Calcium alginate
| |
| Names | |
|---|---|
| IUPAC name
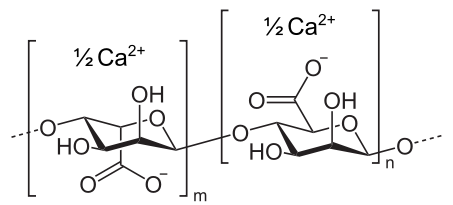
Calcium β-D-mannopyranuronosyl-(1→4)- α-L-gulopyranuronosyl-(1→4)- α-L-gulopyranuronate
| |
| Other names
E404
| |
| Identifiers | |
| ChemSpider | |
| ECHA InfoCard | Lua error in Module:Wikidata at line 879: attempt to index field 'wikibase' (a nil value). Lua error in Module:Wikidata at line 879: attempt to index field 'wikibase' (a nil value). |
| UNII | |
| Properties | |
| (C12H14CaO12)n | |
| Hazards | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
|
WikiDoc Resources for Calcium alginate |
|
Articles |
|---|
|
Most recent articles on Calcium alginate Most cited articles on Calcium alginate |
|
Media |
|
Powerpoint slides on Calcium alginate |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Calcium alginate at Clinical Trials.gov Trial results on Calcium alginate Clinical Trials on Calcium alginate at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Calcium alginate NICE Guidance on Calcium alginate
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Calcium alginate Discussion groups on Calcium alginate Patient Handouts on Calcium alginate Directions to Hospitals Treating Calcium alginate Risk calculators and risk factors for Calcium alginate
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Calcium alginate |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Calcium alginate is a water-insoluble, gelatinous, cream coloured substance that can be created through the addition of aqueous calcium chloride to aqueous sodium alginate. Adding artificial flavours and colours creates a more tasty edible slime. Calcium alginate is also used for entrapment of enzymes and forming artificial seeds in plant tissue culture.
"Alginate" is the term usually used for the salts of alginic acid, but it can also refer to all the derivatives of alginic acid and alginic acid itself; in some publications the term "algin" is used instead of alginate. Alginate is present in the cell walls of brown algae as the calcium, magnesium and sodium salts of alginic acid.
Preparation
Extraction of alginate
To extract the alginate, the seaweed is broken into pieces and stirred with a hot solution of an alkali, usually sodium carbonate. Over a period of about two hours, the alginate dissolves as sodium alginate to give a very thick slurry. This slurry also contains the part of the seaweed that does not dissolve, mainly cellulose. This insoluble residue must be removed from the solution. The solution is too thick (viscous) to be filtered and must be diluted with a very large quantity of water. After dilution, the solution is forced through a filter cloth in a filter press. However, the pieces of undissolved residue are very fine and can quickly clog the filter cloth. Therefore, before filtration is started, a filter aid, such as diatomaceous earth, must be added; this holds most of the fine particles away from the surface of the filter cloth and facilitates filtration. However, filter aid is expensive and can make a significant contribution to costs. To reduce the quantity of filter aid needed, some processors force air into the extract as it is being diluted with water (the extract and diluting water are mixed in an in-line mixer into which air is forced). Fine air bubbles attach themselves to the particles of residue. The diluted extract is left standing for several hours while the air rises to the top, taking the residue particles with it. This frothy mix of air and residue is removed from the top and the solution is withdrawn from the bottom and pumped to the filter.
Preparation of calcium alginate
The goal of the extraction process is to obtain dry, powdered, sodium alginate. The calcium and magnesium salts do not dissolve in water; the sodium salt does. The rationale behind the extraction of alginate from the seaweed is to convert all the alginate salts to the sodium salt, dissolve this in water, and remove the seaweed residue by filtration. The alginate must then be recovered from the aqueous solution. The solution is very dilute and evaporation of the water is not economic. To the Sodium alginate from the initial extraction solution, a calcium salt is added. This causes calcium alginate to form with a fibrous texture; it does not dissolve in water and can be separated from it with relative ease using a metal screen.
Uses
Uses of calcium alginate are:
- in plant tissue culture to produce insoluble artificial seeds
- for immobilizing enzymes by entrapment
- to produce an edible substance
- incorporated into wound dressings (alginate dressings) as a hemostatic
References
- ↑ Gosselin, R.E., H.C. Hodge, R.P. Smith, and M.N. Gleason (1976). Clinical Toxicology of Commercial Products (4th ed.). Baltimore: Williams and Wilkins. p. II-155.
- Pages with script errors
- CS1 maint: Uses authors parameter
- Chemicals without a PubChem CID
- Articles without InChI source
- Articles without EBI source
- Articles without KEGG source
- Chemical articles with unknown parameter in Chembox
- ECHA InfoCard ID from Wikidata
- Articles containing unverified chemical infoboxes
- Edible thickening agents
- Antihemorrhagics