Neuromuscular junction
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
A neuromuscular junction (NMJ) is the synapse or junction of the axon terminal of a motoneuron with the motor end plate, the highly-excitable region of muscle fiber plasma membrane responsible for initiation of action potentials across the muscle's surface, ultimately causing the muscle to contract. In vertebrates, the signal passes through the neuromuscular junction via the neurotransmitter acetylcholine.
Anatomy
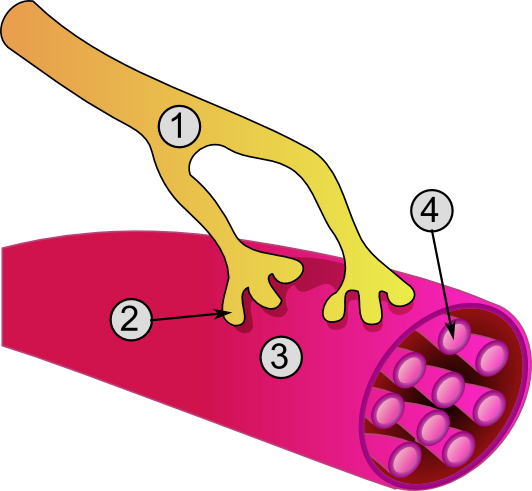
1. Axon
2. Motor end-plate
3. Muscle fiber
4. Myofibril
When a motor neuron enters a muscle, it loses its myelin sheath and splits into many terminal branches. Motor neuron (efferent) axons originating in the spinal cord enter muscle fibers, where they split into many unmyelinated branches. These terminal fibers run along the myocytes to end at the neuromuscular junction, which occupies a depression in the sarcolemma. Each motor neuron can innervate from one to over 2000 [1] muscle fibers, but each muscle fiber receives inputs from only one motor neuron.
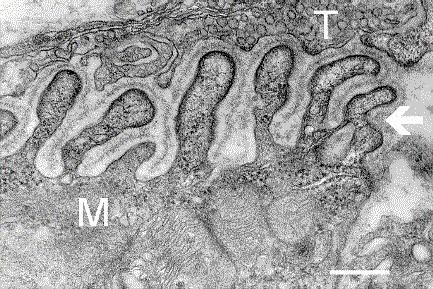
In the terminal bouton of the motor nerve, structures known as presynaptic active zones accumulate synaptic vesicles filled with the neurotransmitter acetylcholine.
On the muscle side of the junction, the muscle fiber is folded into grooves called postjunctional folds that mirror the presynaptic active zones, the spaces between the folds contain acetylcholine receptors.
The muscle surface is covered by the synaptic basal lamina. Postjunctional folds are characteristic of skeletal muscle, particularly in fast muscle fibers.
Mechanism of Action
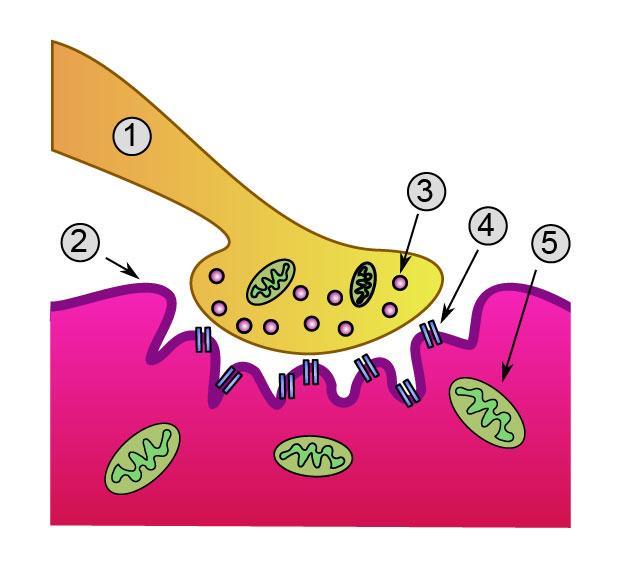
1. Presynaptic terminal
2. Sarcolemma
3. Synaptic vesicle
4. Nicotinic acetylcholine receptor
5. Mitochondrion
Upon the arrival of an action potential at the axon terminal, voltage-dependent calcium channels open and Ca2+ ions flow from the extracellular fluid into the motor neuron's cytosol. This influx of Ca2+ triggers excitation-contraction coupling, a biochemical cascade that causes neurotransmitter-containing vesicles to fuse to the motor neuron's cell membrane and release acetylcholine into the synaptic cleft.
Acetylcholine diffuses across the synaptic cleft and binds to the nicotinic acetylcholine receptors that dot the motor end plate.
The receptors are Ligand-gated ion channels, and when bound by acetylcholine, they open, allowing sodium and potassium ions to flow in and out of the muscle's cytosol, respectively.
Because of the differences in electrochemical gradients across the plasma membrane, more sodium moves in than potassium out, producing a local depolarization of the motor end plate known as an end plate potential (EPP).
This depolarization spreads across the surface of the muscle fiber into transverse tubules, eliciting the release of calcium from the sarcoplasmic reticulum, thus initiating muscle contraction.
The action of acetylcholine is terminated when the enzyme acetylcholinesterase degrades the neurotransmitter.
Development of the Neuromuscular junction
The formation of the neuromuscular junction during embryonic development is well understood.
During development, the growing end of motor neuron axons secrete a protein known as agrin.
This protein binds to several receptors on the surface of skeletal muscle.
The receptor which seems to be required for formation of the neuromuscular junction is called the MuSK protein (Muscle specific kinase).[2]
MuSK is a receptor tyrosine kinase - meaning that it induces cellular signaling by causing the addition of phosphate molecules to particular tyrosines on itself, and on proteins which bind the cytoplasmic domain of the receptor.[3]
Upon activation by its ligand agrin, MuSK signals via two proteins called "Dok-7" and "rapsyn", to induce "clustering" of acetylcholine receptors (AChR).[4]
In addition to the AChR and MuSK, other proteins are then gathered, to form the endplate to the neuromuscular junction. The nerve terminates onto the endplate, forming the NMJ.
Knockout studies
These findings were demonstrated in part by mouse "knockout" studies. In mice which are deficient for either agrin or MuSK, the neuromuscular junction does not form. Further, mice deficient in Dok-7 did not form either acetylcholine receptor clusters or neuromuscular synapses.[5]
Many other proteins also comprise the NMJ, and are required to maintain its integrity.[6]
Neuromuscular junction disorders
In diseases such as myasthenia gravis, the EPP fails to effectively activate the muscle fiber due to an autoimmune reaction against acetylcholine receptors, resulting in muscle weakness and fatigue. [7]
Various toxins, such as botulinum toxin prevent the release of acetylcholine, resulting in muscle paralysis. Myasthenia gravis is caused most commonly by auto-antibodies against the acetylcholine receptor.
It has recently been realized that a second category of gravis is due to auto-antibodies against MuSK.
See also
External links
- Template:McGrawHillAnimation
- Histology image: 21501lca – Histology Learning System at Boston University
Further reading
- Kandel, ER (2000). Principles of Neural Science (4th ed. ed.). New York: McGraw-Hill. ISBN 0-8385-7701-6. Unknown parameter
|coauthors=ignored (help) - Nicholls, J.G. (2001). From Neuron to Brain (4th ed. ed.). Sunderland, MA.: Sinauer Associates. ISBN 0878934391. Unknown parameter
|coauthors=ignored (help) - Engel, A.G. (2004). Myology (3rd ed. ed.). New York: McGraw Hill Professional. ISBN 0-07-137180-X.
References
- ↑ Dale Purves (Editor), George J. Augustine (Editor), David Fitzpatrick (Editor), William C. Hall (Editor), Anthony-Samuel Lamantia (Editor), James O. McNamara (Editor), S. Mark Williams (Editor) (2005). Neurosciences (3 ed.). p. 377. ISBN 978-0878937257. Unknown parameter
|translation=ignored (help) - ↑ DeChiara T, Bowen D, Valenzuela D, Simmons M, Poueymirou W, Thomas S, Kinetz E, Compton D, Rojas E, Park J, Smith C, DiStefano P, Glass D, Burden S, Yancopoulos G (1996). "The receptor tyrosine kinase MuSK is required for neuromuscular junction formation in vivo". Cell. 85 (4): 501–12. PMID 8653786.
- ↑ Valenzuela D, Stitt T, DiStefano P, Rojas E, Mattsson K, Compton D, Nuñez L, Park J, Stark J, Gies D (1995). "Receptor tyrosine kinase specific for the skeletal muscle lineage: expression in embryonic muscle, at the neuromuscular junction, and after injury". Neuron. 15 (3): 573–84. PMID 7546737.
- ↑ Glass D, Bowen D, Stitt T, Radziejewski C, Bruno J, Ryan T, Gies D, Shah S, Mattsson K, Burden S, DiStefano P, Valenzuela D, DeChiara T, Yancopoulos G (1996). "Agrin acts via a MuSK receptor complex". Cell. 85 (4): 513–23. PMID 8653787.
- ↑ Okada K, Inoue A, Okada M, Murata Y, Kakuta S, Jigami T, Kubo S, Shiraishi H, Eguchi K, Motomura M, Akiyama T, Iwakura Y, Higuchi O, Yamanashi Y (2006). "The muscle protein Dok-7 is essential for neuromuscular synaptogenesis". Science. 312 (5781): 1802–5. PMID 16794080. link
- ↑ Strochlic L, Cartaud A, Cartaud J (2005). "The synaptic muscle-specific kinase (MuSK) complex: new partners, new functions". Bioessays. 27 (11): 1129–35. PMID 16237673.
- ↑ Hoch W, McConville J, Helms S, Newsom-Davis J, Melms A, Vincent A (2001). "Auto-antibodies to the receptor tyrosine kinase MuSK in patients with myasthenia gravis without acetylcholine receptor antibodies". Nat Med. 7 (3): 365–8. PMID 11231638.
de:Motorische Endplatte nl:Motorische eindplaat fi:Hermo-lihasliitos Template:WH Template:WikiDoc Sources Template:Jb1