Quinine: Difference between revisions
Adeel Jamil (talk | contribs) No edit summary |
Adeel Jamil (talk | contribs) No edit summary |
||
| Line 1: | Line 1: | ||
{{DrugProjectFormSinglePage | {{DrugProjectFormSinglePage | ||
|authorTag={{AJ}} | |authorTag={{AJ}} | ||
|genericName=Quinine Sulfate | |genericName=[[Quinine Sulfate]] | ||
|aOrAn=a | |aOrAn=a | ||
|drugClass=cinchona alkaloid, antimalarial and anti-Infective Agent, | |drugClass=[[cinchona alkaloid]], [[antimalarial]] and [[anti-Infective]] Agent, | ||
|indicationType=treatment | |indicationType=treatment | ||
|indication=of uncomplicated Plasmodium falciparum malaria | |indication=of uncomplicated [[Plasmodium falciparum]] [[malaria]] | ||
|hasBlackBoxWarning=Yes | |hasBlackBoxWarning=Yes | ||
|adverseReactions=[[cinchonism]] include [[headache]], [[vasodilation]] and [[sweating]], [[nausea]], [[tinnitus]], [[hearing impairment]], [[vertigo]] or [[dizziness]], [[blurred vision]], and disturbance in color perception. | |adverseReactions=[[cinchonism]] include [[headache]], [[vasodilation]] and [[sweating]], [[nausea]], [[tinnitus]], [[hearing impairment]], [[vertigo]] or [[dizziness]], [[blurred vision]], and disturbance in color perception. | ||
|blackBoxWarningTitle=WARNING | |blackBoxWarningTitle=WARNING | ||
|blackBoxWarningBody=* Quinine sulphate use for the treatment or prevention of nocturnal leg cramps may result in serious and life-threatening hematologic reactions, including thrombocytopenia and hemolytic uremic syndrome/thrombotic thrombocytopenic purpura (HUS/TTP). Chronic renal impairment associated with the development of TTP has been reported. The risk associated with Quinine sulphate use in the absence of evidence of its effectiveness in the treatment or prevention of nocturnal leg cramps outweighs any potential benefit | |blackBoxWarningBody=* Quinine sulphate use for the treatment or prevention of nocturnal leg cramps may result in serious and life-threatening [[hematologic]] reactions, including [[thrombocytopenia]] and [[hemolytic uremic syndrome]]/[[thrombotic thrombocytopenic purpura]] ([[HUS]]/[[TTP]]). [[CRF|Chronic renal impairment]] associated with the development of [[TTP]] has been reported. The risk associated with Quinine sulphate use in the absence of evidence of its effectiveness in the treatment or prevention of nocturnal [[leg cramps]] outweighs any potential benefit | ||
<!--FDA-Labeled Indications and Dosage (Adult)--> | <!--FDA-Labeled Indications and Dosage (Adult)--> | ||
| Line 18: | Line 18: | ||
:* Treatment of severe or complicated [[P. falciparum malaria]]. | :* Treatment of severe or complicated [[P. falciparum malaria]]. | ||
:* Prevention of [[malaria]]. | :* Prevention of [[malaria]]. | ||
:* Treatment or prevention of nocturnal leg cramps | :* Treatment or prevention of [[nocturnal]] [[leg cramps]] | ||
====Dosing Information==== | |||
* Treatment of Uncomplicated [[P. falciparum Malaria]] | * Treatment of Uncomplicated [[P. falciparum Malaria]] | ||
* For treatment of uncomplicated [[P. falciparum malaria]] in adults: Orally, 648 mg (two capsules) every 8 hours for 7 days. | * For treatment of uncomplicated [[P. falciparum malaria]] in adults: Orally, 648 mg (two capsules) every 8 hours for 7 days. | ||
* Quinine sulphate should be taken with food to minimize gastric upset. | * Quinine sulphate should be taken with food to minimize [[gastric upset]]. | ||
===== | =====Renal Impairment===== | ||
* In patients with acute uncomplicated [[malaria]] and severe | * In patients with acute uncomplicated [[malaria]] and severe [[CRF|chronic renal impairment]], the following dosage regimen is recommended: one loading dose of 648 mg Quinine sulphate followed 12 hours later by maintenance doses of 324 mg every 12 hours. | ||
* The effects of mild and moderate renal impairment on the safety and [[pharmacokinetic]]s of quinine sulfate are not known. | * The effects of mild and moderate [[renal impairment]] on the safety and [[pharmacokinetic]]s of quinine sulfate are not known. | ||
===== | =====Hepatic Impairment===== | ||
* Adjustment of the recommended dose is not required in mild ([[Child-Pugh A]]) or moderate ([[Child-Pugh B]]) [[hepatic impairment]], but patients should be monitored closely for adverse effects of quinine. Quinine should not be administered in patients with severe ([[Child-Pugh C]]) [[hepatic impairment]]. | * Adjustment of the recommended dose is not required in mild ([[Child-Pugh A]]) or moderate ([[Child-Pugh B]]) [[hepatic impairment]], but patients should be monitored closely for adverse effects of quinine. Quinine should not be administered in patients with severe ([[Child-Pugh C]]) [[hepatic impairment]]. | ||
| Line 52: | Line 52: | ||
|contraindications=Quinine sulphate is contraindicated in patients with the following: | |contraindications=Quinine sulphate is contraindicated in patients with the following: | ||
===== | =====Prolonged QT interval:===== | ||
* One case of a fatal [[ventricular arrhythmia]] was reported in an elderly patient with a [[prolonged QT interval]] at baseline, who received quinine sulfate intravenously for [[P. falciparum malaria]]. | * One case of a [[fatal]] [[ventricular arrhythmia]] was reported in an elderly patient with a [[prolonged QT interval]] at baseline, who received quinine sulfate intravenously for [[P. falciparum malaria]]. | ||
=====[[Glucose-6-phosphate dehydrogenase deficiency]](G6PD):===== | =====[[Glucose-6-phosphate dehydrogenase deficiency]]([[G6PD]]):===== | ||
* [[Hemolysis]] can occur in patients with [[G6PD deficiency]] receiving quinine. | * [[Hemolysis]] can occur in patients with [[G6PD deficiency]] receiving quinine. | ||
=====Known | =====Known hypersensitivity reactions to quinine:===== | ||
* These include, but are not limited to, the following: | * These include, but are not limited to, the following: | ||
:* [[Thrombocytopenia]] | :* [[Thrombocytopenia]] | ||
:* [[Idiopathic thrombocytopenia purpura]] (ITP) and [[Thrombotic thrombocytopenic purpura]] (TTP) | :* [[Idiopathic thrombocytopenia purpura]] ([[ITP]]) and [[Thrombotic thrombocytopenic purpura]] ([[TTP]]) | ||
[[Hemolytic uremic syndrome]] (HUS) | [[Hemolytic uremic syndrome]] (HUS) | ||
:* [[Blackwater fever]] ([[acute intravascular hemolysis]], [[hemoglobinuria]], and [[hemoglobinemia]]) | :* [[Blackwater fever]] ([[acute intravascular hemolysis]], [[hemoglobinuria]], and [[hemoglobinemia]]) | ||
| Line 67: | Line 67: | ||
:* [[Myasthenia gravis]]. Quinine has [[neuromuscular]] blocking activity, and may exacerbate muscle weakness. | :* [[Myasthenia gravis]]. Quinine has [[neuromuscular]] blocking activity, and may exacerbate muscle weakness. | ||
:* [[Optic neuritis]]. Quinine may exacerbate active [[optic neuritis]] | :* [[Optic neuritis]]. Quinine may exacerbate active [[optic neuritis]] | ||
|warnings | |warnings=====Use of Quinine for Treatment or Prevention of Nocturnal Leg Cramps:===== | ||
* Quinine sulphate may cause unpredictable serious and life-threatening [[hematologic]] reactions including [[thrombocytopenia]] and [[hemolytic-uremic syndrome]]/[[thrombotic thrombocytopenic purpura]] ([[HUS]]/[[TTP]]) in addition to [[hypersensitivity]] reactions, [[QT prolongation]], serious [[cardiac arrhythmias]] including [[torsades de pointes]], and other serious adverse events requiring medical intervention and hospitalization. Chronic [[renal impairment]] associated with the development of [[TTP]], and fatalities have also been reported. The risk associated with the use of Quinine sulphate in the absence of evidence of its effectiveness for treatment or prevention of nocturnal leg cramps, outweighs any potential benefit in treating and/or preventing this benign, self-limiting condition. | * Quinine sulphate may cause unpredictable serious and life-threatening [[hematologic]] reactions including [[thrombocytopenia]] and [[hemolytic-uremic syndrome]]/[[thrombotic thrombocytopenic purpura]] ([[HUS]]/[[TTP]]) in addition to [[hypersensitivity]] reactions, [[QT prolongation]], serious [[cardiac arrhythmias]] including [[torsades de pointes]], and other serious adverse events requiring medical intervention and hospitalization. Chronic [[renal impairment]] associated with the development of [[TTP]], and fatalities have also been reported. The risk associated with the use of Quinine sulphate in the absence of evidence of its effectiveness for treatment or prevention of nocturnal [[leg cramps]], outweighs any potential benefit in treating and/or preventing this benign, self-limiting condition. | ||
=====[[Thrombocytopenia]]===== | =====[[Thrombocytopenia]]===== | ||
* Quinine-induced [[thrombocytopenia]] is an immune-mediated disorder. Severe cases of [[thrombocytopenia]] that are fatal or life threatening have been reported, including cases of [[HUS]]/[[TTP]]. Chronic [[renal impairment]] associated with the development of [[TTP]] has also been reported. [[Thrombocytopenia]] usually resolves within a week upon discontinuation of quinine. If quinine is not stopped, a patient is at risk for fatal [[hemorrhage]]. Upon re-exposure to quinine from any source, a patient with quinine-dependent [[antibodies]] could develop [[thrombocytopenia]] that is more rapid in onset and more severe than the original episode. | * Quinine-induced [[thrombocytopenia]] is an immune-mediated disorder. Severe cases of [[thrombocytopenia]] that are fatal or life threatening have been reported, including cases of [[HUS]]/[[TTP]]. Chronic [[renal impairment]] associated with the development of [[TTP]] has also been reported. [[Thrombocytopenia]] usually resolves within a week upon discontinuation of quinine. If quinine is not stopped, a patient is at risk for fatal [[hemorrhage]]. Upon re-exposure to quinine from any source, a patient with quinine-dependent [[antibodies]] could develop [[thrombocytopenia]] that is more rapid in onset and more severe than the original episode. | ||
===== | =====QT Prolongation and Ventricular Arrhythmias:===== | ||
* [[QT interval prolongation]] has been a consistent finding in studies which evaluated [[electrocardiographic]] changes with oral or [[parenteral]] quinine administration, regardless of age, clinical status, or severity of disease. The maximum increase in [[QT interval]] has been shown to correspond with peak quinine plasma concentration. Quinine sulfate has been rarely associated with potentially fatal [[cardiac arrhythmias]], including [[torsades de pointes]], and [[ventricular fibrillation]]. | * [[QT interval prolongation]] has been a consistent finding in studies which evaluated [[electrocardiographic]] changes with oral or [[parenteral]] quinine administration, regardless of age, clinical status, or severity of disease. The maximum increase in [[QT interval]] has been shown to correspond with peak quinine plasma concentration. Quinine sulfate has been rarely associated with potentially fatal [[cardiac arrhythmias]], including [[torsades de pointes]], and [[ventricular fibrillation]]. | ||
* Quinine sulphate has been shown to cause concentration-dependent prolongation of the [[PR]] and [[QRS]] interval. At particular risk are patients with underlying [[structural heart disease]] and preexisting conduction system abnormalities, elderly patients with [[sick sinus syndrome]], patients with [[atrial fibrillation]] with slow ventricular response, patients with [[myocardial ischemia]] or patients receiving drugs known to prolong the [[PR interval]] (e.g. [[verapamil]]) or [[QRS interval]] (e.g. [[flecainide]] or [[quinidine]]). | * Quinine sulphate has been shown to cause concentration-dependent prolongation of the [[PR]] and [[QRS]] interval. At particular risk are patients with underlying [[structural heart disease]] and preexisting conduction system abnormalities, elderly patients with [[sick sinus syndrome]], patients with [[atrial fibrillation]] with slow ventricular response, patients with [[myocardial ischemia]] or patients receiving drugs known to prolong the [[PR interval]] (e.g. [[verapamil]]) or [[QRS interval]] (e.g. [[flecainide]] or [[quinidine]]). | ||
* Quinine sulphate is not recommended for use with other drugs known to cause [[QT prolongation]], including Class IA | * Quinine sulphate is not recommended for use with other drugs known to cause [[QT prolongation]], including [[antiarrhythmic agents|Class IA antiarrhythmic agents]] (e.g., [[quinidine]], [[procainamide]], [[disopyramide]]), and [[antiarrhythmic agents|Class III antiarrhythmic agent]]s (e.g., [[amiodarone]], [[sotalol]], [[dofetilide]]). | ||
* The use of [[macrolide]] [[antibiotic]]s such as [[erythromycin]] should be avoided in patients receiving Quinine sulphate. Fatal [[torsades de pointes]] was reported in an elderly patient who received concomitant quinine, [[erythromycin]], and [[dopamine]]. Although a causal relationship between a specific drug and the arrhythmia was not established in this case, [[erythromycin]] is a [[CYP3A4 inhibitor]] and has been shown to increase quinine plasma levels when used concomitantly. A related [[macrolide]] [[antibiotic]], [[troleandomycin]], has also been shown to increase quinine exposure in a [[pharmacokinetic]] study. | * The use of [[macrolide]] [[antibiotic]]s such as [[erythromycin]] should be avoided in patients receiving Quinine sulphate. Fatal [[torsades de pointes]] was reported in an [[elderly]] patient who received concomitant quinine, [[erythromycin]], and [[dopamine]]. Although a causal relationship between a specific drug and the arrhythmia was not established in this case, [[erythromycin]] is a [[CYP3A4 inhibitor]] and has been shown to increase quinine plasma levels when used concomitantly. A related [[macrolide]] [[antibiotic]], [[troleandomycin]], has also been shown to increase quinine exposure in a [[pharmacokinetic]] study. | ||
* Quinine may inhibit the metabolism of certain drugs that are [[CYP3A4]] substrates and are known to cause [[QT prolongation]], e.g., [[astemizole]], [[cisapride]], [[terfenadine]], [[pimozide]], [[halofantrine]] and [[quinidine]]. [[Torsades de pointes]] has been reported in patients who received concomitant quinine and [[astemizole]]. Therefore, concurrent use of Quinine sulphate with these medications, or drugs with similar properties, should be avoided. | * Quinine may inhibit the [[metabolism]] of certain drugs that are [[CYP3A4]] substrates and are known to cause [[QT prolongation]], e.g., [[astemizole]], [[cisapride]], [[terfenadine]], [[pimozide]], [[halofantrine]] and [[quinidine]]. [[Torsades de pointes]] has been reported in patients who received concomitant quinine and [[astemizole]]. Therefore, concurrent use of Quinine sulphate with these medications, or drugs with similar properties, should be avoided. | ||
* Concomitant administration of Quinine sulphate with the [[antimalarial drug]]s, [[mefloquine]] or [[halofantrine]], may result in [[electrocardiographic]] abnormalities, including [[QT prolongation]], and increase the risk for [[torsades de pointes]] or other serious [[ventricular arrhythmias]]. Concurrent use of Quinine sulphate and [[mefloquine]] may also increase the risk of [[seizure]]s. | * Concomitant administration of Quinine sulphate with the [[antimalarial drug]]s, [[mefloquine]] or [[halofantrine]], may result in [[electrocardiographic]] abnormalities, including [[QT prolongation]], and increase the risk for [[torsades de pointes]] or other serious [[ventricular arrhythmias]]. Concurrent use of Quinine sulphate and [[mefloquine]] may also increase the risk of [[seizure]]s. | ||
* Quinine sulphate should also be avoided in patients with known [[prolongation of QT interval]] and in patients with clinical conditions known to prolong the [[QT interval]], such as uncorrected [[hypokalemia]], [[bradycardia]], and certain cardiac conditions. | * Quinine sulphate should also be avoided in patients with known [[prolongation of QT interval]] and in patients with clinical conditions known to prolong the [[QT interval]], such as uncorrected [[hypokalemia]], [[bradycardia]], and certain [[cardiac]] conditions. | ||
=====Concomitant Use of [[Rifampin]]:===== | =====Concomitant Use of [[Rifampin]]:===== | ||
* Treatment failures may result from the concurrent use of [[rifampin]] with Quinine sulphate, due to decreased plasma concentrations of quinine, and concomitant use of these medications should be avoided. | * Treatment failures may result from the concurrent use of [[rifampin]] with Quinine sulphate, due to decreased plasma concentrations of quinine, and concomitant use of these medications should be avoided. | ||
=====Concomitant Use of | =====Concomitant Use of Neuromuscular Blocking Agents:===== | ||
* The use of [[neuromuscular blocking agent]]s should be avoided in patients receiving Quinine sulphate. In one patient who received [[pancuronium]] during an operative procedure, subsequent administration of quinine resulted in [[respiratory depression]] and [[apnea]]. Although there are no clinical reports with [[succinylcholine]] or [[tubocurarine]], quinine may also potentiate [[neuromuscular]] blockade when used with these drugs. | * The use of [[neuromuscular blocking agent]]s should be avoided in patients receiving Quinine sulphate. In one patient who received [[pancuronium]] during an operative procedure, subsequent administration of quinine resulted in [[respiratory depression]] and [[apnea]]. Although there are no clinical reports with [[succinylcholine]] or [[tubocurarine]], quinine may also potentiate [[neuromuscular]] blockade when used with these drugs. | ||
===== | =====Hypersensitivity===== | ||
* Serious [[hypersensitivity]] reactions reported with quinine sulfate include [[anaphylactic shock]], [[anaphylactoid reactions]], [[urticaria]], serious skin [[rashes]], including [[Stevens-Johnson syndrome]] and [[toxic epidermal necrolysis]], [[angioedema]], [[facial edema]], [[bronchospasm]], and [[pruritus]]. | * Serious [[hypersensitivity]] reactions reported with quinine sulfate include [[anaphylactic shock]], [[anaphylactoid reactions]], [[urticaria]], serious [[skin]] [[rashes]], including [[Stevens-Johnson syndrome]] and [[toxic epidermal necrolysis]], [[angioedema]], [[facial edema]], [[bronchospasm]], and [[pruritus]]. | ||
* A number of other serious adverse reactions reported with quinine, including [[thrombotic thrombocytopenic purpura]] ([[TTP]]) and [[hemolytic uremic syndrome]] ([[HUS]]), [[thrombocytopenia]], [[immune thrombocytopenic purpura]] ([[ITP]]), [[blackwater fever]], [[disseminated intravascular coagulation]], [[leukopenia]], [[neutropenia]], [[granulomatous hepatitis]], and [[acute interstitial nephritis]] may also be due to [[hypersensitivity]] reactions. | * A number of other serious adverse reactions reported with quinine, including [[thrombotic thrombocytopenic purpura]] ([[TTP]]) and [[hemolytic uremic syndrome]] ([[HUS]]), [[thrombocytopenia]], [[immune thrombocytopenic purpura]] ([[ITP]]), [[blackwater fever]], [[disseminated intravascular coagulation]], [[leukopenia]], [[neutropenia]], [[granulomatous hepatitis]], and [[acute interstitial nephritis]] may also be due to [[hypersensitivity]] reactions. | ||
| Line 101: | Line 101: | ||
* Quinine sulphate should be discontinued in case of any signs or symptoms of [[hypersensitivity]]. | * Quinine sulphate should be discontinued in case of any signs or symptoms of [[hypersensitivity]]. | ||
===== | =====Atrial Fibrillation and Flutter:===== | ||
* Quinine sulphate should be used with caution in patients with [[atrial fibrillation]] or [[atrial flutter]]. A paradoxical increase in ventricular response rate may occur with quinine, similar to that observed with quinidine. If [[digoxin]] is used to prevent a rapid ventricular response, serum [[digoxin]] levels should be closely monitored, because [[digoxin]] levels may be increased with use of quinine. | * Quinine sulphate should be used with caution in patients with [[atrial fibrillation]] or [[atrial flutter]]. A paradoxical increase in ventricular response rate may occur with quinine, similar to that observed with quinidine. If [[digoxin]] is used to prevent a rapid ventricular response, serum [[digoxin]] levels should be closely monitored, because [[digoxin]] levels may be increased with use of quinine. | ||
===== | =====Hypoglycemia===== | ||
* Quinine stimulates release of [[insulin]] from the [[pancreas]], and patients, especially [[pregnant]] women, may experience clinically significant [[hypoglycemia]]. | * Quinine stimulates release of [[insulin]] from the [[pancreas]], and patients, especially [[pregnant]] women, may experience clinically significant [[hypoglycemia]]. | ||
|clinicalTrials | |clinicalTrials=====Overall===== | ||
* Quinine can adversely affect almost every body system. The most common adverse events associated with quinine use are a cluster of symptoms called "cinchonism", which occurs to some degree in almost all patients taking quinine. Symptoms of mild cinchonism include headache, vasodilation and sweating, nausea, tinnitus, hearing impairment, vertigo or dizziness, blurred vision, and disturbance in color perception. More severe symptoms of cinchonism are vomiting, diarrhea, abdominal pain, deafness, blindness, and disturbances in cardiac rhythm or conduction. Most symptoms of cinchonism are reversible and resolve with discontinuation of quinine. | * Quinine can adversely affect almost every body system. The most common adverse events associated with quinine use are a cluster of symptoms called "[[cinchonism]]", which occurs to some degree in almost all patients taking quinine. Symptoms of mild [[cinchonism]] include headache, [[vasodilation]] and [[sweating]], [[nausea]], [[tinnitus]], [[hearing impairment]], [[vertigo]] or [[dizziness]], [[blurred vision]], and disturbance in color perception. More severe symptoms of [[cinchonism]] are [[vomiting]], [[diarrhea]], [[abdominal pain]], [[deafness]], [[blindness]], and disturbances in [[cardiac rhythm]] or conduction. Most symptoms of [[cinchonism]] are reversible and resolve with discontinuation of quinine. | ||
* The following ADVERSE REACTIONS have been reported with quinine sulfate. Most of these reactions are thought to be uncommon, but the actual incidence is unknown: | * The following ADVERSE REACTIONS have been reported with quinine sulfate. Most of these reactions are thought to be uncommon, but the actual [[incidence]] is unknown: | ||
=====General:===== | =====General:===== | ||
* Fever, chills, sweating, flushing, asthenia, lupus-like syndrome, and hypersensitivity reactions. | * [[Fever]], [[chills]], [[sweating]], [[flushing]], [[asthenia]], [[lupus]]-like syndrome, and [[hypersensitivity]] reactions. | ||
=====Hematologic:===== | =====Hematologic:===== | ||
* Agranulocytosis, hypoprothrombinemia, thrombocytopenia, disseminated intravascular coagulation, hemolytic anemia; hemolytic uremic syndrome, thrombotic thrombocytopenic purpura, idiopathic thrombocytopenic purpura, petechiae, ecchymosis, hemorrhage, coagulopathy, blackwater fever, leukopenia, neutropenia, pancytopenia, aplastic anemia, and lupus anticoagulant. | * [[Agranulocytosis]], [[hypoprothrombinemia]], [[thrombocytopenia]], [[disseminated intravascular coagulation,]] [[hemolytic anemia]]; [[hemolytic uremic syndrome]], [[thrombotic thrombocytopenic purpura]], [[idiopathic thrombocytopenic purpura]], [[petechiae]], [[ecchymosis]], [[hemorrhage]], [[coagulopathy]], [[blackwater]] [[fever]], [[leukopenia]], [[neutropenia]], [[pancytopenia]], [[aplastic anemia]], and [[lupus anticoagulant]]. | ||
=====Neuropsychiatric:===== | =====Neuropsychiatric:===== | ||
* Headache, diplopia, confusion, altered mental status, seizures, coma, disorientation, tremors, restlessness, ataxia, acute dystonic reaction, aphasia, and suicide. | * [[Headache]], [[diplopia]], [[confusion]], [[altered mental status]], [[seizures]], [[coma]], [[disorientation]], [[tremors]], [[restlessness]], [[ataxia]], [[acute dystonic reaction]], [[aphasia]], and [[suicide]]. | ||
=====Dermatologic:===== | =====Dermatologic:===== | ||
* Cutaneous rashes, including urticarial, papular, or scarlatinal rashes, pruritus, bullous dermatitis, exfoliative dermatitis, erythema multiforme, Stevens-Johnson syndrome, toxic epidermal necrolysis, fixed drug eruption, photosensitivity reactions, allergic contact dermatitis, acral necrosis, and cutaneous vasculitis. | * Cutaneous [[rashes]], including [[urticarial]], [[papular]], or scarlatinal [[rashes]], [[pruritus]], [[bullous]] [[dermatitis]], [[exfoliative dermatitis]], [[erythema multiforme]], [[Stevens-Johnson syndrome]], [[toxic epidermal necrolysis]], fixed drug eruption, [[photosensitivity]] reactions, [[allergic contact dermatitis]], [[acral necrosis]], and cutaneous [[vasculitis]]. | ||
=====Respiratory:===== | =====Respiratory:===== | ||
* Asthma, dyspnea, pulmonary edema. | * [[Asthma]], [[dyspnea]], [[pulmonary edema]]. | ||
=====Cardiovascular:===== | =====Cardiovascular:===== | ||
* Chest pain, vasodilatation, hypotension, postural hypotension, tachycardia, bradycardia, palpitations, syncope, atrioventricular block, atrial fibrillation, irregular rhythm, unifocal premature ventricular contractions, nodal escape beats, U waves, QT prolongation, ventricular fibrillation, ventricular tachycardia, torsades de pointes, and cardiac arrest. | * [[Chest pain]], [[vasodilatation]], [[hypotension]], [[postural hypotension]], [[tachycardia]], [[bradycardia]], [[palpitations]], [[syncope]], [[atrioventricular block]], [[atrial fibrillation]], [[irregular rhythm]], unifocal [[premature ventricular contractions]], [[nodal escape beats]], [[U waves]], [[QT prolongation]], [[ventricular fibrillation]], [[ventricular tachycardia]], [[torsades de pointes,]] and [[cardiac arrest]]. | ||
=====Gastrointestinal:===== | =====Gastrointestinal:===== | ||
* Nausea, vomiting, diarrhea, abdominal pain, gastric irritation, and esophagitis. | * [[Nausea]], [[vomiting]], [[diarrhea]], [[abdominal pain]], [[gastric irritation]], and [[esophagitis]]. | ||
=====Hepatobiliary:===== | =====Hepatobiliary:===== | ||
* Granulomatous hepatitis, hepatitis, jaundice, and abnormal liver function tests. | * [[Granulomatous hepatitis]], [[hepatitis]], [[jaundice]], and abnormal [[liver function tests]]. | ||
=====Metabolic:===== | =====Metabolic:===== | ||
* Hypoglycemia and anorexia. | * [[Hypoglycemia]] and [[anorexia]]. | ||
=====Musculoskeletal:===== | =====Musculoskeletal:===== | ||
* Myalgias and muscle weakness. | * [[Myalgias]] and [[muscle weakness]]. | ||
=====Renal:===== | =====Renal:===== | ||
* Hemoglobinuria, renal failure, renal impairment, and acute interstitial nephritis. | * [[Hemoglobinuria]], [[renal failure]], [[renal impairment]], and [[acute interstitial nephritis]]. | ||
=====Special Senses:===== | =====Special Senses:===== | ||
* Visual disturbances, including blurred vision with scotomata, sudden loss of vision, photophobia, diplopia, night blindness, diminished visual fields, fixed pupillary dilatation, disturbed color vision, optic neuritis, blindness, vertigo, tinnitus, hearing impairment, and deafness. | * Visual disturbances, including [[blurred vision]] with [[scotomata]], sudden [[loss of vision]], [[photophobia]], [[diplopia]], [[night blindness]], diminished [[visual fields]], fixed [[pupillary dilatation]], disturbed [[color vision]], [[optic neuritis]], [[blindness]], [[vertigo]], [[tinnitus]], [[hearing impairment]], and [[deafness]]. | ||
|postmarketing=There is limited information regarding <i>Postmarketing Experience</i> of Quinine sulphate in the drug label. | |postmarketing=There is limited information regarding <i>Postmarketing Experience</i> of Quinine sulphate in the drug label. | ||
|drugInteractions======Effects of Drugs and Other Substances on Quinine Pharmacokinetics===== | |drugInteractions======Effects of Drugs and Other Substances on Quinine Pharmacokinetics===== | ||
* Quinine is a P-gp substrate and is primarily metabolized by CYP3A4. Other enzymes, including CYP1A2, CYP2C8, CYP2C9, CYP2C19, CYP2D6, and CYP2E1 may contribute to the metabolism of quinine. | * Quinine is a P-gp substrate and is primarily metabolized by [[CYP3A4]]. Other [[enzymes]], including [[CYP1A2]], [[CYP2C8]], [[CYP2C9]], [[CYP2C19]], [[CYP2D6]], and [[CYP2E1]] may contribute to the [[metabolism]] of quinine. | ||
* Antacids: | * Antacids: | ||
:* Antacids containing aluminum and/or magnesium may delay or decrease absorption of quinine. Concomitant administration of these antacids with Quinine sulphate should be avoided. | :* [[Antacids]] containing [[aluminum]] and/or [[magnesium]] may delay or decrease absorption of quinine. Concomitant administration of these [[antacids]] with Quinine sulphate should be avoided. | ||
* Antiepileptics (AEDs) (carbamazepine, phenobarbital, and phenytoin): | * Antiepileptics (AEDs) ([[carbamazepine]], [[phenobarbital]], and [[phenytoin]]): | ||
:* Carbamazepine, phenobarbital, and phenytoin are CYP3A4 inducers and may decrease quinine plasma concentrations if used concurrently with Quinine sulphate. | :* [[Carbamazepine]], [[phenobarbital]], and [[phenytoin]] are [[CYP3A4]] inducers and may decrease quinine [[plasma]] concentrations if used concurrently with Quinine sulphate. | ||
* Cholestyramine: | * Cholestyramine: | ||
:* In 8 healthy subjects who received quinine sulfate 600 mg with or without 8 grams of cholestyramine resin, no significant difference in quinine pharmacokinetic parameters was seen. | :* In 8 healthy subjects who received quinine sulfate 600 mg with or without 8 grams of [[cholestyramine]] resin, no significant difference in quinine [[pharmacokinetic]] parameters was seen. | ||
* Cigarette Smoking (CYP1A2 inducer): | * Cigarette Smoking ([[CYP1A2]] inducer): | ||
:* In healthy male heavy smokers, the mean quinine AUC following a single 600 mg dose was 44% lower, the mean Cmax was 18% lower, and the elimination half-life was shorter (7.5 hours versus 12 hours) than in their non-smoking counterparts. However, in malaria patients who received the full 7-day course of quinine therapy, cigarette smoking produced only a 25% decrease in median quinine AUC and a 16.5% decrease in median Cmax, suggesting that the already reduced clearance of quinine in acute malaria could have diminished the metabolic induction effect of smoking. Because smoking did not appear to influence the therapeutic outcome in malaria patients, it is not necessary to increase the dose of quinine in the treatment of acute malaria in heavy cigarette smokers. | :* In healthy male heavy smokers, the mean quinine AUC following a single 600 mg dose was 44% lower, the mean [[Cmax]] was 18% lower, and the elimination half-life was shorter (7.5 hours versus 12 hours) than in their non-smoking counterparts. However, in malaria patients who received the full 7-day course of quinine therapy, cigarette smoking produced only a 25% decrease in median quinine AUC and a 16.5% decrease in median [[Cmax]], suggesting that the already reduced clearance of quinine in acute malaria could have diminished the metabolic induction effect of smoking. Because smoking did not appear to influence the [[therapeutic]] outcome in malaria patients, it is not necessary to increase the dose of quinine in the treatment of [[acute malaria]] in heavy cigarette smokers. | ||
* Grapefruit juice (P-gp/CYP3A4 inhibitor): | * Grapefruit juice (P-gp/[[CYP3A4 inhibitor]]): | ||
:* In a pharmacokinetic study involving 10 healthy subjects, the administration of a single 600 mg dose of quinine sulfate with grapefruit juice (full-strength or half-strength) did not significantly alter the pharmacokinetic parameters of quinine. Quinine sulphate may be taken with grapefruit juice. | :* In a [[pharmacokinetic]] study involving 10 healthy subjects, the administration of a single 600 mg dose of quinine sulfate with grapefruit juice (full-strength or half-strength) did not significantly alter the [[pharmacokinetic]] parameters of quinine. Quinine sulphate may be taken with grapefruit juice. | ||
* Histamine H2-receptor blockers [cimetidine, ranitidine (nonspecific CYP450 inhibitors | * [[H2 antagonist|Histamine H2-receptor blockers]] [[[cimetidine]], [[ranitidine]] (nonspecific [[CYP450 inhibitors|CYP450 inhibitors]]: | ||
:* In healthy subjects who were given a single oral 600 mg dose of quinine sulfate after pretreatment with cimetidine (200 mg three times daily and 400 mg at bedtime for 7 days) or ranitidine (150 mg twice daily for 7 days), the apparent oral clearance of quinine decreased and the mean elimination half-life increased significantly when given with cimetidine but not with ranitidine. Compared to untreated controls, the mean AUC of quinine increased by 20% with ranitidine and by 42% with cimetidine (p<0.05) without a significant change in mean quinine Cmax. When quinine is to be given concomitantly with a | :* In healthy subjects who were given a single oral 600 mg dose of quinine sulfate after pretreatment with [[cimetidine]] (200 mg three times daily and 400 mg at bedtime for 7 days) or [[ranitidine]] (150 mg twice daily for 7 days), the apparent oral clearance of quinine decreased and the mean elimination half-life increased significantly when given with [[cimetidine]] but not with [[ranitidine]]. Compared to untreated controls, the mean AUC of quinine increased by 20% with [[ranitidine]] and by 42% with [[cimetidine]] (p<0.05) without a significant change in mean quinine Cmax. When quinine is to be given concomitantly with a [[H2 antagonist|Histamine H2-receptor blockers]], the use of [[ranitidine]] is preferred over [[cimetidine]]. Although [[cimetidine]] and [[ranitidine]] may be used concomitantly with Quinine sulphate, patients should be monitored closely for adverse events associated with quinine. | ||
* Isoniazid: | * Isoniazid: | ||
:* Isoniazid 300 mg/day pretreatment for 1 week did not significantly alter the pharmacokinetic parameter values of quinine. Adjustment of Quinine sulphate dosage is not necessary when isoniazid is given concomitantly. | :* [[Isoniazid]] 300 mg/day pretreatment for 1 week did not significantly alter the [[pharmacokinetic]] parameter values of quinine. Adjustment of Quinine sulphate dosage is not necessary when isoniazid is given concomitantly. | ||
* Ketoconazole (CYP3A4 inhibitor): | * Ketoconazole (CYP3A4 inhibitor): | ||
:* In a crossover study, healthy subjects (N=9) who received a single oral dose of quinine hydrochloride (500 mg) concomitantly with ketoconazole (100 mg twice daily for 3 days) had a mean quinine AUC that was higher by 45% and a mean oral clearance of quinine that was 31% lower than after receiving quinine alone. Although no change in the Quinine sulphate dosage regimen is necessary with concomitant ketoconazole, patients should be monitored closely for adverse reactions associated with quinine. | :* In a crossover study, healthy subjects (N=9) who received a single oral dose of [[quinine hydrochloride]] (500 mg) concomitantly with [[ketoconazole]] (100 mg twice daily for 3 days) had a mean quinine AUC that was higher by 45% and a mean oral clearance of quinine that was 31% lower than after receiving [[quinine]] alone. Although no change in the Quinine sulphate dosage regimen is necessary with concomitant [[ketoconazole]], patients should be monitored closely for [[adverse reactions]] associated with quinine. | ||
* Macrolide antibiotics (erythromycin, troleandomycin) (CYP3A4 inhibitors): | * Macrolide antibiotics (erythromycin, troleandomycin) (CYP3A4 inhibitors): | ||
:* In a crossover study (N=10), healthy subjects who received a single oral 600 mg dose of quinine sulfate with the macrolide antibiotic, troleandomycin (500 mg every 8 hours) exhibited a 87% higher mean quinine AUC, a 45% lower mean oral clearance of quinine, and a 81% lower formation clearance of the main metabolite, 3-hydroxyquinine, than when quinine was given alone. | :* In a crossover study (N=10), healthy subjects who received a single oral 600 mg dose of quinine sulfate with the [[macrolide antibiotic]], [[troleandomycin]] (500 mg every 8 hours) exhibited a 87% higher mean quinine AUC, a 45% lower mean oral clearance of quinine, and a 81% lower formation clearance of the main [[metabolite]], 3-[[hydroxyquinine]], than when quinine was given alone. | ||
:* Erythromycin was shown to inhibit the in vitro metabolism of quinine in human liver microsomes, an observation confirmed by an in vivo interaction study. In a crossover study (N=10), healthy subjects who received a single oral 500 mg dose of quinine sulfate with erythromycin (600 mg every 8 hours for four days) showed a decrease in quinine oral clearance (CL/F), an increase in half-life, and a decreased metabolite (3-hydroxyquinine) to quinine AUC ratio, as compared to when quinine was given with placebo. | :* [[Erythromycin]] was shown to inhibit the in vitro [[metabolism]] of quinine in human [[liver]] [[microsomes]], an observation confirmed by an in vivo interaction study. In a crossover study (N=10), healthy subjects who received a single oral 500 mg dose of quinine sulfate with [[erythromycin]] (600 mg every 8 hours for four days) showed a decrease in quinine oral clearance (CL/F), an increase in half-life, and a decreased [[metabolite]] (3-hydroxyquinine) to quinine AUC ratio, as compared to when quinine was given with [[placebo]]. | ||
:* Therefore, concomitant administration of macrolide antibiotics such as erythromycin or troleandomycin with Quinine sulphate should be avoided. | :* Therefore, concomitant administration of [[macrolide antibiotics]] such as [[erythromycin]] or [[troleandomycin]] with Quinine sulphate should be avoided. | ||
* Oral contraceptives (estrogen, progestin): | * Oral contraceptives (estrogen, progestin): | ||
:* In 7 healthy females who were using single-ingredient progestin or combination estrogen-containing oral contraceptives, the pharmacokinetic parameters of a single 600 mg dose of quinine sulfate were not altered in comparison to those observed in 7 age-matched female control subjects not using oral contraceptives. | :* In 7 healthy females who were using single-ingredient [[progestin]] or combination estrogen-containing [[oral contraceptives]], the [[pharmacokinetic]] parameters of a single 600 mg dose of quinine sulfate were not altered in comparison to those observed in 7 age-matched female control subjects not using [[oral contraceptives]]. | ||
* Rifampin (CYP3A4 inducer): | * Rifampin (CYP3A4 inducer): | ||
:* In patients with uncomplicated P. falciparum malaria who received quinine sulfate 10 mg/kg concomitantly with rifampin 15 mg/kg/day for 7 days (N=29), the median AUC of quinine between days 3 and 7 of therapy was 75% lower as compared to those who received quinine monotherapy. In healthy subjects (N=9) who received a single oral 600 mg dose of quinine sulfate after 2 weeks of pretreatment with rifampin 600 mg/day, the mean quinine AUC and Cmax decreased by 85% and 55%, respectively. Therefore, the concomitant administration of rifampin with Quinine sulphate should be avoided. | :* In patients with uncomplicated [[P. falciparum malaria]] who received quinine sulfate 10 mg/kg concomitantly with [[rifampin]] 15 mg/kg/day for 7 days (N=29), the median AUC of quinine between days 3 and 7 of therapy was 75% lower as compared to those who received quinine [[monotherapy]]. In healthy subjects (N=9) who received a single oral 600 mg dose of quinine sulfate after 2 weeks of pretreatment with [[rifampin]] 600 mg/day, the mean quinine AUC and [[Cmax]] decreased by 85% and 55%, respectively. Therefore, the concomitant administration of [[rifampin]] with Quinine sulphate should be avoided. | ||
* Ritonavir: | * Ritonavir: | ||
:* In healthy subjects who received a single oral 600 mg dose of quinine sulfate with the 15th dose of ritonavir (200 mg every 12 hours for 9 days), there were 4-fold increases in the mean quinine AUC and Cmax, and an increase in the mean elimination half-life (13.4 hours versus 11.2 hours), compared to when quinine was given alone. Therefore, the concomitant administration of ritonavir with Quinine sulphate capsules should be avoided | :* In healthy subjects who received a single oral 600 mg dose of quinine sulfate with the 15th dose of [[ritonavir]] (200 mg every 12 hours for 9 days), there were 4-fold increases in the mean quinine AUC and [[Cmax]], and an increase in the mean elimination half-life (13.4 hours versus 11.2 hours), compared to when quinine was given alone. Therefore, the concomitant administration of [[ritonavir]] with Quinine sulphate capsules should be avoided. | ||
* Tetracycline: | * Tetracycline: | ||
:* In 8 patients with acute uncomplicated P. falciparum malaria who were treated with oral quinine sulfate (600 mg every 8 hours for 7 days) in combination with oral tetracycline (250 mg every 6 hours for 7 days), the mean plasma quinine concentrations were about two-fold higher than in 8 patients who received quinine monotherapy. Although tetracycline may be concomitantly administered with Quinine sulphate, patients should be monitored closely for adverse reactions associated with quinine sulfate. | :* In 8 patients with acute uncomplicated [[P. falciparum malaria]] who were treated with oral quinine sulfate (600 mg every 8 hours for 7 days) in combination with oral [[tetracycline]] (250 mg every 6 hours for 7 days), the mean [[plasma]] quinine concentrations were about two-fold higher than in 8 patients who received quinine [[monotherapy]]. Although [[tetracycline]] may be concomitantly administered with Quinine sulphate, patients should be monitored closely for adverse reactions associated with quinine sulfate. | ||
* Theophylline or aminophylline: | * Theophylline or aminophylline: | ||
:* In 20 healthy subjects who received multiple doses of Quinine sulphate (648 mg every 8 hours × 7 days) with a single 300 mg oral dose of theophylline, the quinine mean Cmax and AUC were increased by 13% and 14% respectively. Although no change in the Quinine sulphate dosage regimen is necessary with concomitant theophylline or aminophylline, patients should be monitored closely for adverse reactions associated with quinine. | :* In 20 healthy subjects who received multiple doses of Quinine sulphate (648 mg every 8 hours × 7 days) with a single 300 mg oral dose of [[theophylline]], the quinine mean [[Cmax]] and AUC were increased by 13% and 14% respectively. Although no change in the Quinine sulphate dosage regimen is necessary with concomitant [[theophylline]] or [[aminophylline]], patients should be monitored closely for adverse reactions associated with quinine. | ||
* Urinary alkalizers (acetazolamide, sodium bicarbonate): | * Urinary alkalizers (acetazolamide, sodium bicarbonate): | ||
| Line 202: | Line 202: | ||
=====Effects of Quinine on the Pharmacokinetics of Other Drugs:===== | =====Effects of Quinine on the Pharmacokinetics of Other Drugs:===== | ||
* Results of in vivo drug interaction studies suggest that quinine has the potential to inhibit the metabolism of drugs that are substrates of CYP3A4 and CYP2D6. Quinine inhibits P-gp and has the potential to affect the transport of drugs that are P-gp substrates. | * Results of in vivo [[drug interaction]] studies suggest that quinine has the potential to inhibit the [[metabolism]] of drugs that are substrates of [[CYP3A4]] and [[CYP2D6]]. Quinine inhibits P-gp and has the potential to affect the transport of drugs that are P-gp substrates. | ||
* Anticonvulsants (carbamazepine, phenobarbital, and phenytoin): | * Anticonvulsants (carbamazepine, phenobarbital, and phenytoin): | ||
:* A single 600 mg oral dose of quinine sulfate increased the mean plasma Cmax, and AUC0–24 of single oral doses of carbamazepine (200 mg) and phenobarbital (120 mg) but not phenytoin (200 mg) in 8 healthy subjects. The mean AUC increases of carbamazepine, phenobarbital and phenytoin were 104%, 81% and 4%, respectively; the mean increases in Cmax were 56%, 53%, and 4%, respectively. Mean urinary recoveries of the three antiepileptics over 24 hours were also profoundly increased by quinine. If concomitant administration with carbamazepine or phenobarbital cannot be avoided, frequent monitoring of anticonvulsant drug concentrations is recommended. Additionally, patients should be monitored closely for adverse reactions associated with these anticonvulsants. | :* A single 600 mg oral dose of quinine sulfate increased the mean plasma [[Cmax]], and AUC0–24 of single oral doses of [[carbamazepine]] (200 mg) and [[phenobarbital]] (120 mg) but not [[phenytoin]] (200 mg) in 8 healthy subjects. The mean AUC increases of [[carbamazepine]], [[phenobarbital]] and [[phenytoin]] were 104%, 81% and 4%, respectively; the mean increases in [[Cmax]] were 56%, 53%, and 4%, respectively. Mean urinary recoveries of the three [[antiepileptics]] over 24 hours were also profoundly increased by quinine. If concomitant administration with [[carbamazepine]] or phenobarbital cannot be avoided, frequent monitoring of [[anticonvulsant]] drug concentrations is recommended. Additionally, patients should be monitored closely for adverse reactions associated with these [[anticonvulsants]]. | ||
* Astemizole (CYP3A4 substrate): | * Astemizole (CYP3A4 substrate): | ||
:* Elevated plasma astemizole concentrations were reported in a subject who experienced torsades de pointes after receiving three doses of quinine sulfate for nocturnal leg cramps concomitantly with chronic astemizole 10 mg/day. The concurrent use of Quinine sulphate with astemizole and other CYP3A4 substrates with QT prolongation potential (e.g., cisapride, terfenadine, halofantrine, pimozide and quinidine) should also be avoided. | :* Elevated [[plasma]] [[astemizole]] concentrations were reported in a subject who experienced [[torsades de pointes]] after receiving three doses of quinine sulfate for nocturnal [[leg cramps]] concomitantly with chronic [[astemizole]] 10 mg/day. The concurrent use of Quinine sulphate with [[astemizole]] and other [[CYP3A4]] substrates with [[QT prolongation]] potential (e.g., [[cisapride]], [[terfenadine]], [[halofantrine]], [[pimozide]] and [[quinidine]]) should also be avoided. | ||
* Atorvastatin (CYP3A4 substrate): | * Atorvastatin (CYP3A4 substrate): | ||
:* Rhabdomyolysis with acute renal failure secondary to myoglobinuria was reported in a patient taking atorvastatin administered with a single dose of quinine. Quinine may increase plasma concentrations of atorvastatin, thereby increasing the risk of myopathy or rhabdomyolysis. Thus, clinicians considering combined therapy of Quinine sulphate with atorvastatin or other HMG-CoA reductase inhibitors ("statins") that are CYP3A4 substrates (e.g., simvastatin, lovastatin) should carefully weigh the potential benefits and risks of each medication. If Quinine sulphate is used concomitantly with any of these statins, lower starting and maintenance doses of the statin should be considered. Patients should also be monitored closely for any signs or symptoms of muscle pain, tenderness, or weakness, particularly during initial therapy. If marked creatine phosphokinase (CPK) elevation occurs or myopathy (defined as muscle aches or muscle weakness in conjunction with CPK values >10 times the upper limit of normal) is diagnosed or suspected, atorvastatin or other statin should be discontinued. | :* [[Rhabdomyolysis]] with [[acute renal failure]] secondary to [[myoglobinuria]] was reported in a patient taking [[atorvastatin]] administered with a single dose of quinine. Quinine may increase plasma [[concentrations]] of [[atorvastatin]], thereby increasing the risk of [[myopathy]] or [[rhabdomyolysis]]. Thus, clinicians considering combined therapy of Quinine sulphate with [[atorvastatin]] or other [[HMG-CoA reductase|HMG-CoA reductase inhibitors]] ("[[statins]]") that are [[CYP3A4]] substrates (e.g., [[simvastatin]], [[lovastatin]]) should carefully weigh the potential benefits and risks of each medication. If Quinine sulphate is used concomitantly with any of these [[statins]], lower starting and maintenance doses of the [[statin]] should be considered. Patients should also be monitored closely for any signs or symptoms of [[muscle pain]], [[tenderness]], or weakness, particularly during initial therapy. If marked [[creatine phosphokinase]] ([[CPK]]) elevation occurs or [[myopathy]] (defined as [[muscle aches]] or [[muscle weakness]] in conjunction with [[CPK]] values >10 times the upper limit of normal) is diagnosed or suspected, [[atorvastatin]] or other [[statin]] should be discontinued. | ||
* Desipramine (CYP2D6 substrate): | * Desipramine (CYP2D6 substrate): | ||
:* Quinine (750 mg/day for 2 days) decreased the metabolism of desipramine in patients who were extensive CYP2D6 metabolizers, but had no effect in patients who were poor CYP2D6 metabolizers. Lower doses (80 mg to 400 mg) of quinine did not significantly affect the pharmacokinetics of other CYP2D6 substrates, namely, debrisoquine, dextromethorphan, and methoxyphenamine. Although clinical drug interaction studies have not been performed, antimalarial doses (greater than or equal to 600 mg) of quinine may inhibit the metabolism of other drugs that are CYP2D6 substrates (e.g., flecainide, debrisoquine, dextromethorphan, metoprolol, paroxetine). Patients taking medications that are CYP2D6 substrates with Quinine sulphate should be monitored closely for adverse reactions associated with these medications. | :* [[Quinine]] (750 mg/day for 2 days) decreased the metabolism of [[desipramine]] in patients who were extensive [[CYP2D6]] metabolizers, but had no effect in patients who were poor [[CYP2D6]] metabolizers. Lower doses (80 mg to 400 mg) of quinine did not significantly affect the [[pharmacokinetics]] of other [[CYP2D6]] substrates, namely, [[debrisoquine]], [[dextromethorphan]], and [[methoxyphenamine]]. Although clinical drug interaction studies have not been performed, [[antimalarial]] doses (greater than or equal to 600 mg) of quinine may inhibit the metabolism of other drugs that are [[CYP2D6]] substrates (e.g., [[flecainide]], [[debrisoquine]], [[dextromethorphan]], [[metoprolol]], [[paroxetine]]). Patients taking medications that are [[CYP2D6]] substrates with Quinine sulphate should be monitored closely for adverse reactions associated with these medications. | ||
* Digoxin (P-gp substrate): | * Digoxin (P-gp substrate): | ||
:* In 4 healthy subjects who received digoxin (0.5 to 0.75 mg/day) during treatment with quinine (750 mg/day), a 33% increase in mean steady state AUC of digoxin and a 35% reduction in the steady state biliary clearance of digoxin were observed compared to digoxin alone. Thus, if Quinine sulphate is administered to patients receiving digoxin, plasma digoxin concentrations should be closely monitored, and the digoxin dose adjusted, as necessary. | :* In 4 healthy subjects who received [[digoxin]] (0.5 to 0.75 mg/day) during treatment with quinine (750 mg/day), a 33% increase in mean steady state AUC of [[digoxin]] and a 35% reduction in the steady state [[biliary]] clearance of [[digoxin]] were observed compared to [[digoxin]] alone. Thus, if Quinine sulphate is administered to patients receiving [[digoxin]], plasma [[digoxin]] concentrations should be closely monitored, and the [[digoxin]] dose adjusted, as necessary. | ||
* Halofantrine: | * Halofantrine: | ||
:* Although not studied clinically, quinine was shown to inhibit the metabolism of halofantrine in vitro using human liver microsomes. Therefore, concomitant administration of Quinine sulphate is likely to increase plasma halofantrine concentrations | :* Although not studied clinically, quinine was shown to inhibit the metabolism of [[halofantrine]] in vitro using human liver [[microsomes]]. Therefore, concomitant administration of Quinine sulphate is likely to increase plasma [[halofantrine]] concentrations. | ||
* Mefloquine: | * Mefloquine: | ||
:* In 7 healthy subjects who received mefloquine (750 mg) at 24 hours before an oral 600 mg dose of quinine sulfate, the AUC of mefloquine was increased by 22% compared to mefloquine alone. In this study, the QTc interval was significantly prolonged in the subjects who received mefloquine and quinine sulfate 24 hours apart. The concomitant administration of mefloquine and Quinine sulphate may produce electrocardiographic abnormalities (including QTc prolongation) and may increase the risk of seizures. | :* In 7 healthy subjects who received [[mefloquine]] (750 mg) at 24 hours before an oral 600 mg dose of quinine sulfate, the AUC of [[mefloquine]] was increased by 22% compared to [[mefloquine]] alone. In this study, the [[QTc interval]] was significantly prolonged in the subjects who received [[mefloquine]] and quinine sulfate 24 hours apart. The concomitant administration of [[mefloquine]] and Quinine sulphate may produce [[electrocardiographic]] abnormalities (including [[QT prolongation|QTc prolongation]]) and may increase the risk of [[seizures]]. | ||
* Midazolam (CYP3A4 substrate): | * Midazolam (CYP3A4 substrate): | ||
:* In 23 healthy subjects who received multiple doses of Quinine sulphate 324 mg three times daily × 7 days with a single oral 2 mg dose of midazolam, the mean AUC and Cmax of midazolam and 1-hydroxymidazolam were not significantly affected. This finding indicates that 7-day dosing with Quinine sulphate 324 mg every 8 hours did not induce the metabolism of midazolam. | :* In 23 healthy subjects who received multiple doses of Quinine sulphate 324 mg three times daily × 7 days with a single oral 2 mg dose of [[midazolam]], the mean AUC and [[Cmax]] of [[midazolam]] and 1-hydroxymidazolam were not significantly affected. This finding indicates that 7-day dosing with Quinine sulphate 324 mg every 8 hours did not induce the [[metabolism]] of [[midazolam]]. | ||
* Neuromuscular blocking agents (pancuronium, succinylcholine, tubocurarine): | * Neuromuscular blocking agents (pancuronium, succinylcholine, tubocurarine): | ||
:* In one report, quinine potentiated neuromuscular blockade in a patient who received pancuronium during an operative procedure, and subsequently (3 hours after receiving pancuronium) received quinine 1800 mg daily. Quinine may also enhance the neuromuscular | :* In one report, quinine potentiated [[neuromuscular junction|neuromuscular blockade]] in a patient who received [[pancuronium]] during an operative procedure, and subsequently (3 hours after receiving [[pancuronium]]) received quinine 1800 mg daily. Quinine may also enhance the [[neuromuscular junction|neuromuscular blockade]] of [[succinylcholine]] and [[tubocurarine]]. | ||
* Ritonavir: | * Ritonavir: | ||
:* In healthy subjects who received a single oral 600 mg dose of quinine sulfate with the 15th dose of ritonavir (200 mg every 12 hours for 9 days), the mean ritonavir AUC, Cmax, and elimination half-life were slightly but not significantly increased compared to when ritonavir was given alone. However, due to the significant effect of ritonavir on quinine pharmacokinetics, the concomitant administration of Quinine sulphate capsules with ritonavir should be avoided. | :* In healthy subjects who received a single oral 600 mg dose of quinine sulfate with the 15th dose of [[ritonavir]] (200 mg every 12 hours for 9 days), the mean [[ritonavir]] AUC, [[Cmax]], and elimination half-life were slightly but not significantly increased compared to when [[ritonavir]] was given alone. However, due to the significant effect of [[ritonavir]] on quinine [[pharmacokinetics]], the concomitant administration of Quinine sulphate capsules with [[ritonavir]] should be avoided. | ||
* Theophylline or aminophylline (CYP1A2 substrate): | * Theophylline or aminophylline (CYP1A2 substrate): | ||
:* In 19 healthy subjects who received multiple doses of Quinine sulphate 648 mg every 8 hours x 7 days with a single 300 mg oral dose of theophylline, the mean theophylline AUC was 10% lower than when theophylline was given alone. There was no significant effect on mean theophylline Cmax. Therefore, if Quinine sulphate is co-administered to patients receiving theophylline or aminophylline, plasma theophylline concentrations should be monitored frequently to ensure therapeutic concentrations. | :* In 19 healthy subjects who received multiple doses of Quinine sulphate 648 mg every 8 hours x 7 days with a single 300 mg oral dose of [[theophylline]], the mean [[theophylline]] AUC was 10% lower than when [[theophylline]] was given alone. There was no significant effect on mean [[theophylline]] [[Cmax]]. Therefore, if Quinine sulphate is co-administered to patients receiving [[theophylline]] or [[aminophylline]], plasma [[theophylline]] concentrations should be monitored frequently to ensure [[therapeutic]] concentrations. | ||
* Warfarin and oral anticoagulants: | * Warfarin and oral anticoagulants: | ||
:* Cinchona alkaloids, including quinine, may have the potential to depress hepatic enzyme synthesis of vitamin K-dependent coagulation pathway proteins and may enhance the action of warfarin and other oral anticoagulants. Quinine may also interfere with the anticoagulant effect of heparin. Thus, in patients receiving these anticoagulants, the prothrombin time (PT), partial thromboplastin time (PTT), or international normalization ratio (INR) should be closely monitored as appropriate, during concurrent therapy with Quinine sulphate. | :* [[Cinchona alkaloids]], including quinine, may have the potential to depress [[hepatic]] [[enzyme]] synthesis of [[vitamin K|vitamin K-dependent]] [[coagulation]] pathway [[proteins]] and may enhance the action of [[warfarin]] and other oral [[anticoagulants]]. Quinine may also interfere with the [[anticoagulant]] effect of [[heparin]]. Thus, in patients receiving these [[anticoagulants]], the [[prothrombin time]] ([[PT]]), [[partial thromboplastin time]] (PTT), or [[international normalization ratio]] ([[INR]]) should be closely monitored as appropriate, during concurrent therapy with Quinine sulphate. | ||
=====Drug/Laboratory Interactions:===== | =====Drug/Laboratory Interactions:===== | ||
* Quinine may produce an elevated value for urinary 17-ketogenic steroids when the Zimmerman method is used. | * Quinine may produce an elevated value for urinary [[steroids|17-ketogenic steroids]] when the Zimmerman method is used. | ||
* Quinine may interfere with urine qualitative dipstick protein assays as well as quantitative methods (e.g., pyrogallol red-molybdate). | * Quinine may interfere with urine qualitative dipstick protein assays as well as quantitative methods (e.g., [[pyrogallol]] red-molybdate). | ||
|FDAPregCat=C | |FDAPregCat=C | ||
|useInPregnancyFDA=* There are extensive published data but few well-controlled studies of Quinine sulphate in pregnant women. Published data on over 1,000 pregnancy exposures to quinine did not show an increase in teratogenic effects over the background rate in the general population; however, the majority of these exposures were not in the first trimester. In developmental and reproductive toxicity studies, central nervous system (CNS) and ear abnormalities and increased fetal deaths occurred in some species when pregnant animals received quinine at doses about 1 to 4 times the human clinical dose. Quinine should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. | |useInPregnancyFDA=* There are extensive published data but few well-controlled studies of Quinine sulphate in [[pregnant]] women. Published data on over 1,000 [[pregnancy]] exposures to quinine did not show an increase in [[teratogenic]] effects over the background rate in the general population; however, the majority of these exposures were not in the first [[trimester]]. In developmental and [[reproductive]] [[toxicity]] studies, [[central nervous system]] ([[CNS]]) and ear abnormalities and increased [[fetal]] deaths occurred in some species when [[pregnant]] animals received quinine at doses about 1 to 4 times the human clinical dose. Quinine should be used during [[pregnancy]] only if the potential benefit justifies the potential risk to the fetus. | ||
* P. falciparum malaria carries a higher risk of morbidity and mortality in pregnant women than in the general population. Pregnant women with P. falciparum malaria have an increased incidence of fetal loss (including spontaneous abortion and stillbirth), preterm labor and delivery, intrauterine growth retardation, low birth weight, and maternal death. Therefore, treatment of malaria in pregnancy is important. | * [[P. falciparum malaria]] carries a higher risk of [[morbidity]] and mortality in [[pregnant]] women than in the general population. [[Pregnant]] women with [[P. falciparum malaria]] have an increased incidence of [[fetal]] loss (including spontaneous [[abortion]] and [[stillbirth]]), [[preterm labor]] and [[delivery]], [[intrauterine growth retardation]], [[low birth weight]], and [[maternal death]]. Therefore, treatment of [[malaria]] in [[pregnancy]] is important. | ||
* Hypoglycemia, due to increased pancreatic secretion of insulin, has been associated with quinine use, particularly in pregnant women. | * [[Hypoglycemia]], due to increased [[pancreatic]] secretion of [[insulin]], has been associated with quinine use, particularly in [[pregnant]] women. | ||
* Quinine crosses the placenta with measurable | * Quinine crosses the [[placenta]] with measurable bloo[[d]] concentrations in the [[fetus]]. In 8 women who delivered live infants 1 to 6 days after starting quinine therapy, [[umbilical cord]] [[plasma]] quinine concentrations were between 1.0 and 4.6 mg/L (mean 2.4 mg/L) and the mean (±SD) ratio of cord plasma to [[maternal]] [[plasma]] quinine concentrations was 0.32 ± 0.14. Quinine levels in the fetus may not be [[therapeutic]]. If [[congenital]] [[malaria]] is suspected after [[delivery]], the infant should be evaluated and treated appropriately. | ||
* A study from Thailand (1999) of women with P. falciparum malaria who were treated with oral quinine sulfate 10 mg/kg 3 times daily for 7 days at anytime in pregnancy reported no significant difference in the rate of stillbirths at >28 weeks of gestation in women treated with quinine (10 of 633 women [1.6%]) as compared with a control group without malaria or exposure to antimalarial drugs during pregnancy (40 of 2201 women [1.8%]). The overall rate of congenital malformations (9 of 633 offspring [1.4%]) was not different for women who were treated with quinine sulfate compared with the control group (38 of 2201 offspring [1.7%]). The spontaneous abortion rate was higher in the control group (10.9%) than in women treated with quinine sulfate (3.5%) [OR = 3.1; 95% CI 2.1-4.7]. An epidemiologic survey that included 104 mother-child pairs exposed to quinine during the first 4 months of pregnancy, found no increased risk of structural birth defects was seen (2 fetal malformations [1.9%]). Rare and isolated case reports describe deafness and optic nerve hypoplasia in children exposed in utero due to maternal ingestion of high doses of quinine. | * A study from Thailand (1999) of women with [[P. falciparum malaria]] who were treated with oral quinine sulfate 10 mg/kg 3 times daily for 7 days at anytime in [[pregnancy]] reported no significant difference in the rate of [[stillbirths]] at >28 weeks of [[gestation]] in women treated with quinine (10 of 633 women [1.6%]) as compared with a control group without [[malaria]] or exposure to [[antimalarial]] drugs during [[pregnancy]] (40 of 2201 women [1.8%]). The overall rate of [[congenital malformations]] (9 of 633 offspring [1.4%]) was not different for women who were treated with quinine sulfate compared with the [[control group]] (38 of 2201 offspring [1.7%]). The [[spontaneous abortion]] rate was higher in the control group (10.9%) than in women treated with quinine sulfate (3.5%) [OR = 3.1; 95% CI 2.1-4.7]. An [[epidemiologic]] survey that included 104 mother-child pairs exposed to quinine during the first 4 months of [[pregnancy]], found no increased risk of structural [[birth defects]] was seen (2 fetal [[malformations]] [1.9%]). Rare and isolated case reports describe deafness and [[optic nerve hypoplasia]] in children exposed in [[utero]] due to [[maternal]] [[ingestion]] of high doses of quinine. | ||
* In animal developmental studies conducted in multiple animal species, pregnant animals received quinine by the subcutaneous or intramuscular route at dose levels similar to the maximum recommended human dose (MRHD; 32 mg/kg/day) based on body surface area (BSA) comparisons. There were increases in fetal death in utero in rabbits at maternal doses ≥ 100 mg/kg/day and in dogs at ≥ 15 mg/kg/day corresponding to dose levels approximately 0.5 and 0.25 times the MRHD respectively based on BSA comparisons. Rabbit offspring had increased rates of degenerated auditory nerve and spiral ganglion and increased rates of CNS anomalies such as anencephaly and microcephaly at a dose of 130 mg/kg/day corresponding to a maternal dose approximately 1.3 times the MRHD based on BSA comparison. Guinea pig offspring had increased rates of hemorrhage and mitochondrial change in the cochlea at maternal doses of 200 mg/kg corresponding to a dose level of approximately 1.4 times the MRHD based on BSA comparison. There were no teratogenic findings in rats at maternal doses up to 300 mg/kg/day and in monkeys at doses up to 200 mg/kg/day corresponding to doses approximately 1 and 2 times the MRHD respectively based on BSA comparisons. | * In animal developmental studies conducted in multiple animal species, pregnant animals received quinine by the [[subcutaneous]] or [[intramuscular]] route at dose levels similar to the maximum recommended human dose ([[MRHD]]; 32 mg/kg/day) based on body surface area (BSA) comparisons. There were increases in fetal death in [[utero]] in rabbits at maternal doses ≥ 100 mg/kg/day and in dogs at ≥ 15 mg/kg/day corresponding to dose levels approximately 0.5 and 0.25 times the [[MRHD]] respectively based on BSA comparisons. Rabbit offspring had increased rates of degenerated [[auditory nerve]] and [[spiral ganglion]] and increased rates of [[CNS anomalies]] such as [[anencephaly]] and [[microcephaly]] at a dose of 130 mg/kg/day corresponding to a maternal dose approximately 1.3 times the [[MRHD]] based on BSA comparison. Guinea pig offspring had increased rates of [[hemorrhage]] and [[mitochondrial]] change in the [[cochlea]] at maternal doses of 200 mg/kg corresponding to a dose level of approximately 1.4 times the [[MRHD]] based on BSA comparison. There were no [[teratogenic]] findings in rats at [[maternal]] doses up to 300 mg/kg/day and in monkeys at doses up to 200 mg/kg/day corresponding to doses approximately 1 and 2 times the MRHD respectively based on BSA comparisons. | ||
* In a pre- postnatal study in rats, an estimated oral dose of quinine sulfate of 20 mg/kg/day corresponding to approximately 0.1 times the MRHD based on BSA comparison resulted in offspring with impaired growth, lower body weights at birth and during the lactation period, and delayed physical development of teeth eruption and eye opening during the lactation period. | * In a pre- [[postnatal]] study in rats, an estimated oral dose of quinine sulfate of 20 mg/kg/day corresponding to approximately 0.1 times the [[MRHD]] based on BSA comparison resulted in offspring with impaired growth, lower body weights at birth and during the [[lactation]] period, and delayed physical development of [[teeth|teeth eruption]] and eye opening during the [[lactation]] period. | ||
|useInPregnancyAUS=* '''Australian Drug Evaluation Committee (ADEC) Pregnancy Category''' | |useInPregnancyAUS=* '''Australian Drug Evaluation Committee (ADEC) Pregnancy Category''' | ||
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Quinine sulphate in women who are pregnant. | There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Quinine sulphate in women who are pregnant. | ||
|useInLaborDelivery=* There is no evidence that quinine causes uterine contractions at the doses recommended for the treatment of malaria. In doses several-times higher than those used to treat malaria, quinine may stimulate the pregnant uterus. | |useInLaborDelivery=* There is no evidence that quinine causes uterine contractions at the doses recommended for the treatment of malaria. In doses several-times higher than those used to treat malaria, quinine may stimulate the pregnant uterus. | ||
|useInNursing=* There is limited information on the safety of quinine in breastfed infants. No toxicity was reported in infants in a single study where oral quinine sulfate (10 mg/kg every 8 hours for 1 to 10 days) was administered to 25 lactating women. It is estimated from this study that breastfed infants would receive less than 2 to 3 mg per day of quinine base (< 0.4% of the maternal dose) via breast milk. | |useInNursing=* There is limited information on the safety of quinine in [[breastfed]] infants. No toxicity was reported in infants in a single study where oral quinine sulfate (10 mg/kg every 8 hours for 1 to 10 days) was administered to 25 lactating women. It is estimated from this study that [[breastfed]] [[infants]] would receive less than 2 to 3 mg per day of quinine base (< 0.4% of the maternal dose) via [[breast milk]]. | ||
* Although quinine is generally considered compatible with breastfeeding, the risks and benefits to infant and mother should be assessed. Caution should be exercised when administered to a nursing woman. | * Although quinine is generally considered compatible with [[breastfeeding]], the risks and benefits to infant and mother should be assessed. Caution should be exercised when administered to a [[nursing]] woman. | ||
* If malaria is suspected in the infant, appropriate evaluation and treatment should be provided. Plasma quinine levels may not be therapeutic in infants of nursing mothers receiving Quinine sulphate. | * If malaria is suspected in the infant, appropriate evaluation and treatment should be provided. Plasma quinine levels may not be [[therapeutic]] in [[infants]] of [[nursing]] mothers receiving Quinine sulphate. | ||
|useInPed=There is no FDA guidance on the use of Quinine sulphate with respect to pediatric patients. | |useInPed=There is no FDA guidance on the use of Quinine sulphate with respect to pediatric patients. | ||
|useInGeri=There is no FDA guidance on the use of Quinine sulphate with respect to geriatric patients. | |useInGeri=There is no FDA guidance on the use of Quinine sulphate with respect to geriatric patients. | ||
| Line 274: | Line 274: | ||
|useInHepaticImpair=* In patients with severe hepatic impairment (Child-Pugh C), quinine oral clearance (CL/F) is decreased, volume of distribution (Vd/F) is increased, and half-life is prolonged, relative to subjects with normal liver function. Therefore, quinine is not indicated in patients with severe hepatic impairment and alternate therapy should be administered. | |useInHepaticImpair=* In patients with severe hepatic impairment (Child-Pugh C), quinine oral clearance (CL/F) is decreased, volume of distribution (Vd/F) is increased, and half-life is prolonged, relative to subjects with normal liver function. Therefore, quinine is not indicated in patients with severe hepatic impairment and alternate therapy should be administered. | ||
* Close monitoring is recommended for patients with mild (Child-Pugh A) or moderate (Child-Pugh B) hepatic impairment, as exposure to quinine may be increased relative to subjects with normal liver function | * Close monitoring is recommended for patients with mild ([[Child-Pugh A]]) or moderate ([[Child-Pugh B]]) [[hepatic impairment]], as exposure to quinine may be increased relative to subjects with normal [[liver function]] | ||
|useInReproPotential=There is no FDA guidance on the use of Quinine sulphate in women of reproductive potentials and males. | |useInReproPotential=There is no FDA guidance on the use of Quinine sulphate in women of [[reproductive]] potentials and males. | ||
|useInImmunocomp=There is no FDA guidance one the use of Quinine sulphate in patients who are immunocompromised. | |useInImmunocomp=There is no FDA guidance one the use of Quinine sulphate in patients who are immunocompromised. | ||
<!--Administration and Monitoring--> | <!--Administration and Monitoring--> | ||
|administration=* Oral | |administration=* Oral | ||
|monitoring=== | |monitoring===+=Hepatic Impairment===== | ||
* Adjustment of the recommended dose is not required in mild (Child-Pugh A) or moderate (Child-Pugh B) hepatic impairment, but patients should be monitored closely for adverse effects of quinine. Quinine should not be administered in patients with severe (Child-Pugh C) hepatic impairment. | * Adjustment of the recommended dose is not required in mild ([[Child-Pugh A]]) or moderate ([[Child-Pugh B]]) [[hepatic impairment]], but patients should be monitored closely for adverse effects of quinine. Quinine should not be administered in patients with severe ([[Child-Pugh C]]) [[hepatic impairment]]. | ||
* In otherwise healthy subjects with mild hepatic impairment (Child-Pugh A; N=10), who received a single 500 mg dose of quinine sulfate, there was no significant difference in quinine pharmacokinetic parameters or exposure to the primary metabolite, 3-hydroxyquinine as compared to healthy controls (N=10). In otherwise healthy subjects with moderate hepatic impairment (Child-Pugh B; N=9) who received a single oral 600 mg dose of quinine sulfate, the mean AUC increased by 55% without a significant change in mean Cmax, as compared to healthy volunteer controls (N=6). In subjects with hepatitis, the absorption of quinine was prolonged, the elimination half-life was increased, the apparent volume of distribution was higher, but there was no significant difference in weight-adjusted clearance. Therefore, in patients with mild to moderate hepatic impairment, dosage adjustment is not needed, but patients should be monitored closely for adverse effects of quinine. | * In otherwise healthy subjects with mild [[hepatic impairment]] ([[Child-Pugh A]]; N=10), who received a single 500 mg dose of quinine sulfate, there was no significant difference in quinine [[pharmacokinetic]] parameters or exposure to the primary metabolite, 3-hydroxyquinine as compared to healthy controls (N=10). In otherwise healthy subjects with moderate [[hepatic impairment]] ([[Child-Pugh B]]; N=9) who received a single oral 600 mg dose of quinine sulfate, the mean AUC increased by 55% without a significant change in mean [[Cmax]], as compared to healthy volunteer controls (N=6). In subjects with hepatitis, the absorption of quinine was prolonged, the elimination half-life was increased, the apparent volume of distribution was higher, but there was no significant difference in weight-adjusted clearance. Therefore, in patients with mild to moderate [[hepatic impairment]], dosage adjustment is not needed, but patients should be monitored closely for adverse effects of quinine. | ||
=====Atrial Fibrillation and Flutter===== | =====Atrial Fibrillation and Flutter===== | ||
* Quinine sulphate should be used with caution in patients with atrial fibrillation or atrial flutter. A paradoxical increase in ventricular response rate may occur with quinine, similar to that observed with quinidine. If digoxin is used to prevent a rapid ventricular response, serum digoxin levels should be closely monitored, because digoxin levels may be increased with use of quinine | * Quinine sulphate should be used with caution in patients with [[atrial fibrillation]] or [[atrial flutter]]. A paradoxical increase in [[ventricular]] response rate may occur with quinine, similar to that observed with [[quinidine]]. If [[digoxin]] is used to prevent a rapid [[ventricular]] response, serum [[digoxin]] levels should be closely monitored, because [[digoxin]] levels may be increased with use of quinine | ||
=====Histamine H2-receptor blockers i.e cimetidine, ranitidine (nonspecific CYP450 inhibitors)====== | =====Histamine H2-receptor blockers i.e cimetidine, ranitidine (nonspecific CYP450 inhibitors)====== | ||
* In healthy subjects who were given a single oral 600 mg dose of quinine sulfate after pretreatment with cimetidine (200 mg three times daily and 400 mg at bedtime for 7 days) or ranitidine (150 mg twice daily for 7 days), the apparent oral clearance of quinine decreased and the mean elimination half-life increased significantly when given with cimetidine but not with ranitidine. Compared to untreated controls, the mean AUC of quinine increased by 20% with ranitidine and by 42% with cimetidine (p<0.05) without a significant change in mean quinine Cmax. When quinine is to be given concomitantly with a histamine H2-receptor blocker, the use of ranitidine is preferred over cimetidine. Although cimetidine and ranitidine may be used concomitantly with Quinine sulphate, patients should be monitored closely for adverse events associated with quinine. | * In healthy subjects who were given a single oral 600 mg dose of quinine sulfate after pretreatment with [[cimetidine]] (200 mg three times daily and 400 mg at bedtime for 7 days) or [[ranitidine]] (150 mg twice daily for 7 days), the apparent oral clearance of quinine decreased and the mean elimination half-life increased significantly when given with cimetidine but not with [[ranitidine]]. Compared to untreated controls, the mean AUC of quinine increased by 20% with [[ranitidine]] and by 42% with [[cimetidine]] (p<0.05) without a significant change in mean quinine Cmax. When quinine is to be given concomitantly with a [[histamine antagonist|histamine H2-receptor blocker]], the use of [[ranitidine]] is preferred over [[cimetidine]]. Although [[cimetidine]] and [[ranitidine]] may be used concomitantly with Quinine sulphate, patients should be monitored closely for adverse events associated with quinine. | ||
=====Ketoconazole (CYP3A4 inhibitor)===== | =====Ketoconazole (CYP3A4 inhibitor)===== | ||
Revision as of 21:20, 2 March 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Adeel Jamil, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING
See full prescribing information for complete Boxed Warning.
* Quinine sulphate use for the treatment or prevention of nocturnal leg cramps may result in serious and life-threatening hematologic reactions, including thrombocytopenia and hemolytic uremic syndrome/thrombotic thrombocytopenic purpura (HUS/TTP). Chronic renal impairment associated with the development of TTP has been reported. The risk associated with Quinine sulphate use in the absence of evidence of its effectiveness in the treatment or prevention of nocturnal leg cramps outweighs any potential benefit
|
Overview
Quinine is a cinchona alkaloid, antimalarial and anti-Infective Agent, that is FDA approved for the treatment of of uncomplicated Plasmodium falciparum malaria. There is a Black Box Warning for this drug as shown here. Common adverse reactions include cinchonism include headache, vasodilation and sweating, nausea, tinnitus, hearing impairment, vertigo or dizziness, blurred vision, and disturbance in color perception..
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- Quinine sulphate is an antimalarial drug indicated only for treatment of uncomplicated Plasmodium falciparum malaria. Quinine sulfate has been shown to be effective in geographical regions where resistance to chloroquine has been documented.
- Quinine sulphate oral capsules are not approved for:
- Treatment of severe or complicated P. falciparum malaria.
- Prevention of malaria.
- Treatment or prevention of nocturnal leg cramps
Dosing Information
- Treatment of Uncomplicated P. falciparum Malaria
- For treatment of uncomplicated P. falciparum malaria in adults: Orally, 648 mg (two capsules) every 8 hours for 7 days.
- Quinine sulphate should be taken with food to minimize gastric upset.
Renal Impairment
- In patients with acute uncomplicated malaria and severe chronic renal impairment, the following dosage regimen is recommended: one loading dose of 648 mg Quinine sulphate followed 12 hours later by maintenance doses of 324 mg every 12 hours.
- The effects of mild and moderate renal impairment on the safety and pharmacokinetics of quinine sulfate are not known.
Hepatic Impairment
- Adjustment of the recommended dose is not required in mild (Child-Pugh A) or moderate (Child-Pugh B) hepatic impairment, but patients should be monitored closely for adverse effects of quinine. Quinine should not be administered in patients with severe (Child-Pugh C) hepatic impairment.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
- Babesiosis
- Malaria, Uncomplicated, Plasmodium vivax
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Quinine sulphate in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Quinine sulphate in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Quinine sulphate in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Quinine sulphate in pediatric patients.
Contraindications
Quinine sulphate is contraindicated in patients with the following:
Prolonged QT interval:
- One case of a fatal ventricular arrhythmia was reported in an elderly patient with a prolonged QT interval at baseline, who received quinine sulfate intravenously for P. falciparum malaria.
Glucose-6-phosphate dehydrogenase deficiency(G6PD):
- Hemolysis can occur in patients with G6PD deficiency receiving quinine.
Known hypersensitivity reactions to quinine:
- These include, but are not limited to, the following:
Hemolytic uremic syndrome (HUS)
- Blackwater fever (acute intravascular hemolysis, hemoglobinuria, and hemoglobinemia)
- Known hypersensitivity to mefloquine or quinidine: cross-sensitivity to quinine has been documented.
- Myasthenia gravis. Quinine has neuromuscular blocking activity, and may exacerbate muscle weakness.
- Optic neuritis. Quinine may exacerbate active optic neuritis
Warnings
|
WARNING
See full prescribing information for complete Boxed Warning.
* Quinine sulphate use for the treatment or prevention of nocturnal leg cramps may result in serious and life-threatening hematologic reactions, including thrombocytopenia and hemolytic uremic syndrome/thrombotic thrombocytopenic purpura (HUS/TTP). Chronic renal impairment associated with the development of TTP has been reported. The risk associated with Quinine sulphate use in the absence of evidence of its effectiveness in the treatment or prevention of nocturnal leg cramps outweighs any potential benefit
|
Use of Quinine for Treatment or Prevention of Nocturnal Leg Cramps:=
- Quinine sulphate may cause unpredictable serious and life-threatening hematologic reactions including thrombocytopenia and hemolytic-uremic syndrome/thrombotic thrombocytopenic purpura (HUS/TTP) in addition to hypersensitivity reactions, QT prolongation, serious cardiac arrhythmias including torsades de pointes, and other serious adverse events requiring medical intervention and hospitalization. Chronic renal impairment associated with the development of TTP, and fatalities have also been reported. The risk associated with the use of Quinine sulphate in the absence of evidence of its effectiveness for treatment or prevention of nocturnal leg cramps, outweighs any potential benefit in treating and/or preventing this benign, self-limiting condition.
Thrombocytopenia
- Quinine-induced thrombocytopenia is an immune-mediated disorder. Severe cases of thrombocytopenia that are fatal or life threatening have been reported, including cases of HUS/TTP. Chronic renal impairment associated with the development of TTP has also been reported. Thrombocytopenia usually resolves within a week upon discontinuation of quinine. If quinine is not stopped, a patient is at risk for fatal hemorrhage. Upon re-exposure to quinine from any source, a patient with quinine-dependent antibodies could develop thrombocytopenia that is more rapid in onset and more severe than the original episode.
QT Prolongation and Ventricular Arrhythmias:
- QT interval prolongation has been a consistent finding in studies which evaluated electrocardiographic changes with oral or parenteral quinine administration, regardless of age, clinical status, or severity of disease. The maximum increase in QT interval has been shown to correspond with peak quinine plasma concentration. Quinine sulfate has been rarely associated with potentially fatal cardiac arrhythmias, including torsades de pointes, and ventricular fibrillation.
- Quinine sulphate has been shown to cause concentration-dependent prolongation of the PR and QRS interval. At particular risk are patients with underlying structural heart disease and preexisting conduction system abnormalities, elderly patients with sick sinus syndrome, patients with atrial fibrillation with slow ventricular response, patients with myocardial ischemia or patients receiving drugs known to prolong the PR interval (e.g. verapamil) or QRS interval (e.g. flecainide or quinidine).
- Quinine sulphate is not recommended for use with other drugs known to cause QT prolongation, including Class IA antiarrhythmic agents (e.g., quinidine, procainamide, disopyramide), and Class III antiarrhythmic agents (e.g., amiodarone, sotalol, dofetilide).
- The use of macrolide antibiotics such as erythromycin should be avoided in patients receiving Quinine sulphate. Fatal torsades de pointes was reported in an elderly patient who received concomitant quinine, erythromycin, and dopamine. Although a causal relationship between a specific drug and the arrhythmia was not established in this case, erythromycin is a CYP3A4 inhibitor and has been shown to increase quinine plasma levels when used concomitantly. A related macrolide antibiotic, troleandomycin, has also been shown to increase quinine exposure in a pharmacokinetic study.
- Quinine may inhibit the metabolism of certain drugs that are CYP3A4 substrates and are known to cause QT prolongation, e.g., astemizole, cisapride, terfenadine, pimozide, halofantrine and quinidine. Torsades de pointes has been reported in patients who received concomitant quinine and astemizole. Therefore, concurrent use of Quinine sulphate with these medications, or drugs with similar properties, should be avoided.
- Concomitant administration of Quinine sulphate with the antimalarial drugs, mefloquine or halofantrine, may result in electrocardiographic abnormalities, including QT prolongation, and increase the risk for torsades de pointes or other serious ventricular arrhythmias. Concurrent use of Quinine sulphate and mefloquine may also increase the risk of seizures.
- Quinine sulphate should also be avoided in patients with known prolongation of QT interval and in patients with clinical conditions known to prolong the QT interval, such as uncorrected hypokalemia, bradycardia, and certain cardiac conditions.
Concomitant Use of Rifampin:
- Treatment failures may result from the concurrent use of rifampin with Quinine sulphate, due to decreased plasma concentrations of quinine, and concomitant use of these medications should be avoided.
Concomitant Use of Neuromuscular Blocking Agents:
- The use of neuromuscular blocking agents should be avoided in patients receiving Quinine sulphate. In one patient who received pancuronium during an operative procedure, subsequent administration of quinine resulted in respiratory depression and apnea. Although there are no clinical reports with succinylcholine or tubocurarine, quinine may also potentiate neuromuscular blockade when used with these drugs.
Hypersensitivity
- Serious hypersensitivity reactions reported with quinine sulfate include anaphylactic shock, anaphylactoid reactions, urticaria, serious skin rashes, including Stevens-Johnson syndrome and toxic epidermal necrolysis, angioedema, facial edema, bronchospasm, and pruritus.
- A number of other serious adverse reactions reported with quinine, including thrombotic thrombocytopenic purpura (TTP) and hemolytic uremic syndrome (HUS), thrombocytopenia, immune thrombocytopenic purpura (ITP), blackwater fever, disseminated intravascular coagulation, leukopenia, neutropenia, granulomatous hepatitis, and acute interstitial nephritis may also be due to hypersensitivity reactions.
- Quinine sulphate should be discontinued in case of any signs or symptoms of hypersensitivity.
Atrial Fibrillation and Flutter:
- Quinine sulphate should be used with caution in patients with atrial fibrillation or atrial flutter. A paradoxical increase in ventricular response rate may occur with quinine, similar to that observed with quinidine. If digoxin is used to prevent a rapid ventricular response, serum digoxin levels should be closely monitored, because digoxin levels may be increased with use of quinine.
Hypoglycemia
- Quinine stimulates release of insulin from the pancreas, and patients, especially pregnant women, may experience clinically significant hypoglycemia.
Adverse Reactions
Clinical Trials Experience
Overall=
- Quinine can adversely affect almost every body system. The most common adverse events associated with quinine use are a cluster of symptoms called "cinchonism", which occurs to some degree in almost all patients taking quinine. Symptoms of mild cinchonism include headache, vasodilation and sweating, nausea, tinnitus, hearing impairment, vertigo or dizziness, blurred vision, and disturbance in color perception. More severe symptoms of cinchonism are vomiting, diarrhea, abdominal pain, deafness, blindness, and disturbances in cardiac rhythm or conduction. Most symptoms of cinchonism are reversible and resolve with discontinuation of quinine.
- The following ADVERSE REACTIONS have been reported with quinine sulfate. Most of these reactions are thought to be uncommon, but the actual incidence is unknown:
General:
Hematologic:
- Agranulocytosis, hypoprothrombinemia, thrombocytopenia, disseminated intravascular coagulation, hemolytic anemia; hemolytic uremic syndrome, thrombotic thrombocytopenic purpura, idiopathic thrombocytopenic purpura, petechiae, ecchymosis, hemorrhage, coagulopathy, blackwater fever, leukopenia, neutropenia, pancytopenia, aplastic anemia, and lupus anticoagulant.
Neuropsychiatric:
- Headache, diplopia, confusion, altered mental status, seizures, coma, disorientation, tremors, restlessness, ataxia, acute dystonic reaction, aphasia, and suicide.
Dermatologic:
- Cutaneous rashes, including urticarial, papular, or scarlatinal rashes, pruritus, bullous dermatitis, exfoliative dermatitis, erythema multiforme, Stevens-Johnson syndrome, toxic epidermal necrolysis, fixed drug eruption, photosensitivity reactions, allergic contact dermatitis, acral necrosis, and cutaneous vasculitis.
Respiratory:
Cardiovascular:
- Chest pain, vasodilatation, hypotension, postural hypotension, tachycardia, bradycardia, palpitations, syncope, atrioventricular block, atrial fibrillation, irregular rhythm, unifocal premature ventricular contractions, nodal escape beats, U waves, QT prolongation, ventricular fibrillation, ventricular tachycardia, torsades de pointes, and cardiac arrest.
Gastrointestinal:
Hepatobiliary:
- Granulomatous hepatitis, hepatitis, jaundice, and abnormal liver function tests.
Metabolic:
- Hypoglycemia and anorexia.
Musculoskeletal:
- Myalgias and muscle weakness.
Renal:
Special Senses:
- Visual disturbances, including blurred vision with scotomata, sudden loss of vision, photophobia, diplopia, night blindness, diminished visual fields, fixed pupillary dilatation, disturbed color vision, optic neuritis, blindness, vertigo, tinnitus, hearing impairment, and deafness.
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Quinine sulphate in the drug label.
Drug Interactions
Effects of Drugs and Other Substances on Quinine Pharmacokinetics
- Quinine is a P-gp substrate and is primarily metabolized by CYP3A4. Other enzymes, including CYP1A2, CYP2C8, CYP2C9, CYP2C19, CYP2D6, and CYP2E1 may contribute to the metabolism of quinine.
- Antacids:
- Antiepileptics (AEDs) (carbamazepine, phenobarbital, and phenytoin):
- Carbamazepine, phenobarbital, and phenytoin are CYP3A4 inducers and may decrease quinine plasma concentrations if used concurrently with Quinine sulphate.
- Cholestyramine:
- In 8 healthy subjects who received quinine sulfate 600 mg with or without 8 grams of cholestyramine resin, no significant difference in quinine pharmacokinetic parameters was seen.
- Cigarette Smoking (CYP1A2 inducer):
- In healthy male heavy smokers, the mean quinine AUC following a single 600 mg dose was 44% lower, the mean Cmax was 18% lower, and the elimination half-life was shorter (7.5 hours versus 12 hours) than in their non-smoking counterparts. However, in malaria patients who received the full 7-day course of quinine therapy, cigarette smoking produced only a 25% decrease in median quinine AUC and a 16.5% decrease in median Cmax, suggesting that the already reduced clearance of quinine in acute malaria could have diminished the metabolic induction effect of smoking. Because smoking did not appear to influence the therapeutic outcome in malaria patients, it is not necessary to increase the dose of quinine in the treatment of acute malaria in heavy cigarette smokers.
- Grapefruit juice (P-gp/CYP3A4 inhibitor):
- In a pharmacokinetic study involving 10 healthy subjects, the administration of a single 600 mg dose of quinine sulfate with grapefruit juice (full-strength or half-strength) did not significantly alter the pharmacokinetic parameters of quinine. Quinine sulphate may be taken with grapefruit juice.
- Histamine H2-receptor blockers [[[cimetidine]], ranitidine (nonspecific CYP450 inhibitors:
- In healthy subjects who were given a single oral 600 mg dose of quinine sulfate after pretreatment with cimetidine (200 mg three times daily and 400 mg at bedtime for 7 days) or ranitidine (150 mg twice daily for 7 days), the apparent oral clearance of quinine decreased and the mean elimination half-life increased significantly when given with cimetidine but not with ranitidine. Compared to untreated controls, the mean AUC of quinine increased by 20% with ranitidine and by 42% with cimetidine (p<0.05) without a significant change in mean quinine Cmax. When quinine is to be given concomitantly with a Histamine H2-receptor blockers, the use of ranitidine is preferred over cimetidine. Although cimetidine and ranitidine may be used concomitantly with Quinine sulphate, patients should be monitored closely for adverse events associated with quinine.
- Isoniazid:
- Isoniazid 300 mg/day pretreatment for 1 week did not significantly alter the pharmacokinetic parameter values of quinine. Adjustment of Quinine sulphate dosage is not necessary when isoniazid is given concomitantly.
- Ketoconazole (CYP3A4 inhibitor):
- In a crossover study, healthy subjects (N=9) who received a single oral dose of quinine hydrochloride (500 mg) concomitantly with ketoconazole (100 mg twice daily for 3 days) had a mean quinine AUC that was higher by 45% and a mean oral clearance of quinine that was 31% lower than after receiving quinine alone. Although no change in the Quinine sulphate dosage regimen is necessary with concomitant ketoconazole, patients should be monitored closely for adverse reactions associated with quinine.
- Macrolide antibiotics (erythromycin, troleandomycin) (CYP3A4 inhibitors):
- In a crossover study (N=10), healthy subjects who received a single oral 600 mg dose of quinine sulfate with the macrolide antibiotic, troleandomycin (500 mg every 8 hours) exhibited a 87% higher mean quinine AUC, a 45% lower mean oral clearance of quinine, and a 81% lower formation clearance of the main metabolite, 3-hydroxyquinine, than when quinine was given alone.
- Erythromycin was shown to inhibit the in vitro metabolism of quinine in human liver microsomes, an observation confirmed by an in vivo interaction study. In a crossover study (N=10), healthy subjects who received a single oral 500 mg dose of quinine sulfate with erythromycin (600 mg every 8 hours for four days) showed a decrease in quinine oral clearance (CL/F), an increase in half-life, and a decreased metabolite (3-hydroxyquinine) to quinine AUC ratio, as compared to when quinine was given with placebo.
- Therefore, concomitant administration of macrolide antibiotics such as erythromycin or troleandomycin with Quinine sulphate should be avoided.
- Oral contraceptives (estrogen, progestin):
- In 7 healthy females who were using single-ingredient progestin or combination estrogen-containing oral contraceptives, the pharmacokinetic parameters of a single 600 mg dose of quinine sulfate were not altered in comparison to those observed in 7 age-matched female control subjects not using oral contraceptives.
- Rifampin (CYP3A4 inducer):
- In patients with uncomplicated P. falciparum malaria who received quinine sulfate 10 mg/kg concomitantly with rifampin 15 mg/kg/day for 7 days (N=29), the median AUC of quinine between days 3 and 7 of therapy was 75% lower as compared to those who received quinine monotherapy. In healthy subjects (N=9) who received a single oral 600 mg dose of quinine sulfate after 2 weeks of pretreatment with rifampin 600 mg/day, the mean quinine AUC and Cmax decreased by 85% and 55%, respectively. Therefore, the concomitant administration of rifampin with Quinine sulphate should be avoided.
- Ritonavir:
- In healthy subjects who received a single oral 600 mg dose of quinine sulfate with the 15th dose of ritonavir (200 mg every 12 hours for 9 days), there were 4-fold increases in the mean quinine AUC and Cmax, and an increase in the mean elimination half-life (13.4 hours versus 11.2 hours), compared to when quinine was given alone. Therefore, the concomitant administration of ritonavir with Quinine sulphate capsules should be avoided.
- Tetracycline:
- In 8 patients with acute uncomplicated P. falciparum malaria who were treated with oral quinine sulfate (600 mg every 8 hours for 7 days) in combination with oral tetracycline (250 mg every 6 hours for 7 days), the mean plasma quinine concentrations were about two-fold higher than in 8 patients who received quinine monotherapy. Although tetracycline may be concomitantly administered with Quinine sulphate, patients should be monitored closely for adverse reactions associated with quinine sulfate.
- Theophylline or aminophylline:
- In 20 healthy subjects who received multiple doses of Quinine sulphate (648 mg every 8 hours × 7 days) with a single 300 mg oral dose of theophylline, the quinine mean Cmax and AUC were increased by 13% and 14% respectively. Although no change in the Quinine sulphate dosage regimen is necessary with concomitant theophylline or aminophylline, patients should be monitored closely for adverse reactions associated with quinine.
- Urinary alkalizers (acetazolamide, sodium bicarbonate):
- Urinary alkalinizing agents may increase plasma quinine concentrations.
Effects of Quinine on the Pharmacokinetics of Other Drugs:
- Results of in vivo drug interaction studies suggest that quinine has the potential to inhibit the metabolism of drugs that are substrates of CYP3A4 and CYP2D6. Quinine inhibits P-gp and has the potential to affect the transport of drugs that are P-gp substrates.
- Anticonvulsants (carbamazepine, phenobarbital, and phenytoin):
- A single 600 mg oral dose of quinine sulfate increased the mean plasma Cmax, and AUC0–24 of single oral doses of carbamazepine (200 mg) and phenobarbital (120 mg) but not phenytoin (200 mg) in 8 healthy subjects. The mean AUC increases of carbamazepine, phenobarbital and phenytoin were 104%, 81% and 4%, respectively; the mean increases in Cmax were 56%, 53%, and 4%, respectively. Mean urinary recoveries of the three antiepileptics over 24 hours were also profoundly increased by quinine. If concomitant administration with carbamazepine or phenobarbital cannot be avoided, frequent monitoring of anticonvulsant drug concentrations is recommended. Additionally, patients should be monitored closely for adverse reactions associated with these anticonvulsants.
- Astemizole (CYP3A4 substrate):
- Elevated plasma astemizole concentrations were reported in a subject who experienced torsades de pointes after receiving three doses of quinine sulfate for nocturnal leg cramps concomitantly with chronic astemizole 10 mg/day. The concurrent use of Quinine sulphate with astemizole and other CYP3A4 substrates with QT prolongation potential (e.g., cisapride, terfenadine, halofantrine, pimozide and quinidine) should also be avoided.
- Atorvastatin (CYP3A4 substrate):
- Rhabdomyolysis with acute renal failure secondary to myoglobinuria was reported in a patient taking atorvastatin administered with a single dose of quinine. Quinine may increase plasma concentrations of atorvastatin, thereby increasing the risk of myopathy or rhabdomyolysis. Thus, clinicians considering combined therapy of Quinine sulphate with atorvastatin or other HMG-CoA reductase inhibitors ("statins") that are CYP3A4 substrates (e.g., simvastatin, lovastatin) should carefully weigh the potential benefits and risks of each medication. If Quinine sulphate is used concomitantly with any of these statins, lower starting and maintenance doses of the statin should be considered. Patients should also be monitored closely for any signs or symptoms of muscle pain, tenderness, or weakness, particularly during initial therapy. If marked creatine phosphokinase (CPK) elevation occurs or myopathy (defined as muscle aches or muscle weakness in conjunction with CPK values >10 times the upper limit of normal) is diagnosed or suspected, atorvastatin or other statin should be discontinued.
- Desipramine (CYP2D6 substrate):
- Quinine (750 mg/day for 2 days) decreased the metabolism of desipramine in patients who were extensive CYP2D6 metabolizers, but had no effect in patients who were poor CYP2D6 metabolizers. Lower doses (80 mg to 400 mg) of quinine did not significantly affect the pharmacokinetics of other CYP2D6 substrates, namely, debrisoquine, dextromethorphan, and methoxyphenamine. Although clinical drug interaction studies have not been performed, antimalarial doses (greater than or equal to 600 mg) of quinine may inhibit the metabolism of other drugs that are CYP2D6 substrates (e.g., flecainide, debrisoquine, dextromethorphan, metoprolol, paroxetine). Patients taking medications that are CYP2D6 substrates with Quinine sulphate should be monitored closely for adverse reactions associated with these medications.
- Digoxin (P-gp substrate):
- In 4 healthy subjects who received digoxin (0.5 to 0.75 mg/day) during treatment with quinine (750 mg/day), a 33% increase in mean steady state AUC of digoxin and a 35% reduction in the steady state biliary clearance of digoxin were observed compared to digoxin alone. Thus, if Quinine sulphate is administered to patients receiving digoxin, plasma digoxin concentrations should be closely monitored, and the digoxin dose adjusted, as necessary.
- Halofantrine:
- Although not studied clinically, quinine was shown to inhibit the metabolism of halofantrine in vitro using human liver microsomes. Therefore, concomitant administration of Quinine sulphate is likely to increase plasma halofantrine concentrations.
- Mefloquine:
- In 7 healthy subjects who received mefloquine (750 mg) at 24 hours before an oral 600 mg dose of quinine sulfate, the AUC of mefloquine was increased by 22% compared to mefloquine alone. In this study, the QTc interval was significantly prolonged in the subjects who received mefloquine and quinine sulfate 24 hours apart. The concomitant administration of mefloquine and Quinine sulphate may produce electrocardiographic abnormalities (including QTc prolongation) and may increase the risk of seizures.
- Midazolam (CYP3A4 substrate):
- In 23 healthy subjects who received multiple doses of Quinine sulphate 324 mg three times daily × 7 days with a single oral 2 mg dose of midazolam, the mean AUC and Cmax of midazolam and 1-hydroxymidazolam were not significantly affected. This finding indicates that 7-day dosing with Quinine sulphate 324 mg every 8 hours did not induce the metabolism of midazolam.
- Neuromuscular blocking agents (pancuronium, succinylcholine, tubocurarine):
- In one report, quinine potentiated neuromuscular blockade in a patient who received pancuronium during an operative procedure, and subsequently (3 hours after receiving pancuronium) received quinine 1800 mg daily. Quinine may also enhance the neuromuscular blockade of succinylcholine and tubocurarine.
- Ritonavir:
- In healthy subjects who received a single oral 600 mg dose of quinine sulfate with the 15th dose of ritonavir (200 mg every 12 hours for 9 days), the mean ritonavir AUC, Cmax, and elimination half-life were slightly but not significantly increased compared to when ritonavir was given alone. However, due to the significant effect of ritonavir on quinine pharmacokinetics, the concomitant administration of Quinine sulphate capsules with ritonavir should be avoided.
- Theophylline or aminophylline (CYP1A2 substrate):
- In 19 healthy subjects who received multiple doses of Quinine sulphate 648 mg every 8 hours x 7 days with a single 300 mg oral dose of theophylline, the mean theophylline AUC was 10% lower than when theophylline was given alone. There was no significant effect on mean theophylline Cmax. Therefore, if Quinine sulphate is co-administered to patients receiving theophylline or aminophylline, plasma theophylline concentrations should be monitored frequently to ensure therapeutic concentrations.
- Warfarin and oral anticoagulants:
- Cinchona alkaloids, including quinine, may have the potential to depress hepatic enzyme synthesis of vitamin K-dependent coagulation pathway proteins and may enhance the action of warfarin and other oral anticoagulants. Quinine may also interfere with the anticoagulant effect of heparin. Thus, in patients receiving these anticoagulants, the prothrombin time (PT), partial thromboplastin time (PTT), or international normalization ratio (INR) should be closely monitored as appropriate, during concurrent therapy with Quinine sulphate.
Drug/Laboratory Interactions:
- Quinine may produce an elevated value for urinary 17-ketogenic steroids when the Zimmerman method is used.
- Quinine may interfere with urine qualitative dipstick protein assays as well as quantitative methods (e.g., pyrogallol red-molybdate).
Use in Specific Populations
Pregnancy
- There are extensive published data but few well-controlled studies of Quinine sulphate in pregnant women. Published data on over 1,000 pregnancy exposures to quinine did not show an increase in teratogenic effects over the background rate in the general population; however, the majority of these exposures were not in the first trimester. In developmental and reproductive toxicity studies, central nervous system (CNS) and ear abnormalities and increased fetal deaths occurred in some species when pregnant animals received quinine at doses about 1 to 4 times the human clinical dose. Quinine should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
- P. falciparum malaria carries a higher risk of morbidity and mortality in pregnant women than in the general population. Pregnant women with P. falciparum malaria have an increased incidence of fetal loss (including spontaneous abortion and stillbirth), preterm labor and delivery, intrauterine growth retardation, low birth weight, and maternal death. Therefore, treatment of malaria in pregnancy is important.
- Hypoglycemia, due to increased pancreatic secretion of insulin, has been associated with quinine use, particularly in pregnant women.
- Quinine crosses the placenta with measurable blood concentrations in the fetus. In 8 women who delivered live infants 1 to 6 days after starting quinine therapy, umbilical cord plasma quinine concentrations were between 1.0 and 4.6 mg/L (mean 2.4 mg/L) and the mean (±SD) ratio of cord plasma to maternal plasma quinine concentrations was 0.32 ± 0.14. Quinine levels in the fetus may not be therapeutic. If congenital malaria is suspected after delivery, the infant should be evaluated and treated appropriately.
- A study from Thailand (1999) of women with P. falciparum malaria who were treated with oral quinine sulfate 10 mg/kg 3 times daily for 7 days at anytime in pregnancy reported no significant difference in the rate of stillbirths at >28 weeks of gestation in women treated with quinine (10 of 633 women [1.6%]) as compared with a control group without malaria or exposure to antimalarial drugs during pregnancy (40 of 2201 women [1.8%]). The overall rate of congenital malformations (9 of 633 offspring [1.4%]) was not different for women who were treated with quinine sulfate compared with the control group (38 of 2201 offspring [1.7%]). The spontaneous abortion rate was higher in the control group (10.9%) than in women treated with quinine sulfate (3.5%) [OR = 3.1; 95% CI 2.1-4.7]. An epidemiologic survey that included 104 mother-child pairs exposed to quinine during the first 4 months of pregnancy, found no increased risk of structural birth defects was seen (2 fetal malformations [1.9%]). Rare and isolated case reports describe deafness and optic nerve hypoplasia in children exposed in utero due to maternal ingestion of high doses of quinine.
- In animal developmental studies conducted in multiple animal species, pregnant animals received quinine by the subcutaneous or intramuscular route at dose levels similar to the maximum recommended human dose (MRHD; 32 mg/kg/day) based on body surface area (BSA) comparisons. There were increases in fetal death in utero in rabbits at maternal doses ≥ 100 mg/kg/day and in dogs at ≥ 15 mg/kg/day corresponding to dose levels approximately 0.5 and 0.25 times the MRHD respectively based on BSA comparisons. Rabbit offspring had increased rates of degenerated auditory nerve and spiral ganglion and increased rates of CNS anomalies such as anencephaly and microcephaly at a dose of 130 mg/kg/day corresponding to a maternal dose approximately 1.3 times the MRHD based on BSA comparison. Guinea pig offspring had increased rates of hemorrhage and mitochondrial change in the cochlea at maternal doses of 200 mg/kg corresponding to a dose level of approximately 1.4 times the MRHD based on BSA comparison. There were no teratogenic findings in rats at maternal doses up to 300 mg/kg/day and in monkeys at doses up to 200 mg/kg/day corresponding to doses approximately 1 and 2 times the MRHD respectively based on BSA comparisons.
- In a pre- postnatal study in rats, an estimated oral dose of quinine sulfate of 20 mg/kg/day corresponding to approximately 0.1 times the MRHD based on BSA comparison resulted in offspring with impaired growth, lower body weights at birth and during the lactation period, and delayed physical development of teeth eruption and eye opening during the lactation period.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Quinine sulphate in women who are pregnant.
Labor and Delivery
- There is no evidence that quinine causes uterine contractions at the doses recommended for the treatment of malaria. In doses several-times higher than those used to treat malaria, quinine may stimulate the pregnant uterus.
Nursing Mothers
- There is limited information on the safety of quinine in breastfed infants. No toxicity was reported in infants in a single study where oral quinine sulfate (10 mg/kg every 8 hours for 1 to 10 days) was administered to 25 lactating women. It is estimated from this study that breastfed infants would receive less than 2 to 3 mg per day of quinine base (< 0.4% of the maternal dose) via breast milk.
- Although quinine is generally considered compatible with breastfeeding, the risks and benefits to infant and mother should be assessed. Caution should be exercised when administered to a nursing woman.
- If malaria is suspected in the infant, appropriate evaluation and treatment should be provided. Plasma quinine levels may not be therapeutic in infants of nursing mothers receiving Quinine sulphate.
Pediatric Use
There is no FDA guidance on the use of Quinine sulphate with respect to pediatric patients.
Geriatic Use
There is no FDA guidance on the use of Quinine sulphate with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Quinine sulphate with respect to specific gender populations.
Race
There is no FDA guidance on the use of Quinine sulphate with respect to specific racial populations.
Renal Impairment
- Clearance of quinine is decreased in patients with severe chronic renal failure. The dosage and dosing frequency should be reduced
Hepatic Impairment
- In patients with severe hepatic impairment (Child-Pugh C), quinine oral clearance (CL/F) is decreased, volume of distribution (Vd/F) is increased, and half-life is prolonged, relative to subjects with normal liver function. Therefore, quinine is not indicated in patients with severe hepatic impairment and alternate therapy should be administered.
- Close monitoring is recommended for patients with mild (Child-Pugh A) or moderate (Child-Pugh B) hepatic impairment, as exposure to quinine may be increased relative to subjects with normal liver function
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Quinine sulphate in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Quinine sulphate in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
Monitoring
+=Hepatic Impairment===
- Adjustment of the recommended dose is not required in mild (Child-Pugh A) or moderate (Child-Pugh B) hepatic impairment, but patients should be monitored closely for adverse effects of quinine. Quinine should not be administered in patients with severe (Child-Pugh C) hepatic impairment.
- In otherwise healthy subjects with mild hepatic impairment (Child-Pugh A; N=10), who received a single 500 mg dose of quinine sulfate, there was no significant difference in quinine pharmacokinetic parameters or exposure to the primary metabolite, 3-hydroxyquinine as compared to healthy controls (N=10). In otherwise healthy subjects with moderate hepatic impairment (Child-Pugh B; N=9) who received a single oral 600 mg dose of quinine sulfate, the mean AUC increased by 55% without a significant change in mean Cmax, as compared to healthy volunteer controls (N=6). In subjects with hepatitis, the absorption of quinine was prolonged, the elimination half-life was increased, the apparent volume of distribution was higher, but there was no significant difference in weight-adjusted clearance. Therefore, in patients with mild to moderate hepatic impairment, dosage adjustment is not needed, but patients should be monitored closely for adverse effects of quinine.
Atrial Fibrillation and Flutter
- Quinine sulphate should be used with caution in patients with atrial fibrillation or atrial flutter. A paradoxical increase in ventricular response rate may occur with quinine, similar to that observed with quinidine. If digoxin is used to prevent a rapid ventricular response, serum digoxin levels should be closely monitored, because digoxin levels may be increased with use of quinine
Histamine H2-receptor blockers i.e cimetidine, ranitidine (nonspecific CYP450 inhibitors)=
- In healthy subjects who were given a single oral 600 mg dose of quinine sulfate after pretreatment with cimetidine (200 mg three times daily and 400 mg at bedtime for 7 days) or ranitidine (150 mg twice daily for 7 days), the apparent oral clearance of quinine decreased and the mean elimination half-life increased significantly when given with cimetidine but not with ranitidine. Compared to untreated controls, the mean AUC of quinine increased by 20% with ranitidine and by 42% with cimetidine (p<0.05) without a significant change in mean quinine Cmax. When quinine is to be given concomitantly with a histamine H2-receptor blocker, the use of ranitidine is preferred over cimetidine. Although cimetidine and ranitidine may be used concomitantly with Quinine sulphate, patients should be monitored closely for adverse events associated with quinine.
Ketoconazole (CYP3A4 inhibitor)
- In a crossover study, healthy subjects (N=9) who received a single oral dose of quinine hydrochloride (500 mg) concomitantly with ketoconazole (100 mg twice daily for 3 days) had a mean quinine AUC that was higher by 45% and a mean oral clearance of quinine that was 31% lower than after receiving quinine alone. Although no change in the Quinine sulphate dosage regimen is necessary with concomitant ketoconazole, patients should be monitored closely for adverse reactions associated with quinine.
- Tetracycline: In 8 patients with acute uncomplicated P. falciparum malaria who were treated with oral quinine sulfate (600 mg every 8 hours for 7 days) in combination with oral tetracycline (250 mg every 6 hours for 7 days), the mean plasma quinine concentrations were about two-fold higher than in 8 patients who received quinine monotherapy. Although tetracycline may be concomitantly administered with Quinine sulphate, patients should be monitored closely for adverse reactions associated with quinine sulfate.
- Theophylline or aminophylline: In 20 healthy subjects who received multiple doses of Quinine sulphate (648 mg every 8 hours × 7 days) with a single 300 mg oral dose of theophylline, the quinine mean Cmax and AUC were increased by 13% and 14% respectively. Although no change in the Quinine sulphate dosage regimen is necessary with concomitant theophylline or aminophylline, patients should be monitored closely for adverse reactions associated with quinine.
- Anticonvulsants (carbamazepine, phenobarbital, and phenytoin): A single 600 mg oral dose of quinine sulfate increased the mean plasma Cmax, and AUC0–24 of single oral doses of carbamazepine (200 mg) and phenobarbital (120 mg) but not phenytoin (200 mg) in 8 healthy subjects. The mean AUC increases of carbamazepine, phenobarbital and phenytoin were 104%, 81% and 4%, respectively; the mean increases in Cmax were 56%, 53%, and 4%, respectively. Mean urinary recoveries of the three antiepileptics over 24 hours were also profoundly increased by quinine. If concomitant administration with carbamazepine or phenobarbital cannot be avoided, frequent monitoring of anticonvulsant drug concentrations is recommended. Additionally, patients should be monitored closely for adverse reactions associated with these anticonvulsants.
- Atorvastatin (CYP3A4 substrate): Rhabdomyolysis with acute renal failure secondary to myoglobinuria was reported in a patient taking atorvastatin administered with a single dose of quinine. Quinine may increase plasma concentrations of atorvastatin, thereby increasing the risk of myopathy or rhabdomyolysis. Thus, clinicians considering combined therapy of Quinine sulphate with atorvastatin or other HMG-CoA reductase inhibitors ("statins") that are CYP3A4 substrates (e.g., simvastatin, lovastatin) should carefully weigh the potential benefits and risks of each medication. If Quinine sulphate is used concomitantly with any of these statins, lower starting and maintenance doses of the statin should be considered. Patients should also be monitored closely for any signs or symptoms of muscle pain, tenderness, or weakness, particularly during initial therapy. If marked creatine phosphokinase (CPK) elevation occurs or myopathy (defined as muscle aches or muscle weakness in conjunction with CPK values >10 times the upper limit of normal) is diagnosed or suspected, atorvastatin or other statin should be discontinued.
- Desipramine (CYP2D6 substrate): Quinine (750 mg/day for 2 days) decreased the metabolism of desipramine in patients who were extensive CYP2D6 metabolizers, but had no effect in patients who were poor CYP2D6 metabolizers. Lower doses (80 mg to 400 mg) of quinine did not significantly affect the pharmacokinetics of other CYP2D6 substrates, namely, debrisoquine, dextromethorphan, and methoxyphenamine. Although clinical drug interaction studies have not been performed, antimalarial doses (greater than or equal to 600 mg) of quinine may inhibit the metabolism of other drugs that are CYP2D6 substrates (e.g., flecainide, debrisoquine, dextromethorphan, metoprolol, paroxetine). Patients taking medications that are CYP2D6 substrates with Quinine sulphate should be monitored closely for adverse reactions associated with these medications.
- Digoxin (P-gp substrate): In 4 healthy subjects who received digoxin (0.5 to 0.75 mg/day) during treatment with quinine (750 mg/day), a 33% increase in mean steady state AUC of digoxin and a 35% reduction in the steady state biliary clearance of digoxin were observed compared to digoxin alone. Thus, if Quinine sulphate is administered to patients receiving digoxin, plasma digoxin concentrations should be closely monitored, and the digoxin dose adjusted, as necessary.
- Theophylline or aminophylline (CYP1A2 substrate): In 19 healthy subjects who received multiple doses of Quinine sulphate 648 mg every 8 hours x 7 days with a single 300 mg oral dose of theophylline, the mean theophylline AUC was 10% lower than when theophylline was given alone. There was no significant effect on mean theophylline Cmax. Therefore, if Quinine sulphate is co-administered to patients receiving theophylline or aminophylline, plasma theophylline concentrations should be monitored frequently to ensure therapeutic concentrations.
- Warfarin and oral anticoagulants: Cinchona alkaloids, including quinine, may have the potential to depress hepatic enzyme synthesis of vitamin K-dependent coagulation pathway proteins and may enhance the action of warfarin and other oral anticoagulants. Quinine may also interfere with the anticoagulant effect of heparin. Thus, in patients receiving these anticoagulants, the prothrombin time (PT), partial thromboplastin time (PTT), or international normalization ratio (INR) should be closely monitored as appropriate, during concurrent therapy with Quinine sulphate.
IV Compatibility
There is limited information regarding IV Compatibility of Quinine sulphate in the drug label.
Overdosage
- Quinine overdose can be associated with serious complications, including visual impairment, hypoglycemia, cardiac arrhythmias, and death. Visual impairment can range from blurred vision and defective color perception, to visual field constriction and permanent blindness. Cinchonism occurs in virtually all patients with quinine overdose. Symptoms range from headache, nausea, vomiting, abdominal pain, diarrhea, tinnitus, vertigo, hearing impairment, sweating, flushing, and blurred vision, to deafness, blindness, serious cardiac arrhythmias, hypotension, and circulatory collapse. Central nervous system toxicity (drowsiness, disturbances of consciousness, ataxia, convulsions, respiratory depression and coma) has also been reported with quinine overdose, as well as pulmonary edema and adult respiratory distress syndrome.
- Most toxic reactions are dose-related; however, some reactions may be idiosyncratic because of the variable sensitivity of patients to the toxic effects of quinine. A lethal dose of quinine has not been clearly defined, but fatalities have been reported after the ingestion of 2 to 8 grams in adults.
- Quinine, like quinidine, has Class I antiarrhythmic properties. The cardiotoxicity of quinine is due to its negative inotropic action, and to its effect on cardiac conduction, resulting in decreased rates of depolarization and conduction, and increased action potential and effective refractory period. ECG changes observed with quinine overdose include sinus tachycardia, PR prolongation, T wave inversion, bundle branch block, an increased QT interval, and a widening of the QRS complex. Quinine's alpha-blocking properties may result in hypotension and further exacerbate myocardial depression by decreasing coronary perfusion. Quinine overdose has been also associated with hypotension, cardiogenic shock, and circulatory collapse, ventricular arrhythmias, including ventricular tachycardia, ventricular fibrillation, idioventricular rhythm, and torsades de pointes, as well as bradycardia, and atrioventricular block.
- Quinine is rapidly absorbed, and attempts to remove residual quinine sulfate from the stomach by gastric lavage may not be effective. Multiple-dose activated charcoal has been shown to decrease plasma quinine concentrations.
- Forced acid diuresis, hemodialysis, charcoal column hemoperfusion, and plasma exchange were not found to be effective in significantly increasing quinine elimination in a series of 16 patients.
Pharmacology
Mechanism of Action
- Quinine is an antimalarial agent
Structure
- Quinine sulphate (quinine sulfate) is a cinchona alkaloid chemically described as cinchonan-9-ol, 6'-methoxy-, (8α, 9R)-, sulfate (2:1) (salt), dihydrate with a molecular formula of (C20H24N2O2)2•H2SO4•2H2O and a molecular weight of 782.96.
The structural formula of quinine sulfate is:

Pharmacodynamics
- QTc interval prolongation was studied in a double-blind, multiple dose, placebo- and positive-controlled crossover study in young (N=13, 20 to 39 years) and elderly (N=13, 65 to 78 years) subjects. After 7 days of dosing with Quinine sulphate 648 mg three times daily, the maximum mean (95% upper confidence bound) differences in QTcI from placebo after baseline correction was 27.7 (32.2) ms.
- Prolongation of the PR and QRS interval was also noted in subjects receiving Quinine sulphate. The maximum mean (95% upper confidence bound) difference in PR from placebo after baseline-correction was 14.5 (18.0) ms. The maximum mean (95% upper confidence bound) difference in QRS from placebo after baseline-correction was 11.5 (13.3) ms.
Pharmacokinetics
Absorption:
- The oral bioavailability of quinine is 76 to 88% in healthy adults. Quinine exposure is higher in patients with malaria than in healthy subjects. After a single oral dose of quinine sulfate, the mean quinine Tmax was longer, and mean AUC and Cmax were higher in patients with uncomplicated P. falciparum malaria than in healthy subjects, as shown in Table 1 below.
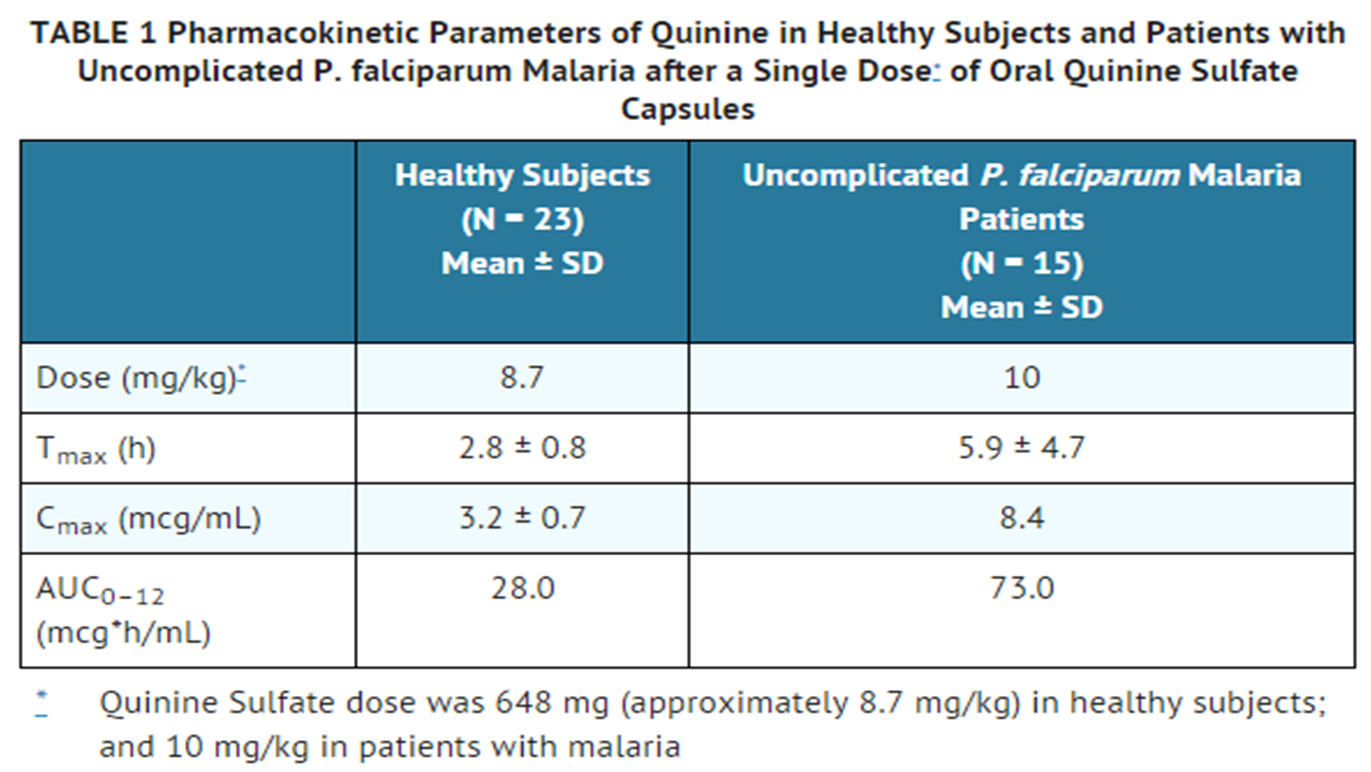
- Quinine sulphate capsules may be administered without regard to meals. When a single oral 324 mg capsule of Quinine sulphate was administered to healthy subjects (N=26) with a standardized high-fat breakfast, the mean Tmax of quinine was prolonged to about 4.0 hours, but the mean Cmax and AUC0-24h were similar to those achieved when Quinine sulphate capsule was given under fasted conditions.
Distribution:
- In patients with malaria, the volume of distribution (Vd/F) decreases in proportion to the severity of the infection. In published studies with healthy subjects who received a single oral 600 mg dose of quinine sulfate, the mean Vd/F ranged from 2.5 to 7.1 L/kg.
- Quinine is moderately protein-bound in blood in healthy subjects, ranging from 69 to 92%. During active malarial infection, protein binding of quinine is increased to 78 to 95%, corresponding to the increase in α1-acid glycoprotein that occurs with malaria infection.
- Intra-erythrocytic levels of quinine are approximately 30 to 50% of the plasma concentration.
- Quinine penetrates relatively poorly into the cerebrospinal fluid (CSF) in patients with cerebral malaria, with CSF concentration approximately 2 to 7% of plasma concentration.
- In one study, quinine concentrations in placental cord blood and breast milk were approximately 32% and 31%, respectively, of quinine concentrations in maternal plasma. The estimated total dose of quinine secreted into breast milk was less than 2 to 3 mg per day.
Metabolism:
- Quinine is metabolized almost exclusively via hepatic oxidative cytochrome P450 (CYP) pathways, resulting in four primary metabolites, 3-hydroxyquinine, 2´-quinone, O-desmethylquinine, and 10,11-dihydroxydihydroquinine. Six secondary metabolites result from further biotransformation of the primary metabolites. The major metabolite, 3-hydroxyquinine, is less active than the parent drug.
- In vitro studies using human liver microsomes and recombinant P450 enzymes have shown that quinine is metabolized mainly by CYP3A4. Depending on the in vitro experimental conditions, other enzymes, including CYP1A2, CYP2C8, CYP2C9, CYP2C19, CYP2D6, and CYP2E1 were shown to have some role in the metabolism of quinine.
Elimination/Excretion:
- Quinine is eliminated primarily via hepatic biotransformation. Approximately 20% of quinine is excreted unchanged in urine. Because quinine is reabsorbed when the urine is alkaline, renal excretion of the drug is twice as rapid when the urine is acidic than when it is alkaline.
- In various published studies, healthy subjects who received a single oral 600 mg dose of quinine sulfate exhibited a mean plasma clearance ranging from 0.08 to 0.47 L/h/kg (median value: 0.17 L/h/kg) with a mean plasma elimination half-life of 9.7 to 12.5 hours.
- In 15 patients with uncomplicated malaria who received a 10 mg/kg oral dose of quinine sulfate, the mean total clearance of quinine was slower (approximately 0.09 L/h/kg) during the acute phase of the infection, and faster (approximately 0.16 L/h/kg) during the recovery or convalescent phase.
- Extracorporeal Elimination:
- Administration of multiple-dose activated charcoal (50 grams administered 4 hours after quinine dosing followed by 3 further doses over the next 12 hours) decreased the mean quinine elimination half-life from 8.2 to 4.6 hours, and increased the mean quinine clearance by 56% (from 11.8 L/h to 18.4 L/h) in 7 healthy adult subjects who received a single oral 600 mg dose of quinine sulfate. Likewise, in 5 symptomatic patients with acute quinine poisoning who received multiple-dose activated charcoal (50 grams every 4 hours), the mean quinine elimination half-life was shortened to 8.1 hours in comparison to a half-life of approximately 26 hours in patients who did not receive activated charcoal [see OVERDOSAGE (10)].
- In 6 patients with quinine poisoning, forced acid diuresis did not change the half-life of quinine elimination (25.1 ± 4.6 hours vs. 26.5 ± 5.8 hours), or the amount of unchanged quinine recovered in the urine, in comparison to 8 patients not treated in this manner.
- Specific Populations:
- Pediatric Patients:
- The pharmacokinetics of quinine in children (1.5 to 12 years old) with uncomplicated P. falciparum malaria appear to be similar to that seen in adults with uncomplicated malaria. Furthermore, as seen in adults, the mean total clearance and the volume of distribution of quinine were reduced in pediatric patients with malaria as compared to the healthy pediatric controls. Table 2 below provides a comparison of the mean ± SD pharmacokinetic parameters of quinine in pediatric patients vs. healthy pediatric controls.
- Pediatric Patients:
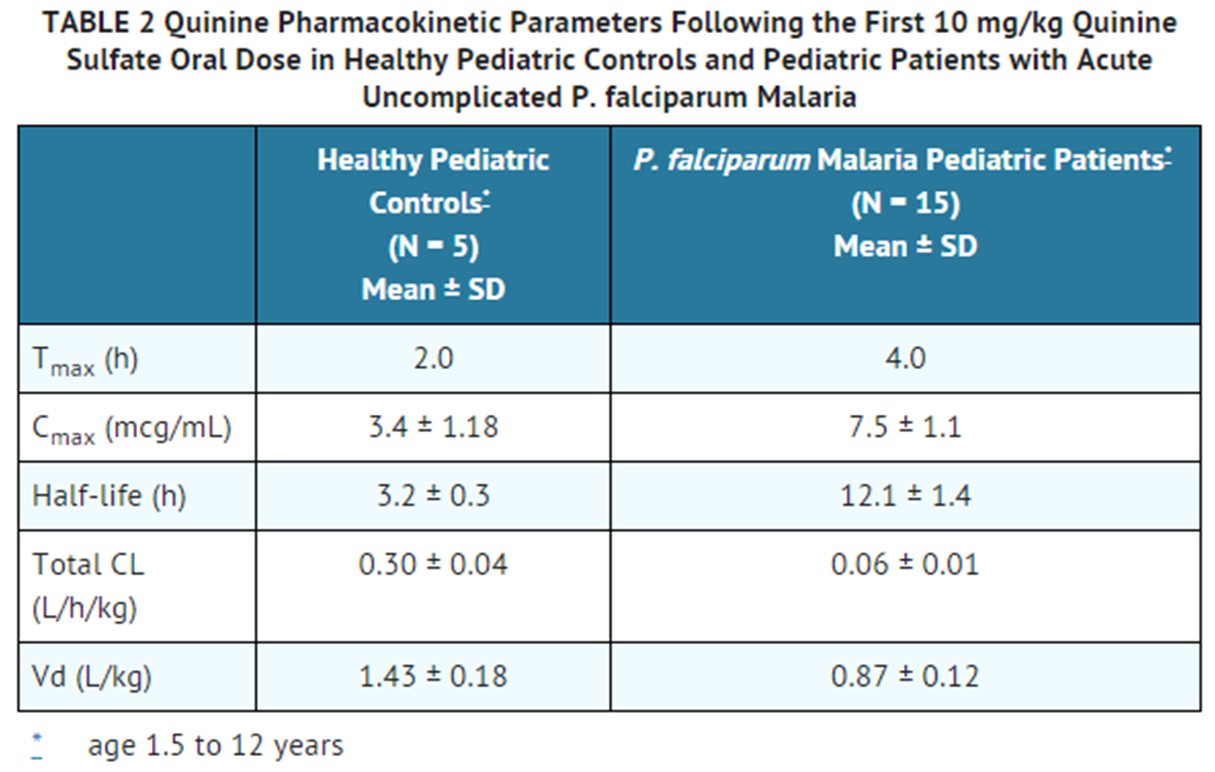
- Geriatric Patients:
- Following a single oral dose of 600 mg quinine sulfate, the mean AUC was about 38% higher in 8 healthy elderly subjects (65 to 78 years old) than in 12 younger subjects (20 to 35 years old). The mean Tmax and Cmax were similar in elderly and younger subjects after a single oral dose of quinine sulfate 600 mg. The mean oral clearance of quinine was significantly decreased, and the mean elimination half-life was significantly increased in elderly subjects compared with younger subjects (0.06 vs. 0.08 L/h/kg, and 18.4 hours vs. 10.5 hours, respectively). Although there was no significant difference in the renal clearance of quinine between the two age groups, elderly subjects excreted a larger proportion of the dose in urine as unchanged drug than younger subjects (16.6% vs. 11.2%).
- After a single 648 mg dose or at steady state, following quinine sulfate 648 mg given three times daily for 7 days, no difference in the rate and extent of absorption or clearance of quinine was seen between 13 elderly subjects (65 to 78 years old) and 14 young subjects (20 to 39 years old). The mean elimination half-life was 20% longer in the elderly subjects (24.0 hours) than in younger subjects (20.0 hours). The steady state Cmax (±SD) and AUC0-8 (±SD) for healthy volunteers are 6.8 ± 1.24 mcg/mL and 48.8 ± 9.15 mcg*h/mL, respectively, following 7 days of oral quinine sulfate 648 mg three times daily. The steady state pharmacokinetic parameters in healthy elderly subjects were similar to the pharmacokinetic parameters in healthy young subjects.
- Geriatric Patients:
- Renal Impairment:
- Following a single oral 600 mg dose of quinine sulfate in otherwise healthy subjects with severe chronic renal failure not receiving any form of dialysis (mean serum creatinine = 9.6 mg/dL), the median AUC was higher by 195% and the median Cmax was higher by 79% than in subjects with normal renal function (mean serum creatinine = 1 mg/dL). The mean plasma half-life in subjects with severe chronic renal impairment was prolonged to 26 hours compared to 9.7 hours in the healthy controls. Computer assisted modeling and simulation indicates that in patients with malaria and severe chronic renal failure, a dosage regimen consisting of one loading dose of 648 mg Quinine sulphate followed 12 hours later by a maintenance dosing regimen of 324 mg every 12 hours will provide adequate systemic exposure to quinine. The effects of mild and moderate renal impairment on the pharmacokinetics and safety of quinine sulfate are not known.
- Renal Impairment:
- Negligible to minimal amounts of circulating quinine in the blood are removed by hemodialysis or hemofiltration. In subjects with chronic renal failure (CRF) on hemodialysis, only about 6.5% of quinine is removed in 1 hour. Plasma quinine concentrations do not change during or shortly after hemofiltration in subjects with CRF [see OVERDOSAGE (10)].
- Hepatic Impairment:
- In otherwise healthy subjects with mild hepatic impairment (Child-Pugh A; N=10), who received a single 500 mg dose of quinine sulfate, there was no significant difference in quinine pharmacokinetic parameters or exposure to the primary metabolite, 3-hydroxyquinine as compared to healthy controls (N=10). In otherwise healthy subjects with moderate hepatic impairment (Child-Pugh B; N=9) who received a single oral 600 mg dose of quinine sulfate, the mean AUC increased by 55% without a significant change in mean Cmax, as compared to healthy volunteer controls (N=6). In subjects with hepatitis, the absorption of quinine was prolonged, the elimination half-life was increased, the apparent volume of distribution was higher, but there was no significant difference in weight-adjusted clearance. Therefore, in patients with mild to moderate hepatic impairment, dosage adjustment is not needed, but patients should be monitored closely for adverse effects of quinine.
- In subjects with severe hepatic impairment (Child-Pugh C; N=10), quinine oral clearance (CL/F) was reduced as was formation of the primary 3-hydroxyquinine metabolite. Volume of distribution (Vd/F) was higher and the plasma elimination half-life was increased. Therefore, quinine is not indicated in this population and alternate therapy should be administered.
- Hepatic Impairment:
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- Carcinogenesis:
- Carcinogenicity studies of quinine have not been conducted.
- Mutagenesis:
- Genotoxicity studies of quinine were positive in the Ames bacterial mutation assay with metabolic activation and in the sister chromatid exchange assay in mice. The sex-linked recessive lethal test performed in Drosophila, the in vivo mouse micronucleus assay, and the chromosomal aberration assay in mice and Chinese hamsters were negative.
- Impairment of Fertility:
- Published studies indicate that quinine produces testicular toxicity in mice at a single intraperitoneal dose of 300 mg/kg corresponding to a dose of approximately 0.75 times the maximum recommended human dose (MRHD; 32 mg/kg/day) and in rats at an intramuscular dose of 10 mg/kg/day, 5 days/week, for 8 weeks corresponding to a daily dose of approximately 0.05 times the MRHD based on body surface area (BSA) comparisons. The findings include atrophy or degeneration of the seminiferous tubules, decreased sperm count and motility, and decreased testosterone levels in the serum and testes. There was no effect on testes weight in studies of oral doses of up to 500 mg/kg/day in mice and 700 mg/kg/day in rats (approximately 1.2 and 3.5 times the MRHD respectively based on BSA comparisons). In a published study in 5 men receiving 600 mg of quinine TID for one week, sperm motility was decreased and percent sperm with abnormal morphology was increased; sperm count and serum testosterone were unaffected.
Clinical Studies
- Quinine has been used worldwide for hundreds of years in the treatment of malaria. Thorough searches of the published literature identified over 1300 references to the treatment of malaria with quinine, and from these, 21 randomized, active-controlled studies were identified which evaluated oral quinine monotherapy or combination therapy for treatment of uncomplicated P. falciparum malaria. Over 2900 patients from malaria-endemic areas were enrolled in these studies, and more than 1400 patients received oral quinine. The following conclusions were drawn from review of these studies:
- In areas where multi-drug resistance of P. falciparum is increasing, such as Southeast Asia, cure rates with 7 days of oral quinine monotherapy were at least 80%; while cure rates for 7 days of oral quinine combined with an antimicrobial agent (tetracycline or clindamycin) were greater than 90%. In areas where multi-drug resistance of the parasite was not as widespread, cure rates with 7 days of quinine monotherapy ranged from 86 to 100%. Cure was defined as initial clearing of parasitemia within 7 days without recrudescence by day 28 after treatment initiation. P. falciparum malaria that is clinically resistant to quinine has been reported in some areas of South America, Southeast Asia, and Bangladesh, and quinine may not be as effective in those areas.
- Completion of a 7-day oral quinine treatment regimen may be limited by drug intolerance, and shorter courses (3 days) of quinine combination therapy have been used. However, the published data from randomized, controlled clinical trials for shorter regimens of oral quinine in conjunction with tetracycline, doxycycline, or clindamycin for treatment of uncomplicated P. falciparum malaria is limited, and these shorter course combination regimens may not be as effective as the longer regimens.
How Supplied
- Quinine sulphate capsules USP, 324 mg are available as clear/clear capsules imprinted AR 102:

Storage
- Store at 20° to 25°C (68° to 77°F).
Images
Drug Images
{{#ask: Page Name::Quinine |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Quinine |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Dosing Instructions
- Patients should be instructed to:
- Take all of the medication as directed.
- Take no more of the medication than the amount prescribed.
- Take with food to minimize possible gastrointestinal irritation.
- If a dose is missed, patients should also be instructed not to double the next dose. If more than 4 hours has elapsed since the missed dose, the patient should wait and take the next dose as previously scheduled.
Precautions with Alcohol
- Alcohol-Quinine sulphate interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Quiphile®
- Quinamm®
- Qualaquin®
Look-Alike Drug Names
There is limited information regarding Quinine Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ 1.0 1.1 sulphate-quinine-342696#showall "Quinine sulphate (quinine) dosing, indications, interactions, adverse effects, and more" Check
|url=value (help). Medscape Reference. WebMD. Retrieved 29 January 2014.
{{#subobject:
|Page Name=Quinine
|Pill Name=No image.jpg
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Label Page=Quinine |Label Name=Quinine drug label01.png
}}
{{#subobject:
|Label Page=Quinine |Label Name=Quinine drug label02.png
}}
{{#subobject:
|Label Page=Quinine |Label Name=
}}