Aprotinin
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Ammu Susheela, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
Boxed Warning
See full prescribing information for complete Boxed Warning.
* Aprotinin administration may cause fatal anaphylactic or anaphylactoid reactions. Fatal reactions have occurred with an initial (test) dose as well as with any of the components of the dose regimen.
|
Overview
Aprotinin is a protease inhibitor that is FDA approved for the treatment of prophylactic use to reduce perioperative blood loss and the need for blood transfusion in patients undergoing cardiopulmonary bypass in the course of coronary artery bypass graft surgery who are at an increased risk for blood loss and blood transfusion. There is a Black Box Warning for this drug as shown here. Common adverse reactions include anaphylactoid reactions, sepsis, hemoperitoneum, dyspepsia, gastrointestinal hemorrhage, pulmonary hypertension, pulmonary thrombosis, hyperglycemia, arthralgia.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- Aprotinin is indicated for prophylactic use to reduce perioperative blood loss and the need for blood transfusion in patients undergoing cardiopulmonary bypass in the course of coronary artery bypass graft surgery who are at an increased risk for blood loss and blood transfusion.
- Aprotinin given prophylactically in both Regimen A and Regimen B (half Regimen A) to patients undergoing CABG surgery significantly reduced the donor blood transfusion requirement relative to placebo treatment. In low risk patients there is no difference in efficacy between regimen A and B. Therefore, the dosage used (A vs. B) is at the discretion of the practitioner.
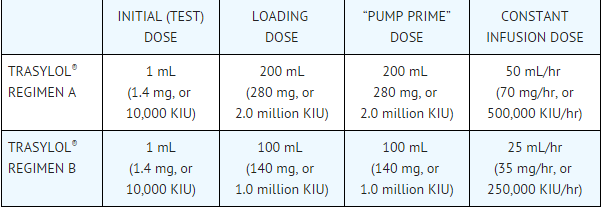
- Aprotinin is supplied as a solution containing 10,000 KIU/mL, which is equal to 1.4 mg/mL. All intravenous doses of Aprotinin should be administered through a central line. DO NOT ADMINISTER ANY OTHER DRUG USING THE SAME LINE. Both regimens include a 1 mL initial (test) dose, a loading dose, a dose to be added while recirculating the priming fluid of the cardiopulmonary bypass circuit (“pump prime” dose), and a constant infusion dose. To avoid physical incompatibility of aprotinin and heparin when adding to the pump prime solution, each agent must be added during recirculation of the pump prime to assure adequate dilution prior to admixture with the other component. Regimens A and B, both incorporating a 1 mL initial (test) dose, are described in the table below:
- The 1 ml initial (test) dose should be administered intravenously at least 10 minutes before the loading dose. With the patient in a supine position, the loading dose is given slowly over 20-30 minutes, after induction of anesthesia but prior to sternotomy. In patients with known previous exposure to aprotinin, the loading dose should be given just prior to cannulation.
- When the loading dose is complete, it is followed by the constant infusion dose, which is continued until surgery is complete and the patient leaves the operating room. The “pump prime” dose is added to the recirculating priming fluid of the cardiopulmonary bypass circuit, by replacement of an aliquot of the priming fluid, prior to the institution of cardiopulmonary bypass. Total doses of more than 7 million KIU have not been studied in controlled trials.
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit. Discard any unused portion.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Aprotinin in adult patients.
Non–Guideline-Supported Use
- Operation on musculoskeletal system [1]
- Pancreatitis [2]
- Surgical procedure on thorax [3]
- Transplantation of liver[4]
- Vascular surgery procedure, Peripheral [5]
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Aprotinin FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Aprotinin in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Aprotinin in pediatric patients.
Contraindications
Hypersensitivity to aprotinin
- Administration of aprotinin to patients with a known or suspected previous aprotinin exposure during the last 12 months is contraindicated. For patients with known or suspected history of exposure to aprotinin greater than 12 months previously. Aprotinin may also be a component of some fibrin sealant products and the use of these products should be included in the patient history.
Warnings
|
Boxed Warning
See full prescribing information for complete Boxed Warning.
* Aprotinin administration may cause fatal anaphylactic or anaphylactoid reactions. Fatal reactions have occurred with an initial (test) dose as well as with any of the components of the dose regimen.
|
- Anaphylactic or anaphylactoid reactions have occurred with aprotinin administration, including fatal reactions in association with the initial (test) dose.
- The initial (test) dose does not fully predict a patient’s risk for a hypersensitivity reaction, including a fatal reaction. Fatal hypersensitivity reactions have occurred among patients who tolerated an initial (test) dose.
- Hypersensitivity reactions often manifest as anaphylactic/anaphylactoid reactions with hypotension the most frequently reported sign of the hypersensitivity reaction. The hypersensitivity reaction can progress to anaphylactic shock with circulatory failure. If a hypersensitivity reaction occurs during injection or infusion of aprotinin, administration should be stopped immediately and emergency treatment should be initiated.
- Even when a second exposure to aprotinin has been tolerated without symptoms, a subsequent administration may result in severe hypersensitivity/anaphylactic reactions.
- Aprotinin should be administered only in operative settings where cardiopulmonary bypass can be rapidly initiated. Before initiating treatment with aprotinin, the recommendations below should be followed to manage a potential hypersensitivity or anaphylactic reaction
- Have standard emergency treatments for hypersensitivity or anaphylactic reactions readily available in the operating room (e.g., epinephrine, corticosteroids). 2) Administration of the initial (test) dose and loading dose should be done only when the patient is intubated and when conditions for rapid cannulation and initiation of cardiopulmonary bypass are present. 3) Delay the addition of aprotinin into the pump prime solution until after the loading dose has been safely administered.
- Re-exposure to aprotinin
- Administration of aprotinin, especially to patients who have received aprotinin in the past, requires a careful risk/benefit assessment because an allergic reaction may occur. Although the majority of cases of anaphylaxis occur upon re-exposure within the first 12 months, there are also case reports of anaphylaxis occurring upon re-exposure after more than 12 months.
- In a retrospective review of 387 European patient records with documented re-exposure to aprotinin, the incidence of hypersensitivity/anaphylactic reactions was 2.7%. Two patients who experienced hypersensitivity/anaphylactic reactions subsequently died, 24 hours and 5 days after surgery, respectively. The relationship of these 2 deaths to aprotinin is unclear. This retrospective review also showed that the incidence of a hypersensitivity or anaphylactic reaction following re-exposure is increased when the re-exposure occurs within 6 months of the initial administration (5.0% for re-exposure within 6 months and 0.9% for re-exposure greater than 6 months). Other smaller studies have shown that in case of re-exposure, the incidence of hypersensitivity/anaphylactic reactions may reach the five percent level.
- An analysis of all spontaneous reports from the Bayer Global database covering a period from 1985 to March 2006 revealed that of 291 possibly associated spontaneous cases of hypersensitivity (fatal: n=52 and non-fatal: n=239), 47% (138/291) of hypersensitivity cases had documented previous exposure to aprotinin. Of the 138 cases with documented previous exposure, 110 had information on the time of the previous exposure. Ninety-nine of the 110 cases had previous exposure within the prior 12 months.
Renal Dysfunction
- Aprotinin administration increases the risk for renal dysfunction and may increase the need for dialysis in the perioperative period. This risk may be especially increased for patients with pre-existing renal impairment or those who receive aminoglycoside antibiotics or drugs that alter renal function. Data from Bayer’s global pool of placebo-controlled studies in patients undergoing coronary artery bypass graft (CABG) surgery showed that the incidence of serum creatinine elevations >0.5 mg/dL above pre-treatment levels was statistically higher at 9.0% (185/2047) in the high-dose aprotinin (Regimen A) group compared with 6.6% (129/1957) in the placebo group.
- In the majority of instances, post-operative renal dysfunction was not severe and was reversible. However, renal dysfunction may progress to renal failure and the incidence of serum creatinine elevations >2.0 mg/dL above baseline was slightly higher in the high-dose aprotinin group (1.1% vs. 0.8%). Careful consideration of the balance of benefits versus potential risks is advised before administering aprotinin to patients with impaired renal function (creatinine clearance < 60 mL/min) or those with other risk factors for renal dysfunction (such as perioperative administration of aminogylcoside or products that alter renal function).
Adverse Reactions
Clinical Trials Experience
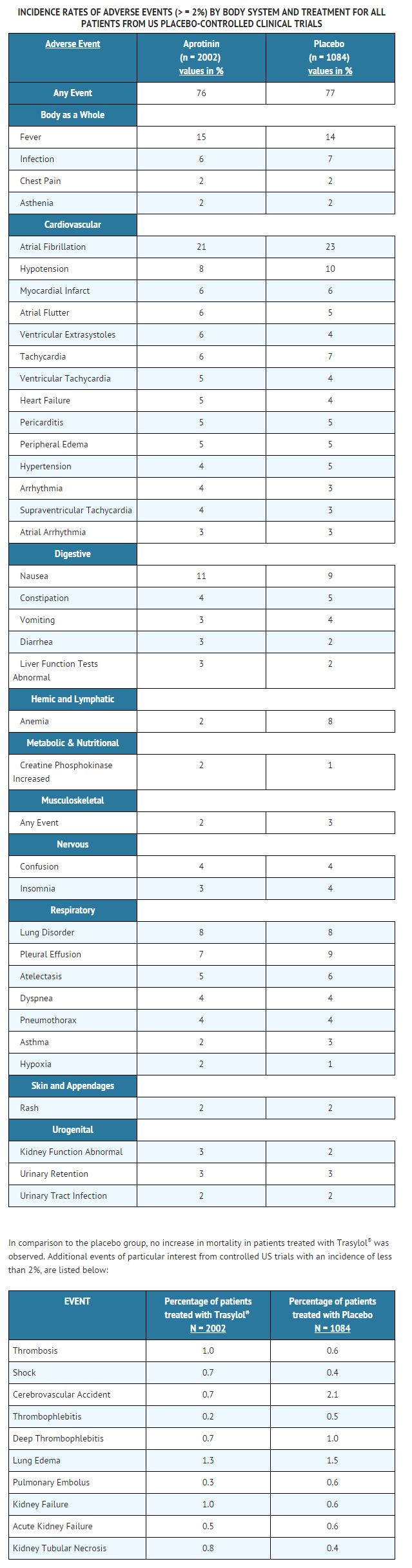
- Studies of patients undergoing CABG surgery, either primary or repeat, indicate that aprotinin is generally well tolerated. The adverse events reported are frequent sequelae of cardiac surgery and are not necessarily attributable to aprotinin therapy. Adverse events reported, up to the time of hospital discharge, from patients in US placebo-controlled trials are listed in the following table. The table lists only those events that were reported in 2% or more of the aprotinin treated patients without regard to causal relationship.
Postmarketing Experience
Body as a Whole
- Sepsis, death, multi-system organ failure, immune system disorder, hemoperitoneum.
Cardiovascular
- Ventricular fibrillation, heart arrest, bradycardia, congestive heart failure, hemorrhage, bundle branch block, myocardial ischemia, ventricular tachycardia, heart block, pericardial effusion, ventricular arrhythmia, shock, pulmonary hypertension.
Digestive
Hematologic and Lymphatic
- Although thrombosis was not reported more frequently in aprotinin versus placebo-treated patients in controlled trials, it has been reported in uncontrolled trials, compassionate use trials, and spontaneous post-marketing reporting. These reports of thrombosis encompass the following terms: thrombosis, occlusion, arterial thrombosis, pulmonary thrombosis, coronary occlusion, embolus, pulmonary embolus, thrombophlebitis, deep thrombophlebitis, cerebrovascular accident, cerebral embolism. Other hematologic events reported include leukocytosis, thrombocytopenia, coagulation disorder (which includes disseminated intravascular coagulation), decreased prothrombin.
Metabolic and Nutritional
- Hyperglycemia, hypokalemia, hypervolemia, acidosis.
Musculoskeletal
Nervous
- Agitation, dizziness, anxiety, convulsion.
Respiratory
Skin
- Skin discoloration.
Urogenital
- Oliguria, kidney failure, acute kidney failure, Acute tubular necrosis.
Myocardial Infarction
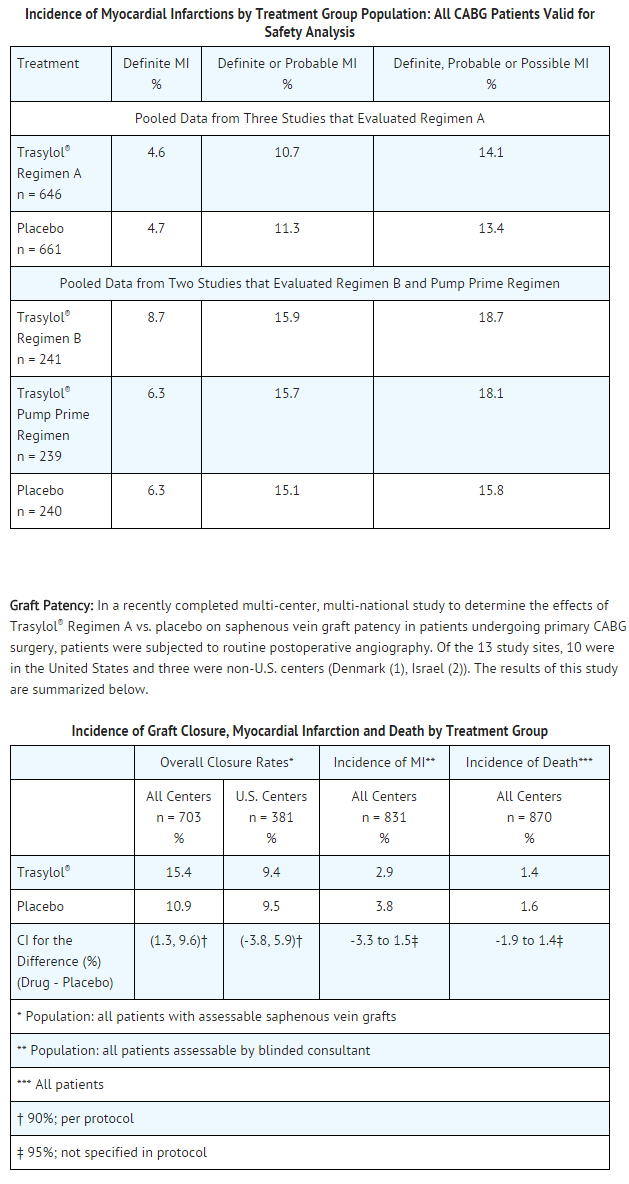
- In the pooled analysis of all patients undergoing CABG surgery, there was no significant difference in the incidence of investigator-reported myocardial infarction (MI) in aprotinin treated patients as compared to placebo treated patients. However, because no uniform criteria for the diagnosis of myocardial infarction were utilized by investigators, this issue was addressed prospectively in three later studies (two studies evaluated Regimen A, Regimen B and Pump Prime Regimen; one study evaluated only Regimen A), in which data were analyzed by a blinded consultant employing an algorithm for possible, probable or definite MI.
- Utilizing this method, the incidence of definite myocardial infarction was 5.9% in the aprotinin-treated patients versus 4.7% in the placebo treated patients. This difference in the incidence rates was not statistically significant. Data from these three studies are summarized below.
- Although there was a statistically significantly increased risk of graft closure for aprotinin treated patients compared to patients who received placebo (p=0.035), further analysis showed a significant treatment by site interaction for one of the non-U.S. sites vs. the U.S. centers. When the analysis of graft closures was repeated for U.S. centers only, there was no statistically significant difference in graft closure rates in patients who received aprotinin vs. placebo.
- These results are the same whether analyzed as the proportion of patients who experienced at least one graft closure postoperatively or as the proportion of grafts closed. There were no differences between treatment groups in the incidence of myocardial infarction as evaluated by the blinded consultant (2.9% aprotinin vs. 3.8% placebo) or of death (1.4% aprotinin vs. 1.6% placebo) in this study.
- Hypersensitivity and anaphylactic reactions during surgery were rarely reported in U.S. controlled clinical studies in patients with no prior exposure to aprotinin (1/1424 patients or <0.1% on aprotinin vs. 1/861 patients or 0.1% on placebo). In case of re-exposure the incidence of hypersensitivity/anaphylactic reactions has been reported to reach the 5% level. A review of 387 European patient records involving re-exposure to aprotinin showed that the incidence of hypersensitivity or anaphylactic reactions was 5.0% for re-exposure within 6 months and 0.9% for re-exposure greater than 6 months.
Laboratory Findings
- Serum Creatinine: Aprotinin administration is associated with a risk for renal dysfunction.
- Serum Transaminases: Data pooled from all patients undergoing CABG surgery in U.S. placebo-controlled trials showed no evidence of an increase in the incidence of postoperative hepatic dysfunction in patients treated with aprotinin.
- The incidence of treatment-emergent increases in ALT (formerly SGPT) > 1.8 times the upper limit of normal was 14% in both the aprotinin and placebo-treated patients (p=0.687), while the incidence of increases > 3 times the upper limit of normal was 5% in both groups (p=0.847).
Other Laboratory Findings
- The incidence of treatment-emergent elevations in plasma glucose, AST (formerly SGOT), LDH, alkaline phosphatase, and CPK-MB was not notably different between aprotinin and placebo treated patients undergoing CABG surgery. Significant elevations in the partial thromboplastin time (PTT) and celite Activated Clotting Time (celite ACT) are expected in aprotinin treated patients in the hours after surgery due to circulating concentrations of aprotinin, which are known to inhibit activation of the intrinsic clotting system by contact with a foreign material (e.g., celite), a method used in these tests.
Drug Interactions
There is limited information regarding Aprotinin Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
- Reproduction studies have been performed in rats at intravenous doses up to 200,000 KIU/kg/day for 11 days, and in rabbits at intravenous doses up to 100,000 KIU/kg/day for 13 days, 2.4 and 1.2 times the human dose on a mg/kg basis and 0.37 and 0.36 times the human mg/m2 dose.
- They have revealed no evidence of impaired fertility or harm to the fetus due to aprotinin. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Aprotinin in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Aprotinin during labor and delivery.
Nursing Mothers
Not applicable
Pediatric Use
Safety and effectiveness in pediatric patient(s) have not been established.
Geriatic Use
- Of the total of 3083 subjects in clinical studies of aprotinin, 1100 (35.7 percent) were 65 and over, while 297 (9.6 percent) were 75 and over. Of patients 65 years and older, 479 (43.5 percent) received Regimen A and 237 (21.5 percent) received Regimen B. No overall differences in safety or effectiveness were observed between these subjects and younger subjects for either dose regimen, and other reported clinical experience has not identified differences in responses between the elderly and younger patients.
Gender
There is no FDA guidance on the use of Aprotinin with respect to specific gender populations.
Race
There is no FDA guidance on the use of Aprotinin with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Aprotinin in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Aprotinin in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Aprotinin in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Aprotinin in patients who are immunocompromised.
Administration and Monitoring
Administration
- Intravenous
Monitoring
- Aprotinin prolongs whole blood clotting times by a different mechanism than heparin. In the presence of aprotinin, prolongation is dependent on the type of whole blood clotting test employed. If an activated clotting time (ACT) is used to determine the effectiveness of heparin anticoagulation, the prolongation of the ACT by aprotinin may lead to an overestimation of the degree of anticoagulation, thereby leading to inadequate anticoagulation.
- During extended extracorporeal circulation, patients may require additional heparin, even in the presence of ACT levels that appear adequate.
- In patients undergoing CPB with aprotinin therapy, one of the following methods may be employed to maintain adequate anticoagulation
- ACT - An ACT is not a standardized coagulation test, and different formulations of the assay are affected differently by the presence of aprotinin. The test is further influenced by variable dilution effects and the temperature experienced during cardiopulmonary bypass. It has been observed that Kaolin-based ACTs are not increased to the same degree by aprotinin as are diatomaceous earth-based (celite) ACTs. While protocols vary, a minimal celite ACT of 750 seconds or kaolin-ACT of 480 seconds, independent of the effects of hemodilution and hypothermia, is recommended in the presence of aprotinin. Consult the manufacturer of the ACT test regarding the interpretation of the assay in the presence of Trasylol ®.
- Fixed Heparin Dosing where a standard loading dose of heparin, administered prior to cannulation of the heart, plus the quantity of heparin added to the prime volume of the CPB circuit, should total at least 350 IU/kg. Additional heparin should be administered in a fixed-dose regimen based on patient weight and duration of CPB.
- Heparin Titration with Protamine titration, a method that is not affected by aprotinin, can be used to measure heparin levels. A heparin dose response, assessed by protamine titration, should be performed prior to administration of aprotinin to determine the heparin loading dose. Additional heparin should be administered on the basis of heparin levels measured by protamine titration. Heparin levels during bypass should not be allowed to drop below 2.7 U/mL (2.0 mg/kg) or below the level indicated by heparin dose response testing performed prior to administration of aprotinin.
- Protamine Administration
- In patients treated with aprotinin, the amount of protamine administered to reverse heparin activity should be based on the actual amount of heparin administered, and not on the ACT values.
IV Compatibility
There is limited information regarding the compatibility of Aprotinin and IV administrations.
Overdosage
- The maximum amount of aprotinin that can be safely administered in single or multiple doses has not been determined. Doses up to 17.5 million KIU have been administered within a 24 hour period without any apparent toxicity.
- There is one poorly documented case, however, of a patient who received a large, but not well determined, amount of aprotinin (in excess of 15 million KIU) in 24 hours. The patient, who had pre-existing liver dysfunction, developed hepatic and renal failure postoperatively and died. Autopsy showed hepatic necrosis and extensive renal tubular and glomerular necrosis. The relationship of these findings to aprotinin therapy is unclear.
Pharmacology
| |
Aprotinin
| |
| Systematic (IUPAC) name | |
| Aprotinin | |
| Identifiers | |
| CAS number | 9004-04-0 |
| ATC code | B02 |
| PubChem | ? |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 6511.51 g/mol |
| Synonyms | Trasylol, bovine pancreatic trypsin inhibitor |
| Pharmacokinetic data | |
| Bioavailability | 100% (intravenous) |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
X |
| Legal status |
RX/POM |
| Dependence Liability | None |
| Routes | Intravenous |
Mechanism of Action
- Aprotinin is a broad spectrum protease inhibitor which modulates the systemic inflammatory response (SIR) associated with cardiopulmonary bypass (CPB) surgery. SIR results in the interrelated activation of the hemostatic, fibrinolytic, cellular and humoral inflammatory systems. Aprotinin, through its inhibition of multiple mediators [e.g., kallikrein, plasmin] results in the attenuation of inflammatory responses, fibrinolysis, and thrombin generation.
- Aprotinin inhibits pro-inflammatory cytokine release and maintains glycoprotein homeostasis. In platelets, aprotinin reduces glycoprotein loss (e.g., GpIb, GpIIb/IIIa), while in granulocytes it prevents the expression of pro-inflammatory adhesive glycoproteins (e.g., CD11b).
- The effects of aprotinin use in CPB involves a reduction in inflammatory response which translates into a decreased need for allogeneic blood transfusions, reduced bleeding, and decreased mediastinal re-exploration for bleeding.
Structure
- Aprotinin (aprotinin injection), C284H432N84O79S7, is a natural proteinase inhibitor obtained from bovine lung. Aprotinin (molecular weight of 6512 daltons), consists of 58 amino acid residues that are arranged in a single polypeptide chain, cross-linked by three disulfide bridges.
- It is supplied as a clear, colorless, sterile isotonic solution for intravenous administration. Each milliliter contains 10,000 KIU (Kallikrein Inhibitor Units) (1.4 mg/mL) and 9 mg sodium chloride in water for injection. Hydrochloric acid and/or sodium hydroxide is used to adjust the pH to 4.5-6.5.
Pharmacodynamics
There is limited information regarding Aprotinin Pharmacodynamics in the drug label.
Pharmacokinetics
- The studies comparing the pharmacokinetics of aprotinin in healthy volunteers, cardiac patients undergoing surgery with cardiopulmonary bypass, and women undergoing hysterectomy suggest linear pharmacokinetics over the dose range of 50,000 KIU to 2 million KIU. After intravenous (IV) injection, rapid distribution of aprotinin occurs into the total extracellular space, leading to a rapid initial decrease in plasma aprotinin concentration. Following this distribution phase, a plasma half-life of about 150 minutes is observed. At later time points, (i.e., beyond 5 hours after dosing) there is a terminal elimination phase with a half-life of about 10 hours.
- Average steady state intraoperative plasma concentrations were 137 KIU/mL (n=10) after administration of the following dosage regimen: 1 million KIU IV loading dose, 1 million KIU into the pump prime volume, 250,000 KIU per hour of operation as continuous intravenous infusion (Regimen B). Average steady state intraoperative plasma concentrations were 250 KIU/mL in patients (n=20) treated with aprotinin during cardiac surgery by administration of Regimen A (exactly double Regimen B): 2 million KIU IV loading dose, 2 million KIU into the pump prime volume, 500,000 KIU per hour of operation as continuous intravenous infusion.
- Following a single IV dose of radiolabelled aprotinin, approximately 25-40% of the radioactivity is excreted in the urine over 48 hours. After a 30 minute infusion of 1 million KIU, about 2% is excreted as unchanged drug. After a larger dose of 2 million KIU infused over 30 minutes, urinary excretion of unchanged aprotinin accounts for approximately 9% of the dose. Animal studies have shown that aprotinin is accumulated primarily in the kidney. Aprotinin, after being filtered by the glomeruli, is actively reabsorbed by the proximal tubules in which it is stored in phagolysosomes. Aprotinin is slowly degraded by lysosomal enzymes. The physiological renal handling of aprotinin is similar to that of other small proteins, e.g., insulin.
Nonclinical Toxicology
There is limited information regarding Aprotinin Nonclinical Toxicology in the drug label.
Clinical Studies
Repeat Coronary Artery Bypass Graft Patients
- Four placebo-controlled, double-blind studies of aprotinin were conducted in the United States; of 540 randomized patients undergoing repeat coronary artery bypass graft (CABG) surgery, 480 were valid for efficacy analysis.
- The following treatment regimens were used in the studies
- Aprotinin Regimen A (2 million KIU IV loading dose, 2 million KIU into the pump prime volume, and 500,000 KIU per hour of surgery as a continuous intravenous infusion); aprotinin Regimen B (1 million KIU IV loading dose, 1 million KIU into the pump prime volume, and 250,000 KIU per hour of surgery as a continuous intravenous infusion); a pump prime regimen (2 million KIU into the pump prime volume only); and a placebo regimen (normal saline). All patients valid for efficacy in the above studies were pooled by treatment regimen for analyses of efficacy.
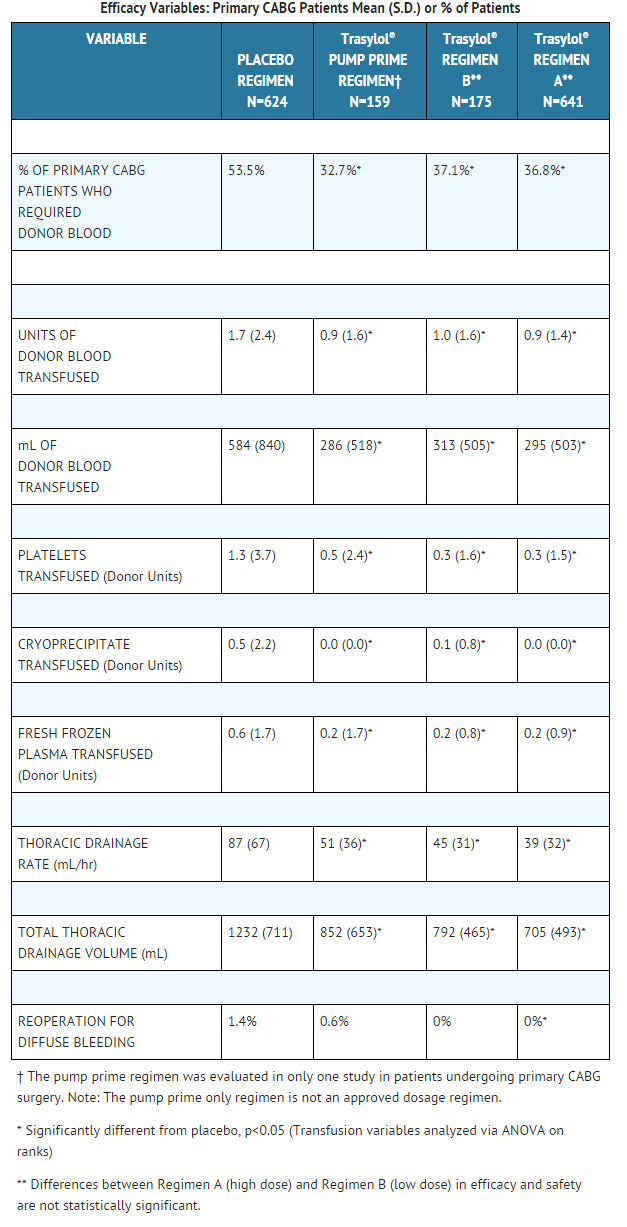
- In this pooled analysis, fewer patients receiving aprotinin, either Regimen A or Regimen B, required any donor blood compared to the pump prime only or placebo regimens. The number of units of donor blood required by patients, the volume (milliliters) of donor blood transfused, the number of units of donor blood products transfused, the thoracic drainage rate, and the total thoracic drainage volumes were also reduced in patients receiving aprotinin as compared to placebo.
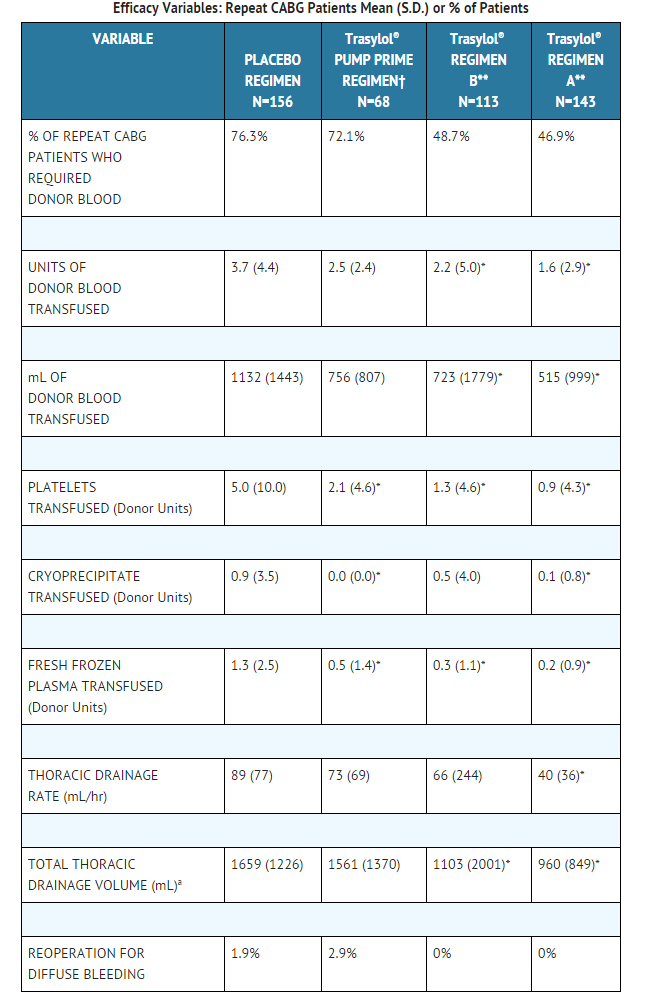
This image is provided by the National Library of Medicine.
Primary Coronary Artery Bypass Graft Patients
- Four placebo-controlled, double-blind studies of aprotinin were conducted in the United States; of 1745 randomized patients undergoing primary CABG surgery, 1599 were valid for efficacy analysis. The dosage regimens used in these studies were identical to those used in the repeat CABG studies described above (Regimens A, B, pump prime, and placebo). All patients valid for efficacy were pooled by treatment regimen.
- In this pooled analysis, fewer patients receiving aprotinin Regimens A, B, and pump prime required any donor blood in comparison to the placebo regimen. The number of units of donor blood required by patients, the volume of donor blood transfused, the number of units of donor blood products transfused, the thoracic drainage rate, and total thoracic drainage volumes were also reduced in patients receiving aprotinin as compared to placebo.
- Additional subgroup analyses showed no diminution in benefit with increasing age. Male and female patients benefited from aprotinin with a reduction in the average number of units of donor blood transfused. Although male patients did better than female patients in terms of the percentage of patients who required any donor blood transfusions, the number of female patients studied was small.
- A double-blind, randomized, Canadian study compared aprotinin Regimen A (n=28) and placebo (n=23) in primary cardiac surgery patients (mainly CABG) requiring cardiopulmonary bypass who were treated with aspirin within 48 hours of surgery. The mean total blood loss (1209.7 mL vs. 2532.3 mL) and the mean number of units of packed red blood cells transfused (1.6 units vs 4.3 units) were significantly less (p<0.008) in the aprotinin group compared to the placebo group.
- In a U.S. randomized study of aprotinin Regimen A and Regimen B versus the placebo regimen in 212 patients undergoing primary aortic and/or mitral valve replacement or repair, no benefit was found for aprotinin in terms of the need for transfusion or the number of units of blood required.
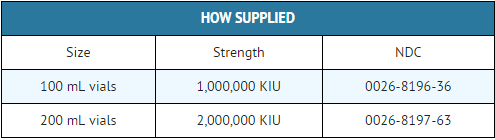
How Supplied
Storage
- Aprotinin should be stored between 2° and 25°C (36° - 77°F).
- Protect from freezing.
Images
Drug Images
{{#ask: Page Name::Aprotinin |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
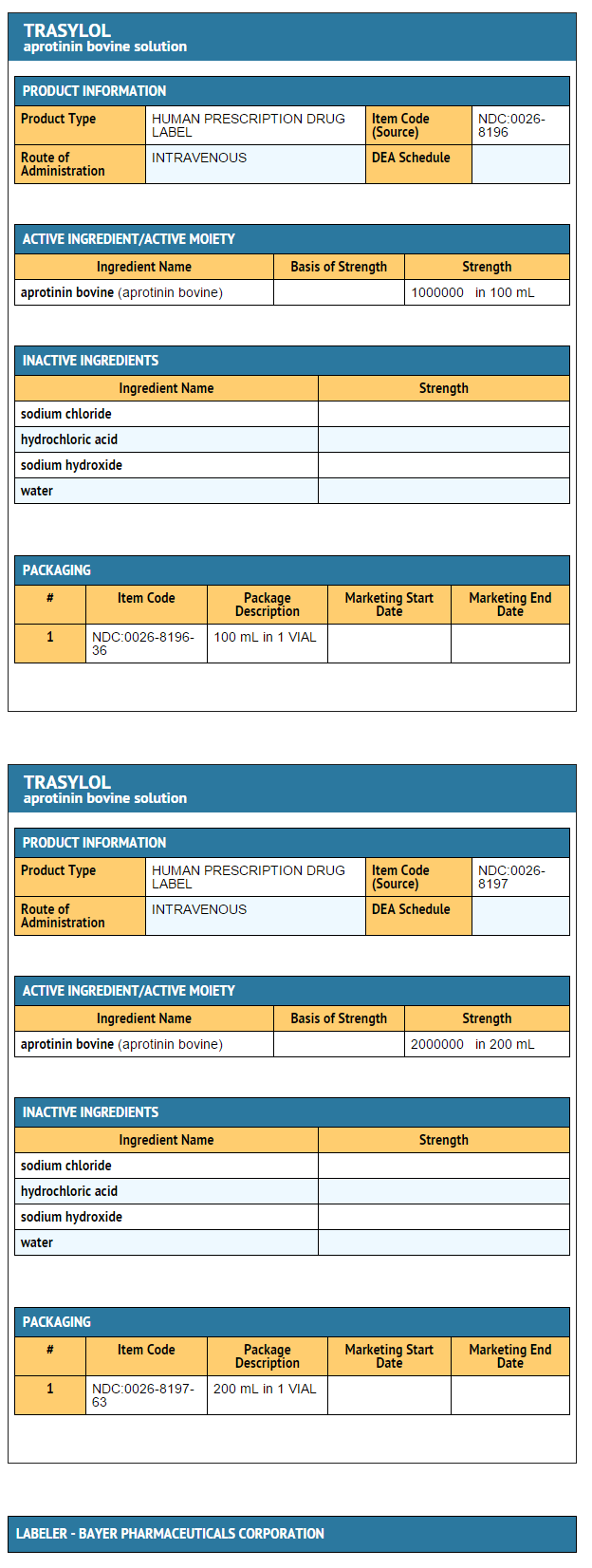
{{#ask: Label Page::Aprotinin |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Aprotinin in the drug label.
Precautions with Alcohol
Alcohol-Aprotinin interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- TRASYLOL[6]
Look-Alike Drug Names
There is limited information regarding Aprotinin Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Jeserschek R, Clar H, Aigner C, Rehak P, Primus B, Windhager R (2003). "Reduction of blood loss using high-dose aprotinin in major orthopaedic surgery: a prospective, double-blind, randomised and placebo-controlled study". J Bone Joint Surg Br. 85 (2): 174–7. PMID 12678347 PMID: 12678347 Check
|pmid=value (help). - ↑ Lasson A (1984). "Acute pancreatitis in man. A clinical and biochemical study of pathophysiology and treatment". Scand J Gastroenterol Suppl. 99: 1–57. PMID 6205440 PMID: 6205440 Check
|pmid=value (help). - ↑ Bedirhan MA, Turna A, Yagan N, Taşçi O (2001). "Aprotinin reduces postoperative bleeding and the need for blood products in thoracic surgery: results of a randomized double-blind study". Eur J Cardiothorac Surg. 20 (6): 1122–7. PMID 11717015 PMID: 11717015 Check
|pmid=value (help). - ↑ Marcel RJ, Stegall WC, Suit CT, Arnold JC, Vera RL, Ramsay MA; et al. (1996). "Continuous small-dose aprotinin controls fibrinolysis during orthotopic liver transplantation". Anesth Analg. 82 (6): 1122–5. PMID 8638778 PMID: 8638778 Check
|pmid=value (help). - ↑ Thompson JF, Roath OS, Francis JL, Webster JH, Chant AD (1990). "Aprotinin in peripheral vascular surgery". Lancet. 335 (8694): 911. PMID 1691424 PMID: 1691424 Check
|pmid=value (help). - ↑ "TRASYLOL- aprotinin bovine solution".