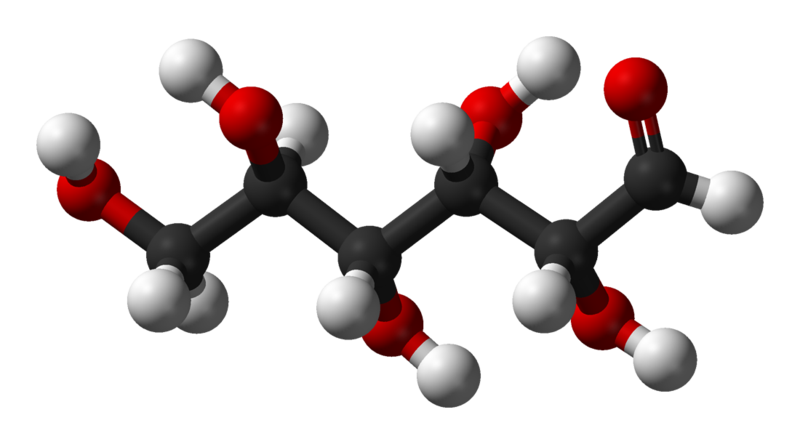
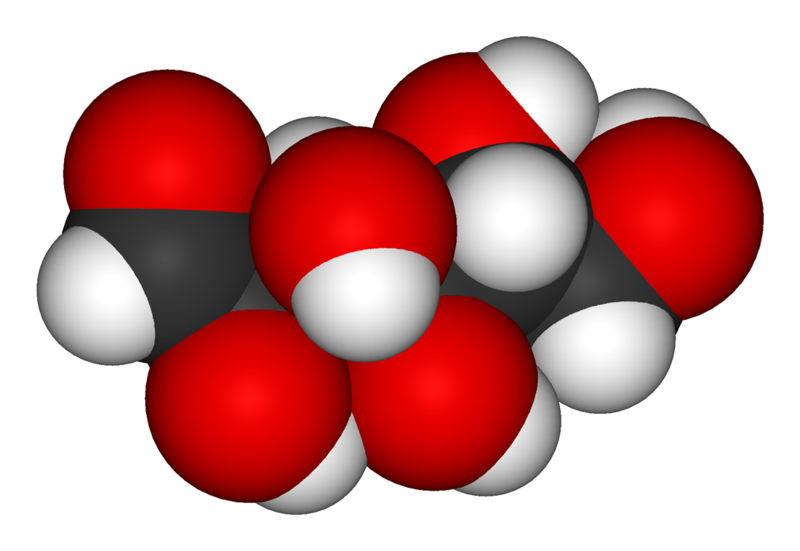
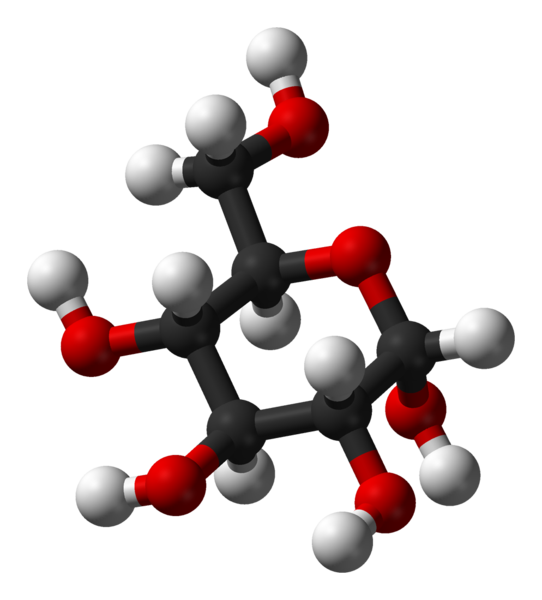
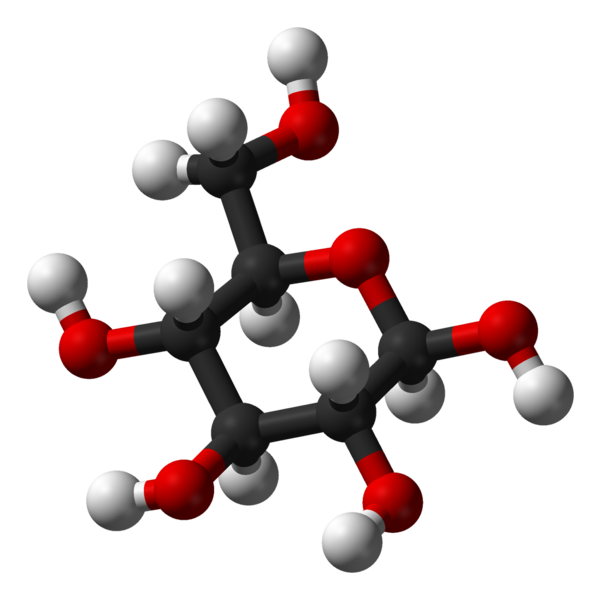
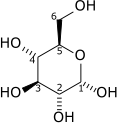
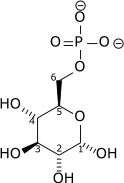
Glucose
|
WikiDoc Resources for Glucose |
|
Articles |
|---|
|
Most recent articles on Glucose |
|
Media |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Glucose at Clinical Trials.gov Clinical Trials on Glucose at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Glucose
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Directions to Hospitals Treating Glucose Risk calculators and risk factors for Glucose
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Glucose |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Glucose (Glc), a monosaccharide (or simple sugar), is an important carbohydrate in biology. The living cell uses it as a source of energy and metabolic intermediate. Glucose is one of the main products of photosynthesis and starts cellular respiration in both prokaryotes and eukaryotes. The name comes from the Greek word glykys (γλυκύς), which means "sweet", plus the suffix "-ose" which denotes a sugar.
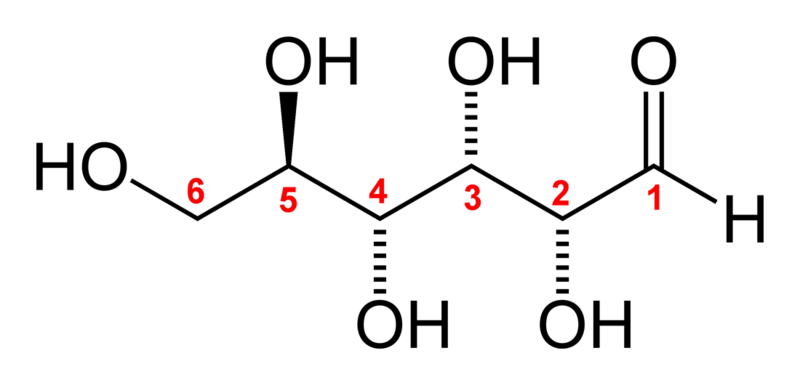
Two stereoisomers of the aldohexose sugars are known as glucose, only one of which (D-glucose) is biologically active. This form (D-glucose) is often referred to as dextrose monohydrate, or, especially in the food industry, simply dextrose (from dextrorotatory glucose[1]). This article deals with the D-form of glucose. The mirror-image of the molecule, L-glucose, cannot be metabolized by cells in the biochemical process known as glycolysis.
Glucose is commonly available in the form of a white substance or as a solid crystal. It can also be commonly found as an aqueous solution.
Structure
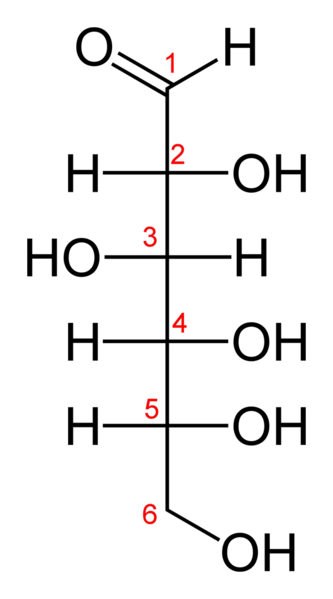
Glucose (C6H12O6) contains six carbon atoms one of which is part of an aldehyde group and is therefore referred to as an aldohexose. In solution, the glucose molecule can exist in an open-chain (acyclic) and ring (cyclic) form (in equilibrium), the latter being the result of a covalent bond between the aldehyde C atom and the C-5 hydroxyl group to form a six-membered cyclic hemiacetal. At pH 7 the cyclic form is predominant. In the solid phase, glucose assumes the cyclic form. As the ring contains five carbon atoms and one oxygen atom, which resembles the structure of pyran, the cyclic form of glucose is also referred to as glucopyranose. In this ring, each carbon is linked to a hydroxyl side group with the exception of the fifth atom, which links to a sixth carbon atom outside the ring, forming a CH2OH group.
Isomers
Aldohexose sugars have 4 chiral centers giving 24 = 16 stereoisomers. These are split into two groups, L and D, with 8 sugars in each. Glucose is one of these sugars, and L and D-glucose are two of the stereoisomers. Only 7 of these are found in living organisms, of which D-glucose (Glu), D-galactose (Gal) and D-mannose (Man) are the most important. These eight isomers (including glucose itself) are all diastereoisomers in relation to each other and all belong to the D-series.
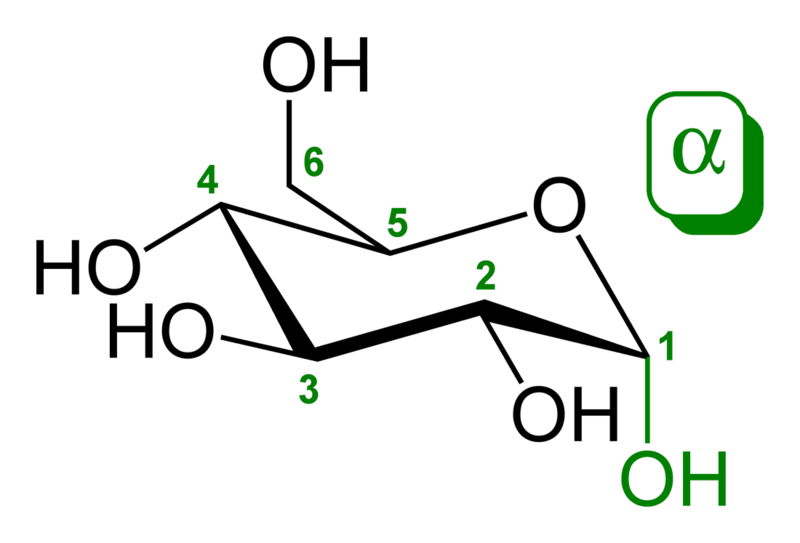
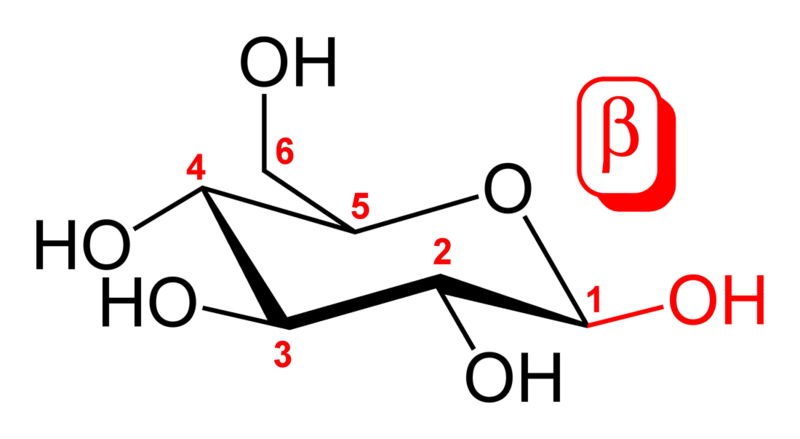
An additional asymmetric center at C-1 (called the anomeric carbon atom) is created when glucose cyclizes and two ring structures, called anomers are formed — α-glucose and β-glucose. These anomers differ structurally with respect to the relative positioning of their hydroxyl group linked to C-1 and the group at C-6, which is termed the reference carbon. When D-glucose is drawn as a Haworth projection or in the standard chair conformation, the designation α means that the hydroxyl group attached to C-1 is positioned trans to the -CH2OH group at C-5, while β means it is cis. Another popular method of distinguishing α from β is by observing whether the C-1 hydroxyl is below or above the plane of the ring, respectively, but this method is an inaccurate definition and may fail if the glucose ring is drawn upside down or in an alternative chair conformation. The α and β forms interconvert over a timescale of hours in aqueous solution, to a final stable ratio of α:β 36:64, in a process called mutarotation.[2]
-
The Fischer projection of the chain form of D-glucose -
The chain form of D-glucose -
α-D-
glucopyranose -
β-D-
glucopyranose -
Chain form: ball-and-stick model -
Chain form: space-filling model -
α-D-
glucopyranose -
β-D-
glucopyranose
Rotamers
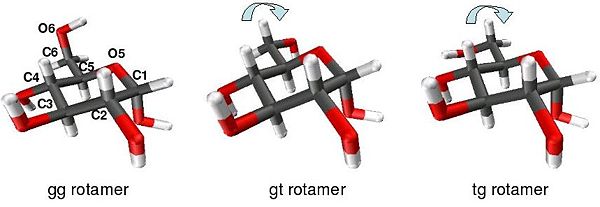
Within the cyclic form of glucose, rotation may occur around the O6-C6-C5-O5 torsion angle, termed the ω-angle, to form three rotamer conformations as shown in the diagram below. Referring to the orientations of the ω-angle and the O6-C6-C5-C4 angle the three stable staggered rotamer conformations are termed gauche-gauche (gg), gauche-trans (gt) and trans-gauche (tg). For methyl α-D-glucopyranose at equilibrium the ratio of molecules in each rotamer conformation is reported as 57:38:5 gg:gt:tg.[3] This tendency for the ω-angle to prefer to adopt a gauche conformation is attributed to the gauche effect.
Production
Natural
- Glucose is one of the products of photosynthesis in plants and some prokaryotes.
- In animals and fungi, glucose is the result of the breakdown of glycogen, a process known as glycogenolysis. In plants the breakdown substrate is starch.
- In animals, glucose is synthesized in the liver and kidneys from non-carbohydrate intermediates, such as pyruvate and glycerol, by a process known as gluconeogenesis.
Commercial
Glucose is produced commercially via the enzymatic hydrolysis of starch. Many crops can be used as the source of starch. Maize, rice, wheat, potato, cassava, arrowroot, and sago are all used in various parts of the world. In the United States, cornstarch (from maize) is used almost exclusively.
This enzymatic process has two stages. Over the course of 1-2 hours near 100 °C, enzymes hydrolyze starch into smaller carbohydrates containing on average 5-10 glucose units each. Some variations on this process briefly heat the starch mixture to 130 °C or hotter one or more times. This heat treatment improves the solubility of starch in water, but deactivates the enzyme, and fresh enzyme must be added to the mixture after each heating.
In the second step, known as "saccharification", the partially hydrolyzed starch is completely hydrolyzed to glucose using the glucoamylase enzyme from the fungus Aspergillus niger. Typical reaction conditions are pH 4.0–4.5, 60 °C, and a carbohydrate concentration of 30–35% by weight. Under these conditions, starch can be converted to glucose at 96% yield after 1–4 days. Still higher yields can be obtained using more dilute solutions, but this approach requires larger reactors and processing a greater volume of water, and is not generally economical. The resulting glucose solution is then purified by filtration and concentrated in a multiple-effect evaporator. Solid D-glucose is then produced by repeated crystallizations.
-
Glucose
-
Glucose tablets
Function
We can speculate on the reasons why glucose, and not another monosaccharide such as fructose (Fru), is so widely used in evolution, the ecosystem, and metabolism. Glucose can form from formaldehyde under abiotic conditions, so it may well have been available to primitive biochemical systems. Probably more important to advanced life is the low tendency of glucose, by comparison to other hexose sugars, to non-specifically react with the amino groups of proteins. This reaction (glycation) reduces or destroys the function of many enzymes. The low rate of glycation is due to glucose's preference for the less reactive cyclic isomer. Nevertheless, many of the long-term complications of diabetes (e.g., blindness, kidney failure, and peripheral neuropathy) are probably due to the glycation of proteins or lipids. In contrast, enzyme-regulated addition of glucose to proteins by glycosylation is often essential to their function.
As an energy source
Glucose is a ubiquitous fuel in biology. It is used as an energy source in most organisms, from bacteria to humans. Use of glucose may be by either aerobic or anaerobic respiration (fermentation). Carbohydrates are the human body's key source of energy, through aerobic respiration, providing approximately 3.75 kilocalories (16 kilojoules) of food energy per gram.[4] Breakdown of carbohydrates (e.g. starch) yields mono- and disaccharides, most of which is glucose. Through glycolysis and later in the reactions of the Citric acid cycle (TCAC), glucose is oxidized to eventually form CO2 and water, yielding energy, mostly in the form of ATP. The insulin reaction, and other mechanisms, regulate the concentration of glucose in the blood. A high fasting blood sugar level is an indication of prediabetic and diabetic conditions.
Glucose is a primary source of energy for the brain, and hence its availability influences psychological processes. When glucose is low, psychological processes requiring mental effort (e.g., self-control) are impaired.[5][6][7]
Glucose in glycolysis
| ||||||||||||||||||||
Use of glucose as an energy source in cells is via aerobic or anaerobic respiration. Both of these start with the early steps of the glycolysis metabolic pathway. The first step of this is the phosphorylation of glucose by hexokinase to prepare it for later breakdown to provide energy.
The major reason for the immediate phosphorylation of glucose by a hexokinase is to prevent diffusion out of the cell. The phosphorylation adds a charged phosphate group so the glucose 6-phosphate cannot easily cross the cell membrane. Irreversible first steps of a metabolic pathway are common for regulatory purposes.
As a precursor
Glucose is critical in the production of proteins and in lipid metabolism. Also, in plants and most animals, it is a precursor for vitamin C (ascorbic acid) production. It is modified for use in these processes by the glycolysis pathway.
Glucose is used as a precursor for the synthesis of several important substances. starch solution Starch, cellulose, and glycogen ("animal starch") are common glucose polymers (polysaccharides). Lactose, the predominant sugar in milk, is a glucose-galactose disaccharide. In sucrose, another important disaccharide, glucose is joined to fructose. These synthesis processes also rely on the phosphorylation of glucose through the first step of glycolysis.
Sources and absorption
All major dietary carbohydrates contain glucose, either as their only building block, as in starch and glycogen, or together with another monosaccharide, as in sucrose and lactose. In the lumen of the duodenum and small intestine, the oligo- and polysaccharides are broken down to monosaccharides by the pancreatic and intestinal glycosidases. Glucose is then transported across the apical membrane of the enterocytes by SLC5A1, and later across their basal membrane by SLC2A2.[8] Some of the glucose goes directly toward fueling brain cells and erythrocytes, while the rest makes its way to the liver and muscles, where it is stored as glycogen, and to fat cells, where it can be used to power reactions which synthesize some fats. Glycogen is the body's auxiliary energy source, tapped and converted back into glucose when there is need for energy.
See also
- Blood glucose or Blood Sugar
- HbA1c
- DMF (potential glucose-based biofuel)
- Glycation
- Glycosylation
- Photosynthesis
- Fructose
ATP
ADP
ATP
ADP
+ +
NAD++ Pi
NADH + H+
NAD++ Pi
NADH + H+ H2O
H2O ADP
ATP
2 × Pyruvate 2 × File:Pyruvat.svg
|
References
- ↑ dextrose - Definition from the Merriam-Webster Online Dictionary
- ↑ McMurry, John (1988). Organic Chemistry. Brooks/Cole. p. 866. ISBN 0534079687.
- ↑ Kirschner, K.N. Woods, R.J. (2001). "Solvent interactions determine carbohydrate conformation". Proc. Natl. Acad. Sci. USA. 98 (19): 10541–10545. PMID 11526221.
- ↑ http://www.fao.org/docrep/006/Y5022E/y5022e04.htm
- ↑ Fairclough, S. H., & Houston, K. (2004). "A metabolic measure of mental effort". Biological Psychology. 66: 177–190.
- ↑ Gailliot, M.T., Baumeister, R.F., DeWall, C.N., Maner, J.K., Plant, E.A., Tice, D.M., Brewer, L.E., & Schmeichel, B.J. (2007). "Self-Control relies on glucose as a limited energy source: Willpower is more than a metaphor". Journal of Personality and Social Psychology. 92: 325–336.
- ↑ Gailliot, M.T., & Baumeister, R.F. (in press). "The physiology of willpower: Linking blood glucose to self-control". Personality and Social Psychology Review. Check date values in:
|year=(help) - ↑ Ferraris, Ronaldo P. (2001). "Dietary and developmental regulation of intestinal sugar transport". Biochemical Journal (360): 265–276. Retrieved 2007-12-21.
ar:غلوكوز be:Глюкоза be-x-old:Глюкоза bs:Glukoza bg:Глюкоза ca:Glucosa cs:Glukóza da:Glukose de:Traubenzucker et:Glükoos eo:Glukozo gl:Glicosa ko:글루코스 hr:Glukoza id:Glukosa is:Glúkósi it:Glucosio he:גלוקוז ka:გლუკოზა la:Glucosium lv:Glikoze lt:Gliukozė hu:Glükóz mk:Глукоза ms:Glukosa nl:Glucose no:Glukose nn:Glukose oc:Glucòsa om:Glucose simple:Glucose sk:Glukóza sl:Glukoza sr:Глукоза sh:Glukoza su:Glukosa fi:Glukoosi sv:Glukos te:గ్లూకోస్ th:กลูโคส uk:Глюкоза