Congestive heart failure treatment of patients with current or prior symptoms of heart failure (Stage C)
| Resident Survival Guide |
| File:Critical Pathways.gif |
| Congestive Heart Failure Microchapters |
|
Pathophysiology |
|---|
|
Differentiating Congestive heart failure from other Diseases |
|
Diagnosis |
|
Treatment |
|
Medical Therapy: |
|
Surgical Therapy: |
|
ACC/AHA Guideline Recommendations
|
|
Specific Groups: |
|
Congestive heart failure treatment of patients with current or prior symptoms of heart failure (Stage C) On the Web |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Mahmoud Sakr, M.D. [2],Seyedmahdi Pahlavani, M.D. [3], Edzel Lorraine Co, D.M.D., M.D. [4]
Overview
Several measures [1] have been listed for patients in Stage A or B as previously mentioned and the Class 1 recommendations for those patients are also appropriate for patients with current or previous symptoms of HF. The effectiveness of diuretics and safety of their usage is an important aspect of the treatment that should be closely monitored along with daily measurement of weight and moderate sodium restriction. Physical activity is highly recommended, although heavy labor or sports shouldn’t be a part of it. A reduction in physical activity promotes physical deconditioning, and an increase of weight which may be associated with more strain on the failing heart.[2] Patients with chronic conditions such as HF are also recommended to be immunized with influenza and pneumococcal vaccines in order to reduce the risk of respiratory infection.
Drugs that should be Avoided or Used with Caution in Patients with HF
Pharmacological therapy should be closely monitored and several classes of drugs should be avoided in case of HF:
- Calcium Channel blockers: There is no direct role of these drugs in the management of HF, due to negative and possible deleterious effect in patients with HF due to systolic dysfunction[3]. Vasoelective Calcium Channel blockers as amlodipine and felodipine have not been linked to adverse effect in HF treatment, but there is no evidence of efficacy for these drugs in the management of HF.[4] However, amlodipine and felodipine appear to be safe for the treatment of concomitant disease in HF patients, such as angina or hypertension.
- Antiarrhythmic agents: Negative inotropic effect exerted by most antiarrhythmic drugs can precipitate HF in patients with reduced LV function. The reduction in LV function can also reduce the elimination of these drugs leading to further drug toxicity. Other antiarrhythmic drugs can induce some proarrhythmic effect, especially class 1 agents and class 3 agents Ibutilide and sotalol;[5] the same class 3 agents in addition to dofetilide can induce torsades to pointes.
Amiodarone is considered the safest of the antiarrhythmic drugs because of its minimal proarrhythmic effect and is generally the preferred drug for treating arrhythmias in HF patients.
- Nonsteroidal anti-inflammatory drugs (NSAID):[6] The administration of non-selective NSAIDs in HF patients is linked with an increased risk of HF exacerbation, increased renal dysfunction, and abnormal responses to ACEIs and diuretics. COX-2 selective inhibitors have not been fully investigated, but observational studies indicate that they may be linked with an increased rate of HF exacerbation and increased mortality.
Aspirin benefits and risks are not well established in patients with HF and Vascular disease (includingCAD). The potential interaction between ACEIs and beta blockers is of great importance. Although no data has proven that aspirin causes more frequent HF exacerbations and interactions with those drugs, health care providers should be aware of the possibility of such risks, but no recommendation for or against aspirin therapy in patients with heart failure can be made before further data are available.
- Oral Hypoglycemic agents:[7] two oral hypoglycemic agents, metformin and thiazolidinediones are considered to be risky in patients with HF.
Metformin - one of the most common side effects of metformin is lactic acidosis, which can be fatal in patients with HF.
Thiazolidinediones -[8] the biggest risk of using Thiazolidinediones is fluid retention which may cause severe worsening of patients with HF.
- Antidepressants: Depression is a common finding between patients suffering from HF that is usually related to high mortality rate and bad prognosis of those patients.[9] Limited data are available on the safety and the risks associated with the usage of antidepressants in patients with heart failure. Health care providers should be aware of major cardiovascular events (as HF, MI, Stroke, cardiovascular death ) that is associated with Tricyclic antidepressants (TCAs) and Selective serotonin reuptake inhibitors (SSRIs).
- Phosphodiesterase inhibitors PDE – The PDE-3 inhibitors as[10] Cilostazol and PDE-4 as [11] Anagrelide should be avoided in patients with HF, because of an increase risk of high-output heart failure and fluid retention that is associated with those drugs.[12]
PDE-5 inhibitors such as sildenafil, vardenafil, and tadalafil, are widely used in the management of erectile dysfunction in men. The use of those agents with any form of nitrate therapy is contraindicated because of severe hypotensive effect that can be life threatening.[13] In a trial where sildenafil and placebo were randomly assigned to 34 HF patients, no significant difference of symptomatic hypotension was observed, but HF patients with borderline low blood pressure and/or low volume status are in risk of severe hypotension and should avoid any PDE-5 inhibitors use.
- Chemotherapy – Cardiotoxic chemotherapeutic agents as Cyclophosphamide, Trastuzumab, Bevacizumab and Anthracyclines, should be avoided in HF patients [14]
- Tumor Necrosis Factor alpha inhibitors TNF-alpha: New onset or worsening of pre-existing heart failure have been linked to TNF-alpha inhibitors.[15] Infliximab has been specifically contraindicated in doses over 5mg/kg in patients with heart failure.
- Antihistamines:[16] some second generation antihistamines as terfenadine and astemizole have been reported to cause long QT syndrome and should not be used in HF patients.
Serum potassium should be closely monitored in HF patients, in order of preventing either hypokalemia or hyperkalemia, which could greatly affect cardiac excitability and conduction, leading to sudden cardiac death.[17] Serum potassium should be maintained between 4.0 to 5.0 mEq per liter range, because low potassium level may affect digitalis and antiarrhythmic drugs treatment, while high potassium level can prevent the use of treatments known to prolong life.[17]
Supervision of HF patients with close monitoring of treatment and diet is a very important aspect of the follow-up process in those individuals. Body weight and medications should be closely monitored, because any minor change in those parameters can have a significant effect over symptoms and hospitalization of patients with HF.[18] Patient education is a crucial aspect of the management of HF, patient and family surveillance over any new change of symptoms or body weight is important in allowing early detection of those changes and implementing new treatment strategies to reduce further complications.[19]
Pharmacological Therapy of HF
Improving symptoms, reducing mortality and slowing or reversing myocardial deterioration are the main goals of pharmacological therapy in HF patients. The therapy should be also directed at preventing arrhythmias, embolic events and other exacerbating factors. Different strategies have been implemented in the treatment of HF, but the ACC/AHA recommendations are based on a combination of 3 types of drugs: a Diuretic, an ACEI or an ARB, and a beta blocker[20]. A review of data from several trials, provide clear evidence supporting the central role of these drugs in HF patient Therapy. The Diuretics play an important role in controlling fluid retention and achieving a euvolemic state. On the other hand, ACEI and a beta blocker should be started and maintained in patients who can tolerate them because of their major effect in controlling symptoms and reducing mortality. Digoxin can be added to this regime as a fourth agent to reduce symptoms, reduce recurrent hospitalization, control great and rhythm, and enhance exercise tolerance.
Diuretics
Different classes of diuretics have been implicated in the treatment of CHF. Loop Diuretics such as furosemide, bumetanide and torsemide, act on the inhibition of sodium and chloride reabsorption in the loop of Henle, whereas thiazide diuretics, metolazone, and potassium sparing diuretics as spironolactone act on the distal portion of the renal tubule. [21]Loop diuretics increase sodium secretion up to 25 % of the filtered load of sodium, enhance free water clearance and maintain their efficacy unless severe renal impairment is present.[22]Thiazide diuretics in contrast, increase the fractional excretion of sodium to a maximum of 10% of the filtered load, decrease free water clearance and lose their efficacy in patients with impaired renal function. For such reasons, loop diuretics are much preferred, unless hypertension with mild fluid retention is present in HF patients, where thiazide diuretics may have a better antihypertensive effect in this case.
Different short-studies demonstrated the efficacy of diuretics in improving various symptoms of HF, reducing jugular venous pressure, pulmonary congestion, peripheral edema and body weight, all within days of initiation of therapy.[23] No long term studies have been conducted so far to reveal the effects of diuretic therapy on morbidity and mortality, but an improvement in cardiac function and exercise tolerance in patients with HF have been demonstrated in intermediate-term trials.[24]
Diuretics produce great symptomatic benefits,[25] much more rapidly than any other drugs for HF, they can adequately control the fluid retention of HF, relieving pulmonary edema and peripheral edema within hours or days. However, Diuretics should not be used alone in controlling Stage C HF. They should be combined with an ACEI and a beta blocker to avoid further clinical deterioration and maintain the HF symptoms under control.[24]
Appropriate dose of diuretics should used in treatment of HF,[26] because low doses will cause fluid retention, which can lead to a reduced response to ACEIs and ARBs and an increase risk of decompensation with the use of beta blockers, while excessive diuretics will lead to volume contraction which can increase the risk of hypotension and renal insufficiency with ACEIs, ARBs and beta blockers.[27]
Loop diuretics are usually the first diuretics to be used to control pulmonary and/or peripheral edema. Furosemide is the most commonly used, but some patients may respond favorably to other agents in this category (as torsemide and Bumetanide) because of superior absorption and longer time of action.[28]
The starting dosage is usually 20 to 40 mg of furosemide or its equivalent, but the exact dosing should always be monitored according to diuresis and other clinical symptoms, since the ultimate goal is to eliminate the evidence of fluid retention such as jugular venous pressure elevation and peripheral edema. Outpatients with HF are usually started with low dose until urine increases and weigh decreases by 0.5 to 1.0 kg daily. Diuretic therapy should be also combined with moderate dietary sodium restriction. Unstable or severe disease, should be controlled with intravenous diuretics( bolus or continuous infusion) and thiazide diuretics can be added for a synergistic effect.[29]
Reducing overall volume may decrease intracardiac filling pressure resulting in a lower cardiac output via the Frank-Starling relationship. This effect is usually a minor effect and does not alter the course of the therapy. On the other hand, an unexplained increase in BUN and creatinine should be closely monitored and suspected as a sign of abnormal tissue perfusion. In this case renal function and other end organ perfusion should be assessed to avoid any concomitant complications.
Angiotensin Converting Enzyme Inhibitors
Patients with moderate to severe HF or asymptomatic left ventricular dysfunction show a great improvement in survival rate with the usage of ACE inhibitors.[30] ACE inhibitors enhance the action of kinins and augment kinin-mediated prostaglandin production, so the effect of ACEIs cannot be solely explained by the suppression of Angiotensin II production. Furthermore, it has been proven that ACEIs modify cardiac remodeling more favorably than ARBs in experimental models of HF.[31] Several studies indicates that ACEIs can alleviate symptoms,[32] improve clinical status and sense of well being in patients with HF.
Another important aspect of ACEIs therapy is the reduction of mortality and hospitalization in such patients.[33] These benefits were noticed in patients with mild, moderate or even severe symptoms of HF, with or without CAD. In general ACEIs should be used together with a beta blocker and should not be used without diuretics in patients with a current or recent history of fluid retention, because of the important role of diuretics in maintaining sodium balance and preventing the development of peripheral and pulmonary edema.[24]
For the reasons mentioned above, ACEI therapy should be started in asymptomatic or symptomatic patients with HF. The beginning therapy should be a low dose ( eg, 2.5 mg of enalapril twice a day, 6.25 mg of captopril three time a day or 5 mg of lisinopril once a day). The dose should be gradually increased in one to two week if the initial therapy is tolerated and try to target a dose of 20 mg of enalapril twice a day, or 50 mg of captopril three times a day or up to 40 mg of lisinopril a day.[34] Plasma potassium and renal function should be assessed one to two weeks after starting or changing a dose and periodically thereafter. Physicians should attempt to target certain doses which have been proved to reduce the risk of cardiovascular events. If these target doses of ACEIs cannot be used or poorly tolerated than intermediate doses should be implemented. [35]
NSAIDs should be avoided since they may cause an increase in adverse effects of ACEIs in patients with HF and a decrease in the favorable effect of ACEIs therapy.[24] Some evidence suggests that aspirin may inhibit the acute hemodynamic benefits of ACEIs.[36] If the patient has a know history of coronary artery disease(CAD) then use of ASA along with ACEIs is recommended. However, but in patients with no history of CAD, there is no evidence to support the use of aspirin.
Treatment of HFrEF Stage C and D
Abbreviations:
ACEI: angiotensin-converting enzyme inhibitor, ARB: angiotensin receptor-blocker, ARNI: angiotensin
receptor-neprilysin inhibitor, BP: blood pressure, bpm: beats per minute, C/I: contraindication, COR: Class of
Recommendation, NT-proBNP: creatinine clearance, NYHA: cardiac resynchronization therapy–device, pts: diagnosis, HF: guideline-directed management and therapy, CrCl: creatinine clearance, CRT-D: cardiac resynchronization therapy–device, Dx: diagnosis, GDMT: guideline-directed management and therapy, HF: heart failure, HFrEF: heart failure with reduced ejection fraction, ISDN/HYD: isosorbide dinitrate hydral-nitrates, K+: potassium, LBBB: left bundle-branch block, LVAD: left ventricular assist device, LVEF: left ventricular ejection fraction, MI: myocardial infarction, NSR: normal sinus rhythm, NYHA: New York Heart Association
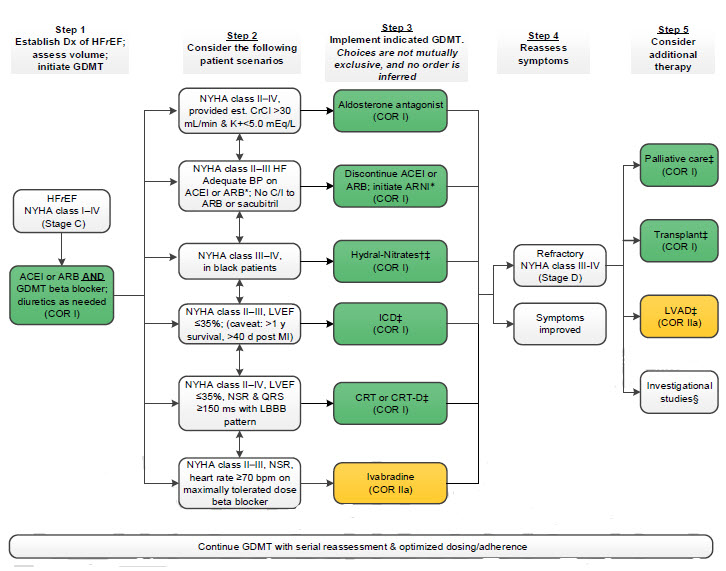
†Hydral-Nitrates green box: The combination of ISDN/HYD with ARNI has not been robustly tested. BP response should be carefully monitored.
§Participation in investigational studies is also appropriate for stage C, NYHA class II and III HF.
2022 AHA/ACC/HFSA Heart Failure Guideline/ 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure/2016 ACC/AHA/HFSA Focused Update on New Pharmacological Therapy for Heart Failure: An Update of the 2013 ACCF/AHA Guideline,2013 ACC/AHA Guideline, 2009 ACC/AHA Focused Update and 2005 Guidelines for the Diagnosis and Management of Heart Failure in the Adult (DO NOT EDIT) [37][38][39]
Pharmacological Treatment for HFrEF (DO NOT EDIT)[37][38][39]
Renin-Angiotensin System Inhibition With ACEi or ARB or ARNi
| Class I |
| "1. In patients with HFrEF and NYHA class II to III symptoms, the use of ARNi is recommended to reduce morbidity and mortality. [40][41][42][43][44](Level of Evidence: A) " |
| "2. In patients with previous or current symptoms of chronic HFrEF, the use of ACEi is beneficial to reduce morbidity and mortality when the use of ARNi is not feasible.[45][46][34][47][48][49][33][50] (Level of Evidence: A) " |
| "3. In patients with previous or current symptoms of chronic HFrEF who are intolerant to ACEi because of cough or angioedema and when the use of ARNi is not feasible, the use of ARB is recommended to reduce morbidity and mortality. [51][52][53][54][55] (Level of Evidence: A) " |
| "5. In patients with chronic symptomatic HFrEF NYHA class II or III who tolerate an ACEi or ARB, replacement by an ARNi is recommended to further reduce morbidity and mortality. [40][41][42][43][44](Level of Evidence: B-R) " |
| Class Value Statement: High Value |
| "4. In patients with previous or current symptoms of chronic HFrEF, in whom ARNi is not feasible, treatment with an ACEi or ARB provides high economic value. [56][57][58][59][4][60][61](Level of Evidence: A) " |
| "6. In patients with chronic symptomatic HFrEF, treatment with an ARNi instead of an ACEi provides high economic value. [62][63][64][65] (Level of Evidence: A) " |
| Class III (Harm) |
| "7. ARNi should not be administered concomitantly with ACEi or within 36 hours of the last dose of an ACEi. [66][67] (Level of Evidence: B-R) " |
| "8. ARNi should not be administered to patients with any history of angioedema.[68][69][70][71] (Level of Evidence: C-LD) " |
| "9. ACEi should not be administered to patients with any history of angioedema. [72][73][74][75] (Level of Evidence: C-LD) " |
Beta Blockers
| Class I |
| "1. In patients with HFrEF, with current or previous symptoms, use of 1 of the 3 beta blockers proven to reduce mortality (eg, bisoprolol, carvedilol,sustained-release metoprolol succinate) is recommended to reduce mortality and hospitalizations. [76][77][78](Level of Evidence: A) " |
| Class Value Statement: High Value |
| "2. In patients with HFrEF, with current or previous symptoms, beta blocker therapy provides high economic values. [56][79][80][81][82] (Level of Evidence: A) " |
Mineralocorticoid Receptor Antagonists (MRAs)
| Class I |
| "1. In patients with HFrEF and NYHA class II to IV symptoms, an MRA (spironolactone or epleronone) is recommended to reduce morbidity and mortality, if eGFR is >30 mL/min/1.73 m2 and serum potassium is <5.0 mEq/L. Careful monitoring of potassium, renal function, and diuretic dosing should be performed at initiation and closely monitored thereafter to minimize risk of hyperkalemia and renal insufficiency. [83][84][85] (Level of Evidence: A) " |
| Value Statement: High Value |
| "2. In patients with HFrEF and NYHA II to IV symptoms, MRA therapy provides high economic value.[56][86][87][88] (Level of Evidence: A) " |
| Class III (Harm) |
| "3. In patients taking MRA whose serum potassium cannot be maintained at <5.5 mEq/L, MRA should be discontinued to avoid life-threatening hyperkalemia. [89][90] (Level of Evidence: B-NR) " |
Sodium-Glucose Contransporter 2 Inhibitors
| Class I |
| "1. In patients with symptomatic chronic HFrEF, SGLT2i are recommended to reduce hospitalization for HF and cardiovascular mortality, irrespective of the presence of type 2 diabetes. [91][92] (Level of Evidence: A) " |
| Value Statement: Intermediate Value |
| "2. In patients with symptomatic chronic HFreF, SGLT2i therapy provides intermediate economic value. [93][94] (Level of Evidence: A) " |
Hydralazine and Isosorbide Dinitrate
| Class I |
| "1. For patients self-identified as African American with NYHA class III-IV HFrEF who are receiving optimal medical therapy, the combination of hydralazine and isosorbide dinitrate is recommended to improve symptoms and reduce morbidity and mortality. [32][95] (Level of Evidence: A) " |
| Value Statement: High Value |
| "2. For patients self-identified as African American with NYHA class III to IV HFrEF who are receiving optimal medical therapy with ACEi or ARB, beta blockers, and MRA, the combination of hydralazine and isosorbide dinitrate provides high economic value.[96] (Level of Evidence: A) " |
| Class IIb |
| "3. In patients with current or previous symptomatic HFrEF who cannot be given first-line agents, such as ARNi, ACEi, or ARB, because of drug intolerance or renal insufficiency, a combination of hydralazine and isosorbide dinitrate might be considered to reduce morbidity and mortality. [97][98] (Level of Evidence: C-LD) " |
Other Drug Treatment
| Class IIb |
| "1. In patients with HF class II to IV symptoms, omega-3 polyunsaturated fatty acid (PUFA) supplementation may be reasonable to use as adjunctive therapy to reduce mortality and cardiovascular hospitalizations. [99][100][101][102] (Level of Evidence: B-R) " |
| "2. In patients with HF who experience hyperkalemia (serum potassium level ≥ 5.5 mEq/L) while taking a renin-angiotensin-aldosterone system inhibitor (RAASi), the effectiveness of potassium binders (patiromer, sodium zirconium cyclosilicate) to improve outcomes by facilitating continuation of RAASi therapy is uncertain. [103][104] (Level of Evidence: B-R) " |
| Class III (No Benefit) |
| "3. In patients with chronic HFrEF without a specific indication (eg, venous thromboembolism [VTE], AF, a previous thromboembolic event, or a cardioembolic source), anticoagulation is not recommended. [105][106][107] (Level of Evidence: B-R) " |
Drugs of Unproven Value or That May Worsen HF
| Class III (No Benefit) |
| "1. In patients with HFrEF, dihydropiridine calcium channel-blocking drugs are not recommended treatment for HF.[108][109] (Level of Evidence: A) " |
| "2. In patients with HFrEF, vitamins, nutritional supplements, and hormonal therapy are not recommended other than to correct specific deficiencies. [110][111][112][113][114][115][116] (Level of Evidence: B-R) " |
| Class III (Harm) |
| "3. In patients with HFrEF, non-dihydropiridine calcium channel-blocking drugs are not recommended. [117][118][119][120] (Level of Evidence: A) " |
| "4.In patients with HFrEF, class IC antiarrhythmic medications and dronedarone may increase the risk of mortality. [121][122][123] (Level of Evidence: A) " |
| "5.In patients with HFrEF, thiazolidinediones increase the risk of worsening HF symptoms and hospitalizations. [124][125][126][127][128](Level of Evidence: A) " |
| "6.In patients with type 2 diabetes and high cardiovascular risk, the dipeptidyl peptidase-4 (DPP-4) inhibitors saxagliptin and alogliptin increase the risk of HF hospitalization and should be avoided in patients with HF. [129][130][131](Level of Evidence: B-R) " |
| "7.In patients with HFrEF, NSAIDs worsen HF symptoms and should be avoided or withdrawn whenever possible. [132][133][134][135] (Level of Evidence: B-NR) " |
| Class I |
| "1. Measures listed as Class I recommendations for patients in stages A and B are also appropriate for patients in Stage C. (Level of Evidence: A, B and C as appropriate)" |
| "2. The clinical strategy of inhibition of the renin-angiotensin system with ACE inhibitors (Level of Evidence:A)[45][46][34][47][49] or ARBs (Level of Evidence: A)[51][52][53][136][40] or ARNI (Level of Evidence: B-R)[77][78][137] in conjunction with evidence based beta blockers and aldosterone antagonists in selected patients is recommended for patients with chronic HFrEF to reduce morbidity and mortality. " |
| "3. Patients with HFrEF and hypertension should be prescribed GDMT titrated to attain systolic blood pressure less than 130 mm Hg. (Level of Evidence: C-EO) " |
| "4. Diuretics and salt restriction are indicated in patients with current or prior symptoms of HFrEF who have evidence of fluid retention. (Level of Evidence: C) " |
| "5. The use of ACE inhibitors is beneficial for patients with prior or current symptoms of chronic HFrEF to reduce morbidity and mortality (Class I, Level of Evidence: A)[45][46][34][47][49]" |
| "6. Beta-blockers (using 1 of the 3 proven to reduce mortality, i.e., bisoprolol, carvedilol, and sustained release metoprolol succinate) are recommended for all stable patients with current or prior symptoms of HFrEF, unless contraindicated.[138][139][140][141][142][143][144][145][146][147][148][149] (Level of Evidence: A) " |
| "7. The use of ARBs to reduce morbidity and mortality is recommended in patients with prior or current symptoms of chronic HFrEF who are intolerant to ACE inhibitors because of cough or angioedema(Class I, Level of Evidence: A)[51][52][53][136][40][54][55] " |
| "8. In patients with chronic symptomatic HFrEF NYHA class II or III who tolerate an ACE inhibitor or ARB, replacement by an ARNI is recommended to further reduce morbidity and mortality. (Class I, Level of Evidence: B-R)[40] " |
| "9. Aldosterone receptor antagonists [or mineralocorticoid receptor antagonists] are recommended in patients with NYHA class II-IV and who have LVEF of 35% or less, unless contraindicated, to reduce morbidity and mortality. Patients with NYHA class II should have a history of prior cardiovascular hospitalization or elevated plasma natriuretic peptide levels to be considered for aldosterone receptor antagonists. Creatinine should be 2.5 mg/dL or less in men or 2.0 mg/dL or less in women (or estimated glomerular filtration rate >30 mL/min/1.73 m2), and potassium should be less than 5.0 mEq/L. Careful monitoring of potassium, renal function, and diuretic dosing should be performed at initiation and closely followed thereafter to minimize risk of hyperkalemia and renal insufficiency.[83][85][150](Level of Evidence: A) " |
| "10. Aldosterone receptor antagonists are recommended to reduce morbidity and mortality following an acute MI in patients who have LVEF of 40% or less who develop symptoms of HF or who have a history of diabetes mellitus, unless contraindicated.[84](Level of Evidence: B) " |
| "11. Patients with chronic HF with permanent/persistent/paroxysmal AF and an additional risk factor for cardioembolic stroke (history of hypertension, diabetes mellitus, previous stroke or transient ischemic attack, or ≥75 years of age) should receive chronic anticoagulant therapy.[151][152](Level of Evidence: A) " |
| "12. The selection of an anticoagulant agent (warfarin, dabigatran, apixaban, or rivaroxaban) for permanent/persistent/paroxysmal AF should be individualized on the basis of risk factors, cost, tolerability, patient preference, potential for drug interactions, and other clinical characteristics, including time in the international normalized ratio therapeutic range if the patient has been taking warfarin. (Level of Evidence: C) " |
| "13. Drugs known to adversely affect the clinical status of patients with current or prior symptoms of HF and reduced LVEF should be avoided or withdrawn whenever possible (e.g., non steroidal anti-inflammatory drugs, most antiarrhythmic drugs, and most calcium channel blocking drugs). [153][154][155][156][157][158][159](Level of Evidence: B) " |
| "14. Exercise training is beneficial as an adjunctive approach to improve clinical status in ambulatory patients with current or prior symptoms of HF and reduced LVEF. [160][161][162][163](Level of Evidence: B) " |
| "15. An implantable cardioverter-defibrillator is recommended as secondary prevention to prolong survival in patients with current or prior symptoms of HF and reduced LVEF who have a history of cardiac arrest, ventricular fibrillation, or hemodynamically destabilizing ventricular tachycardia.[164][165][166](Level of Evidence: A) " |
| "16. Implantable cardioverter-defibrillator therapy is recommended for primary prevention of sudden cardiac death to reduce total mortality in patients with non-ischemic dilated cardiomyopathy or ischemic heart disease at least 40 days post-MI, a LVEF less than or equal to 35%, with NYHA functional class II or III symptoms while receiving chronic optimal medical therapy, and who have reasonable expectation of survival with a good functional status for more than 1 year. [167][168][169][170][171][172][173][174](Level of Evidence: A) " |
| "17. Implantable cardioverter-defibrillator therapy is recommended for primary prevention of sudden cardiac death to reduce total mortality in selected patients at least 40 days post-MI with LVEF of 30% or less, and NYHA class I symptoms while receiving GDMT, who have reasonable expectation of meaningful survival for more than 1 year.[175][176][177](Level of Evidence: B) " |
| "18. CRT is indicated for patients who have LVEF of 35% or less, sinus rhythm, left bundle-branch block (LBBB) with a QRS duration of 150 ms or greater, and NYHA class II, III, or ambulatory IV symptoms on GDMT.. [178][179][180][181][182] [183][184][185][186][187][188][189][190][191][192][193][194] (Level of Evidence: A for NYHA class III/IV), (Level of Evidence: B for NYHA class II ) " |
| "19. Addition of an aldosterone antagonist is reasonable in selected patients with moderately severe to severe symptoms of HF and reduced LVEF who can be carefully monitored for preserved renal function and normal potassium concentration. Creatinine should be less than or equal to 2.5 mg/dL in men or less than or equal to 2.0 mg/dL in women and potassium should be less than 5.0 mEq/L. Under circumstances where monitoring for hyperkalemia or renal dysfunction is not anticipated to be feasible, the risks may outweigh the benefits of aldosterone antagonists. [195][196][197](Level of Evidence: B) " |
| "20. The combination of hydralazine and nitrates is recommended to improve outcomes for patients self-described as African-Americans, with moderate-severe symptoms on optimal therapy with ACE inhibitors, beta blockers and diuretics.[198][199][200](Level of Evidence: B) " |
| Class III (No Benefit) |
| "1. Calcium channel blocking drugs are not recommended as routine treatment for patients with HFrEF.[118][201][108] (Level of Evidence: A) " |
| "2. Anticoagulation is not recommended in patients with chronic HFrEF without AF, a prior thromboembolic event, or a cardioembolic source. [202][106](Level of Evidence: B) " |
| "3. Statins are not beneficial as adjunctive therapy when prescribed solely for the diagnosis of HF in the absence of other indications for their use. [203][204](Level of Evidence: A) " |
| "4. Use of nutritional supplements as treatment for HF is not indicated in patients with current or prior symptoms of HF and reduced LVEF.[205][206] (Level of Evidence: B) " |
| "5. Hormonal therapies other than to replete deficiencies are not recommended and may be harmful to patients with current or prior symptoms of HF and reduced LVEF. (Level of Evidence: C) " |
| "6. CRT is not recommended for patients with NYHA class I or II symptoms and non-LBBB pattern with QRS duration less than 150 ms.[180][181][207](Level of Evidence: B) " |
| "7. CRT is not indicated for patients whose comorbidities and/or frailty limit survival with good functional capacity to less than 1 year.[208](Level of Evidence: C) " |
| Class III (harm) |
| "1. Routine combined use of an ACEI, ARB, and aldosterone antagonist is potentially harmful for patients with HFrEF. (Level of Evidence: C) " |
| "2. ARNI should not be administered concomitantly with ACE inhibitors or within 36 hours of the last dose of an ACE inhibitor. (Level of Evidence: B-R)[66][67] " |
| "3. ARNI should not be administered to patients with a history of angioedema. (Level of Evidence: C-EO) " |
| "4. Inappropriate use of aldosterone receptor antagonists is potentially harmful because of lifethreatening hyperkalemia or renal insufficiency when serum creatinine is more than 2.5 mg/dL in men or more than 2.0 mg/dL in women (or estimated glomerular filtration rate <30 mL/min/1.73 m2), and/or potassium more than 5.0 mEq/L.[90][209] (Level of Evidence: B) " |
| "5. Drugs known to adversely affect the clinical status of patients with current or prior symptoms of HFrEF are potentially harmful and should be avoided or withdrawn whenever possible (e.g., most antiarrhythmic drugs, most calcium channel blocking drugs (except amlodipine), NSAIDs, or thiazolidinediones).[117][124] [210][211][212][213](Level of Evidence: A) " |
| "6. Long-term use of an infusion of a positive inotropic drug may be harmful and is not recommended for patients with current or prior symptoms of HF and reduced LVEF, except as palliation for patients with end-stage disease who cannot be stabilized with standard medical treatment (Stage D). (Level of Evidence: C) " |
| Class IIa |
| "1. It is reasonable to treat patients with atrial fibrillation and HF with a strategy to maintain sinus rhythm or with a strategy to control ventricular rate alone. [214][215][216][217][218](Level of Evidence: A) " |
| "2. Chronic anticoagulation is reasonable for patients with chronic HF who have permanent/persistent/paroxysmal AF but are without an additional risk factor for cardioembolic stroke. [219][220][221][222](Level of Evidence: B) " |
| "3. Maximal exercise testing with or without measurement of respiratory gas exchange is reasonable to facilitate prescription of an appropriate exercise program for patients presenting with HF. (Level of Evidence: C) " |
| "4. Angiotensin II receptor blockers are reasonable to use as alternatives to ACEIs as first-line therapy for patients with mild to moderate HF and reduced LVEF, especially for patients already taking ARBs for other indications.[223][224][225][226][227][228][229][230][231] (Level of Evidence: A) " |
| "5. Ivabradine can be beneficial to reduce HF hospitalization for patients with symptomatic (NYHA class II-III) stable chronic HFrEF (LVEF ≤35%) who are receiving GDEM†, including a beta blocker at maximum tolerated dose, and who are in sinus rhythm with a heart rate of 70 bpm or greater at rest. (Level of Evidence: B-R)[232][233][234][235] " |
| "6. Digitalis can be beneficial in patients with current or prior symptoms of HF and reduced LVEF to decrease hospitalizations for HF.[236][237][238][239][240][241][242] (Level of Evidence: B) " |
| "7. A combination of hydralazine and isosorbide dinitrate can be useful to reduce morbidity or mortality in patients with current or prior symptomatic HFrEF who cannot be given an ACE inhibitor or ARB because of drug intolerance, hypotension, or renal insufficiency, unless contraindicated.[243][244][245][246][247] (Level of Evidence: B)" |
| "8. CRT can be useful for patients who have LVEF of 35% or less, sinus rhythm, a non-LBBB pattern with a QRS duration of 150 ms or greater, and NYHA class III/ambulatory class IV symptoms on GDMT.[182][178][179][181](Level of Evidence: B) " |
| "9. CRT can be useful in patients with AF and LVEF of 35% or less on GDMT if a) the patient requires ventricular pacing or otherwise meets CRT criteria and b) atrioventricular nodal ablation or pharmacological rate control will allow near 100% ventricular pacing with CRT.[248][249](Level of Evidence: C) " |
| "10. CRT can be useful for patients on GDMT who have LVEF of 35% or less, and are undergoing placement of a new or replacement device with anticipated requirement for significant (>40%) ventricular pacing.[250][251][252][253](Level of Evidence: C) " |
| "11. Omega-3 polyunsaturated fatty acid (PUFA) supplementation is reasonable to use as adjunctive therapy in patients with NYHA class II-IV symptoms and HFrEF or HFpEF, unless contraindicated, to reduce mortality and cardiovascular hospitalizations. [99][100](Level of Evidence: B) " |
| †GDEM=Guideline Directed Evaluation and Management (Following Class I Recommendations for Heart Failure) |
| Class IIb |
| "1. The addition of an ARB may be considered in persistently symptomatic patients with reduced LVEF who are already being treated with conventional therapy and in whom an aldosterone antagonist is not indicated or tolerated.[136][254][255][256][257][258][259] (Level of Evidence: B) " |
| "2. CRT may be considered for patients who have LVEF of 35% or less, sinus rhythm, a non-LBBB pattern with QRS duration of 120 to 149 ms, and NYHA class III/ambulatory class IV on GDMT.[181][207](Level of Evidence: B) " |
| "3. CRT may be considered for patients who have LVEF of 35% or less, sinus rhythm, a non-LBBB pattern with a QRS duration of 150 ms or greater, and NYHA class II symptoms on GDMT.[180][181] (Level of Evidence: B) " |
| "4. CRT may be considered for patients who have LVEF of 30% or less, ischemic etiology of HF,
sinus rhythm, LBBB with a QRS duration of 150 ms or greater, and NYHA class I symptoms on GDMT. [180] [181](Level of Evidence: C) " |
Treatment of Patients with HF and preserved ejection fraction HFpEF (DO NOT EDIT) [260]
| Class I |
|
1. Systolic and diastolic blood pressure should be controlled in patients with HFpEF in accordance with published clinical practice guidelines to prevent morbidity. (Class I, Level of Evidence: B) |
| "2. Patients with HFpEF and persistent hypertension after management of volume overload should be prescribed GDMT titrated to attain systolic blood pressure less than 130 mm Hg.[261][262] (Level of Evidence: C-LD)" |
| "3. Physicians should control ventricular rate in patients with HF and normal LVEF and atrial fibrillation. (Level of Evidence: C)" |
| "4. Physicians should use diuretics to control pulmonary congestion and peripheral edema in patients with HF and normal LVEF. (Level of Evidence: C)" |
| Class III (No Benefit) |
| "1. Routine use of nutritional supplements is not recommended for patients with HFpEF. (Level of Evidence: C) " |
| Class IIa |
| "1. Coronary revascularization is reasonable in patients with HF and normal LVEF and coronary artery disease in whom symptomatic or demonstrable myocardial ischemia is judged to be having an adverse effect on cardiac function. (Level of Evidence: C) " |
| "2. Restoration and maintenance of sinus rhythm in patients with atrial fibrillation and HFpEF is reasonable to improve symptoms. (Level of Evidence: C) " |
| "3. The use of beta-adrenergic blocking agents, ACEIs, ARBs, or calcium antagonists in patients with HFpEF and controlled hypertension is reasonable to minimize symptoms of HF. (Level of Evidence: C) " |
| Class IIb |
| "1.The use of ARBs might be considered to decrease hospitalizations for patients with HFpEF.[262](Level of Evidence: B) " |
| "2. The usefulness of digitalis to minimize symptoms of HF in patients with HF and normal LVEF is not well established. (Level of Evidence: C) " |
Related Chapters
External Links
- 2009 focused update: ACCF/AHA Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation [39]
- The ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult [263]
References
- ↑ Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW (2009). "2009 focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation". Circulation. 119 (14): e391–479. doi:10.1161/CIRCULATIONAHA.109.192065. PMID 19324966. Retrieved 2011-04-07. Unknown parameter
|month=ignored (help) - ↑ McKelvie RS, Teo KK, McCartney N, Humen D, Montague T, Yusuf S (1995). "Effects of exercise training in patients with congestive heart failure: a critical review". Journal of the American College of Cardiology. 25 (3): 789–96. doi:10.1016/0735-1097(94)00428-S. PMID 7860930. Retrieved 2011-04-07. Unknown parameter
|month=ignored (help) - ↑ Packer M, Kessler PD, Lee WH (1987). "Calcium-channel blockade in the management of severe chronic congestive heart failure: a bridge too far". Circulation. 75 (6 Pt 2): V56–64. PMID 3552317. Unknown parameter
|month=ignored (help);|access-date=requires|url=(help) - ↑ 4.0 4.1 Reed SD, Friedman JY, Velazquez EJ, Gnanasakthy A, Califf RM, Schulman KA (2004). "Multinational economic evaluation of valsartan in patients with chronic heart failure: results from the Valsartan Heart Failure Trial (Val-HeFT)". American Heart Journal. 148 (1): 122–8. doi:10.1016/j.ahj.2003.12.040. PMID 15215801. Retrieved 2011-04-07. Unknown parameter
|month=ignored (help) - ↑ Torp-Pedersen C, Møller M, Bloch-Thomsen PE, Køber L, Sandøe E, Egstrup K, Agner E, Carlsen J, Videbaek J, Marchant B, Camm AJ (1999). "Dofetilide in patients with congestive heart failure and left ventricular dysfunction. Danish Investigations of Arrhythmia and Mortality on Dofetilide Study Group". The New England Journal of Medicine. 341 (12): 857–65. doi:10.1056/NEJM199909163411201. PMID 10486417. Retrieved 2011-04-07. Unknown parameter
|month=ignored (help) - ↑ Heerdink ER, Leufkens HG, Herings RM, Ottervanger JP, Stricker BH, Bakker A (1998). "NSAIDs associated with increased risk of congestive heart failure in elderly patients taking diuretics". Archives of Internal Medicine. 158 (10): 1108–12. PMID 9605782. Retrieved 2011-04-08. Unknown parameter
|month=ignored (help) - ↑ Gan SC, Barr J, Arieff AI, Pearl RG (1992). "Biguanide-associated lactic acidosis. Case report and review of the literature". Archives of Internal Medicine. 152 (11): 2333–6. PMID 1444694. Retrieved 2011-04-08. Unknown parameter
|month=ignored (help) - ↑ Masoudi FA, Inzucchi SE, Wang Y, Havranek EP, Foody JM, Krumholz HM (2005). "Thiazolidinediones, metformin, and outcomes in older patients with diabetes and heart failure: an observational study". Circulation. 111 (5): 583–90. doi:10.1161/01.CIR.0000154542.13412.B1. PMID 15699279. Retrieved 2011-04-08. Unknown parameter
|month=ignored (help) - ↑ Swenson JR, Doucette S, Fergusson D (2006). "Adverse cardiovascular events in antidepressant trials involving high-risk patients: a systematic review of randomized trials". Canadian Journal of Psychiatry. Revue Canadienne De Psychiatrie. 51 (14): 923–9. PMID 17249635. Unknown parameter
|month=ignored (help);|access-date=requires|url=(help) - ↑ Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, Hiratzka LF, Murphy WR, Olin JW, Puschett JB, Rosenfield KA, Sacks D, Stanley JC, Taylor LM, White CJ, White J, White RA, Antman EM, Smith SC, Adams CD, Anderson JL, Faxon DP, Fuster V, Gibbons RJ, Hunt SA, Jacobs AK, Nishimura R, Ornato JP, Page RL, Riegel B (2006). "ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation". Circulation. 113 (11): e463–654. doi:10.1161/CIRCULATIONAHA.106.174526. PMID 16549646. Retrieved 2011-04-08. Unknown parameter
|month=ignored (help) - ↑ Storen EC, Tefferi A (2001). "Long-term use of anagrelide in young patients with essential thrombocythemia". Blood. 97 (4): 863–6. PMID 11159509. Retrieved 2011-04-08. Unknown parameter
|month=ignored (help) - ↑ "Anagrelide, a therapy for thrombocythemic states: experience in 577 patients. Anagrelide Study Group". The American Journal of Medicine. 92 (1): 69–76. 1992. PMID 1731512. Unknown parameter
|month=ignored (help);|access-date=requires|url=(help) - ↑ Lewis GD, Shah R, Shahzad K, Camuso JM, Pappagianopoulos PP, Hung J, Tawakol A, Gerszten RE, Systrom DM, Bloch KD, Semigran MJ (2007). "Sildenafil improves exercise capacity and quality of life in patients with systolic heart failure and secondary pulmonary hypertension". Circulation. 116 (14): 1555–62. doi:10.1161/CIRCULATIONAHA.107.716373. PMID 17785618. Retrieved 2011-04-08. Unknown parameter
|month=ignored (help) - ↑ Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, Baselga J, Norton L (2001). "Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2". The New England Journal of Medicine. 344 (11): 783–92. doi:10.1056/NEJM200103153441101. PMID 11248153. Retrieved 2011-04-08. Unknown parameter
|month=ignored (help) - ↑ Chung ES, Packer M, Lo KH, Fasanmade AA, Willerson JT (2003). "Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-alpha, in patients with moderate-to-severe heart failure: results of the anti-TNF Therapy Against Congestive Heart Failure (ATTACH) trial". Circulation. 107 (25): 3133–40. doi:10.1161/01.CIR.0000077913.60364.D2. PMID 12796126. Retrieved 2011-04-08. Unknown parameter
|month=ignored (help) - ↑ Yap YG, Camm AJ (2003). "Drug induced QT prolongation and torsades de pointes". Heart (British Cardiac Society). 89 (11): 1363–72. PMC 1767957. PMID 14594906. Retrieved 2011-04-08. Unknown parameter
|month=ignored (help) - ↑ 17.0 17.1 Packer M, Gottlieb SS, Kessler PD (1986). "Hormone-electrolyte interactions in the pathogenesis of lethal cardiac arrhythmias in patients with congestive heart failure. Basis of a new physiologic approach to control of arrhythmia". The American Journal of Medicine. 80 (4A): 23–9. PMID 2871753. Unknown parameter
|month=ignored (help);|access-date=requires|url=(help) - ↑ Rich MW, Beckham V, Wittenberg C, Leven CL, Freedland KE, Carney RM (1995). "A multidisciplinary intervention to prevent the readmission of elderly patients with congestive heart failure". The New England Journal of Medicine. 333 (18): 1190–5. doi:10.1056/NEJM199511023331806. PMID 7565975. Retrieved 2011-04-10. Unknown parameter
|month=ignored (help) - ↑ Philbin EF (1999). "Comprehensive multidisciplinary programs for the management of patients with congestive heart failure". Journal of General Internal Medicine. 14 (2): 130–5. PMID 10051785. Unknown parameter
|month=ignored (help);|access-date=requires|url=(help) - ↑ "Consensus recommendations for the management of chronic heart failure. On behalf of the membership of the advisory council to improve outcomes nationwide in heart failure". The American Journal of Cardiology. 83 (2A): 1A–38A. 1999. PMID 10072251. Unknown parameter
|month=ignored (help);|access-date=requires|url=(help) - ↑ Brater DC (1998). "Diuretic therapy". The New England Journal of Medicine. 339 (6): 387–95. doi:10.1056/NEJM199808063390607. PMID 9691107. Retrieved 2011-04-12. Unknown parameter
|month=ignored (help) - ↑ Cody RJ, Kubo SH, Pickworth KK (1994). "Diuretic treatment for the sodium retention of congestive heart failure". Archives of Internal Medicine. 154 (17): 1905–14. PMID 8074594. Retrieved 2011-04-12. Unknown parameter
|month=ignored (help) - ↑ Patterson JH, Adams KF, Applefeld MM, Corder CN, Masse BR (1994). "Oral torsemide in patients with chronic congestive heart failure: effects on body weight, edema, and electrolyte excretion. Torsemide Investigators Group". Pharmacotherapy. 14 (5): 514–21. PMID 7997385.
|access-date=requires|url=(help) - ↑ 24.0 24.1 24.2 24.3 Richardson A, Bayliss J, Scriven AJ, Parameshwar J, Poole-Wilson PA, Sutton GC (1987). "Double-blind comparison of captopril alone against frusemide plus amiloride in mild heart failure". Lancet. 2 (8561): 709–11. PMID 2888942. Retrieved 2011-04-14. Unknown parameter
|month=ignored (help) - ↑ Packer M, Medina N, Yushak M, Meller J (1983). "Hemodynamic patterns of response during long-term captopril therapy for severe chronic heart failure". Circulation. 68 (4): 803–12. PMID 6352079. Retrieved 2011-04-14. Unknown parameter
|month=ignored (help) - ↑ Risler T, Schwab A, Kramer B, Braun N, Erley C (1994). "Comparative pharmacokinetics and pharmacodynamics of loop diuretics in renal failure". Cardiology. 84 Suppl 2: 155–61. PMID 7954539.
|access-date=requires|url=(help) - ↑ Cody RJ, Franklin KW, Laragh JH (1982). "Postural hypotension during tilt with chronic captopril and diuretic therapy of severe congestive heart failure". American Heart Journal. 103 (4 Pt 1): 480–4. PMID 7039281. Unknown parameter
|month=ignored (help);|access-date=requires|url=(help) - ↑ Vasko MR, Cartwright DB, Knochel JP, Nixon JV, Brater DC (1985). "Furosemide absorption altered in decompensated congestive heart failure". Annals of Internal Medicine. 102 (3): 314–8. PMID 3970471. Unknown parameter
|month=ignored (help);|access-date=requires|url=(help) - ↑ Dormans TP, van Meyel JJ, Gerlag PG, Tan Y, Russel FG, Smits P (1996). "Diuretic efficacy of high dose furosemide in severe heart failure: bolus injection versus continuous infusion". Journal of the American College of Cardiology. 28 (2): 376–82. doi:10.1016/0735-1097(96)00161-1. PMID 8800113. Retrieved 2011-04-14. Unknown parameter
|month=ignored (help) - ↑ "Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions. The SOLVD Investigattors". The New England Journal of Medicine. 327 (10): 685–91. 1992. doi:10.1056/NEJM199209033271003. PMID 1463530. Retrieved 2011-04-22. Unknown parameter
|month=ignored (help) - ↑ Exner DV, Dries DL, Domanski MJ, Cohn JN (2001). "Lesser response to angiotensin-converting-enzyme inhibitor therapy in black as compared with white patients with left ventricular dysfunction". The New England Journal of Medicine. 344 (18): 1351–7. doi:10.1056/NEJM200105033441802. PMID 11333991. Retrieved 2011-04-22. Unknown parameter
|month=ignored (help) - ↑ 32.0 32.1 Carson P, Ziesche S, Johnson G, Cohn JN (1999). "Racial differences in response to therapy for heart failure: analysis of the vasodilator-heart failure trials. Vasodilator-Heart Failure Trial Study Group". Journal of Cardiac Failure. 5 (3): 178–87. PMID 10496190. Retrieved 2011-04-22. Unknown parameter
|month=ignored (help) - ↑ 33.0 33.1 Garg R, Yusuf S (1995). "Overview of randomized trials of angiotensin-converting enzyme inhibitors on mortality and morbidity in patients with heart failure. Collaborative Group on ACE Inhibitor Trials". JAMA : the Journal of the American Medical Association. 273 (18): 1450–6. PMID 7654275. Unknown parameter
|month=ignored (help);|access-date=requires|url=(help) - ↑ 34.0 34.1 34.2 34.3 Packer M, Poole-Wilson PA, Armstrong PW, Cleland JG, Horowitz JD, Massie BM, Rydén L, Thygesen K, Uretsky BF (1999). "Comparative effects of low and high doses of the angiotensin-converting enzyme inhibitor, lisinopril, on morbidity and mortality in chronic heart failure. ATLAS Study Group". Circulation. 100 (23): 2312–8. PMID 10587334. Retrieved 2011-04-22. Unknown parameter
|month=ignored (help) - ↑ Delahaye F, de Gevigney G (2000). "Is the optimal dose of angiotensin-converting enzyme inhibitors in patients with congestive heart failure definitely established?". Journal of the American College of Cardiology. 36 (7): 2096–7. PMID 11127446. Retrieved 2011-04-22. Unknown parameter
|month=ignored (help) - ↑ "Collaborative overview of randomised trials of antiplatelet therapy--I: Prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients. Antiplatelet Trialists' Collaboration". BMJ (Clinical Research Ed.). 308 (6921): 81–106. 1994. PMC 2539220. PMID 8298418. Retrieved 2011-04-22. Unknown parameter
|month=ignored (help) - ↑ 37.0 37.1 Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM; et al. (2022). "2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines". Circulation. 145 (18): e876–e894. doi:10.1161/CIR.0000000000001062. PMID 35363500 Check
|pmid=value (help). - ↑ 38.0 38.1 38.2 Yancy CW, Jessup M, Bozkurt B, Masoudi FA, Butler J, McBride PE; et al. (2013). "2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines". J Am Coll Cardiol. doi:10.1016/j.jacc.2013.05.019. PMID 23747642.
- ↑ 39.0 39.1 39.2 Jessup M, Abraham WT, Casey DE, Feldman AM, Francis GS, Ganiats TG et al. (2009) 2009 focused update: ACCF/AHA Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation 119 (14):1977-2016.DOI:10.1161/CIRCULATIONAHA.109.192064 PMID: 19324967
- ↑ 40.0 40.1 40.2 40.3 40.4 McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR; et al. (2014). "Angiotensin-neprilysin inhibition versus enalapril in heart failure". N Engl J Med. 371 (11): 993–1004. doi:10.1056/NEJMoa1409077. PMID 25176015. Review in: Evid Based Med. 2015 Apr;20(2):61 Review in: Ann Intern Med. 2015 Feb 17;162(4):JC2
- ↑ 41.0 41.1 Wachter R, Senni M, Belohlavek J, Straburzynska-Migaj E, Witte KK, Kobalava Z; et al. (2019). "Initiation of sacubitril/valsartan in haemodynamically stabilised heart failure patients in hospital or early after discharge: primary results of the randomised TRANSITION study". Eur J Heart Fail. 21 (8): 998–1007. doi:10.1002/ejhf.1498. PMID 31134724.
- ↑ 42.0 42.1 Velazquez EJ, Morrow DA, DeVore AD, Duffy CI, Ambrosy AP, McCague K; et al. (2019). "Angiotensin-Neprilysin Inhibition in Acute Decompensated Heart Failure". N Engl J Med. 380 (6): 539–548. doi:10.1056/NEJMoa1812851. PMID 30415601.
- ↑ 43.0 43.1 Desai AS, Solomon SD, Shah AM, Claggett BL, Fang JC, Izzo J; et al. (2019). "Effect of Sacubitril-Valsartan vs Enalapril on Aortic Stiffness in Patients With Heart Failure and Reduced Ejection Fraction: A Randomized Clinical Trial". JAMA. 322 (11): 1077–1084. doi:10.1001/jama.2019.12843. PMC 6749534 Check
|pmc=value (help). PMID 31475296. - ↑ 44.0 44.1 Wang Y, Zhou R, Lu C, Chen Q, Xu T, Li D (2019). "Effects of the Angiotensin-Receptor Neprilysin Inhibitor on Cardiac Reverse Remodeling: Meta-Analysis". J Am Heart Assoc. 8 (13): e012272. doi:10.1161/JAHA.119.012272. PMC 6662364 Check
|pmc=value (help). PMID 31240976. - ↑ 45.0 45.1 45.2 CONSENSUS Trial Study Group (1987). "Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS)". N Engl J Med. 316 (23): 1429–35. doi:10.1056/NEJM198706043162301. PMID 2883575.
- ↑ 46.0 46.1 46.2 SOLVD Investigators. Yusuf S, Pitt B, Davis CE, Hood WB, Cohn JN (1991). "Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure". N Engl J Med. 325 (5): 293–302. doi:10.1056/NEJM199108013250501. PMID 2057034.
- ↑ 47.0 47.1 47.2 Pfeffer MA, Braunwald E, Moyé LA, Basta L, Brown EJ, Cuddy TE; et al. (1992). "Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. Results of the survival and ventricular enlargement trial. The SAVE Investigators". N Engl J Med. 327 (10): 669–77. doi:10.1056/NEJM199209033271001. PMID 1386652.
- ↑ "Effect of ramipril on mortality and morbidity of survivors of acute myocardial infarction with clinical evidence of heart failure. The Acute Infarction Ramipril Efficacy (AIRE) Study Investigators". Lancet. 342 (8875): 821–8. 1993. PMID 8104270.
- ↑ 49.0 49.1 49.2 Køber L, Torp-Pedersen C, Carlsen JE, Bagger H, Eliasen P, Lyngborg K; et al. (1995). "A clinical trial of the angiotensin-converting-enzyme inhibitor trandolapril in patients with left ventricular dysfunction after myocardial infarction. Trandolapril Cardiac Evaluation (TRACE) Study Group". N Engl J Med. 333 (25): 1670–6. doi:10.1056/NEJM199512213332503. PMID 7477219.
- ↑ Woodard-Grice AV, Lucisano AC, Byrd JB, Stone ER, Simmons WH, Brown NJ (2010). "Sex-dependent and race-dependent association of XPNPEP2 C-2399A polymorphism with angiotensin-converting enzyme inhibitor-associated angioedema". Pharmacogenet Genomics. 20 (9): 532–6. doi:10.1097/FPC.0b013e32833d3acb. PMC 2945219. PMID 20625347.
- ↑ 51.0 51.1 51.2 Cohn JN, Tognoni G, Valsartan Heart Failure Trial Investigators (2001). "A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure". N Engl J Med. 345 (23): 1667–75. doi:10.1056/NEJMoa010713. PMID 11759645.
- ↑ 52.0 52.1 52.2 Pfeffer MA, McMurray JJ, Velazquez EJ, Rouleau JL, Køber L, Maggioni AP; et al. (2003). "Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both". N Engl J Med. 349 (20): 1893–906. doi:10.1056/NEJMoa032292. PMID 14610160. Review in: ACP J Club. 2004 Jul-Aug;141(1):3
- ↑ 53.0 53.1 53.2 Konstam MA, Neaton JD, Dickstein K, Drexler H, Komajda M, Martinez FA; et al. (2009). "Effects of high-dose versus low-dose losartan on clinical outcomes in patients with heart failure (HEAAL study): a randomised, double-blind trial". Lancet. 374 (9704): 1840–8. doi:10.1016/S0140-6736(09)61913-9. PMID 19922995. Review in: Evid Based Med. 2010 Apr;15(2):51-2
- ↑ 54.0 54.1 Dominiak M (2008). "[Commentary to the article: ONTARGET Investigators, Yusuf S, Teo KK, Pogue J et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med 2008; 358: 1547-59]". Kardiol Pol. 66 (6): 705–6, discussion 707. PMID 18700309.
- ↑ 55.0 55.1 Telmisartan Randomised AssessmeNt Study in ACE iNtolerant subjects with cardiovascular Disease (TRANSCEND) Investigators. Yusuf S, Teo K, Anderson C, Pogue J, Dyal L; et al. (2008). "Effects of the angiotensin-receptor blocker telmisartan on cardiovascular events in high-risk patients intolerant to angiotensin-converting enzyme inhibitors: a randomised controlled trial". Lancet. 372 (9644): 1174–83. doi:10.1016/S0140-6736(08)61242-8. PMID 18757085. Review in: Ann Intern Med. 2009 Feb 17;150(4):JC2-6
- ↑ 56.0 56.1 56.2 Banka G, Heidenreich PA, Fonarow GC (2013). "Incremental cost-effectiveness of guideline-directed medical therapies for heart failure". J Am Coll Cardiol. 61 (13): 1440–6. doi:10.1016/j.jacc.2012.12.022. PMID 23433562.
- ↑ Dasbach EJ, Rich MW, Segal R, Gerth WC, Carides GW, Cook JR; et al. (1999). "The cost-effectiveness of losartan versus captopril in patients with symptomatic heart failure". Cardiology. 91 (3): 189–94. doi:10.1159/000006908. PMID 10516413.
- ↑ Glick H, Cook J, Kinosian B, Pitt B, Bourassa MG, Pouleur H; et al. (1995). "Costs and effects of enalapril therapy in patients with symptomatic heart failure: an economic analysis of the Studies of Left Ventricular Dysfunction (SOLVD) Treatment Trial". J Card Fail. 1 (5): 371–80. doi:10.1016/s1071-9164(05)80006-5. PMID 12836712.
- ↑ Paul SD, Kuntz KM, Eagle KA, Weinstein MC (1994). "Costs and effectiveness of angiotensin converting enzyme inhibition in patients with congestive heart failure". Arch Intern Med. 154 (10): 1143–9. PMID 8185426.
- ↑ Shekelle P, Morton S, Atkinson S, Suttorp M, Tu W, Heidenreich P; et al. (2003). "Pharmacologic management of heart failure and left ventricular systolic dysfunction: effect in female, black, and diabetic patients, and cost-effectiveness". Evid Rep Technol Assess (Summ) (82): 1–6. PMC 4781559. PMID 14571595.
- ↑ Tsevat J, Duke D, Goldman L, Pfeffer MA, Lamas GA, Soukup JR; et al. (1995). "Cost-effectiveness of captopril therapy after myocardial infarction". J Am Coll Cardiol. 26 (4): 914–9. doi:10.1016/0735-1097(95)00284-1. PMID 7560617.
- ↑ Gaziano TA, Fonarow GC, Claggett B, Chan WW, Deschaseaux-Voinet C, Turner SJ; et al. (2016). "Cost-effectiveness Analysis of Sacubitril/Valsartan vs Enalapril in Patients With Heart Failure and Reduced Ejection Fraction". JAMA Cardiol. 1 (6): 666–72. doi:10.1001/jamacardio.2016.1747. PMID 27438344.
- ↑ Gaziano TA, Fonarow GC, Velazquez EJ, Morrow DA, Braunwald E, Solomon SD (2020). "Cost-effectiveness of Sacubitril-Valsartan in Hospitalized Patients Who Have Heart Failure With Reduced Ejection Fraction". JAMA Cardiol. 5 (11): 1236–1244. doi:10.1001/jamacardio.2020.2822. PMC 7675099 Check
|pmc=value (help). PMID 32785628 Check|pmid=value (help). - ↑ King JB, Shah RU, Bress AP, Nelson RE, Bellows BK (2016). "Cost-Effectiveness of Sacubitril-Valsartan Combination Therapy Compared With Enalapril for the Treatment of Heart Failure With Reduced Ejection Fraction". JACC Heart Fail. 4 (5): 392–402. doi:10.1016/j.jchf.2016.02.007. PMID 27039128.
- ↑ Sandhu AT, Ollendorf DA, Chapman RH, Pearson SD, Heidenreich PA (2016). "Cost-Effectiveness of Sacubitril-Valsartan in Patients With Heart Failure With Reduced Ejection Fraction". Ann Intern Med. 165 (10): 681–689. doi:10.7326/M16-0057. PMID 27571284.
- ↑ 66.0 66.1 Packer M, Califf RM, Konstam MA, Krum H, McMurray JJ, Rouleau JL; et al. (2002). "Comparison of omapatrilat and enalapril in patients with chronic heart failure: the Omapatrilat Versus Enalapril Randomized Trial of Utility in Reducing Events (OVERTURE)". Circulation. 106 (8): 920–6. doi:10.1161/01.cir.0000029801.86489.50. PMID 12186794.
- ↑ 67.0 67.1 Kostis JB, Packer M, Black HR, Schmieder R, Henry D, Levy E (2004). "Omapatrilat and enalapril in patients with hypertension: the Omapatrilat Cardiovascular Treatment vs. Enalapril (OCTAVE) trial". Am J Hypertens. 17 (2): 103–11. doi:10.1016/j.amjhyper.2003.09.014. PMID 14751650.
- ↑ Vardeny O, Miller R, Solomon SD (2014). "Combined neprilysin and renin-angiotensin system inhibition for the treatment of heart failure". JACC Heart Fail. 2 (6): 663–70. doi:10.1016/j.jchf.2014.09.001. PMID 25306450.
- ↑ Messerli FH, Nussberger J (2000). "Vasopeptidase inhibition and angio-oedema". Lancet. 356 (9230): 608–9. doi:10.1016/S0140-6736(00)02596-4. PMID 10968427.
- ↑ Braunwald E (2015). "The path to an angiotensin receptor antagonist-neprilysin inhibitor in the treatment of heart failure". J Am Coll Cardiol. 65 (10): 1029–41. doi:10.1016/j.jacc.2015.01.033. PMID 25766951.
- ↑ Ruilope LM, Dukat A, Böhm M, Lacourcière Y, Gong J, Lefkowitz MP (2010). "Blood-pressure reduction with LCZ696, a novel dual-acting inhibitor of the angiotensin II receptor and neprilysin: a randomised, double-blind, placebo-controlled, active comparator study". Lancet. 375 (9722): 1255–66. doi:10.1016/S0140-6736(09)61966-8. PMID 20236700.
- ↑ Byrd JB, Adam A, Brown NJ (2006). "Angiotensin-converting enzyme inhibitor-associated angioedema". Immunol Allergy Clin North Am. 26 (4): 725–37. doi:10.1016/j.iac.2006.08.001. PMID 17085287.
- ↑ Toh S, Reichman ME, Houstoun M, Ross Southworth M, Ding X, Hernandez AF; et al. (2012). "Comparative risk for angioedema associated with the use of drugs that target the renin-angiotensin-aldosterone system". Arch Intern Med. 172 (20): 1582–9. doi:10.1001/2013.jamainternmed.34. PMID 23147456. Review in: Evid Based Med. 2013 Dec;18(6):e52
- ↑ Makani H, Messerli FH, Romero J, Wever-Pinzon O, Korniyenko A, Berrios RS; et al. (2012). "Meta-analysis of randomized trials of angioedema as an adverse event of renin-angiotensin system inhibitors". Am J Cardiol. 110 (3): 383–91. doi:10.1016/j.amjcard.2012.03.034. PMID 22521308.
- ↑ Rasmussen ER, Pottegård A, Bygum A, von Buchwald C, Homøe P, Hallas J (2019). "Angiotensin II receptor blockers are safe in patients with prior angioedema related to angiotensin-converting enzyme inhibitors - a nationwide registry-based cohort study". J Intern Med. 285 (5): 553–561. doi:10.1111/joim.12867. PMID 30618189.
- ↑ "The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial". Lancet. 353 (9146): 9–13. 1999. PMID 10023943.
- ↑ 77.0 77.1 "Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF)". Lancet. 353 (9169): 2001–7. 1999. PMID 10376614.
- ↑ 78.0 78.1 Packer M, Fowler MB, Roecker EB, Coats AJ, Katus HA, Krum H; et al. (2002). "Effect of carvedilol on the morbidity of patients with severe chronic heart failure: results of the carvedilol prospective randomized cumulative survival (COPERNICUS) study". Circulation. 106 (17): 2194–9. doi:10.1161/01.cir.0000035653.72855.bf. PMID 12390947.
- ↑ Caro JJ, Migliaccio-Walle K, O'Brien JA, Nova W, Kim J, Hauch O; et al. (2005). "Economic implications of extended-release metoprolol succinate for heart failure in the MERIT-HF trial: a US perspective of the MERIT-HF trial". J Card Fail. 11 (9): 647–56. doi:10.1016/j.cardfail.2005.06.433. PMID 16360958.
- ↑ Delea TE, Vera-Llonch M, Richner RE, Fowler MB, Oster G (1999). "Cost effectiveness of carvedilol for heart failure". Am J Cardiol. 83 (6): 890–6. doi:10.1016/s0002-9149(98)01066-2. PMID 10190405.
- ↑ Gregory D, Udelson JE, Konstam MA (2001). "Economic impact of beta blockade in heart failure". Am J Med. 110 Suppl 7A: 74S–80S. doi:10.1016/s0002-9343(98)00387-8. PMID 11334781.
- ↑ Vera-Llonch M, Menzin J, Richner RE, Oster G (2001). "Cost-effectiveness results from the US Carvedilol Heart Failure Trials Program". Ann Pharmacother. 35 (7–8): 846–51. doi:10.1345/aph.10114. PMID 11485131.
- ↑ 83.0 83.1 Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A; et al. (1999). "The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators". N Engl J Med. 341 (10): 709–17. doi:10.1056/NEJM199909023411001. PMID 10471456.
- ↑ 84.0 84.1 Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B; et al. (2003). "Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction". N Engl J Med. 348 (14): 1309–21. doi:10.1056/NEJMoa030207. PMID 12668699. Review in: J Fam Pract. 2003 Aug;52(8):598-9 Review in: ACP J Club. 2003 Sep-Oct;139(2):32
- ↑ 85.0 85.1 Zannad F, McMurray JJ, Krum H, van Veldhuisen DJ, Swedberg K, Shi H; et al. (2011). "Eplerenone in patients with systolic heart failure and mild symptoms". N Engl J Med. 364 (1): 11–21. doi:10.1056/NEJMoa1009492. PMID 21073363. Review in: Evid Based Med. 2011 Aug;16(4):121-2 Review in: J Fam Pract. 2011 Aug;60(8):482-4
- ↑ Glick HA, Orzol SM, Tooley JF, Remme WJ, Sasayama S, Pitt B (2002). "Economic evaluation of the randomized aldactone evaluation study (RALES): treatment of patients with severe heart failure". Cardiovasc Drugs Ther. 16 (1): 53–9. doi:10.1023/a:1015371616135. PMID 12085979.
- ↑ Weintraub WS, Zhang Z, Mahoney EM, Kolm P, Spertus JA, Caro J; et al. (2005). "Cost-effectiveness of eplerenone compared with placebo in patients with myocardial infarction complicated by left ventricular dysfunction and heart failure". Circulation. 111 (9): 1106–13. doi:10.1161/01.CIR.0000157146.86758.BC. PMID 15723981.
- ↑ Zhang Z, Mahoney EM, Kolm P, Spertus J, Caro J, Willke R; et al. (2010). "Cost effectiveness of eplerenone in patients with heart failure after acute myocardial infarction who were taking both ACE inhibitors and beta-blockers: subanalysis of the EPHESUS". Am J Cardiovasc Drugs. 10 (1): 55–63. doi:10.2165/11319940-000000000-00000. PMID 20104935.
- ↑ Hernandez AF, Mi X, Hammill BG, Hammill SC, Heidenreich PA, Masoudi FA; et al. (2012). "Associations between aldosterone antagonist therapy and risks of mortality and readmission among patients with heart failure and reduced ejection fraction". JAMA. 308 (20): 2097–107. doi:10.1001/jama.2012.14795. PMID 23188026.
- ↑ 90.0 90.1 Juurlink DN, Mamdani MM, Lee DS, Kopp A, Austin PC, Laupacis A; et al. (2004). "Rates of hyperkalemia after publication of the Randomized Aldactone Evaluation Study". N Engl J Med. 351 (6): 543–51. doi:10.1056/NEJMoa040135. PMID 15295047.
- ↑ McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA; et al. (2019). "Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction". N Engl J Med. 381 (21): 1995–2008. doi:10.1056/NEJMoa1911303. PMID 31535829. Review in: Ann Intern Med. 2020 Feb 18;172(4):JC16
- ↑ Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P; et al. (2020). "Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure". N Engl J Med. 383 (15): 1413–1424. doi:10.1056/NEJMoa2022190. PMID 32865377 Check
|pmid=value (help). Review in: Ann Intern Med. 2020 Nov 17;173(10):JC51 - ↑ Parizo JT, Goldhaber-Fiebert JD, Salomon JA, Khush KK, Spertus JA, Heidenreich PA; et al. (2021). "Cost-effectiveness of Dapagliflozin for Treatment of Patients With Heart Failure With Reduced Ejection Fraction". JAMA Cardiol. 6 (8): 926–935. doi:10.1001/jamacardio.2021.1437. PMC 8156166 Check
|pmc=value (help). PMID 34037681 Check|pmid=value (help). - ↑ Isaza N, Calvachi P, Raber I, Liu CL, Bellows BK, Hernandez I; et al. (2021). "Cost-effectiveness of Dapagliflozin for the Treatment of Heart Failure With Reduced Ejection Fraction". JAMA Netw Open. 4 (7): e2114501. doi:10.1001/jamanetworkopen.2021.14501. PMC 8317009 Check
|pmc=value (help). PMID 34313742 Check|pmid=value (help). - ↑ Taylor AL, Ziesche S, Yancy C, Carson P, D'Agostino R, Ferdinand K; et al. (2004). "Combination of isosorbide dinitrate and hydralazine in blacks with heart failure". N Engl J Med. 351 (20): 2049–57. doi:10.1056/NEJMoa042934. PMID 15533851. Review in: ACP J Club. 2005 Mar-Apr;142(2):37
- ↑ Angus DC, Linde-Zwirble WT, Tam SW, Ghali JK, Sabolinski ML, Villagra VG; et al. (2005). "Cost-effectiveness of fixed-dose combination of isosorbide dinitrate and hydralazine therapy for blacks with heart failure". Circulation. 112 (24): 3745–53. doi:10.1161/CIRCULATIONAHA.105.563882. PMID 16344404.
- ↑ Cohn JN, Archibald DG, Ziesche S, Franciosa JA, Harston WE, Tristani FE; et al. (1986). "Effect of vasodilator therapy on mortality in chronic congestive heart failure. Results of a Veterans Administration Cooperative Study". N Engl J Med. 314 (24): 1547–52. doi:10.1056/NEJM198606123142404. PMID 3520315.
- ↑ Khazanie P, Liang L, Curtis LH, Butler J, Eapen ZJ, Heidenreich PA; et al. (2016). "Clinical Effectiveness of Hydralazine-Isosorbide Dinitrate Therapy in Patients With Heart Failure and Reduced Ejection Fraction: Findings From the Get With The Guidelines-Heart Failure Registry". Circ Heart Fail. 9 (2): e002444. doi:10.1161/CIRCHEARTFAILURE.115.002444. PMC 4755330. PMID 26867758.
- ↑ 99.0 99.1 Macchia A, Levantesi G, Franzosi MG, Geraci E, Maggioni AP, Marfisi R; et al. (2005). "Left ventricular systolic dysfunction, total mortality, and sudden death in patients with myocardial infarction treated with n-3 polyunsaturated fatty acids". Eur J Heart Fail. 7 (5): 904–9. doi:10.1016/j.ejheart.2005.04.008. PMID 16087142.
- ↑ 100.0 100.1 Tavazzi L, Maggioni AP, Marchioli R, Barlera S, Franzosi MG, Latini R; et al. (2008). "Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial". Lancet. 372 (9645): 1223–30. doi:10.1016/S0140-6736(08)61239-8. PMID 18757090. Review in: Ann Intern Med. 2009 Jan 20;150(2):JC1-11
- ↑ Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB; et al. (2019). "Cardiovascular Risk Reduction with Icosapent Ethyl for Hypertriglyceridemia". N Engl J Med. 380 (1): 11–22. doi:10.1056/NEJMoa1812792. PMID 30415628.
- ↑ Peterson BE, Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA; et al. (2021). "Reduction in Revascularization With Icosapent Ethyl: Insights From REDUCE-IT Revascularization Analyses". Circulation. 143 (1): 33–44. doi:10.1161/CIRCULATIONAHA.120.050276. PMC 7752247 Check
|pmc=value (help). PMID 33148016 Check|pmid=value (help). - ↑ Pitt B, Anker SD, Bushinsky DA, Kitzman DW, Zannad F, Huang IZ; et al. (2011). "Evaluation of the efficacy and safety of RLY5016, a polymeric potassium binder, in a double-blind, placebo-controlled study in patients with chronic heart failure (the PEARL-HF) trial". Eur Heart J. 32 (7): 820–8. doi:10.1093/eurheartj/ehq502. PMC 3069389. PMID 21208974.
- ↑ Anker SD, Kosiborod M, Zannad F, Piña IL, McCullough PA, Filippatos G; et al. (2015). "Maintenance of serum potassium with sodium zirconium cyclosilicate (ZS-9) in heart failure patients: results from a phase 3 randomized, double-blind, placebo-controlled trial". Eur J Heart Fail. 17 (10): 1050–6. doi:10.1002/ejhf.300. PMC 5033065. PMID 26011677.
- ↑ Massie BM, Collins JF, Ammon SE, Armstrong PW, Cleland JG, Ezekowitz M; et al. (2009). "Randomized trial of warfarin, aspirin, and clopidogrel in patients with chronic heart failure: the Warfarin and Antiplatelet Therapy in Chronic Heart Failure (WATCH) trial". Circulation. 119 (12): 1616–24. doi:10.1161/CIRCULATIONAHA.108.801753. PMID 19289640.
- ↑ 106.0 106.1 Homma S, Thompson JL, Pullicino PM, Levin B, Freudenberger RS, Teerlink JR; et al. (2012). "Warfarin and aspirin in patients with heart failure and sinus rhythm". N Engl J Med. 366 (20): 1859–69. doi:10.1056/NEJMoa1202299. PMC 3723382. PMID 22551105. Review in: Ann Intern Med. 2012 Aug 21;157(4):JC2-7 Review in: Evid Based Med. 2013 Apr;18(2):69-70
- ↑ Zannad F, Anker SD, Byra WM, Cleland JGF, Fu M, Gheorghiade M; et al. (2018). "Rivaroxaban in Patients with Heart Failure, Sinus Rhythm, and Coronary Disease". N Engl J Med. 379 (14): 1332–1342. doi:10.1056/NEJMoa1808848. PMID 30146935.
- ↑ 108.0 108.1 Packer M, O'Connor CM, Ghali JK, Pressler ML, Carson PE, Belkin RN; et al. (1996). "Effect of amlodipine on morbidity and mortality in severe chronic heart failure. Prospective Randomized Amlodipine Survival Evaluation Study Group". N Engl J Med. 335 (15): 1107–14. doi:10.1056/NEJM199610103351504. PMID 8813041.
- ↑ Packer M, Carson P, Elkayam U, Konstam MA, Moe G, O'Connor C; et al. (2013). "Effect of amlodipine on the survival of patients with severe chronic heart failure due to a nonischemic cardiomyopathy: results of the PRAISE-2 study (prospective randomized amlodipine survival evaluation 2)". JACC Heart Fail. 1 (4): 308–314. doi:10.1016/j.jchf.2013.04.004. PMID 24621933.
- ↑ Djoussé L, Cook NR, Kim E, Bodar V, Walter J, Bubes V; et al. (2020). "Supplementation With Vitamin D and Omega-3 Fatty Acids and Incidence of Heart Failure Hospitalization: VITAL-Heart Failure". Circulation. 141 (9): 784–786. doi:10.1161/CIRCULATIONAHA.119.044645. PMC 7054158 Check
|pmc=value (help). PMID 31709816. - ↑ Wang T, Liu Z, Fu J, Min Z (2019). "Meta-analysis of vitamin D supplementation in the treatment of chronic heart failure". Scand Cardiovasc J. 53 (3): 110–116. doi:10.1080/14017431.2019.1612084. PMID 31032644.
- ↑ Zittermann A, Ernst JB, Prokop S, Fuchs U, Gruszka A, Dreier J; et al. (2019). "Vitamin D supplementation of 4000 IU daily and cardiac function in patients with advanced heart failure: The EVITA trial". Int J Cardiol. 280: 117–123. doi:10.1016/j.ijcard.2019.01.027. PMID 30654912.
- ↑ Lonn E, Bosch J, Yusuf S, Sheridan P, Pogue J, Arnold JM; et al. (2005). "Effects of long-term vitamin E supplementation on cardiovascular events and cancer: a randomized controlled trial". JAMA. 293 (11): 1338–47. doi:10.1001/jama.293.11.1338. PMID 15769967.
- ↑ Marchioli R, Levantesi G, Macchia A, Marfisi RM, Nicolosi GL, Tavazzi L; et al. (2006). "Vitamin E increases the risk of developing heart failure after myocardial infarction: Results from the GISSI-Prevenzione trial". J Cardiovasc Med (Hagerstown). 7 (5): 347–50. doi:10.2459/01.JCM.0000223257.09062.17. PMID 16645413.
- ↑ Mortensen SA, Rosenfeldt F, Kumar A, Dolliner P, Filipiak KJ, Pella D; et al. (2014). "The effect of coenzyme Q10 on morbidity and mortality in chronic heart failure: results from Q-SYMBIO: a randomized double-blind trial". JACC Heart Fail. 2 (6): 641–9. doi:10.1016/j.jchf.2014.06.008. PMID 25282031.
- ↑ Madmani ME, Yusuf Solaiman A, Tamr Agha K, Madmani Y, Shahrour Y, Essali A; et al. (2014). "Coenzyme Q10 for heart failure". Cochrane Database Syst Rev (6): CD008684. doi:10.1002/14651858.CD008684.pub2. PMID 24049047.
- ↑ 117.0 117.1 "Effect of verapamil on mortality and major events after acute myocardial infarction (the Danish Verapamil Infarction Trial II--DAVIT II)". Am J Cardiol. 66 (10): 779–85. 1990. doi:10.1016/0002-9149(90)90351-z. PMID 2220572.
- ↑ 118.0 118.1 Multicenter Diltiazem Postinfarction Trial Research Group (1988). "The effect of diltiazem on mortality and reinfarction after myocardial infarction". N Engl J Med. 319 (7): 385–92. doi:10.1056/NEJM198808183190701. PMID 2899840.
- ↑ Goldstein RE, Boccuzzi SJ, Cruess D, Nattel S (1991). "Diltiazem increases late-onset congestive heart failure in postinfarction patients with early reduction in ejection fraction. The Adverse Experience Committee; and the Multicenter Diltiazem Postinfarction Research Group". Circulation. 83 (1): 52–60. doi:10.1161/01.cir.83.1.52. PMID 1984898.
- ↑ Figulla HR, Gietzen F, Zeymer U, Raiber M, Hegselmann J, Soballa R; et al. (1996). "Diltiazem improves cardiac function and exercise capacity in patients with idiopathic dilated cardiomyopathy. Results of the Diltiazem in Dilated Cardiomyopathy Trial". Circulation. 94 (3): 346–52. doi:10.1161/01.cir.94.3.346. PMID 8759075.
- ↑ Echt DS, Liebson PR, Mitchell LB, Peters RW, Obias-Manno D, Barker AH; et al. (1991). "Mortality and morbidity in patients receiving encainide, flecainide, or placebo. The Cardiac Arrhythmia Suppression Trial". N Engl J Med. 324 (12): 781–8. doi:10.1056/NEJM199103213241201. PMID 1900101.
- ↑ Waldo AL, Camm AJ, deRuyter H, Friedman PL, MacNeil DJ, Pauls JF; et al. (1996). "Effect of d-sotalol on mortality in patients with left ventricular dysfunction after recent and remote myocardial infarction. The SWORD Investigators. Survival With Oral d-Sotalol". Lancet. 348 (9019): 7–12. doi:10.1016/s0140-6736(96)02149-6. PMID 8691967.
- ↑ Køber L, Torp-Pedersen C, McMurray JJ, Gøtzsche O, Lévy S, Crijns H; et al. (2008). "Increased mortality after dronedarone therapy for severe heart failure". N Engl J Med. 358 (25): 2678–87. doi:10.1056/NEJMoa0800456. PMID 18565860.
- ↑ 124.0 124.1 Lipscombe LL, Gomes T, Lévesque LE, Hux JE, Juurlink DN, Alter DA (2007). "Thiazolidinediones and cardiovascular outcomes in older patients with diabetes". JAMA. 298 (22): 2634–43. doi:10.1001/jama.298.22.2634. PMID 18073359. Review in: ACP J Club. 2008 Jun 17;148(4):13
- ↑ Lago RM, Singh PP, Nesto RW (2007). "Congestive heart failure and cardiovascular death in patients with prediabetes and type 2 diabetes given thiazolidinediones: a meta-analysis of randomised clinical trials". Lancet. 370 (9593): 1129–36. doi:10.1016/S0140-6736(07)61514-1. PMID 17905165. Review in: ACP J Club. 2008 Mar-Apr;148(2):39
- ↑ Dargie HJ, Hildebrandt PR, Riegger GA, McMurray JJ, McMorn SO, Roberts JN; et al. (2007). "A randomized, placebo-controlled trial assessing the effects of rosiglitazone on echocardiographic function and cardiac status in type 2 diabetic patients with New York Heart Association Functional Class I or II Heart Failure". J Am Coll Cardiol. 49 (16): 1696–704. doi:10.1016/j.jacc.2006.10.077. PMID 17448371.
- ↑ Home PD, Pocock SJ, Beck-Nielsen H, Curtis PS, Gomis R, Hanefeld M; et al. (2009). "Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): a multicentre, randomised, open-label trial". Lancet. 373 (9681): 2125–35. doi:10.1016/S0140-6736(09)60953-3. PMID 19501900. Review in: Ann Intern Med. 2009 Oct 20;151(8):JC4-8 Review in: Evid Based Med. 2009 Dec;14(6):168
- ↑ Giles TD, Miller AB, Elkayam U, Bhattacharya M, Perez A (2008). "Pioglitazone and heart failure: results from a controlled study in patients with type 2 diabetes mellitus and systolic dysfunction". J Card Fail. 14 (6): 445–52. doi:10.1016/j.cardfail.2008.02.007. PMID 18672190.
- ↑ Scirica BM, Braunwald E, Raz I, Cavender MA, Morrow DA, Jarolim P; et al. (2014). "Heart failure, saxagliptin, and diabetes mellitus: observations from the SAVOR-TIMI 53 randomized trial". Circulation. 130 (18): 1579–88. doi:10.1161/CIRCULATIONAHA.114.010389. PMID 25189213. Review in: Ann Intern Med. 2015 Apr 21;162(8):JC11
- ↑ Zannad F, Cannon CP, Cushman WC, Bakris GL, Menon V, Perez AT; et al. (2015). "Heart failure and mortality outcomes in patients with type 2 diabetes taking alogliptin versus placebo in EXAMINE: a multicentre, randomised, double-blind trial". Lancet. 385 (9982): 2067–76. doi:10.1016/S0140-6736(14)62225-X. PMID 25765696.
- ↑ Verma S, Goldenberg RM, Bhatt DL, Farkouh ME, Quan A, Teoh H; et al. (2017). "Dipeptidyl peptidase-4 inhibitors and the risk of heart failure: a systematic review and meta-analysis". CMAJ Open. 5 (1): E152–E177. doi:10.9778/cmajo.20160058. PMC 5403656. PMID 28459046.
- ↑ Mamdani M, Juurlink DN, Lee DS, Rochon PA, Kopp A, Naglie G; et al. (2004). "Cyclo-oxygenase-2 inhibitors versus non-selective non-steroidal anti-inflammatory drugs and congestive heart failure outcomes in elderly patients: a population-based cohort study". Lancet. 363 (9423): 1751–6. doi:10.1016/S0140-6736(04)16299-5. PMID 15172772.
- ↑ Hudson M, Richard H, Pilote L (2005). "Differences in outcomes of patients with congestive heart failure prescribed celecoxib, rofecoxib, or non-steroidal anti-inflammatory drugs: population based study". BMJ. 330 (7504): 1370. doi:10.1136/bmj.330.7504.1370. PMC 558290. PMID 15947399.
- ↑ Gislason GH, Rasmussen JN, Abildstrom SZ, Schramm TK, Hansen ML, Fosbøl EL; et al. (2009). "Increased mortality and cardiovascular morbidity associated with use of nonsteroidal anti-inflammatory drugs in chronic heart failure". Arch Intern Med. 169 (2): 141–9. doi:10.1001/archinternmed.2008.525. PMID 19171810. Review in: Ann Intern Med. 2009 Jun 16;150(12):JC6-12
- ↑ Feenstra J, Heerdink ER, Grobbee DE, Stricker BH (2002). "Association of nonsteroidal anti-inflammatory drugs with first occurrence of heart failure and with relapsing heart failure: the Rotterdam Study". Arch Intern Med. 162 (3): 265–70. doi:10.1001/archinte.162.3.265. PMID 11822918.
- ↑ 136.0 136.1 136.2 Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, Michelson EL, Olofsson B, Ostergren J, Yusuf S, Pocock S (2003). "Effects of candesartan on mortality and morbidity in patients with chronic heart failure: the CHARM-Overall programme". Lancet. 362 (9386): 759–66. PMID 13678868.
- ↑ Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL (2013). "2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines". Circulation. 128 (16): e240–327. doi:10.1161/CIR.0b013e31829e8776. PMID 23741058.
- ↑ CIBIS-II Investigators and Committee. The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet. 1999;353:9–13
- ↑ . Hjalmarson A, Goldstein S, Fagerberg B, et al., for the MERIT-HF Study Group. Effects of controlled-release metoprolol on total mortality, hospitalizations, and well-being in patients with heart failure: the Metoprolol CR/XL Randomized Intervention Trial in congestive heart failure (MERIT-HF). JAMA. 2000;283:1295–302
- ↑ A trial of the beta-blocker bucindolol in patients with advanced chronic heart failure. N Engl J Med. 2001;344:1659–67
- ↑ Poole-Wilson PA, Swedberg K, Cleland JG, et al. Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol Or Metoprolol European Trial (COMET): randomised controlled trial. Lancet.2003;362:7 13.
- ↑ Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERITHF). Lancet. 1999;353:2001–7
- ↑ Lechat P, Packer M, Chalon S, et al. Clinical effects of beta-adrenergic blockade in chronic heart failure: a meta-analysis of double-blind, placebo-controlled, randomized trials. Circulation. 1998;98:1184–91.
- ↑ Packer M, Bristow MR, Cohn JN, et al., for the U.S. Carvedilol Heart Failure Study Group. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. N Engl J Med. 1996;334:1349–55.
- ↑ Dargie HJ. Effect of carvedilol on outcome after myocardial infarction in patients with left-ventricular dysfunction: the CAPRICORN randomised trial. Lancet. 2001;357:1385–90.
- ↑ . Cleland JG, Pennell DJ, Ray SG, et al. Myocardial viability as a determinant of the ejection fraction response to carvedilol in patients with heart failure (CHRISTMAS trial): randomised controlled trial. Lancet. 2003;362:14–21
- ↑ Fisher ML, Gottlieb SS, Plotnick GD, et al. Beneficial effects of metoprolol in heart failure associated with coronary artery disease: a randomized trial. J Am Coll Cardiol. 1994;23:943–50
- ↑ Metra M, Nardi M, Giubbini R, et al. Effects of short- and long-term carvedilol administration on rest and exercise hemodynamic variables, exercise capacity and clinical conditions in patients with idiopathic dilated cardiomyopathy. J Am Coll Cardiol. 1994;24:1678–87.
- ↑ Olsen SL, Gilbert EM, Renlund DG, et al. Carvedilol improves left ventricular function and symptoms in chronic heart failure: a doubleblind randomized study. J Am Coll Cardiol. 1995;25:1225–31.
- ↑ Vizzardi E, D'Aloia A, Giubbini R, Bordonali T, Bugatti S, Pezzali N; et al. (2010). "Effect of spironolactone on left ventricular ejection fraction and volumes in patients with class I or II heart failure". Am J Cardiol. 106 (9): 1292–6. doi:10.1016/j.amjcard.2010.06.052. PMID 21029826.
- ↑ Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M; et al. (2011). "Apixaban versus warfarin in patients with atrial fibrillation". N Engl J Med. 365 (11): 981–92. doi:10.1056/NEJMoa1107039. PMID 21870978. Review in: Ann Intern Med. 2012 Jan 17;156(2):JC1-2, JC1-3
- ↑ Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W; et al. (2011). "Rivaroxaban versus warfarin in nonvalvular atrial fibrillation". N Engl J Med. 365 (10): 883–91. doi:10.1056/NEJMoa1009638. PMID 21830957. Review in: Evid Based Med. 2012 Oct;17(5):148-9 Review in: Ann Intern Med. 2012 Jan 17;156(2):JC1-2, JC1-3
- ↑ Heerdink ER, Leufkens HG, Herings RM, et al. NSAIDs associated with increased risk of congestive heart failure in elderly patients taking diuretics. Arch Intern Med. 1998;158:1108–12.
- ↑ . Herchuelz A, Derenne F, Deger F, et al. Interaction between nonsteroidal anti-inflammatory drugs and loop diuretics: modulation by sodiumbalance. J Pharmacol Exp Ther. 1989;248:1175–81.
- ↑ Gottlieb SS, Robinson S, Krichten CM, et al. Renal response to indomethacin in congestive heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. 1992;70:890–3
- ↑ Bank AJ, Kubo SH, Rector TS, et al. Local forearm vasodilation with intra-arterial administration of enalaprilat in humans. Clin Pharmacol Ther. 1991;50:314–21.
- ↑ The Cardiac Arrhythmia Suppression Trial (CAST) Investigators. Preliminary report: effect of encainide and flecainide on mortality in a randomized trial of arrhythmia suppression after myocardial infarction. N Engl J Med. 1989;321:406–12.
- ↑ The Cardiac Arrhythmia Suppression Trial II Investigators. Effect of the antiarrhythmic agent moricizine on survival after myocardial infarction. N Engl J Med. 1992;327:227–33.
- ↑ Pratt CM, Eaton T, Francis M, et al. The inverse relationship between baseline left ventricular ejection fraction and outcome of antiarrhythmic therapy: a dangerous imbalance in the risk-benefit ratio. Am Heart J. 1989;118:433–40.
- ↑ .Nilsson BB, Westheim A, Risberg MA. Effects of group-based highintensity aerobic interval training in patients with chronic heart failure. Am J Cardiol. 2008;102:1361–5.
- ↑ .Mueller L, Myers J, Kottman W, et al. Exercise capacity, physical activity patterns and outcomes six years after cardiac rehabilitation in patients with heart failure. Clin Rehabil. 2007;21:923–31.
- ↑ .Dracup K, Evangelista LS, Hamilton MA, et al. Effects of a home-based exercise program on clinical outcomes in heart failure. Am Heart J. 2007;154:877–83.
- ↑ Jo´nsdo´ttirS, Andersen KK, Sigurosson AF, Sigurosson SB. The effect of physical training in chronic heart failure. Eur J Heart Fail. 2006;8:97–101.
- ↑ Bokhari F, Newman D, Greene M, et al. Long-term comparison of the implantable cardioverter defibrillator versus amiodarone: eleven-year follow-up of a subset of patients in the Canadian Implantable Defibrillator Study (CIDS). Circulation. 2004;110:112–6
- ↑ Mark DB, Nelson CL, Anstrom KJ, et al. Cost-effectiveness of defibrillator therapy or amiodarone in chronic stable heart failure: results from the Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT). Circulation. 2006;114:135–42
- ↑ Bardy GH, Lee KL, Mark DB, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–37
- ↑ Epstein AE, DiMarco JP, Ellenbogen KA, et al. ACC/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices) developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. J Am Coll Cardiol. 2008;51:e1–62.
- ↑ Bardy GH, Lee KL, Mark DB, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–37.
- ↑ Hebert K, McKinnie J, Horswell R, et al. Expansion of heart failure device therapy into a rural indigent population in Louisiana: potential economic and health policy implications. J Card Fail. 2006;12: 689–93.
- ↑ Knight BP, Goyal R, Pelosi F, et al. Outcome of patients with nonischemic dilated cardiomyopathy and unexplained syncope treated with an implantable defibrillator. J Am Coll Cardiol. 1999;33:1964–70.
- ↑ Klein HU, Reek S. The MUSTT study: evaluating testing and treatment. J Interv Card Electrophysiol. 2000;4 Suppl 1:45–50
- ↑ Moss AJ, Zareba W, Hall WJ, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–83.
- ↑ Hohnloser SH, Connolly SJ, Kuck KH, et al. The defibrillator in acute myocardial infarction trial (DINAMIT): study protocol. Am Heart J. 2000;140:735–9.
- ↑ Wilkoff BL, Cook JR, Epstein AE, et al. Dual-chamber pacing or ventricular backup pacing in patients with an implantable defibrillator: the Dual Chamber and VVI Implantable Defibrillator (DAVID) Trial. JAMA. 2002;288:3115–23.
- ↑ Moss AJ, Hall WJ, Cannom DS, Daubert JP, Higgins SL, Klein H; et al. (1996). "Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. Multicenter Automatic Defibrillator Implantation Trial Investigators". N Engl J Med. 335 (26): 1933–40. doi:10.1056/NEJM199612263352601. PMID 8960472.
- ↑ Buxton AE, Lee KL, Fisher JD, Josephson ME, Prystowsky EN, Hafley G (1999). "A randomized study of the prevention of sudden death in patients with coronary artery disease. Multicenter Unsustained Tachycardia Trial Investigators". N Engl J Med. 341 (25): 1882–90. doi:10.1056/NEJM199912163412503. PMID 10601507.
- ↑ Hohnloser SH, Kuck KH, Dorian P, Roberts RS, Hampton JR, Hatala R; et al. (2004). "Prophylactic use of an implantable cardioverter-defibrillator after acute myocardial infarction". N Engl J Med. 351 (24): 2481–8. doi:10.1056/NEJMoa041489. PMID 15590950. Review in: ACP J Club. 2005 May-Jun;142(3):58
- ↑ 178.0 178.1 Bristow MR, Saxon LA, Boehmer J, Krueger S, Kass DA, De Marco T; et al. (2004). "Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure". N Engl J Med. 350 (21): 2140–50. doi:10.1056/NEJMoa032423. PMID 15152059. Review in: ACP J Club. 2004 Nov-Dec;141(3):60
- ↑ 179.0 179.1 Abraham WT, Fisher WG, Smith AL, Delurgio DB, Leon AR, Loh E; et al. (2002). "Cardiac resynchronization in chronic heart failure". N Engl J Med. 346 (24): 1845–53. doi:10.1056/NEJMoa013168. PMID 12063368. Review in: ACP J Club. 2002 Nov-Dec;137(3):82
- ↑ 180.0 180.1 180.2 180.3 Moss AJ, Hall WJ, Cannom DS, Klein H, Brown MW, Daubert JP; et al. (2009). "Cardiac-resynchronization therapy for the prevention of heart-failure events". N Engl J Med. 361 (14): 1329–38. doi:10.1056/NEJMoa0906431. PMID 19723701.
- ↑ 181.0 181.1 181.2 181.3 181.4 181.5 Tang AS, Wells GA, Talajic M, Arnold MO, Sheldon R, Connolly S; et al. (2010). "Cardiac-resynchronization therapy for mild-to-moderate heart failure". N Engl J Med. 363 (25): 2385–95. doi:10.1056/NEJMoa1009540. PMID 21073365. Review in: Evid Based Med. 2011 Oct;16(5):138-9
- ↑ 182.0 182.1 Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L; et al. (2005). "The effect of cardiac resynchronization on morbidity and mortality in heart failure". N Engl J Med. 352 (15): 1539–49. doi:10.1056/NEJMoa050496. PMID 15753115. Review in: ACP J Club. 2005 Sep-Oct;143(2):29
- ↑ Beshai JF, Grimm RA, Nagueh SF, et al. Cardiac-resynchronization therapy in heart failure with narrow QRS complexes. N Engl J Med. 2007;357:2461–71
- ↑ Cleland JG, Daubert JC, Erdmann E, et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539–49.
- ↑ Xiao HB, Roy C, Fujimoto S, et al. Natural history of abnormal conduction and its relation to prognosis in patients with dilated cardiomyopathy. Int J Cardiol. 1996;53:163–70
- ↑ Shamim W, Francis DP, Yousufuddin M, et al. Intraventricular conduction delay: a prognostic marker in chronic heart failure. Int J Cardiol. 1999;70:171–8.
- ↑ Unverferth DV, Magorien RD, Moeschberger ML, et al. Factors influencing the one-year mortality of dilated cardiomyopathy. Am J Cardiol. 1984;54:147–52.
- ↑ Blanc JJ, Etienne Y, Gilard M, et al. Evaluation of different ventricular pacing sites in patients with severe heart failure: results of an acute hemodynamic study. Circulation. 1997;96:3273–7.
- ↑ Kass DA, Chen CH, Curry C, et al. Improved left ventricular mechanics from acute VDD pacing in patients with dilated cardiomyopathy and ventricular conduction delay. Circulation. 1999;99:156773.
- ↑ Toussaint JF, Lavergne T, Ollitraut J, et al. Biventricular pacing in severe heart failure patients reverses electromechanical dyssynchronization from apex to base. Pacing Clin Electrophysiol. 2000;23:1731–4
- ↑ Nelson GS, Berger RD, Fetics BJ, et al. Left ventricular or biventricular pacing improves cardiac function at diminished energy cost in patients with dilated cardiomyopathy and left bundle-branch block. Circulation. 2000;102:3053–9.
- ↑ Abraham WT, Fisher WG, Smith AL, et al. Cardiac resynchronization in chronic heart failure. N Engl J Med. 2002;346:1845–53.
- ↑ Young JB, Abraham WT, Smith AL, et al. Combined cardiac resynchronization and implantable cardioversion defibrillation in advanced chronic heart failure: the MIRACLE ICD Trial. JAMA. 2003;289: 2685–94.
- ↑ McAlister FA, Stewart S, Ferrua S, et al. Multidisciplinary strategies for the management of heart failure patients at high risk for admission: a systematic review of randomized trials. J Am Coll Cardiol. 2004;44:810–9.
- ↑ Juurlink DN, Mamdani M, Kopp A, et al. Drug-drug interactions among elderly patients hospitalized for drug toxicity. JAMA. 2003;289: 1652–8.
- ↑ Juurlink DN, Mamdani MM, Lee DS, et al. Rates of hyperkalemia after publication of the Randomized Aldactone Evaluation Study. N Engl J Med. 2004;351:543–51.
- ↑ Svensson M, Gustafsson F, Galatius S, et al. How prevalent is hyperkalemia and renal dysfunction during treatment with spironolactone in patients with congestive heart failure? J Card Fail. 2004;10:297–303
- ↑ Carson P, Ziesche S, Johnson G, et al., for the Vasodilator-Heart Failure Trial Study Group. Racial differences in response to therapy for heart failure: analysis of the vasodilator-heart failure trials. J Card Fail. 1999;5:178–87.
- ↑ Taylor AL, Ziesche S, Yancy C, et al. Combination of isosorbide dinitrate and hydralazine in blacks with heart failure. N Engl J Med.2004;351:2049–57.
- ↑ Stevenson WG, Tedrow U. Management of atrial fibrillation in patients with heart failure. Heart Rhythm. 2007;4:S28–30.
- ↑ Setaro JF, Zaret BL, Schulman DS, Black HR, Soufer R (1990). "Usefulness of verapamil for congestive heart failure associated with abnormal left ventricular diastolic filling and normal left ventricular systolic performance". Am J Cardiol. 66 (12): 981–6. PMID 2220622.
- ↑ Loh E, Sutton MS, Wun CC, Rouleau JL, Flaker GC, Gottlieb SS; et al. (1997). "Ventricular dysfunction and the risk of stroke after myocardial infarction". N Engl J Med. 336 (4): 251–7. doi:10.1056/NEJM199701233360403. PMID 8995087.
- ↑ Horwich TB, MacLellan WR, Fonarow GC (2004). "Statin therapy is associated with improved survival in ischemic and non-ischemic heart failure". J Am Coll Cardiol. 43 (4): 642–8. doi:10.1016/j.jacc.2003.07.049. PMID 14975476.
- ↑ Gissi-HF Investigators. Tavazzi L, Maggioni AP, Marchioli R, Barlera S, Franzosi MG; et al. (2008). "Effect of rosuvastatin in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial". Lancet. 372 (9645): 1231–9. doi:10.1016/S0140-6736(08)61240-4. PMID 18757089.
- ↑ McMurray JJ, Dunselman P, Wedel H, Cleland JG, Lindberg M, Hjalmarson A; et al. (2010). "Coenzyme Q10, rosuvastatin, and clinical outcomes in heart failure: a pre-specified substudy of CORONA (controlled rosuvastatin multinational study in heart failure)". J Am Coll Cardiol. 56 (15): 1196–204. doi:10.1016/j.jacc.2010.02.075. PMID 20883926.
- ↑ Soukoulis V, Dihu JB, Sole M, Anker SD, Cleland J, Fonarow GC; et al. (2009). "Micronutrient deficiencies an unmet need in heart failure". J Am Coll Cardiol. 54 (18): 1660–73. doi:10.1016/j.jacc.2009.08.012. PMID 19850206.
- ↑ 207.0 207.1 Rickard J, Bassiouny M, Cronin EM, Martin DO, Varma N, Niebauer MJ; et al. (2011). "Predictors of response to cardiac resynchronization therapy in patients with a non-left bundle branch block morphology". Am J Cardiol. 108 (11): 1576–80. doi:10.1016/j.amjcard.2011.07.017. PMID 21890086.
- ↑ Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG; et al. (2009). "2009 Focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines Developed in Collaboration With the International Society for Heart and Lung Transplantation". J Am Coll Cardiol. 53 (15): e1–e90. doi:10.1016/j.jacc.2008.11.013. PMID 19358937.
- ↑ Bozkurt B, Agoston I, Knowlton AA (2003). "Complications of inappropriate use of spironolactone in heart failure: when an old medicine spirals out of new guidelines". J Am Coll Cardiol. 41 (2): 211–4. PMID 12535810.
- ↑ The Multicenter Diltiazem Postinfarction Trial Research Group. The effect of diltiazem on mortality and reinfarction after myocardial infarction. N Engl J Med. 1988;319:385–92.
- ↑ Reed SD, Friedman JY, Velazquez EJ, et al. Multinational economic evaluation of valsartan in patients with chronic heart failure: results from the Valsartan Heart Failure Trial (Val-HeFT). Am Heart J. 2004;148: 122–8.
- ↑ Setaro JF, Zaret BL, Schulman DS, et al. Usefulness of verapamil for congestive heart failure associated with abnormal left ventricular diastolic filling and normal left ventricular systolic performance. Am J Cardiol. 1990;66:981–6.
- ↑ Packer M, O’Connor CM, Ghali JK, et al., for the Prospective Randomized Amlodipine Survival Evaluation Study Group. Effect of amlodipine on morbidity and mortality in severe chronic heart failure. N Engl J Med. 1996;335:1107–14
- ↑ . Stevenson WG, Tedrow U. Management of atrial fibrillation in patients with heart failure. Heart Rhythm. 2007;4:S28 30.
- ↑ . Heist EK, Ruskin JN. Atrial fibrillation and congestive heart failure: risk factors, mechanisms, and treatment. Prog Cardiovasc Dis. 2006;48:256–69.
- ↑ . Roy D, Talajic M, Nattel S, et al. Rhythm control versus rate control for atrial fibrillation and heart failure. N Engl J Med. 2008;358: 2667–77.
- ↑ Wyse DG, Waldo AL, DiMarco JP, et al. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347:1825–33.
- ↑ Van Gelder I, Hagens VE, Bosker HA, et al. A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. N Engl J Med. 2002;347:1834–40.
- ↑ Cairns JA, Connolly S, McMurtry S, Stephenson M, Talajic M, CCS Atrial Fibrillation Guidelines Committee (2011). "Canadian Cardiovascular Society atrial fibrillation guidelines 2010: prevention of stroke and systemic thromboembolism in atrial fibrillation and flutter". Can J Cardiol. 27 (1): 74–90. doi:10.1016/j.cjca.2010.11.007. PMID 21329865.
- ↑ Hughes M, Lip GY, Guideline Development Group, National Clinical Guideline for Management of Atrial Fibrillation in Primary and Secondary Care, National Institute for Health and Clinical Excellence (2008). "Stroke and thromboembolism in atrial fibrillation: a systematic review of stroke risk factors, risk stratification schema and cost effectiveness data". Thromb Haemost. 99 (2): 295–304. doi:10.1160/TH07-08-0508. PMID 18278178.
- ↑ Dries DL, Rosenberg YD, Waclawiw MA, Domanski MJ (1997). "Ejection fraction and risk of thromboembolic events in patients with systolic dysfunction and sinus rhythm: evidence for gender differences in the studies of left ventricular dysfunction trials". J Am Coll Cardiol. 29 (5): 1074–80. PMID 9120162.
- ↑ Freudenberger RS, Hellkamp AS, Halperin JL, Poole J, Anderson J, Johnson G; et al. (2007). "Risk of thromboembolism in heart failure: an analysis from the Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT)". Circulation. 115 (20): 2637–41. doi:10.1161/CIRCULATIONAHA.106.661397. PMID 17485579.
- ↑ Pfeffer MA, McMurray JJ, Velazquez EJ, et al. Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N Engl J Med. 2003;349:1893–906.
- ↑ . Gottlieb SS, Dickstein K, Fleck E, et al. Hemodynamic and neurohormonal effects of the angiotensin II antagonist losartan in patients with congestive heart failure. Circulation. 1993;88:1602–9.
- ↑ Crozier I, Ikram H, Awan N, et al., for the Losartan Hemodynamic Study Group. Losartan in heart failure. Hemodynamic effects and tolerability. Circulation. 1995;91:691–7.
- ↑ Riegger GA, Bouzo H, Petr P, et al., for the Symptom, Tolerability, Response to Exercise Trial of Candesartan Cilexetil in Heart Failure (STRETCH) Investigators. Improvement in exercise tolerance and symptoms of congestive heart failure during treatment with candesartan cilexetil. Circulation. 1999;100:2224–30.
- ↑ Sharma D, Buyse M, Pitt B, et al., for the Losartan Heart Failure Mortality Meta-analysis Study Group. Meta-analysis of observed mortality data from all-controlled, double-blind, multiple-dose studies of losartan in heart failure. Am J Cardiol. 2000;85:187–92.
- ↑ McKelvie RS, Yusuf S, Pericak D, et al., for the RESOLVD Pilot Study Investigators. Comparison of candesartan, enalapril, and their combination in congestive heart failure: randomized evaluation of strategies for left ventricular dysfunction (RESOLVD) pilot study. Circulation. 1999; 100:1056–64.
- ↑ Mazayev VP, Fomina IG, Kazakov EN, et al. Valsartan in heart failure patients previously untreated with an ACE inhibitor. Int J Cardiol. 1998;65:239–46.
- ↑ Cohn JN, Tognoni G. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med. 2001;345: 1667–75.
- ↑ Wong M, Staszewsky L, Latini R, et al. Valsartan benefits left ventricular structure and function in heart failure: Val-HeFT echocardiographic study. J Am Coll Cardiol. 2002;40:970–5.
- ↑ Böhm M, Robertson M, Ford I, Borer JS, Komajda M, Kindermann I, Maack C, Lainscak M, Swedberg K, Tavazzi L (2015). "Influence of Cardiovascular and Noncardiovascular Co-morbidities on Outcomes and Treatment Effect of Heart Rate Reduction With Ivabradine in Stable Heart Failure (from the SHIFT Trial)". Am. J. Cardiol. 116 (12): 1890–7. doi:10.1016/j.amjcard.2015.09.029. PMID 26508709.
- ↑ Swedberg K, Komajda M, Böhm M, Borer JS, Ford I, Dubost-Brama A, Lerebours G, Tavazzi L (2010). "Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study". Lancet. 376 (9744): 875–85. doi:10.1016/S0140-6736(10)61198-1. PMID 20801500.
- ↑ Fox K, Ford I, Steg PG, Tardif JC, Tendera M, Ferrari R (2014). "Ivabradine in stable coronary artery disease without clinical heart failure". N. Engl. J. Med. 371 (12): 1091–9. doi:10.1056/NEJMoa1406430. PMID 25176136.
- ↑ Fox K, Ford I, Steg PG, Tendera M, Ferrari R (2008). "Ivabradine for patients with stable coronary artery disease and left-ventricular systolic dysfunction (BEAUTIFUL): a randomised, double-blind, placebo-controlled trial". Lancet. 372 (9641): 807–16. doi:10.1016/S0140-6736(08)61170-8. PMID 18757088.
- ↑ The Captopril-Digoxin Multicenter Research Group. Comparative effects of therapy with captopril and digoxin in patients with mild to moderate heart failure. JAMA. 1988;259:539–44.
- ↑ Dobbs SM, Kenyon WI, Dobbs RJ. Maintenance digoxin after an episode of heart failure: placebo-controlled trial in outpatients. Br Med J. 1977;1:749–52
- ↑ Lee DC, Johnson RA, Bingham JB, et al. Heart failure in outpatients: a randomized trial of digoxin versus placebo. N Engl J Med. 1982;306: 699–705.
- ↑ Guyatt GH, Sullivan MJ, Fallen EL, et al. A controlled trial of digoxin in congestive heart failure. Am J Cardiol. 1988;61:371–5.
- ↑ . DiBianco R, Shabetai R, Kostuk W, et al. A comparison of oral milrinone, digoxin, and their combination in the treatment of patients with chronic heart failure. N Engl J Med. 1989;320:677–83.
- ↑ Uretsky BF, Young JB, Shahidi FE, et al., for the PROVED Investigative Group. Randomized study assessing the effect of digoxin withdrawal in patients with mild to moderate chronic congestive heart failure: results of the PROVED trial. J Am Coll Cardiol. 1993;22:955–62.
- ↑ Packer M, Gheorghiade M, Young JB, et al. Withdrawal of digoxin from patients with chronic heart failure treated with angiotensin-convertingenzyme inhibitors. RADIANCE Study. N Engl J Med. 1993;329:1–7.
- ↑ Carson P, Ziesche S, Johnson G, et al., for the Vasodilator-Heart Failure Trial Study Group. Racial differences in response to therapy for heart failure: analysis of the vasodilator-heart failure trials. J Card Fail. 1999;5:178–87.
- ↑ Cohn JN. The Vasodilator-Heart Failure Trials (V-HeFT). Mechanistic data from the VA Cooperative Studies. Introduction. Circulation. 1993; 87:VI1–4.
- ↑ Carson P, Ziesche S, Johnson G, et al., for the Vasodilator-Heart Failure Trial Study Group. Racial differences in response to therapy for heart failure: analysis of the vasodilator-heart failure trials. J Card Fail. 1999;5:178–87.
- ↑ Cohn JN, Archibald DG, Ziesche S, et al. Effect of vasodilator therapy on mortality in chronic congestive heart failure. Results of a Veterans Administration Cooperative Study. N Engl J Med. 1986;314:1547–52.
- ↑ Loeb HS, Johnson G, Henrick A, et al., for the V-HeFT VA Cooperative Studies Group. Effect of enalapril, hydralazine plus isosorbide dinitrate, and prazosin on hospitalization in patients with chronic congestive heart failure. Circulation. 1993;87:VI78–87.
- ↑ Brignole M, Gammage M, Puggioni E, Alboni P, Raviele A, Sutton R; et al. (2005). "Comparative assessment of right, left, and biventricular pacing in patients with permanent atrial fibrillation". Eur Heart J. 26 (7): 712–22. doi:10.1093/eurheartj/ehi069. PMID 15618036.
- ↑ Upadhyay GA, Choudhry NK, Auricchio A, Ruskin J, Singh JP (2008). "Cardiac resynchronization in patients with atrial fibrillation: a meta-analysis of prospective cohort studies". J Am Coll Cardiol. 52 (15): 1239–46. doi:10.1016/j.jacc.2008.06.043. PMID 18926327.
- ↑ Wilkoff BL, Cook JR, Epstein AE, Greene HL, Hallstrom AP, Hsia H; et al. (2002). "Dual-chamber pacing or ventricular backup pacing in patients with an implantable defibrillator: the Dual Chamber and VVI Implantable Defibrillator (DAVID) Trial". JAMA. 288 (24): 3115–23. PMID 12495391.
- ↑ Doshi RN, Daoud EG, Fellows C, Turk K, Duran A, Hamdan MH; et al. (2005). "Left ventricular-based cardiac stimulation post AV nodal ablation evaluation (the PAVE study)". J Cardiovasc Electrophysiol. 16 (11): 1160–5. doi:10.1111/j.1540-8167.2005.50062.x. PMID 16302897.
- ↑ Adelstein E, Schwartzman D, Gorcsan J, Saba S (2011). "Predicting hyperresponse among pacemaker-dependent nonischemic cardiomyopathy patients upgraded to cardiac resynchronization". J Cardiovasc Electrophysiol. 22 (8): 905–11. doi:10.1111/j.1540-8167.2011.02018.x. PMID 21332868.
- ↑ Vatankulu MA, Goktekin O, Kaya MG, Ayhan S, Kucukdurmaz Z, Sutton R; et al. (2009). "Effect of long-term resynchronization therapy on left ventricular remodeling in pacemaker patients upgraded to biventricular devices". Am J Cardiol. 103 (9): 1280–4. doi:10.1016/j.amjcard.2009.01.023. PMID 19406272.
- ↑ Velazquez EJ, Pfeffer MA, McMurray JV, Maggioni AP, Rouleau JL, Van de Werf F; et al. (2003). "VALsartan In Acute myocardial iNfarcTion (VALIANT) trial: baseline characteristics in context". Eur J Heart Fail. 5 (4): 537–44. PMID 12921816.
- ↑ Pfeffer MA, McMurray JJ, Velazquez EJ, et al. Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N Engl J Med. 2003;349:1893–906.
- ↑ Granger CB, McMurray JJ, Yusuf S, et al. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function intolerant to angiotensin-converting-enzyme inhibitors: the CHARM-Alternative trial. Lancet. 2003;362:772–6.
- ↑ Gottlieb SS, Dickstein K, Fleck E, et al. Hemodynamic and neurohormonal effects of the angiotensin II antagonist losartan in patients with congestive heart failure. Circulation. 1993;88:1602–9.
- ↑ . Crozier I, Ikram H, Awan N, et al., for the Losartan Hemodynamic Study Group. Losartan in heart failure. Hemodynamic effects and tolerability. Circulation. 1995;91:691–7.
- ↑ Riegger GA, Bouzo H, Petr P, et al., for the Symptom, Tolerability, Response to Exercise Trial of Candesartan Cilexetil in Heart Failure (STRETCH) Investigators. Improvement in exercise tolerance and symptoms of congestive heart failure during treatment with candesartan cilexetil. Circulation. 1999;100:2224–30.
- ↑ Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW, Antman EM, Smith SC Jr, Adams CD, Anderson JL, Faxon DP, Fuster V, Halperin JL, Hiratzka LF, Jacobs AK, Nishimura R, Ornato JP, Page RL, Riegel B; American College of Cardiology; American Heart Association Task Force on Practice Guidelines; American College of Chest Physicians; International Society for Heart and Lung Transplantation; Heart Rhythm Society. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation. 2005 Sep 20; 112(12): e154-235. Epub 2005 Sep 13. PMID 16160202
- ↑ Pfeffer MA, Claggett B, Assmann SF, Boineau R, Anand IS, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, Heitner JF, Lewis EF, O'Meara E, Rouleau JL, Probstfield JL, Shaburishvili T, Shah SJ, Solomon SD, Sweitzer NK, McKinlay SM, Pitt B (2015). "Regional variation in patients and outcomes in the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) trial". Circulation. 131 (1): 34–42. doi:10.1161/CIRCULATIONAHA.114.013255. PMID 25406305.
- ↑ 262.0 262.1 Yusuf S, Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, Michelson EL, Olofsson B, Ostergren J (2003). "Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial". Lancet. 362 (9386): 777–81. doi:10.1016/S0140-6736(03)14285-7. PMID 13678871.
- ↑ Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG et al. (2005) ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation 112 (12):e154-235. DOI:10.1161/CIRCULATIONAHA.105.167586PMID: 16160202
- Pages with reference errors
- Pages with citations using unsupported parameters
- CS1 maint: Multiple names: authors list
- Pages using citations with accessdate and no URL
- CS1 errors: PMID
- CS1 maint: Explicit use of et al.
- CS1 errors: PMC
- Pages with broken file links
- Cardiology
- Disease
- Emergency medicine
- Intensive care medicine
- Medicine
- Up-To-Date
- Up-To-Date cardiology