Rabies
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1], Michael Maddaleni, B.S.{{#meta: itemprop="medicalWebPageAudiences" content="patient"}}{{#meta: itemprop="medicalWebPageSpecialities" content="cardiology"}}{{#meta: itemprop="medicalWebPageInfoTypes" content="symptoms,diagnosis,treatment,causes,prognosis,complications"}} Classification Classic::Classification Atypical::
Overview
|
Rabies Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Rabies On the Web |
|
American Roentgen Ray Society Images of Rabies |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [2] Associate Editor(s)-in-Chief: Mahshid Mir, M.D. [3]
Overview
Rabies is a viral zoonotic disease that causes acute encephalitis (inflammation of the brain) in mammals. In non-vaccinated humans, rabies is almost invariably fatal after neurological symptoms have developed, but prompt post-exposure vaccination may prevent the virus from progressing. The rabies virus is categorized as a Lyssavirus. The molecular biology of rabies consists of bullet shaped virus with helical symmetry that has a length of approximately 180 nm. Rabies typically has its greatest effect on the brain. Rabies is typically defined by encephalitis and myelitis. Various carnivorous animal species have been identified as the source of rabies virus (RV). In Africa and Asia, domestic dogs are the main reservoirs of rabies virus infection. Whereas, in the United States, racoons, foxes, skunks, coyotes, possums and bats are understood to be responsible for the spread of rabies virus. RV infects neurons and leads to the degeneration of the neuronal processes by disrupting cytoskeletal integrity. The differential diagnosis for rabies deals with eliminating diseases with similar symptoms from the diagnosis. There are many viruses that can appear similar to rabies such as encephalitis and the herpes simplex virus. According to the World Health Organization (WHO), human rabies is present in 150 countries and territories and on all continents, except for Antarctica. India has been known to have the highest incidence of rabies. A bite from an infected animal is the biggest risk factor. Rabies is a clinical diagnosis that includes a thorough medical history and a high degree of suspicion. Patients are asymptomatic during the incubation period. Prodromal symptoms may include low-grade fever, chills, malaise, myalgias, weakness, fatigue, anorexia, sore throat, nausea, vomiting and headache. Clinical rabies can present as encephalitic ("furious") rabies or paralytic ("dumb") rabies. Rabies eventually results in progressive encephalopathy, respiratory arrest, coma and death within 10 days of the onset of symptoms. Common physical examination findings of rabies include hyperpyrexia alternating with hypothermia, tachycardia, respiratory collapse, hypersalivation, lacrimation, sweating, dilatation of the pupils and bradycardia. Skin findings may include percussion myoedema, bite marks and bruises. Lumbar puncture may show lymphocytic pleocytosis, CSF protein-elevation and normal glucose concentration. Patients with suspected exposure to rabies or asymptomatic patients can benefit from thorough wound cleaning followed by a combined rabies vaccination and immune globulin administration, these patients have a good prognosis. Human diploid cell rabies vaccines are made using the attenuated Pitman-Moore L503 strain of the virus. Human diploid cell rabies vaccines have been given to more than 1.5 million humans as of 2006. Treatment after exposure, known as post-exposure prophylaxis or "P.E.P.", is highly successful in preventing the disease if administered promptly, within fourteen days after infection. The first step is immediately washing the wound with soap and water, which is very effective at reducing the number of viral particles. In the United States, patients receive one dose of immunoglobulin and five doses of rabies vaccine over a twenty-eight day period. One-half the dose of immunoglobulin is injected in the region of the bite, if possible, with the remainder injected intramuscularly away from the bite.
Historical Perspective
Rabies is a disease that has been classically associated with infected animals, mainly dogs.The first written record of rabies is in the Codex of Eshnunna (ca. 1930 BC - written prior to the Code of Hammurabi), which demonstrates that the owner of a dog showing symptoms of rabies should take preventive measure against bites. Although dogs are viewed as the main culprit of rabies, rabies is also associated with animals such as possums, skunks, and more importantly, bats. In 1885, 9-year-old boy named Joseph Meister was the first person to have received an effective shot for rabies after being bitten by a rabid dog. Louis Pasteur treated the first case of rabies by a weak form of virus (which later became the basis of active immunization for rabies). In the 1950s, people who had been bitten by a rabid animal got 23 shots along the abdomen. Today, the shots are more effective and less painful. They consist of a series of 6 shots given in the arm over a 1 month period. One shot is given around the bite and the rest are given in the arm.
Pathophysiology
The rabies virus is categorized as a Lyssavirus. The molecular biology of rabies consists of bullet shaped virus with helical symmetry that has a length of approximately 180 nm. Rabies typically has its greatest effect on the brain. Rabies is typically defined by encephalitis and myelitis. Various carnivorous animal species have been identified as the source of rabies virus (RV). In Africa and Asia, domestic dogs are the main reservoirs of rabies virus infection. Whereas, in the United States, racoons, foxes, skunks, coyotes, possums and bats are understood to be responsible for the spread of rabies virus.The neuromuscular junction is the major site of entry into neurons. RV infects peripheral nervesand then reaches the central nervous system (CNS) via retrograde axonal transport. The primary mechanism involved in the neuroinvasion of RV is trans-synaptic neuronalspread. RV infects neurons and leads to the degeneration of the neuronal processes by disrupting cytoskeletal integrity. Histopathologic evidence of rabies encephalomyelitis(inflammation) in brain tissue and meninges includes, mononuclear infiltration, perivascular cuffing of lymphocytes or polymorphonuclear cells, lymphocytic foci, Babes nodules consisting of glial cells and Negri bodies.
Causes
The Rabies virus is the cause of rabies.
Differentiating Rabies from other Diseases
The differential diagnosis for rabies deals with eliminating diseases with similar symptoms from the diagnosis. There are many viruses that can appear similar to rabies such as encephalitis and the herpes simplex virus. It is very important to rule out certain diseases such as echovirus and poliovirus. Rabies is a serious disease that needs to be treated quickly if someone is suspected to be infected with the virus.
Epidemiology and Demographics
According to the World Health Organization (WHO), human rabies is present in 150 countries and territories and on all continents, except for Antarctica. India has been known to have the highest incidence of rabies. Twenty-three cases of human rabies have been reported in the United States in the past decade (2008-2017). Many territories, such as the United Kingdom, Ireland, Taiwan, Japan, Hawaii, Mauritius, Barbados and Guam, are free of rabies. Worldwide, 55,000 human deaths occur annually from rabies, with 56 % of deaths estimated to occur in Asia and 44 % in Africa.
Risk Factors
A bite from an infected animal is the biggest risk factor. People that live in an area, or travel to an area that has a large incidence for rabies, are at a high risk for acquiring rabies from a rabid animal. Handling certain wild animals such as bats or raccoons will put a person at a higher risk.
Screening
There is insufficient evidence to recommend routine screening for rabies. Enzyme immunoassay (EIA) is currently under investigation as a convenient test for screening hybridoma supernatants because nanogram amounts of antibody can be detected and up to 100 samples can be tested at the same time.
Natural history, complications and prognosis
If left untreated, rabies runs its course very rapidly. Once symptoms begin to appear, the disease is almost always fatal. The acute period of disease typically ends after 2 to 10 days. Common complications of rabies include, psychosis, seizures, aphasia, muscular twitching, delirium and death. Treatment after exposure (receiving the vaccines), known as post-exposure prophylaxis (PEP), is highly successful in preventing the disease if administered promptly, in general within ten days of infection.
Diagnosis
Diagnostic criteria
Rabies is a clinical diagnosis that includes a thorough medical history and a high degree of suspicion. Laboratory findings that help with the diagnosis of rabies include skin biopsy specimens showing virus-specific immunofluorescent staining, isolation of virus from the samples of saliva and detection of anti-rabies antibodies in serum or cerebrospinal fluid (CSF).
History and symptoms
The symptoms of rabies depend upon the stage of the disease at the time of presentation. Rabies may present during incubation period, prodromal period, acute neurologic period (clinical rabies), or coma. Patients are asymptomatic during the incubation period. Prodromal symptoms may include low-grade fever, chills, malaise, myalgias, weakness, fatigue, anorexia, sore throat, nausea, vomiting and headache. Clinical rabies can present as encephalitic ("furious") rabies or paralytic ("dumb") rabies. Encephalitic rabies is more common and presents as hydrophobia, aerophobia, facial grimace, opisthotonos, autonomic instability, dysarthria, dysphagia, and diplopia. Rabies eventually results in progressive encephalopathy, respiratory arrest, coma and death within 10 days of the onset of symptoms.
Physical examination
Common physical examination findings of rabies include hyperpyrexia alternating with hypothermia, tachycardia, respiratory collapse, hypersalivation, lacrimation, sweating, dilatation of the pupils and bradycardia. Skin findings may include percussion myoedema, bite marks and bruises.
Laboratory findings
Routine lab findings are non-specific and may include peripheral leukocytosis, respiratory alkalosis followed by respiratory acidosis, albuminuria and pyuria. Lumbar puncturemay show lymphocytic pleocytosis, CSF protein-elevation and normal glucose concentration. Serology is useful in assessing the serostatus in immunized humans and animals as it is difficult to document the neutralizing antibody response via immunofluorescence because mostly death occurs prior to mounting a response. Serologic tests include reverse transcriptase polymerase chain reaction (RT-PCR), viral culture, immunofluorescence staining, indirect immunofluorescence, direct fluorescent antibody test (dFA) and virus neutralization assays.
Electrocardiogram
EKG findings may show supraventricular arrhythmias, atrioventricular block, sinus bradycardia, sinus arrest with non-specific ST segment and T-wave changes.
Chest X-ray
CXR may show infiltrates secondary to aspiration, pneumonia, acute respiratory distress syndrome and congestive heart failure.
CT
CT is usually normal. Late findings may include cerebral edema and decreased attenuation in the hippocampus, brain stem, basal ganglia, and periventricular white matter.
MRI
Findings on MRI suggestive of rabies include areas of increased T2 intensity (flare) may be seen in hippocampus, hypothalamus, and brainstem.
Treatment
Medical therapy
Two presentations must be considered in the treatment of rabies. Symptomatic patients with delayed presentation in the emergency department, associated with a low survival rate and treated with "Milwaukee protocol" (which is still being studied) and patients with a suspected exposure to rabies virus or early diagnosed asymptomatic rabies patients. Patients with suspected exposure to rabies or asymptomatic patients can benefit from thorough wound cleaning followed by a combined rabies vaccination and immune globulin administration, these patients have a good prognosis.
Surgery
Immediate gentle irrigation with water or a dilute water povidone-iodine solution has been shown to markedly decrease the risk of bacterial infection. Wound cleansing is especially important in rabies prevention.
Primary prevention
There is no known cure for symptomatic rabies, but it can be prevented by vaccination, both in humans and other animals. Virtually every infection with rabies was a death sentence, until Louis Pasteur and Emile Roux developed the first rabies vaccination in 1885. This vaccine was first used on a human on July 6, 1885 – nine-year old boy Joseph Meister (1876–1940) had been mauled by a rabid dog.Their vaccine consisted of a sample of the virus harvested from infected (and necessarily dead) rabbits, which was weakened by allowing it to dry. Similar nerve tissue-derived vaccines are still used now in some countries, and while they are much cheaper than modern cell culture vaccines, they are not as effective and carry a certain risk of neurological complications.The human diploid cell rabies vaccine (H.D.C.V.) was started in 1967. Human diploid cell rabies vaccines are made using the attenuated Pitman-Moore L503 strain of the virus. Human diploid cell rabies vaccines have been given to more than 1.5 million humans as of 2006. Newer and less expensive purified chicken embryo cell vaccine, and purified Vero cell rabies vaccine are now available. The purified Vero cell rabies vaccine uses the attenuated Wistar strain of the rabies virus, and uses the Vero cell line as its host.
Secondary prevention
Treatment after exposure, known as post-exposure prophylaxis or "P.E.P.", is highly successful in preventing the disease if administered promptly, within fourteen days after infection. The first step is immediately washing the wound with soap and water, which is very effective at reducing the number of viral particles. In the United States, patients receive one dose of immunoglobulin and five doses of rabies vaccine over a twenty-eight day period. One-half the dose of immunoglobulin is injected in the region of the bite, if possible, with the remainder injected intramuscularly away from the bite. Pre-exposure vaccination with human diploid cell Rabies vaccine (HDCV), or purified chick embryo cell (PCEC) vaccine, may be recommended for international travelers based on the local incidence of rabies in the country to be visited, the availability of appropriate antirabies biologicals, and the intended activity and duration of stay of the traveler.
References
Historical Perspective
|
Rabies Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Rabies On the Web |
|
American Roentgen Ray Society Images of Rabies |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [4]; Associate Editor(s)-in-Chief: Syed Hassan A. Kazmi BSc, MD [5] Mahshid Mir, M.D. [6]
Overview
Rabies is a disease that has been classically associated with infected animals, mainly dogs.The first written record of rabies is in the Codex of Eshnunna (ca. 1930 BC - written prior to the Code of Hammurabi), which demonstrates that the owner of a dog showing symptoms of rabies should take preventive measure against bites. Although dogs are viewed as the main culprit of rabies, rabies is also associated with animals such as possums, skunks, and more importantly, bats. In 1885, 9-year-old boy named Joseph Meister was the first person to have received an effective shot for rabies after being bitten by a rabid dog. Louis Pasteur treated the first case of rabies by a weak form of virus (which later became the basis of active immunization for rabies). In the 1950s, people who had been bitten by a rabid animal got 23 shots along the abdomen. Today, the shots are more effective and less painful. They consist of a series of 6 shots given in the arm over a 1 month period. One shot is given around the bite and the rest are given in the arm.
Historical Perspective
- The history of rabies dates back to before the invention of microscopic techniques, although the virus itself was not seen under the electron microscope until the 1960s.
- Rabies in animals was reported in early Babylonian, Greek, and Roman records.
- Rabies was likely brought to the Americas when settlers first came from Europe, bringing rabid animals with them.
- In 1885, 9-year-old boy named Joseph Meister was the first person to have received an effective shot for rabies after being bitten by a rabid dog. Louis Pasteur treated the first case of rabies by a weak form of virus (which later became the basis of active immunization for rabies).
- In the 1950s, people who had been bitten by a rabid animal got 23 shots along the abdomen. Today, the shots are more effective and less painful. They consist of a series of 6 shots given in the arm over a 1 month period. One shot is given around the bite and the rest are given in the arm.
Rabies and animal quarantine
- Although rabies has been known to be endemic in many countries, international transport of animals from one region to the other has been discouraged in order to prevent spread of the disease as a quarantine measure.
- Most developed countries, pioneered by Sweden, now allow restriction-free travel between their territories for pet animals that have demonstrated an adequate immune response to rabies vaccination. Such countries may limit movement to animals from countries where rabies is considered to be under control in pet animals. There are various lists of such countries. The United Kingdom has developed a list, and France has a rather different list, said to be based on a list of the Office International des Epizooties (OIE).
Rabies and dogs
- The association of rabies with dogs is as old as the disease itself.
- The first written record of rabies is in the Codex of Eshnunna (ca. 1930 BC - written prior to the Code of Hammurabi), which demonstrates that the owner of a dog showing symptoms of rabies should take preventive measure against bites. If a person is bitten by a rabid dog and later died, the owner was fined heavily.[1]
Rabies and possums
- The Virginia possum has been known to be a reservoir of rabies, as a result of transfer from other wildlife species such as bats, skunks and the raccoon.
- Cases have been reported all across the United States.[2][3][4]
- In New York state, the Wadsworth Center lists laboratory confirmed cases in possums 5 years out of 10 from 1989 to 1998.
Rabies and bats
- In early 1931, Dr. H. Metivier, a veterinary surgeon, identified bats as the cause of paralytic rabies.
- In September 1931, Dr. J. L. Pawan, a government bacteriologist discovered Negri bodies within the brains of bats.
- In 1934, the Trinidad and Tobago governments began a program of vampire bat control, shooting, netting, trapping and poisoning and vaccination for exposed livestock.
- In 1953, basic research on bats and rabies progressed rapidly under the able direction of Arthur Greenhall, who demonstrated that at least 8 species of bats in Trinidad had been infected with rabies - particularly the Common Vampire Bat, Desmodus rotundus, the rare White-winged Vampire Bat, Diaemus youngi, as well as two abundant species of Fruit Bats: the Seba's Short-tailed Bat or Short-tailed Fruit Bat, Carollia perspicillata, which commonly roosts with Vampires, and the Jamaican Fruit Bat, Artibeus jamaicensis.[5]
- In 1960, Constantine in Frio Cave, Texas, demonstrated non-bite transmission of rabies.[6][7][8]
- In 1996, a single Daubenton's bat was found to be infected with a rabies-like virus usually found only in bats – European Bat Lyssavirus 2 (EBL2) in the United Kingdom, which was thought to be entirely rabies-free before this occurrence. A similar occurrence was reported in September 2002.
- In November 2002 David McRae, a Scottish bat conservationist from Guthrie, became the first human to contract rabies in the United Kingdom since 1902. He died from the disease on November 24 2002.
- In November 2004, Jeanna Giese, a fifteen-year old girl from Fond du Lac, Wisconsin, became the first survivor of rabies, without any vaccine administration.
- On May 12, 2006 Harris County Texas. U.S.A. Health Department officials reported that a teenage boy, Zachary Jones of Humble, Texas, had died of rabies at Texas Children's Hospital in Houston, Texas.
- On November 2, 2006 a 10 year old girl who had been bitten by a bat in Bourbon, Indiana, U.S.A. died of rabies.
- In August of 2006, a 73 year old rural resident located east of Edmonton, Alberta, Canada was bitten by a bat while he slept.
- On August 6, 2006, 950 Girl Scouts were urged to receive rabies shots by the Girl Scouts of America. The nine hundred and fifty girls had attended a camp in Virginia, U.S.A. in July, and had reported seeing bats in their cabins. Even though infections were relatively unlikely, the G.S.A. offered to pay for the shots, at a cost of nearly two million dollars. The Centers for Disease Control reports 27 cases of human rabies caused by the bat variant rabies virus in the United States from 1990 to 2002.[9]
Rabies and Organ Transplantion
- In June 2004, 3 organ transplant recipients were reported dead after transplantion of kidneys and liver from an infected donor from Texarkana.[10] Marijuana and cocaine were found in the donor's urine at the time of his death, according to a report in The New England Journal of Medicine.[11]
"[The surgeons] thought he had suffered a fatal crack-cocaine overdose, which can produce symptoms similar to those of rabies. 'We had an explanation for his condition,' says Dr. Goran Klintmalm, a surgeon who oversees transplantation at Baylor University Medical Center, where the transplants occurred. 'He'd recently smoked crack cocaine. He'd hemorrhaged around the brain. He'd died. That was all we needed to know'. Because of doctor-patient confidentiality rules, doctors involved with this case would not talk about it on the record, but a few did say that if no cocaine was found in the donor's blood, the E.R. doctors might have investigated his symptoms more aggressively instead of assuming he had overdosed. (Because no autopsy was done, doctors have not been able to establish whether the rabies or the drugs actually killed him)".[12]
- In February 2005, three German patients in Mainz and Heidelberg were diagnosed with rabies after receiving various organs and cornea transplants from a female donor.
Rabies Vaccine
- In 1885, Louis Pasteur and Emile Roux developed the first rabies vaccination .This vaccine was first used on a human on July 6, 1885 – nine-year old boy Joseph Meister (1876–1940) had been bitten by a rabid dog.[7] [8]
- The vaccine consisted of attenuated virus harvested from infected (and necessarily dead) rabbits.
- Similar nerve tissue-derived vaccines are still used now in some countries, and while they are much cheaper than modern cell culture vaccines, they are not as effective and carry a certain risk of neurological complications.
- In 1967, the human diploid cell rabies vaccine (H.D.C.V.) was started. The purified Vero cell rabies vaccine uses the attenuated Wistar strain of the rabies virus, and uses the Vero cell line as its host.
Famous Cases
The most famous case of rabies disease that lead to survival for the first time in the history was the case of a girl called Jeanna Giese. The treatment that saved Jeanna Giese, a Wisconsin resident, was an innovated and experimental technique. The basics behind the treatment, which would later be called the Milwaukee protocol, were determined by Dr. Rodney Willoughby, an infectious disease specialist. The theory behind the treatment was to basically shut down her brain by medically inducing a coma. This would serve the purpose of giving her own immune system time to build up antibodies against the virus.[13] In other words, doctors thought it would be possible for Jeanna Giese to survive if they suppressed her brain activity, while allowing enough time for her immune system to attack the rabies.[13]
Although radical, the Milwaukee protocol was most likely the only way for Giese to have a chance for survival. It was also the first time that this method had been used.[13]
Inducing a coma was not the only task doctors performed. They also gave her many antiviral drugs such as ribavarin and amantadine. After approximately a week, tests showed that Giese's immune system was fighting the disease, so they began to cut back on the anesthetics. Doctors also gave Giese supplements for about 6 months after initial treatment. They gave her tetrahyrdobiopterin, which is similar to folic acid. This may have been involved in improving Giese's speech, motor, and other bodily functions.[13]
In June 2011, another child survived rabies infection without receiving the vaccine before appearance of symptoms. Precious Reynolds, an eight-year-old girl from Willow Creek, California, contracted the disease in April 2011 but did not receive medical care until mid-May, after her grandmother took her to the doctor because of influenza-like symptoms that grew so serious, her grandmother said they resembled polio. The hospital said doctors followed the protocol established for Giese. Reynolds was placed in a drug-induced coma and received antiviral medications. She survived after spending two weeks in intensive care undergoing the treatments.[14][15]
References
- ↑ Dunlop, Robert H. (1996). Veterinary Medicine:An Illustrated History. Mosby. ISBN 0-8016-3209-9. Unknown parameter
|coauthors=ignored (help) - ↑ Krebs JW, Smith JS, Rupprecht CE, Childs JE.Rabies surveillance in the United States during 1996. J Am Vet Med Assoc. (1997) 211(12):1525-39. Review. Erratum in: J Am Vet Med Assoc. (1998) 212(8):1280. PMID: 9412679
- ↑ Krebs JW, Smith JS, Rupprecht CE, Childs JE.(1999) Rabies surveillance in the United States during 1998. J Am Vet Med Assoc. (1999) 215(12):1786-98. Erratum in: J Am Vet Med Assoc 2000 216(8):1223
- ↑ Krebs JW, Smith JS, Rupprecht CE, Childs JE.Rabies surveillance in the United States during 1996. J Am Vet Med Assoc. (1997) 211(12):1525-39. Review. Erratum in: J Am Vet Med Assoc. (1998) 212(8):1280. PMID: 9412679
- ↑ Greenhall, Arthur M. 1961. Bats in Agriculture. Ministry of Agriculture, Trinidad and Tobago.
- ↑ Constantine, D. G. 1962. "Rabies transmission by nonbite route." Public Health Reports 77, pp. 287–289.
- ↑ Winkler, W. G. 1968. "Airborne Rabies Virus Isolation." Bull. Wildlife Disease Assoc. Vol. 4, April, 1968, pp. 37-40. Available online at: http://www.jwildlifedis.org/cgi/reprint/4/2/37
- ↑ Messenger, Sharon L., Jean S. Smith, and Charles E. Rupprecht. 2002. "Emerging Epidemiology of Bat-Associated Cryptic Cases of Rabies in Humans in the United States." Clinical Infectious Diseases. 2002; 35, pp. 738–747. Available on line at: journals.uchicago.edu
- ↑ "Rabies Surveillance". Centers for Disease Control. 2003. Retrieved 2006-11-10.
- ↑ "Investigation of rabies infections in organ donor and transplant recipients--Alabama, Arkansas, Oklahoma, and Texas, 2004". MMWR Morb Mortal Wkly Rep. 53 (26): 586–9. 2004. PMID 15241303.
- ↑ Srinivasan A, Burton EC, Kuehnert MJ, Rupprecht C, Sutker WL, Ksiazek TG, Paddock CD, Guarner J, Shieh WJ, Goldsmith C, Hanlon CA, Zoretic J, Fischbach B, Niezgoda M, El-Feky WH, Orciari L, Sanchez EQ, Likos A, Klintmalm GB, Cardo D, LeDuc J, Chamberland ME, Jernigan DB, Zaki SR (2005). "Transmission of rabies virus from an organ donor to four transplant recipients". N Engl J Med. 352 (11): 1103–11. PMID 15784663.
- ↑ Reynolds G (2005). "Will Any Organ Do?". The New York Times Magazine (10 July): &ndash, .
- ↑ 13.0 13.1 13.2 13.3 "Medical Mystery: Only One Person Has Survived Rabies without Vaccine--But How?: Scientific American". Retrieved 2012-02-10.
- ↑ "UC Davis Children's Hospital patient becomes third person in U.S. to survive rabies". UC Davis Medical Center. Retrieved 3 May 2012.
- ↑ "Human Rabies --- Indiana and California, 2006".
Pathophysiology
|
Rabies Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Rabies On the Web |
|
American Roentgen Ray Society Images of Rabies |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [9]; Associate Editor(s)-in-Chief: Syed Hassan A. Kazmi BSc, MD [10]
Overview
The rabies virus is categorized as a Lyssavirus. The molecular biology of rabies consists of bullet shaped virus with helical symmetry that has a length of approximately 180 nm. Rabies typically has its greatest effect on the brain. Rabies is typically defined by encephalitis and myelitis. Various carnivorous animal species have been identified as the source of rabies virus (RV). In Africa and Asia, domestic dogs are the main reservoirs of rabies virus infection. Whereas, in the United States, racoons, foxes, skunks, coyotes, possums and bats are understood to be responsible for the spread of rabies virus.The neuromuscular junction is the major site of entry into neurons. RV infects peripheral nerves and then reaches the central nervous system (CNS) via retrograde axonal transport. The primary mechanism involved in the neuroinvasion of RV is trans-synaptic neuronal spread. RV infects neurons and leads to the degeneration of the neuronal processes by disrupting cytoskeletal integrity. Histopathologic evidence of rabies encephalomyelitis (inflammation) in brain tissue and meninges includes, mononuclear infiltration, perivascular cuffing of lymphocytes or polymorphonuclear cells, lymphocytic foci, Babes nodules consisting of glial cells and Negri bodies.
Pathophysiology
The organism causing rabies is called rabies virus (RV), a negative-stranded RNA virus of the rhabdovirus family. Rabies is an acute encephalomyelitis that causes disease in the human host via two features associated with the rabies virus (RV):[1][2]
- Neurotropism
- Neuroinvasiveness
Transmission
Common route of tranmission
- Various carnivorous animal species have been identified as the source of rabies virus (RV)[3][4]
- In Africa and Asia, domestic dogs are the main reservoirs of infection from rabies virus[5]
- In the United States, racoons, foxes, skunks, coyotes, possums and bats rather than dogs spread the infection through bites[6][7][8][9]
- Three stages of rabies have been known to occurr in dogs. The first stage is a one to three day period characterized by behavioral changes and is known as the prodromal stage. The second stage is the excitative stage, which lasts three to four days. It is this stage that is often known as furious rabies due to the tendency of the affected dog to be hyper-reactive to external stimuli and bite at anything near. The third stage is the paralytic stage and is caused by damage to motor neurons. Incoordination is seen due to rear limb paralysis and drooling and difficulty swallowing is caused by paralysis of facial and throat muscles. Death is usually caused by respiratory arrest.[10]
- Transmission of the rabies virus starts when a human is bit by an animal harboring the virus in its salivary glands[11]
- The RV remains cell-free after initial inoculation so, rigorous wound cleaning may reduce the chances of infection[12]
- RV infects peripheral nerves and then reaches the central nervous system (CNS) via retrograde axonal transport
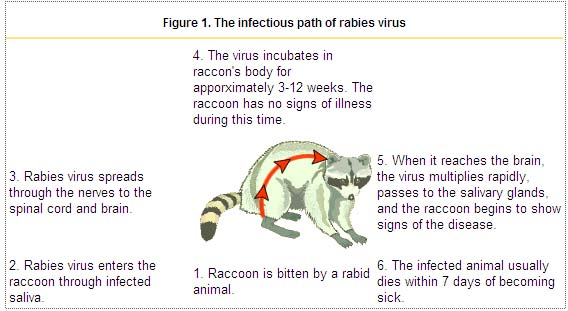
- Less common routes of transmission of rabies virus include:[13][14][15]
- Contamination of mucous membranes (i.e., eyes, nose, mouth)
- Aerosol transmission
- Corneal and other organ transplantation from infected donor
Virology
The rabies virus (RV) belongs to the genus Lyssavirus. This genus of RNA viruses also includes the Aravan virus, Australian bat lyssavirus, Duvenhage virus, European bat lyssavirus 1, European bat lyssavirus 2, Irkut virus, Khujand virus, Lagos bat virus, Mokola virus and West Caucasian bat virus. Lyssaviruses have helical symmetry, so their infectious particles are approximately cylindrical in shape.
- The virus has a bullet-like shape with a length of about 180 nm and a cross-sectional diameter of about 75 nm[16][17]
- One end is rounded or conical and the other end is planar or concave[18]
- The lipoprotein envelope carries knob-like spikes composed of Glycoprotein G. Spikes do not cover the planar end of the virion (virus particle)[19]
- Beneath the envelope is the membrane or matrix (M) protein layer which may be invaginated at the planar end. The core of the virion consists of helically arranged ribonucleoprotein[20]
- The genome is unsegmented linear antisense RNA. Also present in the nucleocapsid are RNA dependent RNA transcriptase and some structural proteins
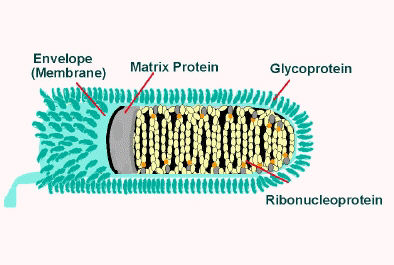
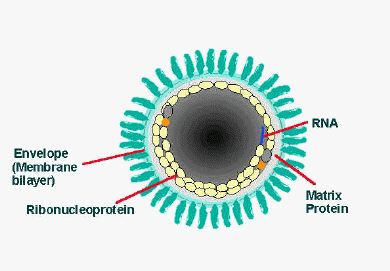
Longitudinal and cross-sectional schematic view of rabies virus
Pathogenesis
Incubation period and eclipse phase
- The incubation period may vary from a few days to several years, but is typically 1 to 3 months[21][22]
- After gaining entry into human host, the RV enters into an eclipse phase, during which, the host immune defenses may confer cell-mediated immunity against viral infection because RV is a good antigen[23][24]
Neuromuscular junction invasion
- The neuromuscular junction is the major site of entry into neurons[25]
- The RV uses the acetylcholine receptors and other receptors such as the neutral cell adhesion molecule (NCAM) to gain entry into the neuron via endocytosis[26]
- Fusion of the viral membrane with endosomal membranes liberates the viral nucleocapsid into the cytosol, where transcription and replication occur[27]
Inter-neuronal spread
- The main mechanism involved in the neuroinvasion of RV is trans-synaptic neuronal spread
- The following proteins lead to the spread of virus between the neurons, once the virus gains entry into the body:[28][29]
- Rabies virus G protein (glycoprotein): RV to spread from the post-synaptic site to the pre-synaptic site
- Rabies virus P protein (a cofactor for RNA polymerase): important determinant of retrograde transport of the virus within axons
CNS invasion
- Trans-synaptic neuronal spread leads to spread of infection to the CNS from the peripheral nerves
- RV forms cytoplasmic inclusion bodies called Negri bodies in the neurons, which are composed of the viral N and P proteins (all viral RNAs genome, antigenome, and every mRNA have been known to be found inside the inclusion bodies- suggesting that they play a role in viral replication and life cycle)[30]
- RV infects neurons and leads to degeneration of the neuronal processes by disrupting cytoskeletal integrity[31]
- The hypothalamus is understood to be affected most severely by RV infection[32]
Microscopic Pathology
Histologic examination of biopsy or autopsy tissues is occasionally useful in diagnosing unsuspected cases of rabies that have not been tested by routine methods. When brain tissue from rabies virus-infected animals are stained with a histologic stain, such as hematoxylin and eosin, evidence of encephalomyelitis may be recognized. Histopathologic evidence of rabies encephalomyelitis (inflammation) in brain tissue and meninges includes the following:[33][34][35][30]
- Mononuclear infiltration
- Perivascular cuffing of lymphocytes or polymorphonuclear cells
- Lymphocytic foci
- Babes nodules consisting of glial cells
- Negri bodies
{{#ev:youtube|NP5CYphae5Y}}
References
- ↑ Mrak RE, Young L (1994). "Rabies encephalitis in humans: pathology, pathogenesis and pathophysiology". J. Neuropathol. Exp. Neurol. 53 (1): 1–10. PMID 8301314.
- ↑ Lafon M (2004). "Subversive neuroinvasive strategy of rabies virus". Arch. Virol. Suppl. (18): 149–59. PMID 15119770.
- ↑ Swanepoel R, Barnard BJ, Meredith CD, Bishop GC, Brückner GK, Foggin CM, Hübschle OJ (1993). "Rabies in southern Africa". Onderstepoort J. Vet. Res. 60 (4): 325–46. PMID 7777317.
- ↑ Bingham J, Foggin CM, Wandeler AI, Hill FW (1999). "The epidemiology of rabies in Zimbabwe. 2. Rabies in jackals (Canis adustus and Canis mesomelas)". Onderstepoort J. Vet. Res. 66 (1): 11–23. PMID 10396757.
- ↑ "www.who.int" (PDF).
- ↑ "CDC - Rabies Surveillance in the U.S.: Wild Animals - Rabies".
- ↑ Constantine DG, Woodall DF. Related Articles, Links Transmission experiments with bat rabies isolates: reactions of certain Carnivora, possum, rodents, and bats to rabies virus of red bat origin when exposed by bat bite or by intrasmuscular inoculation. Am J Vet Res. 1966 Jan;27(116):24-32. No abstract available. PMID: 5913032 [PubMed - indexed for MEDLINE]
- ↑ Constantine DG 1967 Rabies transmission by air in bat caves. US Pub Health Serv, Publ. 1617
- ↑ 1: Am J Vet Res. 1960 May;21:507-10.Links Resistance of the opossum to rabies virus.BEAMER PD, MOHR CO, BARR TR. PMID: 13797881 [PubMed - indexed for MEDLINE]
- ↑ Ettinger, Stephen J.;Feldman, Edward C. (1995). Textbook of Veterinary Internal Medicine (4th ed. ed.). W.B. Saunders Company. ISBN 0-7216-6795-3.
- ↑ The Merck manual of Medical Information. Second Home Edition, (2003), p. 484.
- ↑ "Rabies | Clinical Infectious Diseases | Oxford Academic".
- ↑ "www.microbiologyresearch.org" (PDF).
- ↑ "CDC - Transmission - Rabies".
- ↑ "www.microbiologyresearch.org" (PDF).
- ↑ "Rhabdoviruses: Rabies Virus - Medical Microbiology - NCBI Bookshelf".
- ↑ Iseni F, Barge A, Baudin F, Blondel D, Ruigrok RW (1998). "Characterization of rabies virus nucleocapsids and recombinant nucleocapsid-like structures". J. Gen. Virol. 79 ( Pt 12): 2909–19. doi:10.1099/0022-1317-79-12-2909. PMID 9880004.
- ↑ Hummeler K, Koprowski H, Wiktor TJ (1967). "Structure and development of rabies virus in tissue culture". J. Virol. 1 (1): 152–70. PMC 375516. PMID 4918232.
- ↑ Gaudin Y, Ruigrok RW, Tuffereau C, Knossow M, Flamand A (1992). "Rabies virus glycoprotein is a trimer". Virology. 187 (2): 627–32. PMID 1546457.
- ↑ Mebatsion T, Weiland F, Conzelmann KK (1999). "Matrix protein of rabies virus is responsible for the assembly and budding of bullet-shaped particles and interacts with the transmembrane spike glycoprotein G". J. Virol. 73 (1): 242–50. PMC 103828. PMID 9847327.
- ↑ Charlton KM, Nadin-Davis S, Casey GA, Wandeler AI (1997). "The long incubation period in rabies: delayed progression of infection in muscle at the site of exposure". Acta Neuropathol. 94 (1): 73–7. PMID 9224533.
- ↑ "Rabies | Clinical Infectious Diseases | Oxford Academic".
- ↑ "CDC - Doctors: Transmission - Rabies".
- ↑ Israsena N, Mahavihakanont A, Hemachudha T (2011). "Rabies virus infection and microRNAs". Adv. Virus Res. 79: 329–44. doi:10.1016/B978-0-12-387040-7.00015-9. PMID 21601053.
- ↑ Burrage TG, Tignor GH, Smith AL (1985). "Rabies virus binding at neuromuscular junctions". Virus Res. 2 (3): 273–89. PMID 3890406.
- ↑ Thoulouze MI, Lafage M, Schachner M, Hartmann U, Cremer H, Lafon M (1998). "The neural cell adhesion molecule is a receptor for rabies virus". J. Virol. 72 (9): 7181–90. PMC 109940. PMID 9696812.
- ↑ Finke S, Conzelmann KK (2005). "Replication strategies of rabies virus". Virus Res. 111 (2): 120–31. doi:10.1016/j.virusres.2005.04.004. PMID 15885837.
- ↑ Dietzschold B, Schnell M, Koprowski H (2005). "Pathogenesis of rabies". Curr. Top. Microbiol. Immunol. 292: 45–56. PMID 15981467.
- ↑ "Rabies Virus P Protein Interacts with STAT1 and Inhibits Interferon Signal Transduction Pathways".
- ↑ 30.0 30.1 Lahaye X, Vidy A, Pomier C, Obiang L, Harper F, Gaudin Y, Blondel D (2009). "Functional characterization of Negri bodies (NBs) in rabies virus-infected cells: Evidence that NBs are sites of viral transcription and replication". J. Virol. 83 (16): 7948–58. doi:10.1128/JVI.00554-09. PMC 2715764. PMID 19494013.
- ↑ Li XQ, Sarmento L, Fu ZF (2005). "Degeneration of neuronal processes after infection with pathogenic, but not attenuated, rabies viruses". J. Virol. 79 (15): 10063–8. doi:10.1128/JVI.79.15.10063-10068.2005. PMC 1181611. PMID 16014967.
- ↑ Pleasure SJ, Fischbein NJ (2000). "Correlation of clinical and neuroimaging findings in a case of rabies encephalitis". Arch. Neurol. 57 (12): 1765–9. PMID 11115243.
- ↑ Théodoridès J (1981). "[Histological research on rabies in the 19th century]". Clio Med (in French). 16 (2–3): 83–92. PMID 6176396.
- ↑ Kristensson K, Dastur DK, Manghani DK, Tsiang H, Bentivoglio M (1996). "Rabies: interactions between neurons and viruses. A review of the history of Negri inclusion bodies". Neuropathol. Appl. Neurobiol. 22 (3): 179–87. PMID 8804019.
- ↑ Burton EC, Burns DK, Opatowsky MJ, El-Feky WH, Fischbach B, Melton L, Sanchez E, Randall H, Watkins DL, Chang J, Klintmalm G (2005). "Rabies encephalomyelitis: clinical, neuroradiological, and pathological findings in 4 transplant recipients". Arch. Neurol. 62 (6): 873–82. doi:10.1001/archneur.62.6.873. PMID 15956158.
Epidemiology and Demographics
|
Rabies Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Rabies On the Web |
|
American Roentgen Ray Society Images of Rabies |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [11]; Associate Editor(s)-in-Chief: Syed Hassan A. Kazmi BSc, MD [12]
Overview
According to the World Health Organization (WHO), human rabies is present in 150 countries and territories and on all continents, except for Antarctica. India has been known to have the highest incidence of rabies. Twenty-three cases of human rabies have been reported in the United States in the past decade (2008-2017). Many territories, such as the United Kingdom, Ireland, Taiwan, Japan, Hawaii, Mauritius, Barbados and Guam, are free of rabies. Worldwide, 55,000 human deaths occur annually from rabies, with 56 % of deaths estimated to occur in Asia and 44 % in Africa.
Epidemiology and Demographics
Prevalence
Worldwide
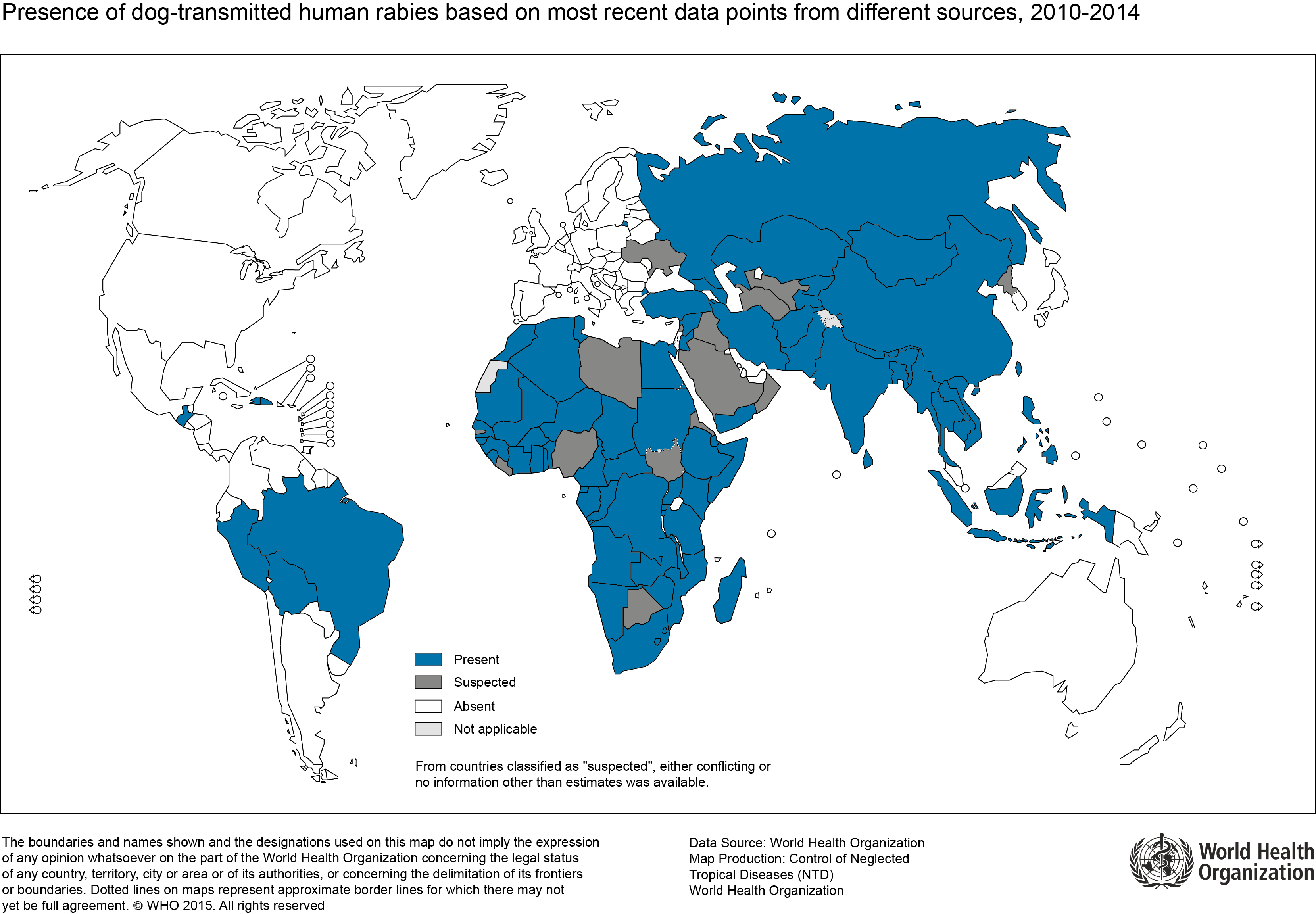
- According to the World Health Organization (WHO), human rabies is present in 150 countries and territories and on all continents, except for Antarctica
- Canine rabies is known to be endemic in parts of Africa, Asia, and Central and South America. Dogs account for over 99 % of all rabies transmission to humans[1][2]
- The following map represents the global estimate of dog-transmitted rabies, based on WHO estimates:
United States
According to CDC, rabies is rare in the United States:[3]
- Human rabies cases in the United States are rare, with only 1 to 3 cases reported annually
- Twenty-three cases of human rabies have been reported in the United States in the past decade (2008-2017). Eight of these were contracted outside of the U.S. and its territories
- In 2001, 49 states, the District of Columbia, and Puerto Rico reported 7,437 cases of rabies in animals and no cases in humans, according to CDC (Hawaii is the only state that has never reported an indigenously acquired rabies case in humans or animals). The total number of reported cases increased by 0.92% from those reported in 2000 (7,369 cases).
Incidence
Worldwide
- A rabies epidemic occurs every 10 years in China[4]
- India is reported to have the highest incidence of rabies[5]
- In Ethopia, the estimated annual incidence in humans is 2.33 cases per 100,000 individuals[6]
- The following table outlines the incidence of rabies in developing countries, according to WHO:[7]
| Country | Number of rabies cases | Major genotype of Rabies virus | Reporting year |
|---|---|---|---|
| Angola | 151 | Genotype 1, 2, 3, 4 | 2010 |
| Botswana | 1794 | Genotype 1, 2, 3, 4 | 2011 |
| Kenya | 3 | Genotype 1, 2, 3, 4 | 2013 |
| Kyrgyzstan | 1 | Genotype 1 | 2012 |
| Lesotho | 15 | Genotype 1, 2, 3, 4 | 2012 |
| Madagascar | 2 | Genotype 1 (European bat Lyssavirus type 1 -EBLV-1, also reported) | 2011 |
| Morocco | 19 | Genotype 1 | 2012 |
| Mozambique | 72 | Genotype 1, 2, 3, 4 | 2011 |
| Namibia | 13 | Genotype 1, 2, 3, 4 | 2010-11 |
| Nepal | 12 | Genotype 1 | 2012 |
| South Africa | 12 | Genotype 1, 2, 3, 4 | 2012 |
| Swaziland | 38 | Genotype 1, 2, 3, 4 | 2011 |
| Tajikistan | 13 | Genotype 1 | 2011 |
| Tunisia | 6 | Genotype 1 | 2013 |
| Vietnam | 102 | Genotype 1 | 2013 |
| Yemen | 30 | Genotype 1 | 2014 |
| Zambia | 5 | Genotype 1, 2, 3, 4 | 2012 |
| Zimbabwe | 2 | Genotype 1, 2, 3, 4 | 2012 |
United States
The following table outlines the cases of rabies in humans in the United States and Puerto Rico from January 2008 through September 2017 by mode of exposure and rabies virus (RV) variant responsible for infection:[8]
| Date of onset | Date of death | State | Age | Gender | Exposure | Virus variant |
|---|---|---|---|---|---|---|
| 5-May-17 | 21-May-17 | VA | 65 | F | Bite | Dog, India |
| 25-Nov-15 | 1-Dec-15 | PR | 54 | M | Bite | Dog-mongoose, Caribbean |
| 17-Sep-15 | 3-Oct-15 | WY | 77 | F | Contact | Bat, Ln |
| 30-Jul-15 | 24-Aug-15 | MA | 65 | M | Bite, Philippines | Dog, Philippines |
| 12-Sep-14 | 26-Sep-14 | MO | 52 | M | Unknown | Bat, Ps |
| 16-May-13 | 11-Jun-13 | TX | 28 | M | Unknown, Guatemala | Dog, Guatemala |
| 31-Jan-13 | 27-Feb-13 | MD | 49 | M | Kidney transplant | Raccoon, eastern United States |
| 6-Jul-12 | 31-Jul-12 | CA | 34 | M | Bite | Bat,Tb |
| 22-Dec-11 | 23-Jan-12 | MA | 63 | M | Contact | Bat, My sp |
| 3-Dec-11 | 19-Dec-11 | SC | 46 | F | Unknown | Bat,Tb |
| 1-Sep-11 | 14-Oct-11 | MA | 40 | M | Contact, Brazil | Dog, Brazil |
| 21-Aug-11 | 1-Sep-11 | NC | 20 | M | Unknown (organ donor)§ | Raccoon, eastern United States |
| 14-Aug-11 | 31-Aug-11 | NY | 25 | M | Contact, Afghanistan | Dog, Afghanistan |
| 30-Jun-11 | 20-Jul-11 | NJ | 73 | F | Bite, Haiti | Dog, Haiti |
| 30-Apr-11 | Survived | CA | 8 | F | Unknown | Unknown |
| 24-Dec-10 | 10-Jan-11 | WI | 70 | M | Unknown | Bat, Ps |
| 2-Aug-10 | 21-Aug-10 | LA | 19 | M | Bite, Mexico | Bat, Dr |
| 23-Oct-09 | 20-Nov-09 | VA | 42 | M | Contact, India | Dog, India |
| 20-Oct-09 | 11-Nov-09 | MI | 55 | M | Contact | Bat, Ln |
| 5-Oct-09 | 20-Oct-09 | IN | 43 | M | Unknown | Bat, Ps |
| 25-Feb-09 | Survived | TX | 17 | F | Contact | Bat, unknown |
| 19-Nov-08 | 30-Nov-08 | MO | 55 | M | Bite | Bat, Ln |
| 16-Mar-08 | 18-Mar-08 | CA | 16 | M | Bite, Mexico | Fox,Tb related |
Legend: Dr = Desmodus rotundus. Ln = Lasionycteris noctivagans. My sp = Myotis species. Ps = Perimyotis subflavus.Tb = Tadarida brasiliensis
Developed countries
- Many territories, such as the United Kingdom, Ireland, Taiwan, Japan, Hawaii, Mauritius, Barbados and Guam, are free of rabies, although there may be a very low prevalence of rabies among bats in the UK
- New Zealand and Australia have never had rabies.[13] However, in Australia, the Australian Bat Lyssavirus occurs normally in both insectivorous and fruit eating bats (flying foxes) from most mainland states. Scientists believe it is present in bat populations throughout the range of flying foxes in Australia
Countries and political units reporting no indigenous cases of rabies during 2005:
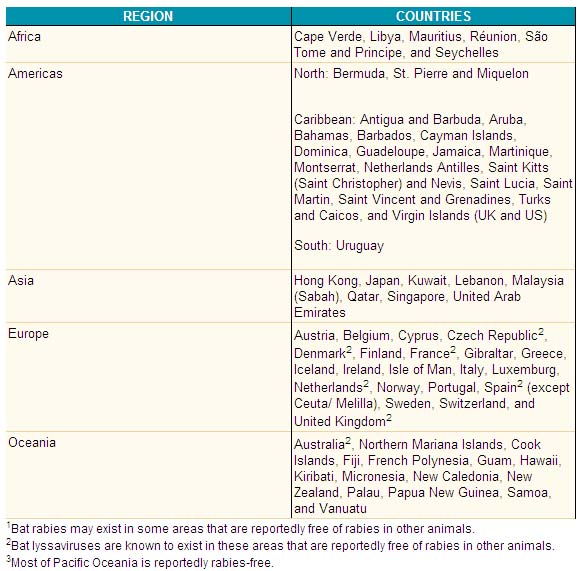
Age
- Worldwide, children under 15 years of age have a higher rabies exposure risk, and most exposures are from dog bites[9]
Case-mortality rate
- Worldwide, 55,000 human deaths occur annually from rabies, with 56 % of deaths estimated to occur in Asia and 44 % in Africa[10]
- An estimated 31,000 human deaths due to rabies occur annually in Asia, with the majority (around 20,000 occuring in India)[11][12][13]
- More than 99% of all human deaths from rabies occur in Africa, Asia, South America and India which report thirty thousand deaths annually.[14] One of the sources of recent flourishing of rabies in the East Asia is the pet boom. China introduced the "One-dog policy" in November 2006 to control the problem.[15]
- The number of human rabies deaths in the United States attributed to rabies has been steadily declining since the 1970’s thanks to animal control and vaccination programs, successful outreach programs, and the availability of modern rabies biologics
| Region | Country | Case-mortality rate per 100,000 patients |
|---|---|---|
| Asia | Bangladesh | 1.1 - 1.8 |
| Bhutan | 2.7 - 7.5 | |
| Cambodia | 2.8 - 11.5 |
Non-human rabies
Wild Animals
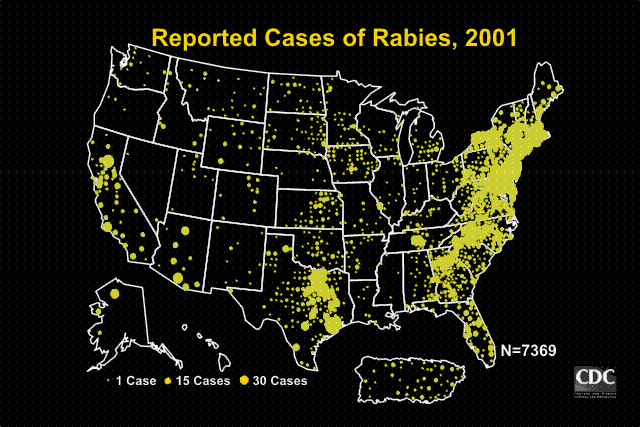
Wild animals accounted for 93% of reported cases of rabies in 2001. Raccoons continued to be the most frequently reported rabid wildlife species (37.2% of all animal cases during 2001), followed by skunks (30.7%), bats (17.2%), foxes (5.9%), and other wild animals, including rodents and lagomorphs (0.7%). Reported cases in raccoons and foxes decreased 0.4% and 3.5% respectively from the totals reported in 2000. Reported cases in skunks, and bats increased 2.6%, and 3.3% respectively from the totals reported in 2000.
Outbreaks of rabies infections in terrestrial mammals like raccoons, skunks, foxes, and coyotes are found in broad geographic regions across the United States. Geographic boundaries of currently recognized reservoirs for rabies in terrestrial mammals are shown on the map below.
Domestic Animals
Domestic species accounted for 6.8% of all rabid animals reported in the United States in 2001. The number of reported rabid domestic animals decreased 2.4% from the 509 cases reported in 2000 to 497 in 2001.
In 2001, cases of rabies in cats increased 8.4%, whereas those in dogs, cattle, horses, sheep and goats, and swine decreased 21.9%, 1.2%, 1.9% and 70.0% respectively compared with those reported in 2000. Rabies cases in cats continue to be more than twice as numerous as those in dogs or cattle. Pennsylvania reported the largest number of rabid domestic animals for any state, followed by New York.
References
- ↑ "apps.who.int" (PDF).
- ↑ Hampson K, Coudeville L, Lembo T, Sambo M, Kieffer A, Attlan M, Barrat J, Blanton JD, Briggs DJ, Cleaveland S, Costa P, Freuling CM, Hiby E, Knopf L, Leanes F, Meslin FX, Metlin A, Miranda ME, Müller T, Nel LH, Recuenco S, Rupprecht CE, Schumacher C, Taylor L, Vigilato MA, Zinsstag J, Dushoff J (2015). "Estimating the global burden of endemic canine rabies". PLoS Negl Trop Dis. 9 (4): e0003709. doi:10.1371/journal.pntd.0003709. PMC 4400070. PMID 25881058.
- ↑ "CDC - Rabies Surveillance in the U.S.: Human Rabies - Rabies".
- ↑ Zhang YZ, Xiong CL, Xiao DL, Jiang RJ, Wang ZX, Zhang LZ, Fu ZF (2005). "Human rabies in China". Emerging Infect. Dis. 11 (12): 1983–4. doi:10.3201/eid1112.040775. PMC 3367615. PMID 16485502.
- ↑ "WHO | Human rabies in India: a problem needing more attention".
- ↑ "Incidence of Rabies in Humans and Domestic Animals and People's Awareness in North Gondar Zone, Ethiopia".
- ↑ "WHO | Epidemiology and burden of disease".
- ↑ "CDC - Rabies Surveillance in the U.S.: Human Rabies - Rabies".
- ↑ "WHO | Rabies".
- ↑ "WHO | Rabies".
- ↑ "WHO | Rabies".
- ↑ "WHO | Human rabies in India: a problem needing more attention".
- ↑ Dutta JK (1999). "Human rabies in India: epidemiological features, management and current methods of prevention". Trop Doct. 29 (4): 196–201. doi:10.1177/004947559902900404. PMID 10578630.
- ↑ "Rabies vaccine". WHO - Immunization, Vaccines and Biologicals. Retrieved 2006-04-20.
- ↑ The Toronto Star "China cracks down on rabid dog menace"
Risk Factors
|
Rabies Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Rabies On the Web |
|
American Roentgen Ray Society Images of Rabies |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [14]; Associate Editor(s)-in-Chief: Syed Hassan A. Kazmi BSc, MD [15]
Overview
The most potent risk factor for the development of rabies is a bite from an infected animal. People that live in an area, or travel to an area that has a large incidence for rabies, are at a high risk for acquiring rabies from a rabid animal. Handling certain wild animals such as bats or raccoons will put a person at a higher risk.
Risk Factors
The following are the risk factors for rabies:[1][2][3][4][5]
- Bites from infected animals
- Exposure to urine or other secretions of infected animals
- Organ transplants from infected donors
- The following individuals may have a higher risk of contracting rabies than the general population:
- International travelers
- Animal control officers
- Spelunkers
- Lab workers
- Injuries to the head and the neck region with open wounds may facilitate quicker transmission of the virus to the brain.
References
- ↑ "WHO | Rabies".
- ↑ Fooks AR, Johnson N, Brookes SM, Parsons G, McElhinney LM (2003). "Risk factors associated with travel to rabies endemic countries". J. Appl. Microbiol. 94 Suppl: 31S–36S. PMID 12675934.
- ↑ Gong Z, He F, Chen Z (2012). "Risk factors for human rabies in China". Zoonoses Public Health. 59 (1): 39–43. doi:10.1111/j.1863-2378.2011.01416.x. PMID 21824368.
- ↑ Dire DJ, Hogan DE, Riggs MW (1994). "A prospective evaluation of risk factors for infections from dog-bite wounds". Acad Emerg Med. 1 (3): 258–66. PMID 7621206.
- ↑ Gautret P, Ribadeau-Dumas F, Parola P, Brouqui P, Bourhy H (2011). "Risk for rabies importation from North Africa". Emerging Infect. Dis. 17 (12): 2187–93. doi:10.3201/eid1712.110300. PMC 3311213. PMID 22185767.
Screening
|
Rabies Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Rabies On the Web |
|
American Roentgen Ray Society Images of Rabies |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [16] Associate Editor(s)-in-Chief: Mahshid Mir, M.D. [17]
Overview
Treatment after exposure, known as post-exposure prophylaxis or "P.E.P.", is highly successful in preventing the disease if administered promptly, within fourteen days after infection. The first step is immediately washing the wound with soap and water, which is very effective at reducing the number of viral particles. In the United States, patients receive one dose of immunoglobulin and five doses of rabies vaccine over a twenty-eight day period. One-half the dose of immunoglobulin is injected in the region of the bite, if possible, with the remainder injected intramuscularly away from the bite. Pre-exposure vaccination with human diploid cell Rabies vaccine (HDCV), or purified chick embryo cell (PCEC) vaccine, may be recommended for international travelers based on the local incidence of rabies in the country to be visited, the availability of appropriate antirabies biologicals, and the intended activity and duration of stay of the traveler.
Secondary Prevention
Post-Exposure Prophylaxis
Treatment after exposure, known as post-exposure prophylaxis or "P.E.P.", is highly successful in preventing the disease if administered promptly, within fourteen days after infection. The first step is immediately washing the wound with soap and water, which is very effective at reducing the number of viral particles. In the United States, patients receive one dose of immunoglobulin and five doses of rabies vaccine over a twenty-eight day period. One-half the dose of immunoglobulin is injected in the region of the bite, if possible, with the remainder injected intramuscularly away from the bite. This is much less painful compared with administering immunoglobulin through the abdominal wall with a large needle, which is how it was done in the past. The first dose of rabies vaccine is given as soon as possible after exposure, with additional doses on days three, seven, fourteen, and twenty-eight after the first. Patients that have previously received pre-exposure vaccination do not receive the immunoglobulin, only the post-exposure vaccinations. Since the widespread vaccination of domestic dogs and cats and the development of effective human vaccines and immunoglobulin treatments, the number of recorded deaths in the U.S. from rabies has dropped from one hundred or more annually in the early twentieth century, to 1–2 per year, mostly caused by bat bites, which may go unnoticed by the victim and hence untreated.
P.E.P. is effective in treating rabies because the virus must travel from the site of infection through the peripheral nervous system (nerves in the body) before infecting the central nervous system (brain and spinal cord) and glands to cause lethal damage. This travel along the nerves is usually slow enough that vaccine and immunoglobulin can be administered to protect the brain and glands from infection. The amount of time this travel requires is dependent on how far the infected area is from the brain: if the victim is bitten in the face, for example, the time between initial infection and infection of the brain is very short and P.E.P. may not be successful.
Prevention for Travellers
Pre-exposure vaccination with human diploid cell Rabies vaccine (HDCV), or purified chick embryo cell (PCEC) vaccine, may be recommended for international travelers based on the local incidence of rabies in the country to be visited, the availability of appropriate antirabies biologicals, and the intended activity and duration of stay of the traveler. Different schedules, alternative routes of administration, and other rabies vaccines besides HDCV and PCEC may be found abroad. Pre-exposure vaccination may be recommended for veterinarians, animal handlers, field biologists, spelunkers, missionaries, and certain laboratory workers. Pre-exposure vaccination does not eliminate the need for additional medical attention after a rabies exposure but simplifies postexposure prophylaxis in populations at risk by eliminating the need for rabies immune globulin (RIG) and by decreasing the number of doses of vaccine required. Pre-exposure vaccination is of particular importance for travelers at risk of exposure to rabies in countries where biologicals are in short supply and locally available rabies vaccines might carry a higher risk of adverse reactions. Pre-exposure vaccination may also provide some degree of protection when there is an unapparent or unrecognized exposure to rabies and when postexposure prophylaxis might be delayed. Planning is needed to ensure compliance in completion of the three pre-exposure vaccine doses, prior to commencing travel.
Travelers should be advised that any animal bite or scratch should receive prompt local treatment by thorough cleansing of the wound with copious amounts of soap and water (and povidone iodine, if available). This local treatment will substantially reduce the risk of rabies. Travelers who might have been exposed to rabies should be advised to always contact local health authorities immediately for advice about postexposure prophylaxis and should also contact their personal physician or state health department as soon as possible thereafter.
Equine rabies immune globulin (ERIG), or purified fractions of ERIG, has been used effectively in some developing countries where human rabies immune globulin (RIG) might not be available. If necessary, such heterologous products are preferable to no RIG administration in human rabies postexposure prophylaxis. The incidence of adverse reactions after the use of these products has been low (0.8%-6.0%), and most of those reactions were minor. However, such products are neither evaluated by U.S. standards nor regulated by the U.S. Food and Drug Administration, and their use cannot be unequivocally recommended at this time. In addition, unpurified antirabies serum of equine origin might still be used in some countries where neither human RIG nor ERIG is available. The use of this antirabies serum is associated with higher rates of serious adverse reactions, including anaphylaxis.
Adverse Reactions
Travelers should be advised that they may experience local reactions after vaccination, such as pain, erythema, swelling, or itching at the injection site, or mild systemic reactions, such as headache, nausea, abdominal pain, muscle aches, and dizziness. Approximately 6% of persons receiving booster vaccinations with HDCV may experience an immune complex-like reaction characterized by urticaria, pruritus, and malaise. Once initiated, rabies postexposure prophylaxis should not be interrupted or discontinued because of local or mild systemic reactions to rabies vaccine.
Rabies and Domestic Skunks in the United States
There is currently no USDA-approved vaccine for the strain of rabies that afflicts skunks. When cases are reported of pet skunks biting a human, the animals are frequently killed in order to be tested for rabies. or humans exposed to the rabies virus must begin post-exposure prophylaxis before the disease can progress to the central nervous system. For this reason, it is necessary to determine whether the animal, in fact, has rabies as quickly as possible. Without a definitive quarantine period in place for skunks, quarantining the animals is not advised as there is no way of knowing how long it may take the animal to show symptoms. Destruction of the skunk is recommended and the brain is then tested for presence of rabies virus.
Skunk owners have recently organized to campaign for USDA approval of both a vaccine and an officially recommended quarantine period for skunks in the United States.
References
Causes
|
Rabies Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Rabies On the Web |
|
American Roentgen Ray Society Images of Rabies |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [18]
| style="background:#Template:Taxobox colour;"|Rabies | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
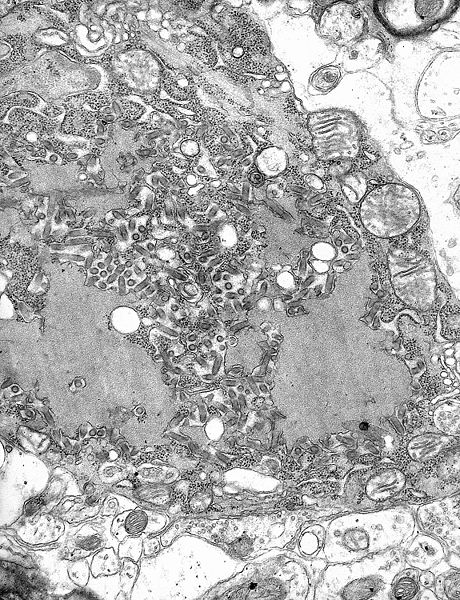 TEM micrograph with numerous rabies virions (small dark-grey rod-like particles) and Negri bodies (the larger pathognomonic cellular inclusions of rabies infection)
| ||||||||||
| style="background:#Template:Taxobox colour;" | Virus classification | ||||||||||
|
The rabies virus is a neurotropic virus that causes rabies in humans and animals. Rabies transmission can occur through the saliva of animals and less commonly through contact with human saliva.
The rabies virus has a cylindrical morphology and is the type species of the Lyssavirus genus of the Rhabdoviridae family. These viruses are enveloped and have a single stranded RNA genome with negative-sense. The genetic information is packaged as a ribonucleoprotein complex in which RNA is tightly bound by the viral nucleoprotein. The RNA genome of the virus encodes five genes whose order is highly conserved. These genes code for nucleoprotein (N), phosphoprotein (P), matrix protein (M), glycoprotein (G) and the viral RNA polymerase (L).[1] The complete genome sequences range from 11,615 to 11,966 nt in length.[2]
All transcription and replication events take place in the cytoplasm inside a specialized “virus factory”, the Negri body (named after Adelchi Negri[3]). These are 2–10 µm in diameter and are typical for a rabies infection and thus have been used as definite histological proof of such infection.[4]
Structure
Lyssaviruses have helical symmetry, so their infectious particles are approximately cylindrical in shape. They are characterized by an extremely broad host spectrum ranging from plants to insects and mammals; human-infecting viruses more commonly have cubic symmetry and take shapes approximating regular polyhedra.
The rabies virus has a bullet like shape with a length of about 180 nm and a cross-sectional diameter of about 75 nm. One end is rounded or conical and the other end is planar or concave. The lipoprotein envelope carries knob-like spikes composed of Glycoprotein G. Spikes do not cover the planar end of the virion (virus particle). Beneath the envelope is the membrane or matrix (M) protein layer which may be invaginated at the planar end. The core of the virion consists of helically arranged ribonucleoprotein.
Life cycle
Template:Viral life cycle After receptor binding, rabies virus enters its host cells through the endosomal transport pathway. Inside the endosome, the low pH value induces the membrane fusion process, thus enabling the viral genome to reach the cytosol. Both processes, receptor binding and membrane fusion, are catalyzed by the glycoprotein G which plays a critical role in pathogenesis (mutant virus without G proteins cannot propagate).[1]
The next step after entry is the transcription of the viral genome by the P-L polymerase (P is an essential cofactor for the L polymerase) in order to make new viral protein. The viral polymerase can only recognize ribonucleoprotein and cannot use free RNA as template. Transcription is regulated by cis-acting sequences on the virus genome and by protein M which is not only essential for virus budding but also regulates the fraction of mRNA production to replication. Later in infection, the activity of the polymerase switches to replication in order to produce full-length positive-strand RNA copies. These complementary RNAs are used as templates to make new negative-strand RNA genomes. They are packaged together with protein N to form ribonucleoprotein which then can form new viruses.[4]
Infection
In September 1931, Joseph Lennox Pawan of Trinidad in the West Indies, a Government Bacteriologist, found Negri bodies in the brain of a bat with unusual habits. In 1932, Pawan first discovered that infected vampire bats could transmit rabies to humans and other animals.[5][6] For a brief history of some of the controversies surrounding the early discoveries relating to rabies in Trinidad, see the brief history by James Waterman.[7]
From the wound of entry, the rabies virus travels quickly along the neural pathways of the peripheral nervous system. The retrograde axonal transport of the rabies virus to the CNS (Central Nervous System) is the key step of pathogenesis during natural infection. The exact molecular mechanism of this transport is unknown although binding of the P protein from rabies virus to the dynein light chain protein DYNLL1 has been shown.[8] P also acts as an interferon antagonist, thus decreasing the immune response of the host.
From the CNS, the virus further spreads to other organs. The salivary glands located in the tissues of the mouth and cheeks receive high concentrations of the virus, thus allowing it to be further transmitted due to projectile salivation. Fatality can occur from two days to five years from the time of initial infection.[9] This however depends largely on the species of animal acting as a reservoir. Most infected mammals die within weeks, while strains of a species such as the African Yellow Mongoose (Cynictis penicillata) might survive an infection asymptomatically for years.[10]
Antigenicity
Upon viral entry into the body and also after vaccination, the body produces virus neutralizing antibodies which bind and inactivate the virus. Specific regions of the G protein have been shown to be most antigenic in leading to the production of virus neutralizing antibodies. These antigenic sites, or epitopes, are categorized into regions I-IV and minor site a. Previous work has demonstrated that antigenic sites II and III are most commonly targeted by natural neutralizing antibodies.[11] Additionally, a monoclonal antibody with neutralizing functionality has been demonstrated to target antigenic site I.[12] Other proteins, such as the nucleoprotein, have been shown to be unable to elicit production of virus neutralizing antibodies.[13] The epitopes which bind neutralizing antibodies are both linear and conformational.[14]
Transmission
All warm-blooded species, including humans, may become infected with the rabies virus and develop symptoms. Birds were first artificially infected with rabies in 1884; however, infected birds are largely if not wholly asymptomatic, and recover.[15] Other bird species have been known to develop rabies antibodies, a sign of infection, after feeding on rabies-infected mammals.[16][17]
The virus has also been adapted to grow in cells of poikilothermic ("cold-blooded") vertebrates.[18][19] Most animals can be infected by the virus and can transmit the disease to humans. Infected bats,[20][21] monkeys, raccoons, foxes, skunks, cattle, wolves, coyotes, dogs, mongooses (normally yellow mongoose)[22] and cats present the greatest risk to humans.
Rabies may also spread through exposure to infected domestic farm animals, groundhogs, weasels, bears, and other wild carnivorans. Small rodents, such as squirrels, hamsters, guinea pigs, gerbils, chipmunks, rats, and mice, and lagomorphs such as rabbits and hares, are almost never found to be infected with rabies and are not known to transmit rabies to humans.[23] The Virginia opossum is resistant but not immune to rabies.[24]
The virus is usually present in the nerves and saliva of a symptomatic rabid animal.[25][26] The route of infection is usually, but not always, by a bite. In many cases, the infected animal is exceptionally aggressive, may attack without provocation, and exhibits otherwise uncharacteristic behavior.[27] This is an example of a viral pathogen modifying the behavior of its host to facilitate its transmission to other hosts.
Transmission between humans is extremely rare. A few cases have been recorded through transplant surgery.[28] After a typical human infection by bite, the virus enters the peripheral nervous system. It then travels along the afferent nerves toward the central nervous system.[29] During this phase, the virus cannot be easily detected within the host, and vaccination may still confer cell-mediated immunity to prevent symptomatic rabies. When the virus reaches the brain, it rapidly causes encephalitis, the prodromal phase, and is the beginning of the symptoms. Once the patient becomes symptomatic, treatment is almost never effective and mortality is over 99%. Rabies may also inflame the spinal cord, producing transverse myelitis.[30][31]
Evolution
All extant rabies viruses appear to have evolved within the last 1500 years.[32] There are seven genotypes of rabies virus. In Eurasia cases are due to three of these—genotype 1 (classical rabies) and to a lesser extent genotypes 5 and 6 (European bat lyssaviruses type-1 and -2).[33] Genotype 1 evolved in Europe in the 17th century and spread to Asia, Africa and the Americas as a result of European exploration and colonization.
Bat rabies in North America appears to have been present since 1281 CE (95% confidence interval: 906–1577 CE).[34]
Application
Rabies virus is used in research for viral neuronal tracing to establish synaptic connections and directionality of synaptic transmission. [35]
See also
References
- ↑ 1.0 1.1 Finke S, Conzelmann KK (August 2005). "Replication strategies of rabies virus". Virus Res. 111 (2): 120–131. doi:10.1016/j.virusres.2005.04.004. PMID 15885837.
- ↑ "Rabies complete genome". NCBI Nucleotide Database. Retrieved 29 May 2013.
- ↑ Template:WhoNamedIt
- ↑ 4.0 4.1 Albertini AA, Schoehn G, Weissenhorn W, Ruigrok RW (January 2008). "Structural aspects of rabies virus replication". Cell. Mol. Life Sci. 65 (2): 282–294. doi:10.1007/s00018-007-7298-1. PMID 17938861.
- ↑ Pawan, J. L. (1936). "Transmission of the Paralytic Rabies in Trinidad of the Vampire Bat: Desmodus rotundus murinus Wagner, 1840". Annals of Tropical Medicine and Parasitology. 30: 137–156. ISSN 0003-4983.
- ↑ Pawan, J. L. (1936). "Rabies in the vampire bat of Trinidad, with special reference to the clinical course and the latency of infection". Ann Trop Med Parasitol. 30: 101–129. ISSN 0003-4983.
- ↑ Waterman, James A. (1965). "The History of the Outbreak of Paralytic Rabies in Trinidad Transmitted by Bats to Human beings and Lower animals from 1925". Caribbean Medical Journal. 26 (1–4): 164–169. ISSN 0374-7042.
- ↑ Raux H, Flamand A, Blondel D (November 2000). "Interaction of the rabies virus P protein with the LC8 dynein light chain". J. Virol. 74 (21): 10212–10216. doi:10.1128/JVI.74.21.10212-10216.2000. PMC 102061. PMID 11024151.
- ↑ "Rabies". University of Northern British Columbia. Retrieved 2008-10-10.
- ↑ Taylor PJ (December 1993). "A systematic and population genetic approach to the rabies problem in the yellow mongoose (Cynictis penicillata)". Onderstepoort J. Vet. Res. 60 (4): 379–87. PMID 7777324.
- ↑ Benmansour A (1991). "Antigenicity of rabies virus glycoprotein". Journal of Virology. 65 (8): 4198–4203. PMC 248855. PMID 1712859.
- ↑ Marissen, WE.; Kramer, RA.; Rice, A.; Weldon, WC.; Niezgoda, M.; Faber, M.; Slootstra, JW.; Meloen, RH.; et al. (Apr 2005). "Novel rabies virus-neutralizing epitope recognized by human monoclonal antibody: fine mapping and escape mutant analysis". J Virol. 79 (8): 4672–8. doi:10.1128/JVI.79.8.4672-4678.2005. PMC 1069557. PMID 15795253.
- ↑ Wiktor, TJ.; György, E.; Schlumberger, D.; Sokol, F.; Koprowski, H. (Jan 1973). "Antigenic properties of rabies virus components". J Immunol. 110 (1): 269–76. PMID 4568184.
- ↑ Bakker, AB.; Marissen, WE.; Kramer, RA.; Rice, AB.; Weldon, WC.; Niezgoda, M.; Hanlon, CA.; Thijsse, S.; et al. (Jul 2005). "Novel human monoclonal antibody combination effectively neutralizing natural rabies virus variants and individual in vitro escape mutants". J Virol. 79 (14): 9062–8. doi:10.1128/JVI.79.14.9062-9068.2005. PMC 1168753. PMID 15994800.
- ↑ Shannon LM, Poulton JL, Emmons RW, Woodie JD, Fowler ME (April 1988). "Serological survey for rabies antibodies in raptors from California". J. Wildl. Dis. 24 (2): 264–7. doi:10.7589/0090-3558-24.2.264. PMID 3286906.
- ↑ Gough PM, Jorgenson RD (1976). "Rabies antibodies in sera of wild birds". Journal of Wildlife Diseases. 12 (3): 392–5. doi:10.7589/0090-3558-12.3.392. PMID 16498885.
- ↑ Jorgenson RD, Gough PM (July 1976). "Experimental rabies in a great horned owl". J. Wildl. Dis. 12 (3): 444–7. doi:10.7589/0090-3558-12.3.444.
- ↑ Wong, Derek. "Rabies". Wong's Virology. Retrieved 19 Mar 2009.
- ↑ Campbell, James B.; Charlton, K.M. (1988). Developments in Veterinary Virology: Rabies. Springer. p. 48. ISBN 0-89838-390-0.
- ↑ Pawan JL (1959). "The transmission of paralytic rabies in Trinidad by the vampire bat (Desmodus rotundus murinus Wagner". Caribbean Medical Journal. 21: 110–36. PMID 13858519.
- ↑ Pawan JL (1959). "Rabies in the vampire bat of Trinidad, with special reference to the clinical course and the latency of infection". Caribbean Medical Journal. 21: 137–56. PMID 14431118.
- ↑ Taylor PJ (December 1993). "A systematic and population genetic approach to the rabies problem in the yellow mongoose (Cynictis penicillata)". The Onderstepoort Journal of Veterinary Research. 60 (4): 379–87. PMID 7777324.
- ↑ "Rabies. Other Wild Animals: Terrestrial carnivores: raccoons, skunks and foxes". Centers for Disease Control and Prevention(CDC). Retrieved 2010-12-23.
- ↑ McRuer DL, Jones KD (May 2009). "Behavioral and nutritional aspects of the Virginian opossum (Didelphis virginiana)". The veterinary clinics of North America. Exotic animal practice. 12 (2): 217–36, viii. doi:10.1016/j.cvex.2009.01.007. PMID 19341950.
- ↑ The Merck Manual, 11th Edition (1983), p. 183
- ↑ The Merck manual of Medical Information. Second Home Edition, (2003), p. 484.
- ↑ Turton, Jenny (2000). "Rabies: a killer disease". National Department of Agriculture.
- ↑ Srinivasan A, Burton EC, Kuehnert MJ, Rupprecht C, Sutker WL, Ksiazek TG, Paddock CD, Guarner J, Shieh WJ, Goldsmith C, Hanlon CA, Zoretic J, Fischbach B, Niezgoda M, El-Feky WH, Orciari L, Sanchez EQ, Likos A, Klintmalm GB, Cardo D, LeDuc J, Chamberland ME, Jernigan DB, Zaki SR (March 2005). "Transmission of rabies virus from an organ donor to four transplant recipients" (PDF). N Engl J Med. 352 (11): 1103–11. doi:10.1056/NEJMoa043018. PMID 15784663.
- ↑ Jackson, Alan C., Wunner, William H. (2002). Rabies. Academic Press. p. 290. ISBN 978-0-12-379077-4.
- ↑ Joanne Lynn, M.D. (October 1997) Transverse Myelitis: Symptoms, Causes and Diagnosis The Transverse Myelitis Association
- ↑ Larry Ernest Davis; Molly K. King; Jessica L. Schultz (15 June 2005). Fundamentals of neurologic disease. Demos Medical Publishing. p. 73. ISBN 978-1-888799-84-2.
- ↑ Nadin-Davis, S. A.; Real, L. A. (2011). "Molecular phylogenetics of the lyssaviruses--insights from a coalescent approach". Adv Virus Res. Advances in Virus Research. 79: 203–238. doi:10.1016/B978-0-12-387040-7.00011-1. ISBN 9780123870407. PMID 21601049.
- ↑ McElhinney, L. M.; Marston, D. A.; Stankov, S; Tu, C.; Black, C.; Johnson, N.; Jiang, Y.; Tordo, N.; Müller, T.; Fooks, A. R. (2008). "Molecular epidemiology of lyssaviruses in Eurasia". Dev Biol (Basel). 131: 125–131. PMID 18634471.
- ↑ Kuzmina, N. A.; Kuzmin, I. V.; Ellison, J. A.; Taylor, S. T.; Bergman, D. L.; Dew, B.; Rupprecht, C. E. (2013). "A reassessment of the evolutionary timescale of bat rabies viruses based upon glycoprotein gene sequences". Virus Genes. Forthcoming (2): 305. doi:10.1007/s11262-013-0952-9.
- ↑ Ginger, M., Haberl M., Conzelmann K.-K., Schwarz M. and Frick A. (2013). Revealing the secrets of neuronal circuits with recombinant rabies virus technology. Front. Neural Circuits. doi:10.3389/fncir.2013.00002
External links
Differential Diagnosis

Editor-In-Chief: C. Michael Gibson, M.S., M.D. [19] Associate Editor(s)-in-Chief: Mahshid Mir, M.D. [20]
Overview
The differential diagnosis for rabies deals with eliminating diseases with similar symptoms from the diagnosis. There are many viruses that can appear similar to rabies such as encephalitis and the herpes simplex virus. It is very important to rule out certain diseases such as echovirus and poliovirus. Rabies is a serious disease that needs to be treated quickly if someone is suspected to be infected with the virus.
Differentiating Rabies From Other Diseases
Rabies should be differentiated from other causes of headache and decrease consciousness:
| Diseases | History and Physical | Diagnostic tests | Other Findings | |||||
|---|---|---|---|---|---|---|---|---|
| Prodromal symptoms | Fever | Headache | LOC | Neuro Onset | Laboratory Findings | Imaging preferance | ||
| Rabies infection[1] | + | + | + | + | Insidious | Antibody detection in serology
Skin biopsy of injured skin |
MRI | Hydrophobia, aerophobia, dysphagia, and localized pain, weakness or paresthesias |
| Meningitis[2][3] | + | + | + | - | Sudden | CSF analysis:
↑ Protein ↓ Glucose |
CT scan: First choice
MRI: Best choice |
Fever, neck, rigidity |
| Encephalitis[4] | + | + | + | + | Sudden | PCR
CSF analysis and culture reveal the responsible micro-organism |
MRI | Accompany a meningoencephalitis, seizures, hemiparesis, cranial nerve palsies, photophobia, nausea |
| Autoimmune encephalitis[5] | - | +/- | + | +/- | Insidious | Autoantibodies present in both serum and CSF | MRI
EEG |
Memory deficit, dyskinesias, seizures, autonomic instability |
| CNS abscess[6] | + | + | + | + | Insidious | CSF analysis:
↓ Glucose ↑ Protein |
MRI is more sensitive and specific | High grade fever, fatigue,nausea, vomiting |
| Poliomyelitis[7] | + | + | + | + | Sudden | PCR of CSF | MRI | Asymmetric paralysis following a flu-like syndrome |
| Neurosyphilis[8] | - | - | + | + | Insidious | CSF VDRL-specifc | MRI & Lumbar puncture | History of unprotected sex or multiple sexual partners, and genital ulcer (chancre)
Blindness, confusion, depression, abnormal gait |
| Tick paralysis (Dermacentor tick)[9] | - | - | +/- | +/- | Insidious | - | - | History of outdoor activity in Northeastern United States
Tick often still latched to the patient at presentation (often in head and neck area) |
| Botulism[10] | - | - | - | - | Sudden | Toxin test, blood, wound, or stool culture | - | Diplopia, Hyporeflexia, Hypotonia, possible respiratory paralysis, Floppy baby syndrome |
| Tetrodotoxin poisoning[11] | - | - | +/- | +/- | Sudden | - | - | History of consumption of puffer fish species |
| Metabolic disturbances (electrolyte imbalance, hypoglycemia)[12] | - | +/- | - | + | Sudden | Hypoglycemia, hypo and hypernatremia | MRI | Confusion, seizure, palpitations, sweating, dizziness, hypoglycemia |
| Electrolyte disturbance[13] | - | - | - | +/- | Insidious | Hypocalcemia, hypomagnesemia, hypo- or hyperkalemia | Possible arrhythmia | |
| Drug toxicity/Neuroleptic malignant syndrome[14] | - | + | - | + | Sudden | Elevated serum creatine kianse
Hypocalcemia, hypomagnesemia, hypo- and hypernatremia, hyperkalemia, and metabolic acidosis |
Generalized slow wave EEG | Causative medications (eg, neuroleptics, antiemetics, concomitant lithium), dopaminergic withdrawal
Mental status change, rigidity, or dysautonomia |
| Organophosphate toxicity[15] | - | - | - | + | Sudden | Clinical suspicion confirmed with RBC AchE activity | - | History of exposure to insecticide or living in farming environment with : Diarrhea, Miosis, Bradycardia, Lacrimation, Emesis, Salivation, Sweating |
| Ischemic stroke[16] | - | - | +/- | + | Sudden | - | MRI for ischemia | Sudden unilateral motor and sensory deficit in a patient with a history of atherosclerotic risk factors (diabetes, hypertension, smoking) or atrial fibrillation |
| Hemorrhagic stroke[17] | - | - | + | + | Sudden | - | CT scan without contrast | Neck stiffness |
| Subdural hemorrhage[18] | - | - | + | + | Sudden | CSF analysis:
Xanthochromia |
CT scan without contrast | Confusion, dizziness, nausea, vomiting |
| Hypertensive encephalopathy[19] | - | - | + | + | Sudden | - | - | Delirium, cortical blindness, cerebral edema, seizure |
| Wernicke’s encephalopathy[20] | - | - | - | + | Sudden | - | - | Ophthalmoplegia, confusion |
| Amyotrophic lateral sclerosis[21] | - | - | +/- | +/- | Insidious | Normal LP (to rule out DDx) | MRI | Patient initially presents with upper motor neuron deficit (spasticity) followed by lower motor neuron deficit (flaccidity) |
| Diffuse gliomatosis[22] | - | - | + | - | Insidious | Cancer specific molecular characteristics
Normal CSF |
MRI (expansile, T2 hyperintense lesion) | Seizures, memory loss, motor weakness, visual symptoms, language deficit, and cognitive and personality changes |
| Central nervous system lymphoma[23] | + | - | + | +/- | Insidious | CSF cytology, flow cytometry, and stereotactic brain biopsy | MRI (parenchymal or leptomeningeal enhancement) | Associated with immunodeficiency
Focal neurological deficits, neuropsychiatric symptoms, signs of raised intracranial pressure, seizures, and ocular symptoms |
The most important differential diagnosis when it comes to the rabies presentations include meningitis and encephalitis. It is important to differentiate different rabies from each one of the causes of meningitis/encephalitis, which may differ based on the season of occurrence and the geographical region.
The most important viruses to rule out are:[24][25]
- Herpes simplex virus type 1
- Varicella-zoster virus
- (Less commonly) Enteroviruses including:
- Coxsackieviruses
- Echoviruses
- Polioviruses
- Human enteroviruses 68 to 71
In addition, consideration should be given to the local epidemiology of encephalitis caused by arboviruses belonging to several taxonomic groups, including:
- Eastern equine encephalitis viruses
- Western equine encephalitis viruses
- St. Louis encephalitis virus
- Powassan virus
- The California encephalitis virus serogroup
- La Crosse virus
New causes of viral encephalitis are also possible, as was evidenced by the recent outbreak in Malaysia of some 300 cases of encephalitis (mortality rate, 40%) caused by Nipah virus, a newly recognized paramyxovirus. Similarly, well-known viruses may be introduced into new locations, as is illustrated by the recent outbreak of encephalitis due to West Nile virus in the eastern United States. Epidemiological factors (e.g., season, geographic location, and the patient’s age, travel history, and possible exposure to animal bites, rodents, and ticks) may help direct the diagnostic workup.
References
- ↑ Baevsky RH, Bartfield JM (1993). "Human rabies: a review". Am J Emerg Med. 11 (3): 279–86. PMID 8489675.
- ↑ Tacon CL, Flower O (2012). "Diagnosis and management of bacterial meningitis in the paediatric population: a review". Emerg Med Int. 2012: 320309. doi:10.1155/2012/320309. PMC 3461291. PMID 23050153.
- ↑ Chadwick DR (2005). "Viral meningitis". Br. Med. Bull. 75-76: 1–14. doi:10.1093/bmb/ldh057. PMID 16474042.
- ↑ Steiner I, Budka H, Chaudhuri A, Koskiniemi M, Sainio K, Salonen O, Kennedy PG (2005). "Viral encephalitis: a review of diagnostic methods and guidelines for management". Eur. J. Neurol. 12 (5): 331–43. doi:10.1111/j.1468-1331.2005.01126.x. PMID 15804262.
- ↑ Lancaster E (2016). "The Diagnosis and Treatment of Autoimmune Encephalitis". J Clin Neurol. 12 (1): 1–13. doi:10.3988/jcn.2016.12.1.1. PMC 4712273. PMID 26754777.
- ↑ Alvis Miranda H, Castellar-Leones SM, Elzain MA, Moscote-Salazar LR (2013). "Brain abscess: Current management". J Neurosci Rural Pract. 4 (Suppl 1): S67–81. doi:10.4103/0976-3147.116472. PMC 3808066. PMID 24174804.
- ↑ Obregón R, Chitnis K, Morry C, Feek W, Bates J, Galway M, Ogden E (2009). "Achieving polio eradication: a review of health communication evidence and lessons learned in India and Pakistan". Bull. World Health Organ. 87 (8): 624–30. PMC 2733260. PMID 19705014.
- ↑ Timmermans M, Carr J (2004). "Neurosyphilis in the modern era". J. Neurol. Neurosurg. Psychiatr. 75 (12): 1727–30. doi:10.1136/jnnp.2004.031922. PMC 1738873. PMID 15548491.
- ↑ Edlow JA, McGillicuddy DC (2008). "Tick paralysis". Infect. Dis. Clin. North Am. 22 (3): 397–413, vii. doi:10.1016/j.idc.2008.03.005. PMID 18755381.
- ↑ Cherington M (2004). "Botulism: update and review". Semin Neurol. 24 (2): 155–63. doi:10.1055/s-2004-830901. PMID 15257512.
- ↑ Hwang DF, Noguchi T (2007). "Tetrodotoxin poisoning". Adv. Food Nutr. Res. 52: 141–236. doi:10.1016/S1043-4526(06)52004-2. PMID 17425946.
- ↑ Josephson MA, Kaul S, Hopkins J, Kvam D, Singh BN (1985). "Hemodynamic effects of intravenous flecainide relative to the level of ventricular function in patients with coronary artery disease". Am. Heart J. 109 (1): 41–5. PMID 3966331.
- ↑ Marcelou-Kinti O, Velegraki-Abel A (1988). "[Resistance to amphotericin B and genotypic resistance to 5-fluorocytosine of serotypes A and B of Candida albicans]". Therapie (in French). 43 (2): 121–2. PMID 3043756.
- ↑ Pelonero AL, Levenson JL, Pandurangi AK (1998). "Neuroleptic malignant syndrome: a review". Psychiatr Serv. 49 (9): 1163–72. doi:10.1176/ps.49.9.1163. PMID 9735957.
- ↑ Minton NA, Murray VS (1988). "A review of organophosphate poisoning". Med Toxicol Adverse Drug Exp. 3 (5): 350–75. PMID 3057326.
- ↑ Baldwin K, Orr S, Briand M, Piazza C, Veydt A, McCoy S (2010). "Acute ischemic stroke update". Pharmacotherapy. 30 (5): 493–514. doi:10.1592/phco.30.5.493. PMID 20412000.
- ↑ Bullard CW (1987). "Issues in low-level radioactive waste management". JAPCA. 37 (11): 1337–41. PMID 3443867.
- ↑ Cenic A, Bhandari M, Reddy K (2005). "Management of chronic subdural hematoma: a national survey and literature review". Can J Neurol Sci. 32 (4): 501–6. PMID 16408582.
- ↑ Schwartz RB (2002). "Hyperperfusion encephalopathies: hypertensive encephalopathy and related conditions". Neurologist. 8 (1): 22–34. PMID 12803657.
- ↑ Flynn A, Macaluso M, D'Empaire I, Troutman MM (2015). "Wernicke's Encephalopathy: Increasing Clinician Awareness of This Serious, Enigmatic, Yet Treatable Disease". Prim Care Companion CNS Disord. 17 (3). doi:10.4088/PCC.14r01738. PMC 4578911. PMID 26644959.
- ↑ Zarei S, Carr K, Reiley L, Diaz K, Guerra O, Altamirano PF, Pagani W, Lodin D, Orozco G, Chinea A (2015). "A comprehensive review of amyotrophic lateral sclerosis". Surg Neurol Int. 6: 171. doi:10.4103/2152-7806.169561. PMC 4653353. PMID 26629397.
- ↑ Chen S, Tanaka S, Giannini C, Morris J, Yan ES, Buckner J, Lachance DH, Parney IF (2013). "Gliomatosis cerebri: clinical characteristics, management, and outcomes". J. Neurooncol. 112 (2): 267–75. doi:10.1007/s11060-013-1058-x. PMC 3907195. PMID 23341100.
- ↑ Wilne SH, Ferris RC, Nathwani A, Kennedy CR (2006). "The presenting features of brain tumours: a review of 200 cases". Arch. Dis. Child. 91 (6): 502–6. doi:10.1136/adc.2005.090266. PMC 2082784. PMID 16547083.
- ↑ Romero JR, Newland JG (2003). "Viral meningitis and encephalitis: traditional and emerging viral agents". Semin Pediatr Infect Dis. 14 (2): 72–82. doi:10.1053/spid.2003.127223. PMID 12881794.
- ↑ Xie Y, Tan Y, Chongsuvivatwong V, Wu X, Bi F, Hadler SC, Jiraphongsa C, Sornsrivichai V, Lin M, Quan Y (2015). "A Population-Based Acute Meningitis and Encephalitis Syndromes Surveillance in Guangxi, China, May 2007-June 2012". PLoS ONE. 10 (12): e0144366. doi:10.1371/journal.pone.0144366. PMC 4669244. PMID 26633824.
Natural History, Complications & Prognosis
Natural History
|
Rabies Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Rabies On the Web |
|
American Roentgen Ray Society Images of Rabies |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [21]
Overview
If left untreated, rabies runs its course very rapidly. Once symptoms begin to appear, the disease is almost always fatal. The acute period of disease typically ends after 2 to 10 days. Common complications of rabies include, psychosis, seizures, aphasia, muscular twitching, delirium and death. Treatment after exposure (receiving the vaccines), known as post-exposure prophylaxis (PEP), is highly successful in preventing the disease if administered promptly, in general within ten days of infection.
Natural History
- Rabies runs its course very rapidly throughout the body. Once symptoms begin to appear, the disease is almost always fatal.[1][2]
- The period between infection and the first flu-like symptoms is normally two to twelve weeks, but can be as long as two years.[3][4][5][6]
- The acute period of disease typically ends after 2 to 10 days.
- To date only six documented cases of human survival from clinical rabies have been reported and each included a history of either pre- or postexposure prophylaxis.
- The few humans who are known to have survived the disease were all left with severe brain damage, with the recent exception of Jeanna Giese.
Complications
Common complications of rabies include:[7][8][9]
Prognosis
Treatment after exposure (receiving the vaccines), known as post-exposure prophylaxis (PEP), is highly successful in preventing the disease if administered promptly, in general within ten days of infection. Begun with little or no delay, PEP is 100% effective against rabies.[10] In the case in which there has been a significant delay in administering PEP, the treatment should be administered regardless of that delay, as it may still be effective.
In unvaccinated humans, rabies is usually fatal after neurological symptoms have developed, but prompt post-exposure vaccination may prevent the virus from progressing. Rabies kills around 55,000 people a year, mostly in Asia and Africa.[11]
Survival data using the Milwaukee protocol are available from the rabies registry.[12] As of 2011, seven people have been saved by this induced coma treatment.
References
- ↑ Hemachudha T, Ugolini G, Wacharapluesadee S, Sungkarat W, Shuangshoti S, Laothamatas J (2013). "Human rabies: neuropathogenesis, diagnosis, and management". Lancet Neurol. 12 (5): 498–513. doi:10.1016/S1474-4422(13)70038-3. PMID 23602163.
- ↑ Hattwick MA (1972). "Reactions to rabies". N. Engl. J. Med. 287 (23): 1204. PMID 5082226.
- ↑ Knobel DL, Cleaveland S, Coleman PG, Fèvre EM, Meltzer MI, Miranda ME, Shaw A, Zinsstag J, Meslin FX (2005). "Re-evaluating the burden of rabies in Africa and Asia". Bull. World Health Organ. 83 (5): 360–8. doi:/S0042-96862005000500012 Check
|doi=value (help). PMC 2626230. PMID 15976877. - ↑ Noah DL, Drenzek CL, Smith JS, Krebs JW, Orciari L, Shaddock J, Sanderlin D, Whitfield S, Fekadu M, Olson JG, Rupprecht CE, Childs JE (1998). "Epidemiology of human rabies in the United States, 1980 to 1996". Ann. Intern. Med. 128 (11): 922–30. PMID 9634432.
- ↑ Rupprecht CE, Hanlon CA, Hemachudha T (2002). "Rabies re-examined". Lancet Infect Dis. 2 (6): 327–43. PMID 12144896.
- ↑ Boland TA, McGuone D, Jindal J, Rocha M, Cumming M, Rupprecht CE, Barbosa TF, de Novaes Oliveira R, Chu CJ, Cole AJ, Kotait I, Kuzmina NA, Yager PA, Kuzmin IV, Hedley-Whyte ET, Brown CM, Rosenthal ES (2014). "Phylogenetic and epidemiologic evidence of multiyear incubation in human rabies". Ann. Neurol. 75 (1): 155–60. doi:10.1002/ana.24016. PMC 4118733. PMID 24038455.
- ↑ Udwadia ZF, Udwadia FE, Katrak SM, Dastur DK, Sekhar M, Lall A, Kumta A, Sane B (1989). "Human rabies: clinical features, diagnosis, complications, and management". Crit. Care Med. 17 (8): 834–6. PMID 2752780.
- ↑ Bhatt DR, Hattwick MA, Gerdsen R, Emmons RW, Johnson HN (1974). "Human rabies. Diagnosis, complications, and management". Am. J. Dis. Child. 127 (6): 862–9. PMID 4600621.
- ↑ Tullu MS, Rodrigues S, Muranjan MN, Bavdekar SB, Kamat JR, Hira PR (2003). "Neurological complications of rabies vaccines". Indian Pediatr. 40 (2): 150–4. PMID 12626831.
- ↑ Jordan Lite (2008-10-08). "Medical Mystery: Only One Person Has Survived Rabies without Vaccine--But How?". Scientific American. Retrieved 2010-01-30.
- ↑ "Rabies". World Health Organization (WHO). September 2011. Retrieved 31 December 2011.
- ↑ "Rabies Registry". Medical College of Wisconsin. Retrieved 29 December 2009.
Complications
There are many complications associated the rabies. Someone that has not been vaccinated against rabies can develop severe complications. Rabies is typically fatal if not treated properly.
- Coma
- Respiratory failure
- CSF lactic acidosis
- Poikilothermia
There are also complications that are seen with rabies, but they are not as common as the previous list. These complications are:
- Ileus
- Hypotension
- Diabetes insipidus
- Cerebral edema
- Hypersalivation
- Hyponatraemia
- Cardiac arrhythmias
Some complications don't occur that often, but they should be mentioned as a possibility:
References
Prognosis
Treatment after exposure (receiving the vaccines), known as post-exposure prophylaxis (PEP), is highly successful in preventing the disease if administered promptly, in general within ten days of infection. Begun with little or no delay, PEP is 100% effective against rabies.[1] In the case in which there has been a significant delay in administering PEP, the treatment should be administered regardless of that delay, as it may still be effective.
In unvaccinated humans, rabies is usually fatal after neurological symptoms have developed, but prompt post-exposure vaccination may prevent the virus from progressing. Rabies kills around 55,000 people a year, mostly in Asia and Africa.[2]
Survival data using the Milwaukee protocol are available from the rabies registry.[3] As of 2011, seven people have been saved by this induced coma treatment.
References
- ↑ Jordan Lite (2008-10-08). "Medical Mystery: Only One Person Has Survived Rabies without Vaccine--But How?". Scientific American. Retrieved 2010-01-30.
- ↑ "Rabies". World Health Organization (WHO). September 2011. Retrieved 31 December 2011.
- ↑ "Rabies Registry". Medical College of Wisconsin. Retrieved 29 December 2009.
Diagnosis
{{#ask:Used To Diagnose::Rabies |?Sort Order |format=list |headers=hide |link=none |sep= | |template=MedicalTestQuery |sort=Sort Order }}
Treatment
{{#ask:Used To Treat::Rabies |?Sort Order |format=list |headers=hide |link=none |sep= | |template=MedicalTreatmentQuery |sort=Sort Order }} {{#ask:Prevents::Rabies |?Sort Order |intro= | |format=list |headers=hide |link=none |sep= | |template=MedicalTreatmentQuery2 |sort=Sort Order }}
{{#set:On The Web = }}