsenic trioxide. Forty patients in the Phase 2 study received the recommended dose of 0.15 mg/kg of which 28 completed both induction and consolidation treatment cycles. An additional 12 patients with relapsed or refractory APL received doses generally similar to the recommended dose. Most patients experienced some drug-related toxicity, most commonly leukocytosis, gastrointestinal (nausea, vomiting, diarrhea, and abdominal pain), fatigue, edema, hyperglycemia, dyspnea, cough, rash or itching, headaches, and dizziness. These adverse effects have not been observed to be permanent or irreversible nor do they usually require interruption of therapy.
- Serious adverse events (SAEs), grade 3 or 4 according to version 2 of the NCI Common Toxicity Criteria, were common. Those SAEs attributed to Arsenic trioxide in the Phase 2 study of 40 patients with refractory or relapsed APL included APL differentiation syndrome (n=3), hyperleukocytosis (n=3), QTc interval ≥ 500 msec (n=16, 1 with torsade de pointes), atrial dysrhythmias (n=2), and hyperglycemia (n=2).
- The following table describes the adverse events that were observed in patients treated for APL with Arsenic trioxide at the recommended dose at a rate of 5% or more. Similar adverse event profiles were seen in the other patient populations who received Arsenic trioxide
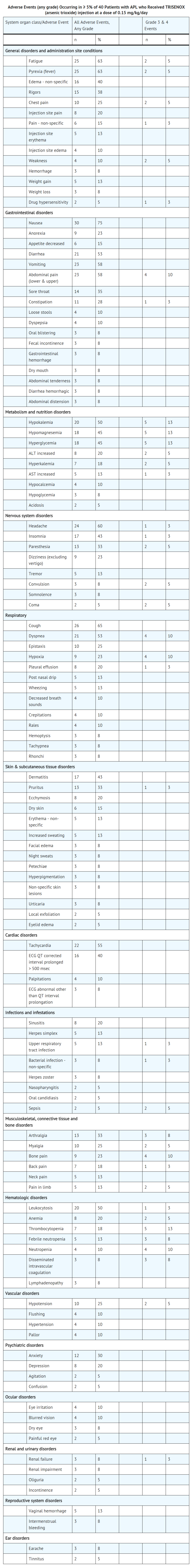
The following additional adverse events were reported as related to Arsenic trioxide treatment in 13 pediatric patients (defined as ages 4 through 20): gastrointestinal (dysphagia, mucosal inflammation/stomatitis, oropharyngeal pain, caecitis), metabolic and nutrition disorders (hyponatremia, hypoalbuminemia, hypophosphatemia, and lipase increased), cardiac failure congestive, respiratory (acute respiratory distress syndrome, lung infiltration, pneumonitis, pulmonary edema, respiratory distress, capillary leak syndrome), neuralgia, and enuresis. Pulmonary edema (n=1) and caecitis (n=1) were considered serious reactions.
Postmarketing Experience
- The following reactions have been reported from clinical trials and/or world-wide post-marketing surveillance. Because they are reported from a population of unknown size, precise estimates of frequency cannot be made.
- Cardiac disorders: ventricular extrasystoles in association with QT prolongation, and ventricular tachycardia in association with QT prolongation
- Nervous system disorders: peripheral neuropathy
- Hematologic disorders: pancytopenia
- Respiratory, thoracic, and mediastinal disorders: A differentiation syndrome, like retinoic acid syndrome, has been reported with the use of Arsenic trioxide for the treatment of malignancies other than APL.
Drug Interactions
- No formal assessments of pharmacokinetic drug-drug interactions between Arsenic trioxide and other drugs have been conducted. The methyltransferases responsible for metabolizing arsenic trioxide are not members of the cytochrome P450 family of isoenzymes.
- In vitro incubation of arsenic trioxide with human liver microsomes showed no inhibitory activity on substrates of the major cytochrome P450 (CYP) enzymes such as 1A2, 2A6, 2B6, 2C8, 2C9, 2C19, 2D6, 2E1, 3A4/5, and 4A9/11. The pharmacokinetics of drugs that are substrates for these CYP enzymes are not expected to be affected by concomitant treatment with arsenic trioxide
- Caution is advised when Arsenic trioxide is coadministered with other medications that can prolong the QT interval (e.g., certain antiarrhythmics or thioridazine) or lead to electrolyte abnormalities (such as diuretics or amphotericin B).
Use in Specific Populations
Pregnancy
- Arsenic Trioxide may cause fetal harm when administered to a pregnant woman. Studies in pregnant mice, rats, hamsters, and primates have shown that inorganic arsenicals cross the placental barrier when given orally or by injection. The reproductive toxicity of arsenic trioxide has been studied in a limited manner. An increase in resorptions, neural-tube defects, anophthalmia and microphthalmia were observed in rats administered 10 mg/kg of arsenic trioxide on gestation day 9 (approximately 10 times the recommended human daily dose on a mg/m² basis). Similar findings occurred in mice administered a 10 mg/kg dose of a related trivalent arsenic, sodium arsenite, (approximately 5 times the projected human dose on a mg/m² basis) on gestation days 6, 7, 8 or 9. Intravenous injection of 2 mg/kg sodium arsenite (approximately equivalent to the projected human daily dose on a mg/m² basis) on gestation day 7 (the lowest dose tested) resulted in neural-tube defects in hamsters.
- There are no studies in pregnant women using Arsenic trioxide. If this drug is used during pregnancy or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential harm to the fetus. One patient who became pregnant while receiving arsenic trioxide had a miscarriage. Women of childbearing potential should be advised to avoid becoming pregnant.
- There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Arsenic Trioxide in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of ArsenicTrioxide during labor and delivery.
Nursing Mothers
- Arsenic is excreted in human milk. Because of the potential for serious adverse reactions in nursing infants from Arsenic Trioxide, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother. .
Pediatric Use
- There are limited clinical data on the pediatric use of ArsenicTrioxide. Of 5 patients below the age of 18 years (age range: 5 to 16 years) treated with ArsenicTrioxide, at the recommended dose of 0.15 mg/kg/day, 3 achieved a complete response.
- In an additional study, the toxicity profile observed in 13 pediatric patients with APL between the ages of 4 and 20 receiving ArsenicTrioxide at 0.15 mg/kg/day was similar to that observed in adult patients .
- Safety and effectiveness in relapsed APL pediatric patients below the age of 4 years have not been studied.
Geriatic Use
There is no FDA guidance on the use of ArsenicTrioxide with respect to geriatric patients.
Gender
There is no FDA guidance on the use of ArsenicTrioxide with respect to specific gender populations.
Race
There is no FDA guidance on the use of ArsenicTrioxide with respect to specific racial populations.
Renal Impairment
- Exposure of arsenic trioxide may be higher in patients with severe renal impairment . Patients with severe renal impairment (creatinine clearance less than 30 mL/min) should be closely monitored for toxicity when these patients are treated with Arsenic trioxide, and a dose reduction may be warranted.
- The use of Arsenic trioxide in patients on dialysis has not been studied.
Hepatic Impairment
- Since limited data are available across all hepatic impairment groups, caution is advised in the use of Arsenic trioxide in patients with hepatic impairment . Patients with severe hepatic impairment (Child-Pugh class C) should be closely monitored for toxicity when these patients are treated with Arsenic trioxide.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of ArsenicTrioxide in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of ArsenicTrioxide in patients who are immunocompromised.
Administration and Monitoring
Administration
- Intravenous
Monitoring
- The patient’s electrolyte, hematologic and coagulation profiles should be monitored at least twice weekly, and more frequently for clinically unstable patients during the induction phase and at least weekly during the consolidation phase. ECGs should be obtained weekly, and more frequently for clinically unstable patients, during induction and consolidation.
IV Compatibility
There is limited information regarding IV Compatibility of ArsenicTrioxide in the drug label.
Overdosage
- If symptoms suggestive of serious acute arsenic toxicity (e.g., convulsions, muscle weakness and confusion) appear, arsenic trioxide injection should be immediately discontinued and chelation therapy should be considered.
Management
- A conventional protocol for acute arsenic intoxication includes dimercaprol administered at a dose of 3 mg/kg intramuscularly every 4 hours until immediate life-threatening toxicity has subsided. Thereafter, penicillamine at a dose of 250 mg orally, up to a maximum frequency of four times per day (≤ 1 g per day), may be given.
Pharmacology
Mechanism of Action
- The mechanism of action of Arsenic trioxide is not completely understood. Arsenic trioxide causes morphological changes and DNA fragmentation characteristic of apoptosis in NB4 human promyelocytic leukemia cells in vitro. Arsenic trioxide also causes damage or degradation of the fusion protein PML/RAR-alpha.
Structure
There is limited information regarding Arsenic trioxide Structure in the drug label.
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Arsenic trioxide in the drug label.
Pharmacokinetics
- The inorganic, lyophilized form of arsenic trioxide, when placed into solution, immediately forms the hydrolysis product arsenious acid (AsIII). AsIII is the pharmacologically active species of arsenic trioxide. Monomethylarsonic acid (MMAV), and dimethylarsinic acid (DMAV) are the main pentavalent metabolites formed during metabolism, in addition to arsenic acid (AsV) a product of AsIII oxidation. The pharmacokinetics of arsenical species ([AsIII], [AsV], [MMAV], [DMAV]) were determined in 6 APL patients following once daily doses of 0.15 mg/kg for 5 days per week. Over the total single dose range of 7 to 32 mg (administered as 0.15 mg/kg), systemic exposure (AUC) appears to be linear. Peak plasma concentrations of arsenious acid (AsIII), the primary active arsenical species were reached at the end of infusion (2 hours). Plasma concentration of AsIII declined in a biphasic manner with a mean elimination half-life of 10 to 14 hours and is characterized by an initial rapid distribution phase followed by a slower terminal elimination phase. The daily exposure to AsIII (mean AUC0-24) was 194 ng·hr/mL (n=5) on Day 1 of Cycle 1 and 332 ng·hr/mL (n=6) on Day 25 of Cycle 1, which represents an approximate 2-fold accumulation. The primary pentavalent metabolites, MMAV and DMAV, are slow to appear in plasma (approximately 10-24 hours after first administration of arsenic trioxide), but, due to their longer half-life, accumulate more upon multiple dosing than does AsIII. The mean estimated terminal elimination half-lives of the metabolites MMAV and DMAV are 32 hours and 72 hours, respectively. Approximate accumulation ranged from 1.4- to 8-fold following multiple dosing as compared to single dose administration. AsV is present in plasma only at relatively low levels.
- Distribution
- The volume of distribution (Vss) for AsIII is large (mean 562 L, N=10) indicating that AsIII is widely distributed throughout body tissues. Vss is also dependent on body weight and increases as body weight increases.
- Metabolism
- Much of the AsIII is distributed to the tissues where it is methylated to the less cytotoxic metabolites, monomethylarsonic acid (MMAV) and dimethylarsinic acid (DMAV) by methyltransferases primarily in the liver. The metabolism of arsenic trioxide also involves oxidation of AsIII to AsV, which may occur in numerous tissues via enzymatic or nonenzymatic processes. AsV is present in plasma only at relatively low levels following administration of arsenic trioxide.
- Excretion
- Approximately 15% of the administered Arsenic trioxide dose is excreted in the urine as unchanged AsIII. The methylated metabolites of AsIII (MMAV, DMAV) are primarily excreted in the urine. The total clearance of AsIII is 49 L/h and the renal clearance is 9 L/h. Clearance is not dependent on body weight or dose administered over the range of 7-32 mg.
The effect of age, gender, or race on the pharmacokinetics of Arsenic trioxide has not been studied.
- Pediatric Patients
- Following IV administration of 0.15 mg/kg/day of arsenic trioxide in 10 APL patients (median age = 13.5 years, range 4-20 years), the daily exposure to AsIII (mean AUC0-24h) was 317 ng·hr/mL on Day 1 of Cycle 1.
- Effect of Renal Impairment
- The effect of renal impairment on the pharmacokinetics of AsIII, AsV, and the pentavalent metabolites MMAV and DMAV was evaluated in 20 patients with advanced malignancies. Patients were classified as having normal renal function (creatinine clearance CrCl > 80 mL/min, n=6), mild renal impairment (CrCl 50-80 mL/min, n=5), moderate renal impairment (CrCl 30-49 mL/min, n=6), or severe renal impairment (CrCl < 30 mL/min, n=3). :*Following twice weekly administration of 0.15 mg/kg over a 2-hour infusion, the mean AUC0-∞ for AsIII was comparable among the normal, mild and moderate renal impairment groups. However, in the severe renal impairment group, the mean AUC0-∞ for AsIII was approximately 48% higher than that in the normal group.
- Systemic exposure to MMAV and DMAV tended to be larger in patients with renal impairment; however, the clinical consequences of this increased exposure are not known. AsV plasma levels were generally below the limit of assay quantitation in patients with impaired renal function. The use of arsenic trioxide in patients on dialysis has not been studied.
- Effect of Hepatic Impairment
- The effect of pharmacokinetics of AsIII, AsV, and the pentavalent metabolites MMAV and DMAV was evaluated following administration of 0.25-0.50 mg/kg of arsenic trioxide in patients with hepatocellular carcinoma. Patients were classified as having normal hepatic function (n=4), mild hepatic impairment (Child-Pugh class A, n=12), moderate hepatic impairment (Child-Pugh class B, n=3), or severe hepatic impairment (Child-Pugh class C, n=1). No clear trend toward an increase in systemic exposure to AsIII, AsV, MMAV or DMAV was observed with decreasing level of hepatic function as assessed by dose-normalized (per mg dose) AUC in the mild and moderate hepatic impairment groups. However, the one patient with severe hepatic impairment had mean dose-normalized AUC0‑24 and Cmax values 40% and 70% higher, respectively, than those patients with normal hepatic function. The mean dose-normalized trough plasma levels for both MMAV and DMAV in this severely hepatically impaired patient were 2.2-fold and 4.7-fold higher, respectively, than those in the patients with normal hepatic function .
Nonclinical Toxicology
- Carcinogenesis, Mutagenesis, Impairment of Fertility
- Arsenic trioxide and trivalent arsenite salts have not been demonstrated to be mutagenic to bacteria, yeast or mammalian cells. Arsenite salts are clastogenic in vitro (human fibroblast, human lymphocytes, Chinese hamster ovary cells, Chinese hamster V79 lung cells). Trivalent arsenic produced an increase in the incidence of chromosome aberrations and micronuclei in bone marrow cells of mice. The effect of arsenic on fertility has not been adequately studied.
Clinical Studies
- Arsenic trioxide has been investigated in 40 relapsed or refractory APL patients, previously treated with an anthracycline and a retinoid regimen, in an open-label, single-arm, non-comparative study. Patients received 0.15 mg/kg/day intravenously over 1 to 2 hours until the bone marrow was cleared of leukemic cells or up to a maximum of 60 days. The CR (absence of visible leukemic cells in bone marrow and peripheral recovery of platelets and white blood cells with a confirmatory bone marrow ≥ 30 days later) rate in this population of previously treated patients was 28 of 40 (70%). *Among the 22 patients who had relapsed less than one year after treatment with ATRA, there were 18 complete responders (82%). Of the 18 patients receiving Arsenic trioxide ≥ one year from ATRA treatment, there were 10 complete responders (55%). The median time to bone marrow remission was 44 days and to onset of CR was 53 days. Three of 5 children, 5 years or older, achieved CR. No children less than 5 years old were treated.
- Three to six weeks following bone marrow remission, 31 patients received consolidation therapy with Arsenic trioxide, at the same dose, for 25 additional days over a period up to 5 weeks. In follow-up treatment, 18 patients received further arsenic trioxide as a maintenance course. Fifteen patients had bone marrow transplants. At last follow-up, 27 of 40 patients were alive with a median follow-up time of 484 days (range 280 to 755) and 23 of 40 patients remained in complete response with a median follow-up time of 483 days (range 280 to 755).
- Cytogenetic conversion to no detection of the APL chromosome rearrangement was observed in 24 of 28 (86%) patients who met the response criteria defined above, in 5 of 5 (100%) patients who met some but not all of the response criteria, and 3 of 7 (43%) of patients who did not respond. Reverse Transcriptase – Polymerase Chain Reaction conversions to no detection of the APL gene rearrangement were demonstrated in 22 of 28 (79%) of patients who met the response criteria, in 3 of 5 (60%) of patients who met some but not all of the response criteria, and in 2 of 7 (29%) of patients who did not respond.
- Responses were seen across all age groups tested, ranging from 6 to 72 years. The ability to achieve a CR was similar for both genders. There were insufficient patients of Black, Hispanic or Asian derivation to estimate relative response rates in these groups, but responses were seen in members of each group.
- Another single center study in 12 patients with relapsed or refractory APL, where patients received arsenic trioxide injection doses generally similar to the recommended dose, had similar results with 9 of 12 (75%) patients attaining a CR.
How Supplied
- Arsenic trioxide injection is supplied as a sterile, clear, colorless solution in 10 mL glass, single-use ampules.
- NDC 63459-400-10 10 mg/10 mL (1 mg/mL) ampule in packages of ten ampules.
Storage
- Store at 25°C (77°F); excursions permitted to 15 - 30°C (59 - 86°F). Do not freeze.
- Do not use beyond expiration date printed on the label.
Images
Drug Images
{{#ask: Page Name::Arsenic trioxide |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Arsenic trioxide |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Arsenic trioxide in the drug label.
Precautions with Alcohol
- Alcohol- Arsenic trioxide interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Trisenox®
Look-Alike Drug Names
There is limited information regarding Arsenic trioxide Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Mathews V, George B, Lakshmi KM, Viswabandya A, Bajel A, Balasubramanian P; et al. (2006). "Single-agent TRISENOX in the treatment of newly diagnosed acute promyelocytic leukemia: durable remissions with minimal toxicity". Blood. 107 (7): 2627–32. doi:10.1182/blood-2005-08-3532. PMID 16352810.
- ↑ Munshi NC, Tricot G, Desikan R, Badros A, Zangari M, Toor A; et al. (2002). "Clinical activity of TRISENOX for the treatment of multiple myeloma". Leukemia. 16 (9): 1835–7. doi:10.1038/sj.leu.2402599. PMID 12200700.
- ↑ Vey N, Bosly A, Guerci A, Feremans W, Dombret H, Dreyfus F; et al. (2006). "Arsenic trioxide in patients with myelodysplastic syndromes: a phase II multicenter study". J Clin Oncol. 24 (16): 2465–71. doi:10.1200/JCO.2005.03.9503. PMID 16651646.
{{#subobject:
|Page Name=Arsenic trioxide
|Pill Name=No image.jpg
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Label Page=Arsenic trioxide |Label Name=ARSENIC04.png
}}
{{#subobject:
|Label Page=Arsenic trioxide |Label Name=ARSENIC05.png
}}
{{#subobject:
|Label Page=Arsenic trioxide |Label Name=ARSENIC06.png
}}