|
|
| (66 intermediate revisions by 8 users not shown) |
| Line 1: |
Line 1: |
| | __NOTOC__ |
| {{Infobox_Disease | | | {{Infobox_Disease | |
| Name = Long QT syndrome | | | Name = Long QT syndrome | |
| Image = SinusRhythmLabels.png | | | Image =Long QT Syndrome.PNG| |
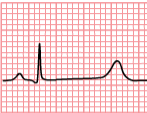
| Caption = Schematic representation of normal ECG trace ''([[sinus rhythm]]),'' with waves, segments, and intervals labeled. | | | Caption = Type 3 Long QT Syndrome | |
| DiseasesDB = 11104 | | | DiseasesDB = 11104 | |
| ICD10 = {{ICD10|I|45|8|i|30}} | | | ICD10 = {{ICD10|I|45|8|i|30}} | |
| Line 9: |
Line 10: |
| OMIM = | | | OMIM = | |
| MedlinePlus = | | | MedlinePlus = | |
| eMedicineSubj = med |
| |
| eMedicineTopic = 1983 |
| |
| MeshID = D008133 | | | MeshID = D008133 | |
| }} | | }} |
| {{SI}} | | {{Long QT Syndrome}} |
| {{WikiDoc Cardiology Network Infobox}}
| |
|
| |
|
| {{CMG}}
| | '''For patient information, click [[Long QT syndrome (patient information)|here]].''' |
| __NOTOC__
| |
| '''Associate Editor-In-Chief:''' {{CZ}} | |
|
| |
|
| {{Editor Help}}
| | '''Long QT syndrome is one of the many causes of [[QT prolongation]]. For a complete review of [[QT prolongation]] in general, click [[QT prolongation|here]].''' |
|
| |
|
| ==Overview==
| | {{CMG}} |
| | |
| The '''long QT syndrome''' ('''LQTS''') is a [[heart]] condition associated with prolongation of repolarisation (recovery) following depolarisation (excitation) of the cardiac [[ventricle (heart)|ventricles]]. It is associated with [[fainting|syncope]] (fainting) and [[sudden death]] due to [[left ventricle|ventricular]] [[cardiac arrhythmia|arrhythmias]]. Arrhythmias in individuals with LQTS are often associated with exercise or excitement. LQTS is associated with the rare, ventricular arrhythmia [[torsade de pointes]], which can deteriorate into [[ventricular fibrillation]] and ultimately death.
| |
| | |
| Individuals with LQTS have a prolongation of the [[QT interval]] on the [[electrocardiogram|ECG]]. The Q wave on the [[electrocardiogram|ECG]] corresponds to ventricular depolarization while the T wave corresponds to ventricular repolarization. The QT interval is measured from the Q point to the end of the [[Electrocardiogram#T wave|T wave]]. While many individuals with LQTS have persistent prolongation of the QT interval, some individuals do not always show the QT prolongation; in these individuals, the QT interval may prolong with the administration of certain medications.
| |
| | |
| | |
| <div align="left">
| |
| <gallery heights="125" widths="125">
| |
| Image:QRSinterval.jpg|The QT interval start at the onset of the Q wave and ends where the tangent line for the steepest part of the T wave intersects with the baseline of the ECG.
| |
| Image:acquired_longQT.jpg|A 12 lead ECG of a patient with acquired long QT syndrome. Notice the QT prolongation. The QTc is about 640ms.
| |
| Image:lqts1-3.png|The three most common forms of LQTS can be recognized by the '''characteristic ECG abnormalities'''
| |
| </gallery>
| |
| </div>
| |
| | |
| | |
| <div align="left">
| |
| <gallery heights="125" widths="125">
| |
| Image:lastigeQT2.png|The ECG does not meet the baseline after the end of the T wave. Still, the crossing of the tangent and baseline should be used for measurements.
| |
| Image:lastigeQT3.png|A bifasic T wave. The tangent to the 'hump' with the largest amplitude is chosen. This can change from beat to beat, making it more important to average several measurements.
| |
| Image:lastigeQT1.png|The T wave is broad, but the tangent crosses the baseline before the T wave joins the baseline. The QT interval would be overestimated when this last definition of the end of the T wave would be used.
| |
| </gallery>
| |
| </div>
| |
| | |
| ==Genetics==
| |
| The two most common types of LQTS are genetic and drug-induced. Genetic LQTS can arise from mutation to one of several genes. These mutations tend to prolong the duration of the [[ventricular action potential]] (APD), thus lengthening the QT interval. LQTS can be inherited in an [[autosomal dominant]] or an [[autosomal recessive]] fashion. The autosomal recessive forms of LQTS tend to have a more severe [[phenotype]], with some variants having associated [[syndactyly]] (LQT8) or congenital neural deafness (LQT1). A number of specific genes loci have been identified that are associated with LQTS. Following is a list of the most common mutations:
| |
| | |
| {| class="wikitable" | |
| | '''Type''' || '''OMIM''' || '''Mutation''' || '''Notes'''
| |
| |-
| |
| | LQT1 || {{OMIM2|192500}} || alpha subunit of the slow delayed rectifier potassium channel ([[KvLQT1]] or KCNQ1) || The current through the heteromeric channel (KvLQT1 + minK) is known as I<sub>Ks</sub>. These mutations often cause LQT by reducing the amount of repolarizing current that is required to terminate the action potential, leading to an increase in the action potential duration (APD). These mutations tend to be the most common yet least severe.
| |
| |-
| |
| | LQT2 || {{OMIM2|152427}} || alpha subunit of the rapid delayed rectifier potassium channel ([[HERG]] + [[MiRP1]]) || Current through this channel is known as I<sub>Kr</sub>. This phenotype is also probably caused by a reduction in repolarizing current.
| |
| |-
| |
| | LQT3 || {{OMIM2|603830}} || alpha subunit of the [[sodium channel]] ([[SCN5A]]) || Current through this channel is commonly referred to as I<sub>Na</sub>. Depolarizing current through the channel late in the action potential is thought to prolong APD. The late current is due to failure of the channel to remain inactivated and hence enter a bursting mode in which significant current can enter when it should not. These mutations are more lethal but less common.
| |
| |-
| |
| | LQT4 || {{OMIM2|600919}} || anchor protein [[Ankyrin B]] || LQT4 is very rare. Ankyrin B anchors the ion channels in the cell.
| |
| |-
| |
| | LQT5 || {{OMIM2|176261}} || beta subunit MinK (or [[KCNE1]]) which coassembles with [[KvLQT1]] || -
| |
| |-
| |
| | LQT6 || {{OMIM2|603796}} || beta subunit MiRP1 (or [[KCNE2]]) which coassembles with [[HERG]] || -
| |
| |-
| |
| | LQT7 || {{OMIM2|170390}} || potassium channel [[KCNJ2]] (or K<sub>ir</sub>2.1) || The current through this channel and KCNJ12 (K<sub>ir</sub>2.2) is called I<sub>K1</sub>. LQT7 leads to [[Andersen-Tawil syndrome]].
| |
| |-
| |
| | LQT8 || {{OMIM2|601005}} || alpha subunit of the [[calcium channel]] Cav1.2 encoded by the gene [[CACNA1c]]. || Leads to [[Timothy's syndrome]].
| |
| |-
| |
| | LQT9 || || [[Caveolin 3]] ||
| |
| |-
| |
| | LQT10 || || [[SCN4B]] ||
| |
| | |
| |}
| |
| | |
| Drug induced LQT is usually a result of treatment by [[Antiarrhythmic agent|anti-arrhythmic]] drugs such as [[amiodarone]] or a number of other drugs that have been reported to cause this problem (e.g. [[cisapride]]). Some [[anti-psychotic]] drugs, such as [[Haloperidol]] and [[Ziprasidone]], have a prolonged QT interval as a rare side effect. Genetic mutations may make one more susceptible to drug induced LQT.
| |
| | |
| ===LQT1===
| |
| LQT1 is the most common type of long QT syndrome, making up about 40 to 55 percent of all cases. The LQT1 [[gene]] is {{gene|KCNQ1}} which has been isolated to [[chromosome]] 11p15.5. KCNQ1 codes for the voltage-gated potassium channel [[KvLQT1]] that is highly expressed in the heart. It is believed that the product of the KCNQ1 gene produces an alpha subunit that interacts with other proteins (particularly the minK beta subunit) to create the I<sub>Ks</sub> ion channel, which is responsible for the delayed potassium rectifier current of the [[cardiac action potential]].
| |
| | |
| Mutations to the KCNQ1 gene can be inherited in an [[autosomal dominant]] or an [[autosomal recessive]] pattern in the same family. In the autosomal recessive mutation of this gene, [[homozygous]] mutations in KVLQT1 leads to severe prolongation of the QT interval (due to near-complete loss of the I<sub>Ks</sub> ion channel), and is associated with increased risk of ventricular arrhythmias and congenital deafness. This variant of LQT1 is known as the [[Jervell and Lange-Nielsen syndrome]].
| |
| | |
| Most individuals with LQT1 show paradoxical prolongation of the QT interval with infusion of [[epinephrine]]. This can also unmark latent carriers of the LQT1 gene.
| |
| | |
| Many [[missense mutation]]s of the LQT1 gene have been identified. These are often associated with a high risk percentage of symptomatic carriers and sudden death.
| |
| | |
| ===LQT2===
| |
| | |
| The LQT2 type is the second most common gene location that is affected in long QT syndrome, making up about 35 to 45 percent of all cases. This form of long QT syndrome most likely involves mutations of the ''human ether-a-go-go related gene'' ([[HERG]]) on chromosome 7. The [[HERG]] gene (also known as KCNH2) is part of the rapid component of the potassium rectifying current (I<sub>Kr</sub>). (The I<sub>Kr</sub> current is mainly responsible for the termination of the [[cardiac action potential]], and therefore the length of the QT interval.) The normally functioning [[HERG]] gene allows protection against early after depolarizations (EADs).
| |
| | |
| Most drugs that cause long QT syndrome do so by blocking the I<sub>Kr</sub> current via the [[HERG]] gene. These include [[erythromycin]], [[terfenadine]], and [[ketoconazole]]. The HERG channel is very sensitive to unintended drug binding due to two [[aromatic]] [[amino acid]]s, the [[tyrosine]] at position 652 and the [[phenylalanine]] at position 656. These amino acid residues are poised so drug binding to them will block the channel from conducting current. Other potassium channels do not have these residues in these positions and are therefore not as prone to blockage.
| |
| | |
| ===LQT3===
| |
| | |
| The LQT3 type of long QT syndrome involves mutation of the gene that encodes the alpha subunit of the [[sodium|Na<sup>+</sup>]] ion channel. This gene is located on chromosome 3p21-24, and is known as [[SCN5A]] (also hH1 and Na<sub>V</sub>1.5). The mutations involved in LQT3 slow the inactivation of the Na<sup>+</sup> channel, resulting in prolongation of the Na<sup>+</sup> influx during depolarization. Paradoxically, the mutant sodium channels inactivate more quickly, and may open repetitively during the action potential.
| |
| | |
| A large number of mutations have been characterized as leading to or predisposing LQT3. Calcium has been suggested as a regulator of SCN5A, and the effects of calcium on SCN5A may begin to explain the mechanism by which some these mutations cause LQT3. Furthermore mutations in [[SCN5A]] can cause [[Brugada syndrome]], Cardiac Conduction disease and [[dilated cardiomyopathy]]. Rarely some affected individuals can have combinations of these diseases.
| |
| | |
| ===LQT5===
| |
| | |
| is an [[autosomal dominant]] relatively uncommon form of LQTS. It involves mutations in the gene KCNE1 which encodes for the potassium channel beta subunit MinK. In its rare homozygous forms it can lead to [[Jervell and Lange-Nielsen syndrome]]
| |
| | |
| ===LQT6===
| |
| | |
| is an [[autosomal dominant]] relatively uncommon form of LQTS. It involves mutations in the gene KCNE2 which encodes for the potassium channel beta subunit MiRP1, constituting part of the I<sub>Kr</sub> repolarizing K<sup>+</sup> current.
| |
|
| |
|
| ===LQT7===
| | {{SK}} LQTS; long QT; congenital long QT; congenital long QT interval |
|
| |
|
| [[Andersen-Tawil syndrome]] is an [[autosomal dominant]] form of LQTS associated with skeletal deformities. It involves mutation in the gene KCNJ2 which encodes for the potassium channel protein Kir 2.1. The syndrome is characterized by Long QT syndrome with ventricular arrhythmias, periodic paralysis and skeletal developmental abnormalities as clinodactyly, low-set ears and [[micrognathia]]. The manifestations are highly variable.<ref>Tristani-Firouzi M, Jensen JL, Donaldson MR, Sansone V, Meola G, Hahn A, Bendahhou S, Kwiecinski H, Fidzianska A, Plaster N, Fu YH, Ptacek LJ, Tawil R. Functional and clinical characterization of KCNJ2 mutations associated with LQT7 (Andersen syndrome). Journal of Clinical Investigation. 2002 Aug;110(3):381-8. PMID 12163457.</ref> | | == [[Long QT Syndrome overview|Overview]] == |
|
| |
|
| ===LQT8=== | | == [[Long QT Syndrome historical perspective|Historical Perspective]]== |
|
| |
|
| Timothy's syndrome is due to mutations in the calcium channel Cav1.2 encoded by the gene CACNA1c. Since the Calcium channel Cav1.2 is abundant in many tissues, patients with Timothy's syndrome have many clinical manifestations including congenital heart disease, autism, syndactyly and immune deficiency.
| | == [[Long QT Syndrome classification|Classification]] == |
|
| |
|
| ===LQT9=== | | == [[Long QT Syndrome pathophysiology|Pathophysiology]] == |
|
| |
|
| This newly discovered variant is caused by mutations in the membrane structural protein, [[caveolin]]<nowiki>-3</nowiki>. Caveolins form specific membrane domains called [[caveolae]] in which among others the Na<sub>V</sub>1.5 [[voltage-gated sodium channel]] sits. Similar to LQT3, these particular mutations increase so-called 'late' sodium current which impairs cellular [[repolarization]].
| | == [[Long QT Syndrome differential diagnosis|Differentiating Long QT Syndrome from other Diseases]] == |
|
| |
|
| ===LQT10=== | | == [[Long QT Syndrome epidemiology and demographics|Epidemiology and Demographics]]== |
|
| |
|
| This novel susceptibility gene for LQT is ''SCN4B'' encoding the protein Na<sub>V</sub>β4, an auxiliary [[subunit]] to the pore-forming Na<sub>V</sub>1.5 (gene: ''SCN5A'') subunit of the [[voltage-gated sodium channel]] of the heart. The mutation leads to a positive shift in inactivation of the sodium current, thus increasing sodium current. Only one mutation in one patient has so far been found.
| | == [[Long QT Syndrome risk stratification|Risk Stratification]] == |
|
| |
|
| ==Associated syndromes== | | == [[Long QT Syndrome screening|Screening]]== |
|
| |
|
| A number of syndromes are associated with LQTS.
| | == [[Long QT Syndrome natural history, complications and prognosis|Natural History, Complications and Prognosis]] == |
| | |
| ===Jervell and Lange-Nielsen syndrome=== | |
| | |
| The [[Jervell and Lange-Nielsen syndrome]] (JLNS) is an [[autosomal recessive]] form of LQTS with associated congenital deafness. It is caused specifically by mutation of the KCNE1 and KCNQ1 genes
| |
| | |
| In untreated individuals with JLNS, about 50 percent die by the age of 15 years due to ventricular arrhythmias.
| |
| | |
| ===Romano-Ward syndrome===
| |
| | |
| [[Romano-Ward syndrome]] is an autosomal dominant form of LQTS that is ''not'' associated with deafness.
| |
| | |
| ==Mechanism of arrhythmia generation== | |
| | |
| All forms of the long QT syndrome involve an abnormal repolarization of the heart. The abnormal repolarization causes differences in the "refractoriness" of the [[myocyte]]s. After-depolarizations (which occur more commonly in LQTS) can be propagated to neighboring cells due to the differences in the [[refractory period|refractory periods]], leading to re-entrant ventricular arrhythmias.
| |
| | |
| It is believed that the so-called early after-depolarizations (EADs) that are seen in LQTS are due to re-opening of L-type calcium channels during the plateau phase of the [[cardiac action potential]]. Since [[adrenergic]] stimulation can increase the activity of these channels, this is an explanation for why the risk of sudden death in individuals with LQTS is increased during increased adrenergic states (ie exercise, excitement) -- especially since repolarization is impaired. Normally during adrenergic states, repolarizing currents will also be enhanced to shorten the action potential. In the absence of this shortening and the presence of increased L-type calcium current, EADs may arise.
| |
| | |
| The so-called delayed after-depolarizations (DADs) are thought to be due to an increased Ca<sup>2+</sup> filling of the [[sarcoplasmic reticulum]]. This overload may cause spontaneous Ca<sup>2+</sup> release during repolarization, causing the released Ca<sup>2+</sup> to exit the cell through the 3Na<sup>+</sup>/Ca<sup>2+</sup>-exchanger which results in a net depolarizing current.
| |
|
| |
|
| == Diagnosis == | | == Diagnosis == |
| | [[Long QT Syndrome history and symptoms|History and Symptoms]] | [[Long QT Syndrome electrocardiogram|Electrocardiogram]] | [[Long QT Syndrome genetic studies|Genetic Studies]] | [[Long QT Syndrome other diagnostic studies|Other Diagnostic Studies]] |
|
| |
|
| The diagnosis of LQTS is not easy since 2.5% of the healthy population have prolonged QT interval, and 10% of LQTS patients have a normal QT interval. A commonly used criterion to diagnose LQTS is the LQTS "diagnostic score" <ref>Schwartz PJ, Moss AJ, Vincent GM, Crampton RS. Diagnostic criteria for the long QT syndrome. An update. Circulation. 1993 Aug;88(2):782-4. PMID 8339437.</ref>. Its based on several criteria giving points to each. With 4 or more points the probability is high for LQTS, and with 1 or less point the probability is low. Two or 3 points indicates intermediate probability.
| | == Treatment == |
|
| |
|
| * QTc (Defined as QT interval / square root of RR interval)
| | ===Medical Therapy=== |
| ** >= 480 msec - 3 points
| |
| ** 460-470 msec - 2 points
| |
| ** 450 msec and male gender - 1 point
| |
| * [[Torsades de Pointes]] ventricular tachycardia - 2 points
| |
| * [[T wave alternans]] - 1 point
| |
| * Notched T wave in at least 3 leads - 1 point
| |
| * Low heart rate for age (children) - 0.5 points
| |
| * Syncope (one cannot receive points both for syncope and Torsades de pointes)
| |
| ** With stress - 2 points
| |
| ** Without stress - 1 point
| |
| * Congenital deafness - 0.5 points
| |
| * Family history (the same family member cannot be counted for LQTS and sudden death)
| |
| ** Other family members with definite LQTS - 1 point
| |
| ** Sudden death in immediate family (members before the age 30) - 0.5 points
| |
|
| |
|
| ==Treatment options== | | ===Surgery=== |
| There are two treatment options in individuals with LQTS: arrhythmia prevention, and arrhythmia termination.
| |
|
| |
|
| ===Arrhythmia prevention=== | | ===Primary Prevention=== |
| Arrhythmia suppression involves the use of medications or surgical procedures that attack the underlying cause of the arrhythmias associated with LQTS. Since the cause of arrhythmias in LQTS is after depolarizations, and these after depolarizations are increased in states of adrenergic stimulation, steps can be taken to blunt adrenergic stimulation in these individuals. These include:
| |
| * Administration of [[beta blocker|beta receptor blocking agents]] which decreases the risk of stress induced arrhythmias. Beta blockers are the first choice in treating Long QT syndrome.
| |
|
| |
|
| In 2004 it has been shown that genotype and QT interval duration are independent predictors of recurrence of life-threatening events during beta-blockers therapy. Specifically the presence of QTc >500ms and LQT2 and LQT3 genotype are associated with the highest incidence of recurrence. In these patients primary prevention with ICD (Implantable Cardioverster Defibrilator) implantaion can be considered.<ref>Priori SG, Napolitano C, Schwartz PJ, Grillo M, Bloise R, Ronchetti E, Moncalvo C, Tulipani C, Veia A, Bottelli G, Nastoli J. Association of long QT syndrome loci and cardiac events among patients treated
| | ===Secondary Prevention=== |
| with beta-blockers. JAMA. 2004 Sep 15;292(11):1341-4. [http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=pubmed&cmd=Retrieve&dopt=AbstractPlus&list_uids=15367556&query_hl=26&itool=pubmed_docsum PMID: 15367556]</ref>
| |
|
| |
|
| * Potassium supplementation. If the potassium content in the blood rises, the action potential shortens and due to this reason it is believed that increasing potassium concentration could minimize the occurrence of arrhythmias. It should work best in LQT2 since the HERG channel is especially sensible to potassium concentration, but the use is experimental and not evidence based.
| | ====Contraindicated medications==== |
| * [[Mexiletine]]. A sodium channel blocker. In LQT3 the problem is that the sodium channel does not close properly. Mexiletine closes these channels and is believed to be usable when other therapies fail. It should be especially effective in LQT3 but there is no evidence based documentation.
| | {{MedCondContrAbs|MedCond = Long QT syndrome|Dofetilide}} |
| * Amputation of the [[cervical sympathetic chain]] (left [[Stellate ganglion|stellectomy]]). This may be used as an add-on therapy to beta blockers but modern therapy mostly favors ICD implantation if beta blocker therapy fails.
| |
|
| |
|
| ===Arrhythmia termination=== | | ==Case Studies== |
| Arrhythmia termination involves stopping a life-threatening arrhythmia once it has already occurred. The only effective form of arrhythmia termination in individuals with LQTS is placement of an [[implantable cardioverter-defibrillator]] (ICD). ICD are commonly used in patients with syncopes despite beta blocker therapy, and in patients who have experienced a cardiac arrest.
| | :[[Long QT Syndrome case study one|Case #1]] |
|
| |
|
| With better knowledge of the genetics underlying the long QT syndrome, more precise treatments will be readily available.<ref>Compton SJ, Lux RL, Ramsey MR, Strelich KR, Sanguinetti MC, Green LS, Keating MT, Mason JW. Genetically defined therapy of inherited long-QT syndrome. Correction of abnormal repolarization by potassium. Circulation. 1996 Sep 1;94(5):1018-22. PMID 8790040</ref>
| | ==Related Chapters== |
| | |
| == Risk stratification == | |
| | |
| The risk for untreated LQTS patients having events (syncopes or cardiac arrest) can be predicted from their genotype (LQT1-8), gender and corrected QT interval.<ref>Risk Stratification in the Long-QT Syndrome: N Engl J Med 2003; 349:908-909, Aug 28, 2003. PMID 12944579.</ref>
| |
| | |
| * High risk (>50%)
| |
| | |
| QTc>500 msec LQT1 & LQT2 & LQT3(males)
| |
| | |
| * Intermediate risk (30-50%)
| |
| | |
| QTc>500 msec LQT3(females)
| |
| | |
| QTc<500 msec LQT2(females)& LQT3
| |
| | |
| * Low risk (<30%)
| |
|
| |
| QTc<500 msec LQT1 & LQT2 (males)
| |
| | |
| ==References==
| |
| <references/>
| |
| | |
| ==See also==
| |
| * [[Cardiac action potential]] | | * [[Cardiac action potential]] |
| * [[Short QT syndrome]] | | * [[Short QT syndrome]] |
|
| |
| ==External links==
| |
|
| |
| * [http://videos.med.wisc.edu/videoInfo.php?videoid=266 VIDEO - Mechanistic Insight into a Rare Cardiovascular Disease: Congenital Long QT Syndrome 2007]
| |
| * {{DMOZ|Health/Conditions_and_Diseases/Cardiovascular_Disorders/Heart_Disease/Arrhythmia/Long_QT_Syndrome/}}
| |
|
| |
|
| {{Electrocardiography}} | | {{Electrocardiography}} |
| {{Circulatory system pathology}} | | {{Circulatory system pathology}} |
| {{SIB}}
| |
|
| |
| [[de:QT-Syndrom]]
| |
| [[es:Síndrome del QT largo]]
| |
| [[he:תסמונת Long QT]]
| |
| [[nl:Lange-QT-syndroom]]
| |
| [[zh:長QT症]]
| |
| [[ja:QT延長症候群]]
| |
| [[tr:Uzamış QT sendromu]]
| |
|
| |
|
|
| |
|
| | [[Category:Best pages]] |
| [[Category:Cardiology]] | | [[Category:Cardiology]] |
| [[Category:Electrophysiology]] | | [[Category:Electrophysiology]] |