Dermatofibrosarcoma protuberans: Difference between revisions
Sara Mohsin (talk | contribs) No edit summary |
|||
| (117 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
__NOTOC__ | __NOTOC__ | ||
{{ | {{Dermatofibrosarcoma protuberans}} | ||
{{CMG}} | {{CMG}} {{AE}} {{S.M.}}, {{KS}}, {{Faizan}} | ||
{{SK}} | {{SK}} Darier-Ferrand tumor, Darier-Hoffmann tumor, Dermatofibrosarcoma, DFSP, Intermediate fibrous histiocytoma, Borderline fibrous histiocytoma | ||
==Overview== | ==Overview== | ||
Dermatofibrosarcoma protuberans ([[DFSP]]) is a [[rare]] non-[[hereditary]] [[neoplasm]] of the [[dermis]] layer of the [[skin]] which is sometimes described as having the tentacles [[Growth|growing]] into the surrounding [[fat]], [[muscle]] and even [[bone]] and is therefore, [[Classification|classified]] as a [[soft tissue]] [[sarcoma]]. In many respects, the [[disease]] [[Behavior|behaves]] as a [[benign]] [[tumor]], but in 2-5% of [[Case-based reasoning|cases]] it can [[Metastasis|metastasize]], so it should be considered to have a [[malignant]] [[potential]]. Over 95% of [[DFSP]] [[tumors]] have the [[chromosomal translocation]] t(17;22). The [[Translocations|translocation]] [[Fusion gene|fuses]] the [[collagen]] [[gene]] ([[COL1A1]]) with the [[PDGF|platelet-derived growth factor]] [[gene]]. The [[fibroblast]], the [[Cell (biology)|cell]] of [[origin]] of this [[tumor]], [[Expression|expresses]] the [[fusion gene]] in the belief that it is [[collagen]]. However, the [[Result|resulting]] [[fusion protein]] is [[Process (anatomy)|processed]] into mature [[PDGF|platelet-derived growth factor]] which is a [[Potential|potent]] [[growth factor]]. [[Fibroblasts]] contain the [[Receptor (biochemistry)|receptor]] for this [[growth factor]]. Thus, the [[Cell (biology)|cell]] "thinks" it is [[Product (biology)|producing]] a [[Structure factor|structural]] [[protein]], but in fact [[Product (biology)|produces]] a self-[[Stimulated emission|stimulatory]] [[growth]] [[Signal (biology)|signal]]. The [[Cell division|cell divides]] rapidly and a [[tumor]] forms. In dermatofibrosarcoma protuberans, the [[tumor]] has a tendency to return after being removed. However, it does not often [[metastasize]] to other parts of the [[Human body|body]]. | |||
==Classification== | |||
There are several variants of dermatofibrosarcoma protuberans in which different [[Cell (biology)|cell]] types are involved in the [[tumor]]. | |||
<ref name="pmid30775318">{{cite journal| author=Lee SW, Zaesim A, Jackson A, Borkat M| title=Fibrosarcomatous dermatofibrosarcoma protuberans from scar following trauma. | journal=Autops Case Rep | year= 2018 | volume= 8 | issue= 4 | pages= e2018039 | pmid=30775318 | doi=10.4322/acr.2018.039 | pmc=6360829 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=30775318 }} </ref><ref name="pmid17890911">{{cite journal| author=Mentzel T, Schärer L, Kazakov DV, Michal M| title=Myxoid dermatofibrosarcoma protuberans: clinicopathologic, immunohistochemical, and molecular analysis of eight cases. | journal=Am J Dermatopathol | year= 2007 | volume= 29 | issue= 5 | pages= 443-8 | pmid=17890911 | doi=10.1097/DAD.0b013e318145413c | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=17890911 }} </ref><ref name="pmid17721193">{{cite journal| author=Reimann JD, Fletcher CD| title=Myxoid dermatofibrosarcoma protuberans: a rare variant analyzed in a series of 23 cases. | journal=Am J Surg Pathol | year= 2007 | volume= 31 | issue= 9 | pages= 1371-7 | pmid=17721193 | doi=10.1097/PAS.0b013e31802ff7e7 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=17721193 }} </ref> | |||
{| class="wikitable" | |||
|+Different variants of dermatofibrosarcoma protuberans | |||
! style="background: #4479BA; width: 200px;" | {{fontcolor|#FFF|Variant subtype}} | |||
! style="background: #4479BA; width: 400px;" | {{fontcolor|#FFF|Details}} | |||
|- | |||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |'''[[Pigmented lesions|Pigmented]] dermatofibrosarcoma protuberans (Bednar [[tumor]])''' | |||
| | |||
* Contains dark-[[Color|colored]], [[Pigmented lesions|pigmented]] [[Cells (biology)|cells]] known as [[melanin]]-containing [[dendritic cells]] | |||
*[[Red-Al|Red]] or [[brown]] in [[color]] | |||
*Comprises of 1%-5% of all DFSP [[Case-based reasoning|cases]] | |||
|- | |||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |'''Myxoid dermatofibrosarcoma protuberans [[tumor]]''' | |||
| | |||
* Contains an [[abnormal]] type of [[connective tissue]] known as myxoid [[stroma]] | |||
*Uncommon and difficult to [[diagnose]] | |||
|- | |||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |'''[[Juvenile (organism)|Juvenile]] dermatofibrosarcoma protuberans ([[Giant cell fibroblastoma]])''' | |||
| | |||
* Called as [[Juvenile (organism)|juvenile]] because it typically [[Affect|affects]] [[children]] and [[Adolescent|adolescents]] | |||
* Contains [[giant cells]] in the [[tumor]] | |||
|- | |||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |[[Fibrosarcoma|Fibrosarcomatous]] (FS) Dermatofibrosarcoma protuberans | |||
| | |||
*[[Appearance|Appears]] as regions [[Lookahead|looking]] familiar to [[fibrosarcoma]] in different types of DFSP | |||
*More aggressive type of [[soft tissue sarcoma]] | |||
*More likely to [[metastasize]] than other types of DFSP | |||
|} | |||
==Pathophysiology== | ==Pathophysiology== | ||
[[ | ===Epigenetics=== | ||
In normal cells, the COL1A1 gene provides instructions for making part of a large molecule called type I | * Dermatofibrosarcoma protuberans is [[Association (statistics)|associated]] with the [[genetic]] [[rearrangement]] (i.e. [[Chromosomal translocation|translocation]]) between [[chromosomes]] 17 and 22<ref>http://ghr.nlm.nih.gov/condition/dermatofibrosarcoma-protuberans</ref><ref name="pmid31353504">{{cite journal| author=Yokoyama D, Kunisada M, Nakamura K, Takemori C, Tajima S, Sudo T et al.| title=Case of two lesions of dermatofibrosarcoma protuberans revealing identical COL1A1-PDGFB fusion gene: Skin metastasis or multicentric lesions? | journal=J Dermatol | year= 2019 | volume= | issue= | pages= | pmid=31353504 | doi=10.1111/1346-8138.15028 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=31353504 }} </ref><ref name="pmid16980946">{{cite journal| author=Abbott JJ, Erickson-Johnson M, Wang X, Nascimento AG, Oliveira AM| title=Gains of COL1A1-PDGFB genomic copies occur in fibrosarcomatous transformation of dermatofibrosarcoma protuberans. | journal=Mod Pathol | year= 2006 | volume= 19 | issue= 11 | pages= 1512-8 | pmid=16980946 | doi=10.1038/modpathol.3800695 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=16980946 }} </ref><ref name="pmid29140881">{{cite journal| author=Jahanseir K, Xing D, Greipp PT, Sukov WR, Keeney GL, Howitt BE et al.| title=PDGFB Rearrangements in Dermatofibrosarcoma Protuberans of the Vulva: A Study of 11 Cases Including Myxoid and Fibrosarcomatous Variants. | journal=Int J Gynecol Pathol | year= 2018 | volume= 37 | issue= 6 | pages= 537-546 | pmid=29140881 | doi=10.1097/PGP.0000000000000472 | pmc=5951727 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=29140881 }} </ref><ref name="pmid23347652">{{cite journal| author=Ha SY, Lee SE, Kwon MJ, Kim YJ, Lee EH, Seo J et al.| title=PDGFB rearrangement in dermatofibrosarcoma protuberans: correlation with clinicopathologic characteristics and clinical implications. | journal=Hum Pathol | year= 2013 | volume= 44 | issue= 7 | pages= 1300-9 | pmid=23347652 | doi=10.1016/j.humpath.2012.09.021 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=23347652 }} </ref><ref name="pmid21111450">{{cite journal| author=Segura S, Salgado R, Toll A, Martín-Ezquerra G, Yébenes M, Sáez A et al.| title=Identification of t(17;22)(q22;q13) (COL1A1/PDGFB) in dermatofibrosarcoma protuberans by fluorescence in situ hybridization in paraffin-embedded tissue microarrays. | journal=Hum Pathol | year= 2011 | volume= 42 | issue= 2 | pages= 176-84 | pmid=21111450 | doi=10.1016/j.humpath.2010.07.015 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=21111450 }} </ref><ref name="pmid10565681">{{cite journal| author=Wang J, Hisaoka M, Shimajiri S, Morimitsu Y, Hashimoto H| title=Detection of COL1A1-PDGFB fusion transcripts in dermatofibrosarcoma protuberans by reverse transcription-polymerase chain reaction using archival formalin-fixed, paraffin-embedded tissues. | journal=Diagn Mol Pathol | year= 1999 | volume= 8 | issue= 3 | pages= 113-9 | pmid=10565681 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=10565681 }} </ref><ref name="pmid12641779">{{cite journal| author=Gökden N, Dehner LP, Zhu X, Pfeifer JD| title=Dermatofibrosarcoma protuberans of the vulva and groin: detection of COL1A1-PDGFB fusion transcripts by RT-PCR. | journal=J Cutan Pathol | year= 2003 | volume= 30 | issue= 3 | pages= 190-5 | pmid=12641779 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=12641779 }} </ref><ref name="pmid17950782">{{cite journal| author=Patel KU, Szabo SS, Hernandez VS, Prieto VG, Abruzzo LV, Lazar AJ et al.| title=Dermatofibrosarcoma protuberans COL1A1-PDGFB fusion is identified in virtually all dermatofibrosarcoma protuberans cases when investigated by newly developed multiplex reverse transcription polymerase chain reaction and fluorescence in situ hybridization assays. | journal=Hum Pathol | year= 2008 | volume= 39 | issue= 2 | pages= 184-93 | pmid=17950782 | doi=10.1016/j.humpath.2007.06.009 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=17950782 }} </ref><ref name="pmid20061935">{{cite journal| author=Kutzner H, Mentzel T, Palmedo G, Hantschke M, Rütten A, Paredes BE et al.| title=Plaque-like CD34-positive dermal fibroma ("medallion-like dermal dendrocyte hamartoma"): clinicopathologic, immunohistochemical, and molecular analysis of 5 cases emphasizing its distinction from superficial, plaque-like dermatofibrosarcoma protuberans. | journal=Am J Surg Pathol | year= 2010 | volume= 34 | issue= 2 | pages= 190-201 | pmid=20061935 | doi=10.1097/PAS.0b013e3181c7cf11 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=20061935 }} </ref><ref name="pmid9591728">{{cite journal| author=Mentzel T, Beham A, Katenkamp D, Dei Tos AP, Fletcher CD| title=Fibrosarcomatous ("high-grade") dermatofibrosarcoma protuberans: clinicopathologic and immunohistochemical study of a series of 41 cases with emphasis on prognostic significance. | journal=Am J Surg Pathol | year= 1998 | volume= 22 | issue= 5 | pages= 576-87 | pmid=9591728 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=9591728 }} </ref> | ||
The abnormally fused COL1A1-PDGFB gene provides instructions for making an abnormal combined (fusion) [[protein]] that | *t(17;22)(q21;q13.1) [[Lead|leads]] to [[Fusion gene|fusion]] of a part of [[COL1A1]] [[gene]] from [[Chromosome 17 (human)|chromosome 17]] with a part of the [[PDGFB gene]] from [[chromosome 22]]<ref name="pmid16625088">{{cite journal| author=Abbott JJ, Oliveira AM, Nascimento AG| title=The prognostic significance of fibrosarcomatous transformation in dermatofibrosarcoma protuberans. | journal=Am J Surg Pathol | year= 2006 | volume= 30 | issue= 4 | pages= 436-43 | pmid=16625088 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=16625088 }} </ref><ref name="pmid12502925">{{cite journal| author=Terrier-Lacombe MJ, Guillou L, Maire G, Terrier P, Vince DR, de Saint Aubain Somerhausen N et al.| title=Dermatofibrosarcoma protuberans, giant cell fibroblastoma, and hybrid lesions in children: clinicopathologic comparative analysis of 28 cases with molecular data--a study from the French Federation of Cancer Centers Sarcoma Group. | journal=Am J Surg Pathol | year= 2003 | volume= 27 | issue= 1 | pages= 27-39 | pmid=12502925 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=12502925 }} </ref><ref name="pmid17890911">{{cite journal| author=Mentzel T, Schärer L, Kazakov DV, Michal M| title=Myxoid dermatofibrosarcoma protuberans: clinicopathologic, immunohistochemical, and molecular analysis of eight cases. | journal=Am J Dermatopathol | year= 2007 | volume= 29 | issue= 5 | pages= 443-8 | pmid=17890911 | doi=10.1097/DAD.0b013e318145413c | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=17890911 }} </ref><ref name="pmid12502925">{{cite journal| author=Terrier-Lacombe MJ, Guillou L, Maire G, Terrier P, Vince DR, de Saint Aubain Somerhausen N et al.| title=Dermatofibrosarcoma protuberans, giant cell fibroblastoma, and hybrid lesions in children: clinicopathologic comparative analysis of 28 cases with molecular data--a study from the French Federation of Cancer Centers Sarcoma Group. | journal=Am J Surg Pathol | year= 2003 | volume= 27 | issue= 1 | pages= 27-39 | pmid=12502925 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=12502925 }} </ref><ref name="pmid17950782">{{cite journal| author=Patel KU, Szabo SS, Hernandez VS, Prieto VG, Abruzzo LV, Lazar AJ et al.| title=Dermatofibrosarcoma protuberans COL1A1-PDGFB fusion is identified in virtually all dermatofibrosarcoma protuberans cases when investigated by newly developed multiplex reverse transcription polymerase chain reaction and fluorescence in situ hybridization assays. | journal=Hum Pathol | year= 2008 | volume= 39 | issue= 2 | pages= 184-93 | pmid=17950782 | doi=10.1016/j.humpath.2007.06.009 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=17950782 }} </ref><ref name="pmid11420709">{{cite journal| author=Simon MP, Navarro M, Roux D, Pouysségur J| title=Structural and functional analysis of a chimeric protein COL1A1-PDGFB generated by the translocation t(17;22)(q22;q13.1) in Dermatofibrosarcoma protuberans (DP). | journal=Oncogene | year= 2001 | volume= 20 | issue= 23 | pages= 2965-75 | pmid=11420709 | doi=10.1038/sj.onc.1204426 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=11420709 }} </ref><ref name="pmid8988177">{{cite journal| author=Simon MP, Pedeutour F, Sirvent N, Grosgeorge J, Minoletti F, Coindre JM et al.| title=Deregulation of the platelet-derived growth factor B-chain gene via fusion with collagen gene COL1A1 in dermatofibrosarcoma protuberans and giant-cell fibroblastoma. | journal=Nat Genet | year= 1997 | volume= 15 | issue= 1 | pages= 95-8 | pmid=8988177 | doi=10.1038/ng0197-95 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=8988177 }} </ref><ref name="pmid9739023">{{cite journal| author=O'Brien KP, Seroussi E, Dal Cin P, Sciot R, Mandahl N, Fletcher JA et al.| title=Various regions within the alpha-helical domain of the COL1A1 gene are fused to the second exon of the PDGFB gene in dermatofibrosarcomas and giant-cell fibroblastomas. | journal=Genes Chromosomes Cancer | year= 1998 | volume= 23 | issue= 2 | pages= 187-93 | pmid=9739023 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=9739023 }} </ref><ref name="pmid12034531">{{cite journal| author=Maire G, Martin L, Michalak-Provost S, Gattas GJ, Turc-Carel C, Lorette G et al.| title=Fusion of COL1A1 exon 29 with PDGFB exon 2 in a der(22)t(17;22) in a pediatric giant cell fibroblastoma with a pigmented Bednar tumor component. Evidence for age-related chromosomal pattern in dermatofibrosarcoma protuberans and related tumors. | journal=Cancer Genet Cytogenet | year= 2002 | volume= 134 | issue= 2 | pages= 156-61 | pmid=12034531 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=12034531 }} </ref><ref name="pmid10963386">{{cite journal| author=Vanni R, Faa G, Dettori T, Melis GB, Dumanski JP, O'Brien KP| title=A case of dermatofibrosarcoma protuberans of the vulva with a COL1A1/PDGFB fusion identical to a case of giant cell fibroblastoma. | journal=Virchows Arch | year= 2000 | volume= 437 | issue= 1 | pages= 95-100 | pmid=10963386 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=10963386 }} </ref><ref name="pmid18069662">{{cite journal| author=Macarenco RS, Zamolyi R, Franco MF, Nascimento AG, Abott JJ, Wang X et al.| title=Genomic gains of COL1A1-PDFGB occur in the histologic evolution of giant cell fibroblastoma into dermatofibrosarcoma protuberans. | journal=Genes Chromosomes Cancer | year= 2008 | volume= 47 | issue= 3 | pages= 260-5 | pmid=18069662 | doi=10.1002/gcc.20530 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=18069662 }} </ref> | ||
* The [[translocation]] is found on one or more extra [[chromosomes]] that can be either the [[normal]] linear or [[Circular DNA|circular]] in [[Shape parameter|shape]] | |||
*[[Circular DNA|Circular]] extra [[chromosomes]] are known as supernumerary [[Ring chromosome|ring chromosomes]] (which form after a [[chromosome]] breaks in two places and the ends of the [[chromosomal]] [[Arm|arms]] [[Fusion gene|fuse]] together to form a [[Circular DNA|circular]] [[Structure factor|structure]]) | |||
* Other [[genes]] from [[Chromosome 17|chromosomes 17]] and [[Chromosome 22|22]] can be found on the extra [[chromosomes]], but the role of these [[genes]] in the [[Development (biology)|development]] of this [[condition]] is unclear | |||
*[[Translocation]] is [[acquired]] during a [[Person|person's]] [[Lifetime prevalence|lifetime]] and the [[chromosomes]] containing the [[translocation]] are present only in the [[Tumor cell|tumor cells]], this type of [[genetic]] [[Change detection|change]] is known as [[somatic mutation]] | |||
* In [[normal]] [[Cells (biology)|cells]], the [[COL1A1]] [[gene]] provides instructions for [[MakeBot|making]] part of a large [[molecule]] called [[type I collagen]], which [[Strength training|strengthens]] and [[Support|supports]] many [[tissues]] in the [[Human body|body]] | |||
* The [[PDGFB gene]] provides instructions for making one [[Version (eye)|version]] ([[isoform]]) of the [[Platelet-derived growth factor|platelet derived growth factor]] ([[Platelet-derived growth factor|PDGF]]) [[protein]] which by [[Attachment (psychology)|attaching]] to its [[receptor]], becomes activated and [[Stimulated emission|stimulates]] many [[cellular]] [[Process (anatomy)|processes]], including [[cell growth]] and [[Division (biology)|division]] ([[proliferation]]) and [[maturation]] ([[differentiation]]) | |||
* The [[Abnormal|abnormally]] [[Fusion gene|fused]] [[COL1A1]]-[[PDGFB gene]] provides instructions for making an [[abnormal]] combined ([[Fusion protein|fusion]]) [[protein]] that ultimately [[Function (biology)|functions]] like the [[PDGFB]] [[protein]] | |||
* This [[gene fusion]] [[Lead|leads]] to the [[Product (biology)|production]] of an [[Excess risk|excessive]] [[Amount of substance|amount]] of [[protein]] that [[Function (biology)|functions]] like the [[PDGFB]] [[protein]] | |||
* In [[Excess risk|excess]], this [[fusion protein]] [[Stimulated emission|stimulates]] [[Cells (biology)|cells]] to [[proliferate]] and [[differentiate]] [[Abnormal|abnormally]], [[Lead|leading]] to the [[tumor]] [[Formation matrix|formation]] as seen in dermatofibrosarcoma protuberans | |||
*[[COL1A1]]-[[PDGFB gene|PDGFB]] [[fusion gene]] is found in more than 90% of the dermatofibrosarcoma protuberans [[Case-based reasoning|cases]] | |||
*In the remaining 10% of the [[Case-based reasoning|cases]], [[Change detection|changes]] in some other unidentified [[genes]] may be [[Association (statistics)|associated]] with this [[condition]] such as complex t(5;8) involving the CSPG2 and PTK2B genes<ref name="pmid18253748">{{cite journal| author=Bianchini L, Maire G, Guillot B, Joujoux JM, Follana P, Simon MP et al.| title=Complex t(5;8) involving the CSPG2 and PTK2B genes in a case of dermatofibrosarcoma protuberans without the COL1A1-PDGFB fusion. | journal=Virchows Arch | year= 2008 | volume= 452 | issue= 6 | pages= 689-96 | pmid=18253748 | doi=10.1007/s00428-008-0580-2 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=18253748 }} </ref> | |||
== | ===Gross Pathology=== | ||
[[Gross anatomy|Gross features]] of dermatofibrosarcoma protuberans include: | |||
*[[Nodular]], [[Polypoidy|polypoid]] or [[plaque]]-like [[mass]] | |||
*Centered in the [[dermis]] | |||
*Can also occur in deep [[soft tissue]] | |||
*[[Mean]] [[Size consistency|size]] is 5 [[Centimeter|cm]] | |||
*Gray-[[White (mutation)|white]], or [[brown]]/[[black]] in [[color]] (if [[melanocytes]] are present) | |||
*May [[Appearance|appear]] to be circumscribed | |||
*[[Rare|Rarely]] shows: | |||
**[[Hemorrhage]] | |||
**[[Necrosis]] | |||
===Histopathology=== | |||
[[Microscopic]] [[Features (pattern recognition)|features]] include: | |||
* Non-circumscribed | |||
* Highly [[cellular]] with [[Cells (biology)|cells]] having following [[Characteristic function (probability theory)|characteristics]]: | |||
** Monomorphic | |||
** Thin | |||
**[[Spindle cells|Spindly]] | |||
** Scant [[eosinophilic]] [[cytoplasm]] | |||
** Hyperchromatic [[nuclei]] (resembling [[neurofibroma]]) | |||
* Tight storiform [[pattern]] ([[Cells (biology)|cells]] [[Radiating fibers|radiating]] in spokes at right [[Angle|angles]] around a [[central]] [[Point (geometry)|point]] that often contains a [[Blood vessel|vessel]]) [[Infiltration (medical)|infiltrating]] deeply into [[subcutaneous tissue]] and entraping [[fat cells]] to form a [[Characteristic function (probability theory)|characteristic]] honeycomb [[pattern]] | |||
*[[Area|Areas]] of [[Fasciculus|fascicular]] [[growth]] (seen in some [[Case-based reasoning|cases]]) | |||
* The early [[plaque]] stage may lack the particular storiform [[pattern]] | |||
* Many non-atypical [[mitotic]] figures may be present | |||
* Non-[[Polarization|polarized]], thin [[collagen]] | |||
* Mild [[pleomorphism]] (not [[Significant figure|significant]]) | |||
* Focal [[atypia]] | |||
* May coexist with [[giant cell]] fibroblastoma | |||
* Absent or [[rare]] [[histiocytes]] | |||
* Following [[Cell (biology)|cell]] types are absent: | |||
**[[Histiocyte]]-like [[Cells (biology)|cells]] | |||
**[[Foam cells]] | |||
**[[Giant cells]] | |||
** Other [[inflammatory cells]] | |||
* Different '''variants''' include: | |||
**[[Atrophic]] ([[Depress|depressed]] [[lesion]]) | |||
**[[Collagenous]] (with [[central]] thick [[collagen]] bundles) | |||
**[[Granular cell]] ([[S100A1|S100]] negative) | |||
** Myxoid | |||
** Palisading | |||
**[[Pigmented lesions|Pigmented]] | |||
** Sclerosing | |||
{| | |||
| | |||
[[File:Dermatofibrosarcoma protuberans (2) recurrence.jpg|thumb|200px|none|Histopathological image of dermatofibrosarcoma protuberans. Local recurrence long after the first excision. At higher magnification. H&E stain [https://commons.wikimedia.org/wiki/Category:Histopathology_of_dermatofibrosarcoma_protuberans#/media/File:Dermatofibrosarcoma_protuberans_(2)_recurrence.JPG Source: Wikimedia Commons]]] | |||
| | |||
[[File:Dermatofibrosarcoma protuberans (3) recurrence.jpg|thumb|200px|none|Histopathological image of dermatofibrosarcoma protuberans. Local recurrence long after the first excision. H&E stain.[https://commons.wikimedia.org/wiki/Category:Histopathology_of_dermatofibrosarcoma_protuberans#/media/File:Dermatofibrosarcoma_protuberans_(3)_recurrence.JPG Source: Wikimedia Commons]]] | |||
| | |||
[[File:Dermatofibrosarcoma protuberans (1) recurrence.jpg|thumb|200px|none| Histopathological image of dermatofibrosarcoma protuberans. Local recurrence long after the first excision. H&E stain.[https://commons.wikimedia.org/wiki/Category:Histopathology_of_dermatofibrosarcoma_protuberans#/media/File:Dermatofibrosarcoma_protuberans_(1)_recurrence.JPG Source: Wikimedia Commons]]] | |||
| | |||
[[File:1280px-SkinTumors-P9270828.jpg|thumb|200px|none|Dermatofibrosarcoma protuberans (DFSP )[https://commons.wikimedia.org/wiki/Category:Histopathology_of_dermatofibrosarcoma_protuberans#/media/File:SkinTumors-P9270828.JPG Source: Wikimedia Commons]]] | |||
| | |||
|} | |||
== | ===Cytology=== | ||
Cytology of dermatofibrosarcoma has following characteristics:<ref name="pmid15048962">{{cite journal| author=Klijanienko J, Caillaud JM, Lagacé R| title=Fine-needle aspiration of primary and recurrent dermatofibrosarcoma protuberans. | journal=Diagn Cytopathol | year= 2004 | volume= 30 | issue= 4 | pages= 261-5 | pmid=15048962 | doi=10.1002/dc.20024 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=15048962 }} </ref><ref name="pmid12478683">{{cite journal| author=Domanski HA, Gustafson P| title=Cytologic features of primary, recurrent, and metastatic dermatofibrosarcoma protuberans. | journal=Cancer | year= 2002 | volume= 96 | issue= 6 | pages= 351-61 | pmid=12478683 | doi=10.1002/cncr.10760 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=12478683 }} </ref> | |||
* [[Homogeneous]] | |||
* Isolated [[spindle cells]] | |||
* [[Tissue]] [[Fragmentation (biology)|fragments]] often present with storiform [[pattern]] | |||
* [[Fibrillation|Fibrillary]] [[stromal]] [[Fragmentation (biology)|fragments]] | |||
* Naked [[nuclei]] | |||
* Slight to moderate [[atypia]] (occasionally)<ref name="pmid15830369">{{cite journal| author=Domanski HA| title=FNA diagnosis of dermatofibrosarcoma protuberans. | journal=Diagn Cytopathol | year= 2005 | volume= 32 | issue= 5 | pages= 299-302 | pmid=15830369 | doi=10.1002/dc.20238 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=15830369 }} </ref> | |||
===Electron microscopy=== | |||
*[[Stellate cell|Stellate]] or [[spindle cells]] are present which have long, slender, ramified [[Cell (biology)|cell]] [[Process (anatomy)|processes]] which are [[Joint|joined]] by [[Primitive (integral)|primitive]] junctions, often with subplasmalemmal [[Density|densities]]<ref name="pmid15249855">{{cite journal| author=Li N, McNiff J, Hui P, Manfioletti G, Tallini G| title=Differential expression of HMGA1 and HMGA2 in dermatofibroma and dermatofibrosarcoma protuberans: potential diagnostic applications, and comparison with histologic findings, CD34, and factor XIIIa immunoreactivity. | journal=Am J Dermatopathol | year= 2004 | volume= 26 | issue= 4 | pages= 267-72 | pmid=15249855 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=15249855 }} </ref><ref name="pmid18705829">{{cite journal| author=Mori T, Misago N, Yamamoto O, Toda S, Narisawa Y| title=Expression of nestin in dermatofibrosarcoma protuberans in comparison to dermatofibroma. | journal=J Dermatol | year= 2008 | volume= 35 | issue= 7 | pages= 419-25 | pmid=18705829 | doi=10.1111/j.1346-8138.2008.00496.x | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=18705829 }} </ref> | |||
* Multivesicular buds are also present commonly<ref name="pmid16971353">{{cite journal| author=Dominguez-Malagon H, Valdez-Carrillo Mdel C, Cano-Valdez AM| title=Dermatofibroma and dermatofibrosarcoma protuberans: a comparative ultrastructural study. | journal=Ultrastruct Pathol | year= 2006 | volume= 30 | issue= 4 | pages= 283-91 | pmid=16971353 | doi=10.1080/01913120600820468 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=16971353 }} </ref><ref name="pmid11172295">{{cite journal| author=Kahn HJ, Fekete E, From L| title=Tenascin differentiates dermatofibroma from dermatofibrosarcoma protuberans: comparison with CD34 and factor XIIIa. | journal=Hum Pathol | year= 2001 | volume= 32 | issue= 1 | pages= 50-6 | pmid=11172295 | doi=10.1053/hupa.2001.21137 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=11172295 }} </ref> | |||
== | ===Immunohistochemistry=== | ||
*[[Immunhistochemistry|Immunhistochemical]] [[staining]] of dermatofibrosarcoma protuberans shows: | |||
**Positive [[staining]] for: | |||
***[[CD34]] (strong in 95%) | |||
***[[Vimentin]] | |||
***Focal [[actin]] | |||
***ApoD<ref name="pmid15252314">{{cite journal| author=West RB, Harvell J, Linn SC, Liu CL, Prapong W, Hernandez-Boussard T et al.| title=Apo D in soft tissue tumors: a novel marker for dermatofibrosarcoma protuberans. | journal=Am J Surg Pathol | year= 2004 | volume= 28 | issue= 8 | pages= 1063-9 | pmid=15252314 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=15252314 }} </ref><ref name="pmid17885669">{{cite journal| author=Lisovsky M, Hoang MP, Dresser KA, Kapur P, Bhawan J, Mahalingam M| title=Apolipoprotein D in CD34-positive and CD34-negative cutaneous neoplasms: a useful marker in differentiating superficial acral fibromyxoma from dermatofibrosarcoma protuberans. | journal=Mod Pathol | year= 2008 | volume= 21 | issue= 1 | pages= 31-8 | pmid=17885669 | doi=10.1038/modpathol.3800971 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=17885669 }} </ref><ref name="pmid20062007">{{cite journal| author=Bandarchi B, Ma L, Marginean C, Hafezi S, Zubovits J, Rasty G| title=D2-40, a novel immunohistochemical marker in differentiating dermatofibroma from dermatofibrosarcoma protuberans. | journal=Mod Pathol | year= 2010 | volume= 23 | issue= 3 | pages= 434-8 | pmid=20062007 | doi=10.1038/modpathol.2009.176 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=20062007 }} </ref> | |||
***[[Bcl2]] | |||
***NKI-[[C3 (complement)|C3]]<ref name="pmid1553911">{{cite journal| author=Ma CK, Zarbo RJ, Gown AM| title=Immunohistochemical characterization of atypical fibroxanthoma and dermatofibrosarcoma protuberans. | journal=Am J Clin Pathol | year= 1992 | volume= 97 | issue= 4 | pages= 478-83 | pmid=1553911 | doi=10.1093/ajcp/97.4.478 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=1553911 }} </ref> | |||
***CD99<ref name="pmid18201229">{{cite journal| author=Diwan AH, Skelton HG, Horenstein MG, Kelly DR, Barrett TL, Bussian AH et al.| title=Dermatofibrosarcoma protuberans and giant cell fibroblastoma exhibit CD99 positivity. | journal=J Cutan Pathol | year= 2008 | volume= 35 | issue= 7 | pages= 647-50 | pmid=18201229 | doi=10.1111/j.1600-0560.2007.00872.x | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=18201229 }} </ref> | |||
***[[CD99]] | |||
***[[Nestin (protein)|Nestin]] | |||
***[[Tenascin]] | |||
***[[CD163]] | |||
***Factor XIIIa | |||
***[[CD10]] | |||
**Negative [[staining]] for: | |||
***Factor XIIIa (usually) | |||
***[[Keratin]] | |||
***EMA | |||
***[[S100A1|S100]] | |||
***HMB45 | |||
***[[Desmin]] | |||
***[[CD117]]<ref name="pmid17944726">{{cite journal| author=Labonte S, Hanna W, Bandarchi-Chamkhaleh B| title=A study of CD117 expression in dermatofibrosarcoma protuberans and cellular dermatofibroma. | journal=J Cutan Pathol | year= 2007 | volume= 34 | issue= 11 | pages= 857-60 | pmid=17944726 | doi=10.1111/j.1600-0560.2007.00731.x | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=17944726 }} </ref> | |||
Dermatofibrosarcoma protuberans | ==Epidemiology and Demographics== | ||
* Dermatofibrosarcoma protuberans is [[Estimate|estimated]] to occur in 1 in 100,000 to 1-5 in 1 million people per [[year]] | |||
* It usually occurs in [[Adult|adults]] of 20 - 40 [[Year|years]] of [[age]] | |||
* It [[Affect|affects]] twice more commonly the [[Black|blacks]] than whites in [[United States|US]]<ref name="pmid17141362">{{cite journal| author=Criscione VD, Weinstock MA| title=Descriptive epidemiology of dermatofibrosarcoma protuberans in the United States, 1973 to 2002. | journal=J Am Acad Dermatol | year= 2007 | volume= 56 | issue= 6 | pages= 968-73 | pmid=17141362 | doi=10.1016/j.jaad.2006.09.006 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=17141362 }} </ref> | |||
* It can also occur in [[infants]] and [[children]]<ref name="pmid18096453">{{cite journal| author=Reddy C, Hayward P, Thompson P, Kan A| title=Dermatofibrosarcoma protuberans in children. | journal=J Plast Reconstr Aesthet Surg | year= 2009 | volume= 62 | issue= 6 | pages= 819-23 | pmid=18096453 | doi=10.1016/j.bjps.2007.11.009 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=18096453 }} </ref><ref name="pmid20952167">{{cite journal| author=Zaraa I, Ben abdallah M, Driss M, Trojjet S, Ben Sassi M, El Euch D et al.| title=[Dermatofibrosarcoma protuberans in children]. | journal=Arch Pediatr | year= 2011 | volume= 18 | issue= 1 | pages= 23-7 | pmid=20952167 | doi=10.1016/j.arcped.2010.09.010 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=20952167 }} </ref><ref name="pmid17310000">{{cite journal| author=Maire G, Fraitag S, Galmiche L, Keslair F, Ebran N, Terrier-Lacombe MJ et al.| title=A clinical, histologic, and molecular study of 9 cases of congenital dermatofibrosarcoma protuberans. | journal=Arch Dermatol | year= 2007 | volume= 143 | issue= 2 | pages= 203-10 | pmid=17310000 | doi=10.1001/archderm.143.2.203 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=17310000 }} </ref> | |||
* It has a [[Stable element|stable]] [[incidence]] which is highest among [[Womens Pack|women]]<ref name="pmid26730971">{{cite journal| author=Kreicher KL, Kurlander DE, Gittleman HR, Barnholtz-Sloan JS, Bordeaux JS| title=Incidence and Survival of Primary Dermatofibrosarcoma Protuberans in the United States. | journal=Dermatol Surg | year= 2016 | volume= 42 Suppl 1 | issue= | pages= S24-31 | pmid=26730971 | doi=10.1097/DSS.0000000000000300 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=26730971 }} </ref><ref name="pmid22691126">{{cite journal| author=Kuzel P, Metelitsa AI, Dover DC, Salopek TG| title=Epidemiology of dermatofibrosarcoma protuberans in Alberta, Canada, from 1988 to 2007. | journal=Dermatol Surg | year= 2012 | volume= 38 | issue= 9 | pages= 1461-8 | pmid=22691126 | doi=10.1111/j.1524-4725.2012.02482.x | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=22691126 }} </ref> | |||
* Worse [[Survival analysis|survival]] is [[Association (statistics)|associated]] with: | |||
** Increased [[age]] | |||
**[[Male]] [[Sex (activity)|sex]] | |||
**[[Black]] [[race]] | |||
**[[Anatomic]] [[Location parameter|location]] of the [[limbs]] and [[head]] | |||
== | ==Risk Factors== | ||
*Common [[risk factors]] for the [[development]] of dermatofibrosarcoma include: | |||
**A [[scar]] [[Development|developing]] [[after surgery]] or a [[burn]] | |||
**[[Female]] [[Sex (activity)|sex]] | |||
**[[Age]] of 30-50 [[Year|years]] | |||
**African-American [[race]] (Bendar or [[Pigmented lesions|pigmented]] variant) | |||
**[[Pregnancy]] | |||
==Diagnosis== | |||
== | ===History and symptoms=== | ||
*[[Symptoms]] include:<ref name="pmid30287997">{{cite journal| author=Maji S, Paul MJ, Sen S| title=Dermatofibrosarcoma Protuberans of the Breast-a Rare Entity. | journal=Indian J Surg Oncol | year= 2018 | volume= 9 | issue= 3 | pages= 351-354 | pmid=30287997 | doi=10.1007/s13193-017-0684-8 | pmc=6154368 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=30287997 }} </ref> | |||
**Flat or a slightly raised [[skin]] [[Patched|patch]] (first [[Sign (medicine)|sign]]) | |||
**1 to 5 [[Centimeter|centimeters]] in [[diameter]] | |||
**Rubbery (hard to [[touch]]) | |||
**Gives the [[appearance]] of a [[scar]] or a [[Wrinkle|wrinkled]] [[skin]] [[Patched|patch]] | |||
**[[Skin]]-[[Color|colored]], [[Violet (plant)|violet]] or [[Red-Al|reddish]]-[[brown]] | |||
**[[Soft tissue|Soft]], [[Depress|depressed]] [[skin]] [[area]] ([[Rare|rarely]], makes it difficult to [[diagnose]]) | |||
**[[Growth|Grows]] very [[Slow|slowly]] over the [[period]] of [[Year|years]] | |||
====Common sites of involvement==== | |||
*[[Tumors]] commonly involve the following [[Human body|body]] parts: | |||
**[[Torso]]/[[trunk]] (most common) | |||
**[[Arm|Arms]] | |||
**[[Legs]] | |||
**[[Head]] | |||
**[[Neck]] | |||
== | ===Physical examination=== | ||
*On [[physical examination]], it can be [[Feeling|felt]] as a small, [[Purple haze|purplish]], [[Red-Al|reddish]], or [[flesh]]-[[Color|colored]], flat or raised [[skin]] [[Patched|patch]] or [[Nodule (medicine)|nodule]] almost 1-5 [[Centimeter|centimeters]] in [[diameter]] | |||
====Skin==== | |||
=====Extremities===== | |||
{| | |||
| | |||
[[Image:Dermatofibrosarcoma protuberans02.jpg|thumb|200px|none|Dermatofibrosarcoma protuberans.[http://www.atlasdermatologico.com.br/ Source: Dermatology Atlas]]] | |||
| | |||
[[Image:Dermatofibrosarcoma protuberans01.jpg|thumb|200px|none| Dermatofibrosarcoma protuberans.[http://www.atlasdermatologico.com.br/ Source: Dermatology Atlas]]] | |||
| | |||
[[Image:Dermatofibrosarcoma protuberans05.jpg|thumb|200px|none|Dermatofibrosarcoma protuberans.[http://www.atlasdermatologico.com.br/ Source: Dermatology Atlas]]] | |||
| | |||
|} | |||
=====Trunk===== | |||
{| | |||
| | |||
[[Image:Dermatofibrosarcoma protuberans03.jpg|thumb|200px|none|Dermatofibrosarcoma protuberans.[http://www.atlasdermatologico.com.br/ Source: Dermatology Atlas]]] | |||
| | |||
[[Image:Dermatofibrosarcoma protuberans04.jpg|thumb|200px|none| Dermatofibrosarcoma protuberans.[http://www.atlasdermatologico.com.br/ Source: Dermatology Atlas]]] | |||
| | |||
[[Image:Dermatofibrosarcoma protuberans08.jpg|thumb|200px|none|Dermatofibrosarcoma protuberans.[http://www.atlasdermatologico.com.br/ Source: Dermatology Atlas]]] | |||
| | |||
[[Image:Dermatofibrosarcoma protuberans09.jpg|thumb|200px|none|Dermatofibrosarcoma protuberans.[http://www.atlasdermatologico.com.br/ Source: Dermatology Atlas]]] | |||
| | |||
|} | |||
===Diagnostic studies=== | |||
*These include:<ref name="pmid12146044">{{cite journal| author=Zee SY, Wang Q, Jones CM, Abadi MA| title=Fine needle aspiration cytology of dermatofibrosarcoma protuberans presenting as a breast mass. A case report. | journal=Acta Cytol | year= 2002 | volume= 46 | issue= 4 | pages= 741-3 | pmid=12146044 | doi=10.1159/000326988 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=12146044 }} </ref><ref name="pmid10579001">{{cite journal| author=Filipowicz EA, Ventura KC, Pou AM, Logrono R| title=FNAC in the diagnosis of recurrent dermatofibrosarcoma protuberans of the forehead. A case report. | journal=Acta Cytol | year= 1999 | volume= 43 | issue= 6 | pages= 1177-80 | pmid=10579001 | doi=10.1159/000331376 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=10579001 }} </ref> | |||
**[[FNA|FNAC]] <ref name="pmid12478683">{{cite journal| author=Domanski HA, Gustafson P| title=Cytologic features of primary, recurrent, and metastatic dermatofibrosarcoma protuberans. | journal=Cancer | year= 2002 | volume= 96 | issue= 6 | pages= 351-61 | pmid=12478683 | doi=10.1002/cncr.10760 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=12478683 }} </ref><ref name="pmid15830369">{{cite journal| author=Domanski HA| title=FNA diagnosis of dermatofibrosarcoma protuberans. | journal=Diagn Cytopathol | year= 2005 | volume= 32 | issue= 5 | pages= 299-302 | pmid=15830369 | doi=10.1002/dc.20238 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=15830369 }} </ref><ref name="pmid11176051">{{cite journal| author=Zamecnik M| title=Fibrosarcomatous dermatofibrosarcoma protuberans with giant rosettes. | journal=Am J Dermatopathol | year= 2001 | volume= 23 | issue= 1 | pages= 41-5 | pmid=11176051 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=11176051 }} </ref><ref name="pmid15048962">{{cite journal| author=Klijanienko J, Caillaud JM, Lagacé R| title=Fine-needle aspiration of primary and recurrent dermatofibrosarcoma protuberans. | journal=Diagn Cytopathol | year= 2004 | volume= 30 | issue= 4 | pages= 261-5 | pmid=15048962 | doi=10.1002/dc.20024 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=15048962 }} </ref> | |||
**[[Biopsy]] | |||
**[[Immunohistochemistry]] | |||
***[[CD34]] [[staining]] <ref name="pmid9700370">{{cite journal| author=Harvell JD, Kilpatrick SE, White WL| title=Histogenetic relations between giant cell fibroblastoma and dermatofibrosarcoma protuberans. CD34 staining showing the spectrum and a simulator. | journal=Am J Dermatopathol | year= 1998 | volume= 20 | issue= 4 | pages= 339-45 | pmid=9700370 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=9700370 }} </ref><ref name="pmid20061935">{{cite journal| author=Kutzner H, Mentzel T, Palmedo G, Hantschke M, Rütten A, Paredes BE et al.| title=Plaque-like CD34-positive dermal fibroma ("medallion-like dermal dendrocyte hamartoma"): clinicopathologic, immunohistochemical, and molecular analysis of 5 cases emphasizing its distinction from superficial, plaque-like dermatofibrosarcoma protuberans. | journal=Am J Surg Pathol | year= 2010 | volume= 34 | issue= 2 | pages= 190-201 | pmid=20061935 | doi=10.1097/PAS.0b013e3181c7cf11 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=20061935 }} </ref> | |||
{| | |||
| | |||
[[File:Dermatofibrosarcoma protuberans (5) CD34.jpg|thumb|300px|none|Histopathological image of dermatofibrosarcoma protuberans. Local recurrence long after the first excision. CD34 immunostain.[https://commons.wikimedia.org/wiki/Category:Histopathology_of_dermatofibrosarcoma_protuberans#/media/File:Dermatofibrosarcoma_protuberans_(5)_CD34.JPG Source: Wikimedia Commons]]] | |||
| | |||
|} | |||
**[[Magnetic resonance imaging|MRI]] | |||
**[[CT scan]] | |||
{| | |||
| | |||
[[File:Dermatofibrosarcoma protuberans CT cor.jpg|thumb|400px|none|CT image demonstrating a dermatofibrosarcoma protuberans [https://commons.wikimedia.org/wiki/Category:Dermatofibrosarcoma_protuberans#/media/File:Dermatofibrosarcoma_protuberans_CT_cor.jpg Source: Wikimedia Commons]]] | |||
| | |||
[[Image:Dermatofibrosarcoma-protuberans-001.jpg|thumb|400px|none|CT image demonstrating a dermatofibrosarcoma protuberans in the right groin]] | |||
| | |||
|} | |||
Dermatofibrosarcoma-protuberans- | ==Treatment== | ||
*[[Treatments|Treatment]] includes:<ref name="pmid31251395">{{cite journal| author=Huis In 't Veld EA, van Houdt WJ| title=Reply to Follow-up after treatment of dermatofibrosarcoma protuberans. | journal=Cancer | year= 2019 | volume= | issue= | pages= | pmid=31251395 | doi=10.1002/cncr.32341 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=31251395 }} </ref><ref name="pmid16416612">{{cite journal| author=Loss L, Zeitouni NC| title=Management of scalp dermatofibrosarcoma protuberans. | journal=Dermatol Surg | year= 2005 | volume= 31 | issue= 11 Pt 1 | pages= 1428-33 | pmid=16416612 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=16416612 }} </ref><ref name="pmid8646467">{{cite journal| author=Dawes KW, Hanke CW| title=Dermatofibrosarcoma protuberans treated with Mohs micrographic surgery: cure rates and surgical margins. | journal=Dermatol Surg | year= 1996 | volume= 22 | issue= 6 | pages= 530-4 | pmid=8646467 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=8646467 }} </ref><ref name="pmid10870053">{{cite journal| author=Bowne WB, Antonescu CR, Leung DH, Katz SC, Hawkins WG, Woodruff JM et al.| title=Dermatofibrosarcoma protuberans: A clinicopathologic analysis of patients treated and followed at a single institution. | journal=Cancer | year= 2000 | volume= 88 | issue= 12 | pages= 2711-20 | pmid=10870053 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=10870053 }} </ref><ref name="pmid14983497">{{cite journal| author=DuBay D, Cimmino V, Lowe L, Johnson TM, Sondak VK| title=Low recurrence rate after surgery for dermatofibrosarcoma protuberans: a multidisciplinary approach from a single institution. | journal=Cancer | year= 2004 | volume= 100 | issue= 5 | pages= 1008-16 | pmid=14983497 | doi=10.1002/cncr.20051 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=14983497 }} </ref><ref name="pmid15221986">{{cite journal| author=Snow SN, Gordon EM, Larson PO, Bagheri MM, Bentz ML, Sable DB| title=Dermatofibrosarcoma protuberans: a report on 29 patients treated by Mohs micrographic surgery with long-term follow-up and review of the literature. | journal=Cancer | year= 2004 | volume= 101 | issue= 1 | pages= 28-38 | pmid=15221986 | doi=10.1002/cncr.20316 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=15221986 }} </ref> | |||
{| class="wikitable" | |||
|+Treatment options for dermatofibrosarcoma protuberans | |||
!style="background: #4479BA; width: 200px;" | {{fontcolor|#FFF|Treatment option}} | |||
!style="background: #4479BA; width: 200px;" | {{fontcolor|#FFF|Details}} | |||
|- | |||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |'''[[Surgery]]''' | |||
| | |||
*[[Mohs micrographic surgery]] | |||
*[[Excision repair|Excisional]] [[surgery]] includes: | |||
**Removal of [[subcutaneous fat]] (i.e. [[Wide and fast|wide]] [[local]] [[excision]] with 2 - 3 [[Centimetre|cm]] margins) | |||
**3-[[Dimensional analysis|dimensional]] [[histological]] evaluation of margins (necessarily recommended in order to [[Prevention (medical)|prevent]] the [[tumor]] [[Recurrence plot|recurrence]]) | |||
|- | |||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |[[Targeted therapy|'''Targeted therapy''']] | |||
| | |||
* [[Imatinib]]-[[STI571]] ([[tyrosine kinase inhibitor]]) [[therapy]] (may be [[Effective method|effective]] in [[CD117]]- [[tumors]]) is the [[Gold standard (test)|gold standard]] [[Treatments|treatment]] of [[Recurrence plot|recurrent]] or [[inoperable]] [[tumor]] | |||
* As [[imatinib]] [[Inhibition|inhibits]] [[PDGFB]], may be [[Effective method|effective]] for [[tumors]] positive for the t(17;22) [[Translocations|translocation]] | |||
|- | |||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |'''[[Chemotherapy]]''' | |||
| | |||
* Less beneficial than [[targeted therapy]] | |||
|- | |||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |'''[[Radiotherapy]]''' | |||
| | |||
* May be required sometimes | |||
|} | |||
==Differential Diagnosis== | |||
Dermatofibrosarcoma protuberans must be [[Differentiate|differentiated]] must be [[Differentiate|differentiated]] from the following:<ref name="libre">Neurofibroma. Libre Pathology 2015. http://librepathology.org/wiki/index.php/Neurofibroma#cite_note-pmid15486243-2 Accessed on November 17, 2015 </ref><ref>http://surgpathcriteria.stanford.edu/peripheral-nerve/neurofibroma/</ref><ref>http://surgpathcriteria.stanford.edu/peripheral-nerve/neurofibroma/</ref> | |||
*[[Neurofibroma]] | |||
*[[Schwannoma]] | |||
* [[Ganglioneuroma]] | |||
* [[Dermal]] neurotized [[melanocytic nevus]] | |||
* Myxoid [[liposarcoma]] | |||
* [[Solitary]] circumscribed [[neuroma]]/palisaded [[Encapsulated organisms|encapsulated]] [[neuroma]] | |||
* [[Trauma|Traumatic]] [[neuroma]] | |||
* [[Superficial]] angiomyxoma | |||
* [[Nerve sheath]] [[myxoma]] | |||
* [[Malignant peripheral nerve sheath tumor|Malignant peripheral nerve sheath tumor (MPNST)]]/[[malignant]] [[schwannoma]] | |||
* [[Lipoma|Spindle cell lipoma]] | |||
* [[Leiomyoma]] | |||
* [[Inflammatory]] myofibroblastic [[tumor]] | |||
* [[Fibroepithelial polyp]]/[[acrochordon]] (aka [[Skin tags|skin tag]] or [[Soft tissue|soft]] [[fibroma]]) | |||
{| class="wikitable" | |||
|+Differentiating neurofibroma from other diseases | |||
! style="background:#4479BA; color: #FFFFFF;" align="center" + |Disease entity | |||
! style="background:#4479BA; color: #FFFFFF;" align="center" + |Etiology (Genetic or others) | |||
! style="background:#4479BA; color: #FFFFFF;" align="center" + |Histopathological findings | |||
! style="background:#4479BA; color: #FFFFFF;" align="center" + |Immunohistochemical staining | |||
! style="background:#4479BA; color: #FFFFFF;" align="center" + |Risk factors | |||
! style="background:#4479BA; color: #FFFFFF;" align="center" + |Common site of involvement | |||
! style="background:#4479BA; color: #FFFFFF;" align="center" + |Clinical manifestations | |||
! style="background:#4479BA; color: #FFFFFF;" align="center" + |Other associated features | |||
|- | |||
| style="background:#DCDCDC;" align="center" + |'''[[Neurofibroma]]'''<ref name="RodriguezFolpe2012">{{cite journal|last1=Rodriguez|first1=Fausto J.|last2=Folpe|first2=Andrew L.|last3=Giannini|first3=Caterina|last4=Perry|first4=Arie|title=Pathology of peripheral nerve sheath tumors: diagnostic overview and update on selected diagnostic problems|journal=Acta Neuropathologica|volume=123|issue=3|year=2012|pages=295–319|issn=0001-6322|doi=10.1007/s00401-012-0954-z}}</ref><ref name="ChoiKomurov2017">{{cite journal|last1=Choi|first1=Kwangmin|last2=Komurov|first2=Kakajan|last3=Fletcher|first3=Jonathan S.|last4=Jousma|first4=Edwin|last5=Cancelas|first5=Jose A.|last6=Wu|first6=Jianqiang|last7=Ratner|first7=Nancy|title=An inflammatory gene signature distinguishes neurofibroma Schwann cells and macrophages from cells in the normal peripheral nervous system|journal=Scientific Reports|volume=7|issue=1|year=2017|issn=2045-2322|doi=10.1038/srep43315}}</ref><ref name="LiaoBooker2018">{{cite journal|last1=Liao|first1=Chung-Ping|last2=Booker|first2=Reid C.|last3=Brosseau|first3=Jean-Philippe|last4=Chen|first4=Zhiguo|last5=Mo|first5=Juan|last6=Tchegnon|first6=Edem|last7=Wang|first7=Yong|last8=Clapp|first8=D. Wade|last9=Le|first9=Lu Q.|title=Contributions of inflammation and tumor microenvironment to neurofibroma tumorigenesis|journal=Journal of Clinical Investigation|volume=128|issue=7|year=2018|pages=2848–2861|issn=0021-9738|doi=10.1172/JCI99424}}</ref><ref name="StaserYang2010">{{cite journal|last1=Staser|first1=K.|last2=Yang|first2=F.-C.|last3=Clapp|first3=D. W.|title=Mast cells and the neurofibroma microenvironment|journal=Blood|volume=116|issue=2|year=2010|pages=157–164|issn=0006-4971|doi=10.1182/blood-2009-09-242875}}</ref><ref name="MuirNeubauer2001">{{cite journal|last1=Muir|first1=David|last2=Neubauer|first2=Debbie|last3=Lim|first3=Ingrid T.|last4=Yachnis|first4=Anthony T.|last5=Wallace|first5=Margaret R.|title=Tumorigenic Properties of Neurofibromin-Deficient Neurofibroma Schwann Cells|journal=The American Journal of Pathology|volume=158|issue=2|year=2001|pages=501–513|issn=00029440|doi=10.1016/S0002-9440(10)63992-2}}</ref><ref name="WilkinsonManson2004">{{cite journal|last1=Wilkinson|first1=Lana M.|last2=Manson|first2=David|last3=Smith|first3=Charles R.|title=Best Cases from the AFIP|journal=RadioGraphics|volume=24|issue=suppl_1|year=2004|pages=S237–S242|issn=0271-5333|doi=10.1148/rg.24si035170}}</ref><ref name="BernthalJones2013">{{cite journal|last1=Bernthal|first1=Nicholas|last2=Jones|first2=Kevin|last3=Monument|first3=Michael|last4=Liu|first4=Ting|last5=Viskochil|first5=David|last6=Randall|first6=R.|title=Lost in Translation: Ambiguity in Nerve Sheath Tumor Nomenclature and Its Resultant Treatment Effect|journal=Cancers|volume=5|issue=4|year=2013|pages=519–528|issn=2072-6694|doi=10.3390/cancers5020519}}</ref><ref name="StaserYang2010">{{cite journal|last1=Staser|first1=K.|last2=Yang|first2=F.-C.|last3=Clapp|first3=D. W.|title=Mast cells and the neurofibroma microenvironment|journal=Blood|volume=116|issue=2|year=2010|pages=157–164|issn=0006-4971|doi=10.1182/blood-2009-09-242875}}</ref><ref name="MautnerFriedrich2003">{{cite journal|last1=Mautner|first1=V. F.|last2=Friedrich|first2=R. E.|last3=von Deimling|first3=A.|last4=Hagel|first4=C.|last5=Korf|first5=B.|last6=Knöfel|first6=M. T.|last7=Wenzel|first7=R.|last8=Fünsterer|first8=C.|title=Malignant peripheral nerve sheath tumours in neurofibromatosis type 1: MRI supports the diagnosis of malignant plexiform neurofibroma|journal=Neuroradiology|volume=45|issue=9|year=2003|pages=618–625|issn=0028-3940|doi=10.1007/s00234-003-0964-6}}</ref><ref name="ShenHarper1996">{{cite journal|last1=Shen|first1=M H|last2=Harper|first2=P S|last3=Upadhyaya|first3=M|title=Molecular genetics of neurofibromatosis type 1 (NF1).|journal=Journal of Medical Genetics|volume=33|issue=1|year=1996|pages=2–17|issn=1468-6244|doi=10.1136/jmg.33.1.2}}</ref><ref name="RubinGutmann2005">{{cite journal|last1=Rubin|first1=Joshua B.|last2=Gutmann|first2=David H.|title=Neurofibromatosis type 1 — a model for nervous system tumour formation?|journal=Nature Reviews Cancer|volume=5|issue=7|year=2005|pages=557–564|issn=1474-175X|doi=10.1038/nrc1653}}</ref><ref name="Gray1990">{{cite journal|last1=Gray|first1=Mark H.|title=Immunohistochemical Demonstration of Factor XIIIa Expression in Neurofibromas|journal=Archives of Dermatology|volume=126|issue=4|year=1990|pages=472|issn=0003-987X|doi=10.1001/archderm.1990.01670280056009}}</ref> | |||
| | |||
Can be sporadic or as a part of [[Neurofibromatosis 1]] and 2 | |||
* ''[[Neurofibromatosis type I|NF1]] [[gene]] located at [[chromosomal]] region [[CCL7|17q11.2]], [[Code|codes]] for''[[neurofibromin]] | |||
* Functional part of [[neurofibromin]] GAP (or [[GTPase-activating proteins|GTPase-activating protein]]) accelerates the [[Conversion (logic)|conversion]] of the active [[GTP-binding protein|GTP]]-bound [[RAS]] to its inactive GDP-[[Bound state|bound]] form | |||
* Loss of ''[[RAS]]'' [[control]]<nowiki/>leads to increased [[Activity (chemistry)|activity]] of other [[Signaling pathway|signaling pathways]]<nowiki/>including ''[[C-Raf|RAF]]'', ''[[Extracellular signal-regulated kinases|ERK1/2]]'', ''[[Phosphoinositide 3-kinase|PI3K]]'', ''[[PAK1|PAK]], [[MAPK]], [[SCF-complex|SCF]]/[[c-kit]]'' and ''[[Mammalian target of rapamycin|mTOR-S6 kinase]]'' | |||
| | |||
* Uniphasic, low to moderate cellularity | |||
* No peripheral perineural [[capsule]] | |||
* [[Random]] [[pattern]], only [[rare]] palisading | |||
* No well formed verocy bodies | |||
* Hypocellular with abundant [[mucinous]]/myxoid [[matrix]] without hypercellular [[Area|areas]] | |||
* Frequent [[mast cells]] | |||
* Contains [[neural]] [[fibroblasts]] and fibrillary or shredded carrot [[collagen]] | |||
* [[Random]] [[proliferation]] of [[Schwann cells]] and scattered admixed [[axons]] | |||
* No [[Nevi|nevoid cells]] | |||
* No [[epithelial]] component | |||
* [[Diffuse]] [[growth]] [[pattern]] | |||
* Scant [[cytoplasm]] | |||
* Wavy [[spindle cells]] with buckled [[nuclei]] | |||
* Pseudomeissnerian [[Body|bodies]] representing specific [[differentiation]] may be present | |||
* Lacks storiform [[pattern]] | |||
[[Neurofibroma]] with [[degenerative]] [[atypia]] ("ancient [[Change detection|change]]") has following [[microscopic]] [[Features (pattern recognition)|features]]: | |||
* [[Localized disease|Localized]] [[Cells (biology)|cells]] with large [[pleomorphic]] [[nuclei]], [[cytoplasmic]] [[nuclear]] [[inclusions]], smudgy [[chromatin]], and inconspicuous [[nuclei]] | |||
* Absent or very low [[Mitotic|mitotic activity]] | |||
* Low to moderate cellularity | |||
|Positive for: | |||
* [[S100A12|S100]] (weaker) | |||
* [[SOX10]] | |||
* [[Neurofilament]] (and Bielshowsky) | |||
* [[GFAP]] | |||
* [[CD34]] (stronger) | |||
* Factor XIIIa | |||
* [[Calretinin]] (focal) | |||
* MBP ([[myelin]]-[[Basic (chemistry)|basic]] [[protein]]) | |||
Negative for: | |||
* EMA (except in plexiform [[Neurofibroma|neurofibromas]]) | |||
| | |||
* [[Neurofibromatosis type I|Neurofibromatosis 1]] | |||
* [[Neurofibromatosis 2]](multiple [[Neurofibroma|neurofibromas]], [[meningiomas]] of the [[brain]]<nowiki/>or [[spinal cord]], and [[ependymomas]] of the [[spinal cord]]) | |||
| | |||
* Can occur anywhere | |||
* [[Diffuse]] [[Neurofibroma|neurofibromas]] commonly involve [[scalp]] | |||
| | |||
* | |||
* Soft [[Mass|masses]]/[[Bumps on skin|bumps on or under skin]] ([[internal]] or [[superficial]]) | |||
* [[Transient]] [[itching]] ([[mast cells]] release [[histamine]]) | |||
* [[Transient]] [[pain]] | |||
* [[Numbness]] and [[tingling]] in the affected [[area]] | |||
* Severe [[bleeding]] (sign of [[tumor]] [[growth]]) | |||
* [[Physical therapy|Physical]] disfiguration | |||
* [[Cognitive]] [[disability]] | |||
* [[Stinging in the eye|Stinging]] | |||
* [[Neurological]] [[Deficits in Attention, Motor control and Perception|deficits]] | |||
* [[Change detection|Changes]] in [[Movement disorder|movement]] ([[clumsiness]] in [[hands]], trouble [[walking]]) | |||
* [[Bowel]] [[incontinence]] | |||
* [[Scoliosis]] (an [[abnormal]] [[Curvature of spine|curvature of the spine]], if the [[tumor]] creates [[muscular]] [[imbalance]] or erodes [[bones]] of the [[spine]]) | |||
* Following [[symptoms]] may occur with [[genitourinary tract]] involvement (rarely): | |||
** [[Urinary tract infection]] (most common [[clinical]] manifestation) | |||
** [[Urinary retention]] | |||
** [[Urinary frequency]] | |||
** [[Urgency]] | |||
** [[Hematuria]] | |||
** Pelvic mass | |||
** [[Hydronephrosis)|Hydronephrosis]] | |||
** [[Urinary incontinence]] (decreased [[Urinary bladder|bladder]] capacity or [[compliance]]) | |||
** [[Appearance|Appears]] as a focal [[mass]] or [[diffuse]] [[Urinary bladder|bladder]] wall thickening in case of a [[plexiform neurofibroma]] | |||
| | |||
* [[Nerve]] often not identified, incorporates [[nerve]], [[axons]] often present in [[lesion]] | |||
* Seldom [[cystic]] | |||
* Frequently multiple | |||
* Widespread [[soft tissue]] [[Infiltration (medical)|infiltration]] | |||
* Tends to displace [[Adnexal and skin appendage neoplasms|adnexa]] | |||
* <2cm in [[diameter]] | |||
* [[Lack (manque)|Lacks]] [[Distinctive feature|distinct]] [[Lobule|lobulation]] | |||
* [[Lack (manque)|Lacks]] [[fat]] | |||
* Affects [[Individual growth|individuals]] between 20-40 [[Year|years]] of [[age]] | |||
* Men and women are [[Equalism|equally]] affected | |||
* [[Plexiform neurofibroma]] are thought to be [[Congenital disorder|congenital]] and occur earlier in [[life]] | |||
|- | |||
| style="background:#DCDCDC;" align="center" + |'''[[Schwannoma]]'''<ref>Schwannoma. Dr Tim Luijkx and Dr Sara Wein et al. http://radiopaedia.org/articles/schwannoma</ref><ref name="wiki">Vestibular Schwannoma. Wikipedia(2015) https://en.wikipedia.org/wiki/Vestibular_schwannoma Accessed on October 2 2015</ref><ref name="pmid2612565">{{cite journal |vauthors=Giordano J, Rogers LV |title=Peripherally administered serotonin 5-HT3 receptor antagonists reduce inflammatory pain in rats |journal=[[European Journal of Pharmacology]] |volume=170 |issue=1-2 |pages=83–6 |year=1989 |pmid=2612565 |doi= |url= |issn= |accessdate=2015-11-20}}</ref><ref name="pmid2588243">{{cite journal |vauthors=Kolvenbach H, Lauven PM, Schneider B, Kunath U |title=Repetitive intercostal nerve block via catheter for postoperative pain relief after thoracotomy |journal=[[The Thoracic and Cardiovascular Surgeon]] |volume=37 |issue=5 |pages=273–6 |year=1989 |pmid=2588243 |doi=10.1055/s-2007-1020331 |url=http://www.thieme-connect.com/DOI/DOI?10.1055/s-2007-1020331 |issn= |accessdate=2015-11-20}}</ref><ref name="pmid3735913">{{cite journal |vauthors=Opaleva-Stegantseva VA, Ivanov AG, Gavrilina IA, Khar'kov EI, Ratovskaia VI |title=[Incidence of sudden death cases in acute coronary insufficiency and acute myocardial infarction at the pre-hospital stage in Krasnoyarsk] |language=Russian |journal=[[Kardiologiia]] |volume=26 |issue=5 |pages=23–6 |year=1986 |pmid=3735913 |doi= |url= |issn= |accessdate=2015-11-20}}</ref> | |||
| | |||
* Loss of [[Function (biology)|function]] of the [[tumor suppressor gene]] '''[[Merlin (protein)|merlin]]''' (schwannomin) | |||
* Direct [[Genetics (journal)|genetic]] [[Change detection|change]] involving the ''[[NF2 gene|NF2]]'' [[gene]] on [[chromosome 22]] | |||
* Can occur spontaneously | |||
* [[Mutations]] and biallelic inactivation of ''[[SMARCB1]] ([[Spinal cord|spinal]] [[schwannomas]])'' | |||
| | |||
* [[Encapsulated organisms|Encapsulated]] | |||
* Aggregates of [[Spindle cells|spindled cells]] with indistinct [[cytoplasm]] and elongated [[nuclei]] with [[Blunt end|blunt]] pointed ends | |||
* Ancient changes may show [[nuclear]] [[pleomorphism]] and occasionally [[nuclear]] [[inclusions]] as well | |||
* Infrequent [[extracellular]] [[collagen]] | |||
* [[Biphasic]]: majority entirely, and compactly hypercellular '''Antoni A''' & myxoid hypocellular '''Antoni B''' [[Area|areas]] (may be absent in small [[tumors]]) | |||
* [[Nuclear]] palisading evident around fibrillary [[Process (anatomy)|process]] ('''Verocay bodies''') in [[cellular]] [[Area|areas]] | |||
* Large, irregularly [[Spaced out|spaced]] [[vessels]] prominent in Antoni B [[Area|areas]] | |||
* Narrow, elongated and wavy [[Cells (biology)|cells]] with tapered ends, [[Interspersed repeat|interspersed]] with [[Collagen|collagen fibers]] | |||
* [[Tumor cell|Tumor cells]] with ill defined [[cytoplasm]], [[dense]] [[chromatin]] | |||
* Often displays [[degenerative]] [[nuclear]] [[atypia]] (ancient [[Change detection|change]]) | |||
* [[Rare]] [[Mitotic|mitotic figures]] | |||
* [[Blood vessels]] may show gaping [[tortuous]] [[Luminal|lumina]] having thickened hyalinized walls; may have [[thrombi]] | |||
* Dilated [[vessels]] surrounded/invested by [[hemorrhage]] | |||
* Foamy [[macrophages]] | |||
* [[Lymphoid]] aggregates | |||
* Amianthoid [[Fiber|fibers]] or [[collagenous]] spherules: large [[nodular]] [[Mass|masses]] of [[collagen]] with [[Radiating fibers|radiating]] [[Edge detection|edges]] | |||
* No [[axons]] except where [[nerve]] is attached | |||
* [[Malignant]] [[transformation]] may have [[malignant]] [[Epithelioid cell|epithelioid cells]] and rarely shows [[Divergent evolution|divergent]] [[differentiation]] as [[angiosarcoma]]-like [[Area|areas]] | |||
|Positive for: | |||
* [[S-100]] | |||
* [[SOX10]] | |||
* [[CD56]] | |||
* Podoplanin | |||
* [[CD34]] (weak) | |||
* [[Neurofilament]] (and Bielshowsky) | |||
* Factor XIIIa (focal) | |||
* [[Calretinin]] | |||
* [[GFAP]] | |||
* EMA ([[capsule]]) highlights the perineural [[fibroblasts]] | |||
* [[Laminin]] | |||
* [[Type IV collagen]] | |||
* [[Vimentin]] | |||
* [[CD68]] | |||
Negative for: | |||
* [[Cytokeratin]] | |||
* [[Desmin]] | |||
* [[SMA]] | |||
| | |||
* [[Neurofibromatosis type II|NF-2]] associated | |||
* [[Schwannomatosis]] | |||
* [[Carney complex]] | |||
| | |||
* [[Upper limbs]] | |||
* [[Head]] and [[neck]] [[area]] ([[oral cavity]], [[Orbit (anatomy)|orbit]] and [[salivary glands]]) | |||
* Deeply seated [[tumors]] are mainly in: | |||
** [[Posterior mediastinum]] | |||
** [[Retroperitoneum]] | |||
* [[Posterior]] [[Spine|spinal]] roots | |||
* [[Bone]] | |||
* [[Gastrointestinal tract]] | |||
* [[Pancreas]] | |||
* [[Liver]] | |||
* [[Thyroid]] | |||
* [[Adrenal glands]] | |||
* [[Lymph nodes]] | |||
* [[Penis]] (rarely) | |||
* [[Vulva]] (rarely) | |||
| | |||
[[Symptoms]] of [[schwannoma]] depend on the [[Location parameter|location]] of the [[tumor]]: | |||
*Intracranial [[schwannoma]]: | |||
**[[Acoustic neuroma]] (most common): | |||
***[[Sensorineural]] [[hearing]] loss | |||
***[[Vertigo]] | |||
***[[Tinnitus]] | |||
***[[Facial weakness]] | |||
***[[Facial]] [[numbness]] and [[tingling]] | |||
***[[Headaches]] | |||
***[[Dizziness]] | |||
***[[Difficulty swallowing]] and [[hoarseness]] | |||
***[[Taste]] changes | |||
***[[Confusion]] | |||
**[[Trigeminal]] [[schwannoma]]: | |||
***[[Trigeminal nerve]] [[dysfunction]] | |||
**[[Facial nerve]] [[schwannoma]]: | |||
***[[Facial nerve]] [[dysfunction]] | |||
**[[Jugular foramen]] [[schwannoma]]: | |||
***[[Hearing loss]] | |||
***[[Tinnitus]] | |||
***[[Dysphagia]] | |||
***[[Ataxia]] | |||
***[[Hoarseness]] | |||
**[[Hypoglossal nerve|Hypoglossal]] [[schwannomas]]: | |||
***[[Hypoglossal nerve]] [[dysfunction]] | |||
*[[Spine|Spinal]] [[Schwannoma|schwannoma:]] | |||
**[[Back pain]] | |||
**[[Urinary incontinence]] | |||
**[[Urinary retention]] | |||
**[[Clumsiness]] | |||
**[[Weakness]] | |||
**[[Paresthesias]] | |||
*[[Intercostal nerve]] [[schwannoma]]: | |||
**Usually [[asymptomatic]] | |||
*[[Intramuscular]] [[schwannoma]]: | |||
**Painless [[mass]] | |||
| | |||
* [[Nerve]] often identifiable | |||
* [[Eccentric Lesion|Eccentric]] to [[nerve]], [[axons]] generally absent within [[lesion]] | |||
* Occasionally [[cystic]] | |||
* Can [[Causes|cause]] other [[neoplasms]] including: | |||
**[[Meningioma]] | |||
**[[Mesothelioma]] | |||
**[[Glioma|Glioma multiforme]] | |||
**[[Breast Cancer|Breast cancer]] | |||
**[[Colorectal Cancer|Colorectal cancer]] | |||
**[[Renal cell carcinoma|Kidney (clear cell type) carcinoma]] | |||
**[[Hepatocellular carcinoma]] | |||
**[[Prostatic cancer]] | |||
**[[Dermal]] [[cancer]] | |||
</ | * [[Affect|Affects]] individuals between 20-50 years of [[age]] | ||
* [[Men]] and women are equally [[Affect|affected]] | |||
|- | |||
| style="background:#DCDCDC;" align="center" + |'''Palisaded encapsulated [[neuroma]] (PEN) /[[solitary]] circumscribed [[neuroma]]'''<ref name="pmid17414438">{{cite journal| author=Misago N, Inoue T, Narisawa Y| title=Unusual benign myxoid nerve sheath lesion: myxoid palisaded encapsulated neuroma (PEN) or nerve sheath myxoma with PEN/PEN-like features? | journal=Am J Dermatopathol | year= 2007 | volume= 29 | issue= 2 | pages= 160-4 | pmid=17414438 | doi=10.1097/01.dad.0000256688.91974.09 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=17414438 }} </ref> | |||
| | |||
* Spontaneous [[development]] | |||
* [[RET gene|RET]] [[proto-oncogene]] [[genetic mutations]] ([[inherited]] PEN) | |||
| | |||
* [[Solitary]] [[dermal]] or [[subcutaneous]] [[tumor]] | |||
* [[Encapsulated organisms|Encapsulated]] by [[perineurium]] | |||
* Club-like [[extension]] in the [[subcutaneous tissue]] | |||
* Moderately [[cellular]] [[lesion]] with [[proliferation]] of [[schwann cells]] and [[axons]] | |||
* [[Nuclear]] palisading may be present | |||
* Rare [[mast cells]] | |||
* [[Silver staining|'''Silver''' stains]] show the [[axons]] traversing the [[Schwann cells]] | |||
|Positive for: | |||
* EMA | |||
* [[S100A1|S100]] ([[schwann cells]]) | |||
* [[Neurofilament]] ([[axons]]) | |||
* [[Collagen, type IV, alpha 1|Collagen type IV]] | |||
* EMA ([[perineurium]]) | |||
* [[Neuron-Specific Enolase (NSE)|Neuron-specific Enolase]] | |||
* [[CD57]] (Leu-7) | |||
* [[Myelin basic protein|Myelin basic proteins]] | |||
Negative for: | |||
* [[GFAP]] | |||
| | |||
* Positive [[family history]] of [[tumor]] occurrence | |||
* [[Multiple mucosal neuroma syndrome]] | |||
* [[Multiple endocrine neoplasia syndrome]] ([[MEN 2B]]) | |||
|90% [[lesions]] affect the [[face]] involving: | |||
* [[Eyelid]] | |||
* [[Nose]] | |||
* [[Oral mucosa]] | |||
Remaining 10% can occur anywhere in [[body]] involving: | |||
* [[Shoulder]] | |||
* [[Arm]] | |||
* [[Hand]] | |||
* [[Foot]] | |||
* [[Glans penis|Glans of penis]] | |||
| | |||
* Small, [[solitary]], raised, [[Domes|dome-shaped]], firm, flesh-colored painless [[nodule]] on [[skin]] | |||
* Cosmetic issues due to [[facial]] involvement | |||
* [[Scar]] after [[surgery]] | |||
| | |||
* [[Benign]] [[tumor]] of the [[nerve fibers]] | |||
* [[Affect|Affects]] middle [[Age|aged]] people (40-60 years) | |||
* No known [[familial]] [[Association (statistics)|association]] | |||
* [[Affect|Affects]] [[females]] more frequently than [[males]] | |||
|- | |||
| style="background:#DCDCDC;" align="center" + |'''[[Traumatic neuroma]]'''<ref name="pmid9745184">{{cite journal| author=Lee EJ, Calcaterra TC, Zuckerbraun L| title=Traumatic neuromas of the head and neck. | journal=Ear Nose Throat J | year= 1998 | volume= 77 | issue= 8 | pages= 670-4, 676 | pmid=9745184 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=9745184 }} </ref><ref name="pmid27179535">{{cite journal| author=Hanna SA, Catapano J, Borschel GH| title=Painful pediatric traumatic neuroma: surgical management and clinical outcomes. | journal=Childs Nerv Syst | year= 2016 | volume= 32 | issue= 7 | pages= 1191-4 | pmid=27179535 | doi=10.1007/s00381-016-3109-z | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=27179535 }} </ref><ref name="pmid18599222">{{cite journal| author=Foltán R, Klíma K, Spacková J, Sedý J| title=Mechanism of traumatic neuroma development. | journal=Med Hypotheses | year= 2008 | volume= 71 | issue= 4 | pages= 572-6 | pmid=18599222 | doi=10.1016/j.mehy.2008.05.010 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=18599222 }} </ref><ref name="pmid28915703">{{cite journal| author=Yao C, Zhou X, Zhao B, Sun C, Poonit K, Yan H| title=Treatments of traumatic neuropathic pain: a systematic review. | journal=Oncotarget | year= 2017 | volume= 8 | issue= 34 | pages= 57670-57679 | pmid=28915703 | doi=10.18632/oncotarget.16917 | pmc=5593675 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=28915703 }} </ref> | |||
| | |||
* Tangle of [[neural]] [[Fiber|fibers]] and [[connective tissue]] that [[Development|develops]] following a [[peripheral nerve]] [[injury]] | |||
* Interruption in [[Continuity correction|continuity]] of [[nerve]] [[Causality|causing]] [[wallerian degeneration]] (loss of [[axons]] in [[proximal]] stump and [[retraction]] of [[axons]] in [[distal]] [[Segment (linguistics)|segment]]), followed by exuberant [[regeneration]] of [[nerve]] and [[Formation matrix|formation]] of [[mass]] of [[Schwann cells]], [[axons]] and [[fibrous]] [[Cells (biology)|cells]] | |||
| | |||
* Numerous well formed small [[nerve]] twigs | |||
* Limited [[soft tissue]] [[Infiltration (medical)|infiltration]] | |||
* Contains [[axons]] in haphazardly arranged [[nerves]] within mature [[collagenous]] [[scar]] with entrapped [[smooth muscle]] | |||
|Positive for: | |||
* [[S100A1|S100]] | |||
| | |||
* History of [[trauma]] to a [[nerve]] (especially during a [[surgery]]) | |||
* [[Cone biopsy]] ([[rare]] [[Complications|complication]]) | |||
* 55% of [[hysterectomy]] [[patients]] have microneuromas, associated with [[childbirth]] | |||
|Most common [[oral]] [[Location parameter|locations]] are: | |||
* [[Tongue]] | |||
* Near [[mental foramen]] of [[mouth]] | |||
[[Rare|Rarely]] involves: | |||
* [[Head]] | |||
* [[Neck]] | |||
| | |||
* Firm, [[oval]], whitish, [[Slow|slowly]] [[Growth|growing]], [[palpable]] [[nodule]] on [[skin]] (no discoloration of [[skin]] on the top of [[nodule]]) | |||
* </=2cm in [[Size consistency|size]] | |||
* [[Trauma|Traumatic]] [[neuropathic]] [[pain]] with the presence of a [[Typical set|typical]] [[trigger point]] in the [[area]] of a [[neuroma]] (especially with the [[pressure]] application) [[Causality|causing]] the [[patient]] to feel burning, stabbing, [[raw]], gnawing or sickening [[sensations]] | |||
* [[Paresthesias|Paresthesia]] over the [[Injured reserve list|injured]] [[area]] | |||
* [[Dysesthesia]] ([[painful]] [[hypersensitivity]] to normal [[light]] [[tactile]] [[Stimulants|stimuli]]) | |||
* [[Function (biology)|Functional]] [[impairment]] | |||
* [[Psychological]] [[distress]] (severely decreasing the [[quality of life]]) | |||
|Also known as: | |||
* [[Amputation]] [[neuroma]] | |||
* [[Traumatic neuroma|Pseudoneuroma]] | |||
|- | |||
| style="background:#DCDCDC;" align="center" + |'''Neurotized [[melanocytic nevus]]'''<ref name="pmid1693815">{{cite journal| author=Gray MH, Smoller BR, McNutt NS, Hsu A| title=Neurofibromas and neurotized melanocytic nevi are immunohistochemically distinct neoplasms. | journal=Am J Dermatopathol | year= 1990 | volume= 12 | issue= 3 | pages= 234-41 | pmid=1693815 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=1693815 }} </ref><ref name="pmid22742554">{{cite journal| author=Chen Y, Klonowski PW, Lind AC, Lu D| title=Differentiating neurotized melanocytic nevi from neurofibromas using Melan-A (MART-1) immunohistochemical stain. | journal=Arch Pathol Lab Med | year= 2012 | volume= 136 | issue= 7 | pages= 810-5 | pmid=22742554 | doi=10.5858/arpa.2011-0335-OA | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=22742554 }} </ref><ref name="pmid25657396">{{cite journal| author=Singh N, Chandrashekar L, Kar R, Sylvia MT, Thappa DM| title=Neurotized congenital melanocytic nevus resembling a pigmented neurofibroma. | journal=Indian J Dermatol | year= 2015 | volume= 60 | issue= 1 | pages= 46-50 | pmid=25657396 | doi=10.4103/0019-5154.147789 | pmc=4318062 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=25657396 }} </ref><ref name="pmid1690969">{{cite journal| author=Gray MH, Smoller BR, McNutt NS, Hsu A| title=Immunohistochemical demonstration of factor XIIIa expression in neurofibromas. A practical means of differentiating these tumors from neurotized melanocytic nevi and schwannomas. | journal=Arch Dermatol | year= 1990 | volume= 126 | issue= 4 | pages= 472-6 | pmid=1690969 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=1690969 }} </ref> | |||
| | |||
* [[Melanin|Melan]]-A (Mart-1) [[gene]] | |||
* [[Defect]] in [[embryologic]] [[development]] [[Causes|causing]] fast [[proliferation]] [[rate]] of [[melanocytes]] (during first twelve weeks of [[pregnancy]]) | |||
| | |||
* Neurotized [[Nevus]] is a type of [[mole]] in which [[melanocytes]] are in the [[dermis]] with accompanying [[fibrosis]] | |||
* [[Biphasic]] consisting of [[malignant melanoma]] and mature appearing [[neural]] component | |||
* [[Superficial]] classic [[Nevoid melanoma|nevoid]] [[melanocytes]] (i.e. [[melanocytes]] appear like [[spindle cells]] resembling a [[nerve]]; and hence, called a neurotized [[nevus]]) | |||
* [[Congenital]] and nested [[growth]] [[Pattern|patterns]] | |||
* More abundant [[cytoplasm]] | |||
* Tends to surround [[adnexa]] | |||
* Scattered nests of type A or B [[nevus]] [[Cells (biology)|cells]], surrounded by [[basement membrane]], present in the [[papillary]] [[dermis]] of [[lesions]] (otherwise indistinguishable from [[Neurofibroma|neurofibromas]]) | |||
|Positive for: | |||
* [[S100A1|S-100]] | |||
* MelanA (MART-1) | |||
Negative for: | |||
* Factor XIIIa | |||
* Leu-7 | |||
* [[Glial fibrillary acidic protein|GFAP]] | |||
* [[Myelin basic protein|MBP]] | |||
| | |||
* [[Sun exposure]] ([[Ultraviolet light|ultraviolet light)]] | |||
* [[Hormonal]] [[Change detection|changes]] during: | |||
** [[Pregnancy]] | |||
** [[Diabetes]] | |||
== | * Fair-[[Skin|skinned]] individuals (Caucasians of America and Europe) | ||
=== | * Positive [[family history]] of [[mole]] | ||
==== | |Can occur anywhere in [[body]], mostly involving following [[Area|areas]]: | ||
===== | * [[Head]] | ||
< | * [[Neck]] | ||
| | |||
* Slowly growing, [[benign]], [[oval]] or round, well-circumscribed [[macule]], [[papule]] or [[Nodule (medicine)|nodule]] | |||
* [[Color]] varies from [[skin]] [[color]] to [[light]] [[brown]] to [[black]] | |||
* Cosmetic concerns | |||
|_ | |||
|- | |||
| style="background:#DCDCDC;" align="center" + |'''[[Cutaneous]] [[myxoma]] ([[Superficial]] angiomyxoma)'''<ref>https://www.sciencedirect.com/topics/medicine-and-dentistry/cutaneous-myxoma</ref><ref name="AlaitiNelson2000">{{cite journal|last1=Alaiti|first1=Samer|last2=Nelson|first2=Fern P.|last3=Ryoo|first3=Jei W.|title=Solitary cutaneous myxoma|journal=Journal of the American Academy of Dermatology|volume=43|issue=2|year=2000|pages=377–379|issn=01909622|doi=10.1067/mjd.2000.101878}}</ref><ref name="Carney1986">{{cite journal|last1=Carney|first1=J. Aidan|title=Cutaneous Myxomas|journal=Archives of Dermatology|volume=122|issue=7|year=1986|pages=790|issn=0003-987X|doi=10.1001/archderm.1986.01660190068018}}</ref><ref name="IidaEgi2019">{{cite journal|last1=Iida|first1=Ken|last2=Egi|first2=Takeshi|last3=Shigi|first3=Masato|last4=Sogabe|first4=Yusuke|last5=Ohashi|first5=Hirotsugu|title=Cutaneous Myxoma of Multiple Lesions|journal=Plastic and Reconstructive Surgery - Global Open|volume=7|issue=2|year=2019|pages=e2040|issn=2169-7574|doi=10.1097/GOX.0000000000002040}}</ref> | |||
| | |||
* [[Sporadic Epithelial ovarian tumors|Sporadic]] | |||
* Associated with: | |||
**[[Carney's syndrome]] ([[autosomal dominant]] [[condition]] associated with [[abnormalities]] in [[chromosomes]] 2p and 17q, especially [[mutation]] in the ''[[PRKAR1A|PRKAR1α]]'' [[gene]] on the [[chromosome]] 17q22–q24 [[locus]]) | |||
**NAME [[syndrome]] | |||
**LAMB [[syndrome]] | |||
| | |||
* Predominantly involves [[dermis]] and [[subcutis]] | |||
* Multilobulated, poorly circumscribed | |||
* [[Alcian blue]] positive, and [[hyaluronidase]] sensitive myxoid [[stroma]]/[[acellular]] [[mucin]] pools forming [[cleft]]-like spaces | |||
* Scattered bland [[Stellate cell|stellate]] to [[Spindle cells|spindled cells]] with multiple [[oval]] [[nuclei]] | |||
* [[Rare|Rarely]], [[pleomorphism]], and [[mitotic]] figures seen | |||
* Occasional intranuclear pseudoinclusions | |||
* Many thin-walled small [[blood vessels]] | |||
* Frequent [[neutrophils]] | |||
* Entrapped [[epithelial]] component in 20-30% of cases: | |||
**[[Keratinous]] [[cyst]] | |||
**Thin strands of [[squamous epithelium]] | |||
**Basaloid buds | |||
|Positive for: | |||
* [[CD34]] | |||
* [[Smooth muscle]] [[actin]] (90%) | |||
* [[Muscle]] specific [[actin]] (67%) | |||
* Factor XIIIa (60%) | |||
* [[Vimentin]] | |||
* [[S100|S-100]] (rarely, 40%) | |||
* Factor VIIIa ([[variable]]) | |||
Negative for: | |||
* [[Cytokeratin]] | |||
* [[Desmin]] | |||
* [[Glial fibrillary acidic protein|GFAP]] | |||
* [[ER]] | |||
* [[PR]] | |||
|Associated with '''[[Carney complex|Carney's complex/syndrome]]''' which includes following: | |||
*'''[[Myxomas]]:''' | |||
**[[Cutaneous]] | |||
**[[External ear]] | |||
**[[Heart]] | |||
**[[Breast]] myxoid [[fibroadenoma]] | |||
*'''[[Cutaneous]] [[Melanocytic nevus|melanocytic]] [[lesions]]:''' | |||
**Lentigines | |||
**[[Blue nevus]] | |||
*'''[[Endocrine]] [[hyperplasia]] and [[neoplasia]]''': | |||
**[[Pituitary]] | |||
**[[Thyroid]] | |||
**[[Adrenal cortex]] | |||
**[[Testis]] [[large cell]] calcifying [[Sertoli cell]] [[tumor]] | |||
*'''Psammomatous [[Melanotic cancer|melanotic]] [[schwannoma]]''' | |||
May be associated with '''NAME''' or '''LAMB [[syndrome]]''' | |||
| | |||
* [[Trunk]] | |||
* [[Limbs]] | |||
* [[Head]]/[[face]] ([[eyelids]] and [[external ear]] [[Canal (anatomy)|canal]] in [[Carney's syndrome]]) | |||
* [[Neck]] | |||
* [[Perineal]] | |||
* [[Nipples]] | |||
* [[Buttocks]] | |||
| | |||
* [[Solitary]] or multiple flesh-[[Color|colored]] [[nodules]] | |||
* 1-5cm in [[diameter]] | |||
| | |||
* Sometimes, may be the earliest manifestation of [[Carney complex]] | |||
* [[Affect|Affects]] [[men]] more frequently than women | |||
* Involves mostly middle-[[Age|aged]] [[population]] | |||
|- | |||
| style="background:#DCDCDC;" align="center" + |'''[[Nerve sheath]] [[myxoma]]'''<ref name="pmid16327434">{{cite journal| author=Fetsch JF, Laskin WB, Miettinen M| title=Nerve sheath myxoma: a clinicopathologic and immunohistochemical analysis of 57 morphologically distinctive, S-100 protein- and GFAP-positive, myxoid peripheral nerve sheath tumors with a predilection for the extremities and a high local recurrence rate. | journal=Am J Surg Pathol | year= 2005 | volume= 29 | issue= 12 | pages= 1615-24 | pmid=16327434 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=16327434 }} </ref><ref name="pmid30820132">{{cite journal| author=Yadav SK, Singh S, Sarin N, Naeem R, Pruthi SK| title=Nerve Sheath Myxoma of Scalp: A Rare Site of Presentation. | journal=Int J Trichology | year= 2019 | volume= 11 | issue= 1 | pages= 34-37 | pmid=30820132 | doi=10.4103/ijt.ijt_45_18 | pmc=6385516 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=30820132 }} </ref><ref name="pmid26023558">{{cite journal| author=Bhat A, Narasimha A, C V, Vk S| title=Nerve sheath myxoma: report of a rare case. | journal=J Clin Diagn Res | year= 2015 | volume= 9 | issue= 4 | pages= ED07-9 | pmid=26023558 | doi=10.7860/JCDR/2015/10911.5810 | pmc=4437072 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=26023558 }} </ref><ref name="pmid17498433">{{cite journal| author=Avninder S, Ramesh V, Vermani S| title=Benign nerve sheath myxoma (myxoid neurothekeoma) in the leg. | journal=Dermatol Online J | year= 2007 | volume= 13 | issue= 2 | pages= 14 | pmid=17498433 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=17498433 }} </ref><ref name="pmid24470676">{{cite journal| author=Kim BW, Won CH, Chang SE, Lee MW| title=A case of nerve sheath myxoma on finger. | journal=Indian J Dermatol | year= 2014 | volume= 59 | issue= 1 | pages= 99-101 | pmid=24470676 | doi=10.4103/0019-5154.123526 | pmc=3884944 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=24470676 }} </ref><ref name="pmid4091218">{{cite journal| author=Pulitzer DR, Reed RJ| title=Nerve-sheath myxoma (perineurial myxoma). | journal=Am J Dermatopathol | year= 1985 | volume= 7 | issue= 5 | pages= 409-21 | pmid=4091218 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=4091218 }} </ref> | |||
| | |||
* Unknown [[etiology]] | |||
| | |||
* [[Tumors]] involve the [[dermis]] and/or [[subcutis]] | |||
* [[Distinctive feature|Distinct]] multinodular/multilobular [[Mass|masses]] | |||
* Markedly hypocellular with abundant myxoid [[stroma]] | |||
* Peripheral [[fibrous]] [[Borderline|border]] made up of [[Collagen, type IV, alpha 1|collagen IV]] | |||
* [[Schwann cells]] may show the following different [[Features (pattern recognition)|features]]: | |||
** [[Cytoplasmic]]-[[nuclear]] [[Invagination|invaginations]] | |||
** Small [[epithelioid]] [[Schwann cells]] in corded, nested, and/or syncytial-like aggregates | |||
** [[Schwann cells]] with a ring-like [[appearance]] | |||
** [[Scattering|Scattered]] [[Spindle cells|spindled]] and [[Stellate cell|stellate]]-shaped [[Schwann cells]] | |||
|Positive for: | |||
* [[S100A1|S-100]] | |||
* [[Glial fibrillary acidic protein]] | |||
* [[Neuron-Specific Enolase (NSE)|Neuron specific enolase]] | |||
* [[CD57]] | |||
* [[Epithelial]] [[membrane]] [[antigen]] | |||
* [[CD34]] | |||
|_ | |||
|Can occur anywhere in [[body]]: | |||
*Most commonly involves [[extremities]] especially: | |||
**[[Fingers]] | |||
**[[Knees]] | |||
*[[Rare|Rarely]] involves: | |||
**[[Scalp]]/[[head]] | |||
**[[Trunk]] | |||
| | |||
* Painless [[skin]] [[mass]] or [[Nodule (medicine)|nodule]] | |||
* Occasionally [[painful]] to [[touch]] | |||
* [[Skin]] over the [[nodule]] is pink, [[Soft tissue|soft]], and usually intact (no [[ulceration]]) | |||
* 0.5-2 cm in size | |||
* Cosmetic issue (when present in [[head]] and [[neck]] region) | |||
| | |||
* First described by Harkin and Reed in 1969 | |||
* Peak [[incidence]] in the fourth decade of [[life]] | |||
* [[Strong]] predilection for the [[extremities]] | |||
* Also known as: | |||
** Classical [[Nerve]] Sheath [[Myxoma]] | |||
** [[Cutaneous]] [[Lobular]] Neuro [[Myxoma]] | |||
** [[Myxomatous]] Perineuroma | |||
|- | |||
| style="background:#DCDCDC;" align="center" + |'''[[Malignant peripheral nerve sheath tumor]] ([[MPNST]])/[[malignant]] [[schwannoma]]'''<ref name="Valeyrie-AllanoreIsmaili2005">{{cite journal|last1=Valeyrie-Allanore|first1=L.|last2=Ismaili|first2=N.|last3=Bastuji-Garin|first3=S.|last4=Zeller|first4=J.|last5=Wechsler|first5=J.|last6=Revuz|first6=J.|last7=Wolkenstein|first7=P.|title=Symptoms associated with malignancy of peripheral nerve sheath tumours: a retrospective study of 69 patients with neurofibromatosis 1|journal=British Journal of Dermatology|volume=153|issue=1|year=2005|pages=79–82|issn=0007-0963|doi=10.1111/j.1365-2133.2005.06558.x}}</ref><ref name="pmid12011145">{{cite journal| author=Evans DG, Baser ME, McGaughran J, Sharif S, Howard E, Moran A| title=Malignant peripheral nerve sheath tumours in neurofibromatosis 1. | journal=J Med Genet | year= 2002 | volume= 39 | issue= 5 | pages= 311-4 | pmid=12011145 | doi= | pmc=1735122 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=12011145 }} </ref><ref name="pmid24174807">{{cite journal| author=Panigrahi S, Mishra SS, Das S, Dhir MK| title=Primary malignant peripheral nerve sheath tumor at unusual location. | journal=J Neurosci Rural Pract | year= 2013 | volume= 4 | issue= Suppl 1 | pages= S83-6 | pmid=24174807 | doi=10.4103/0976-3147.116480 | pmc=3808069 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=24174807 }} </ref><ref name="pmid17705563">{{cite journal| author=Ferrari A, Bisogno G, Carli M| title=Management of childhood malignant peripheral nerve sheath tumor. | journal=Paediatr Drugs | year= 2007 | volume= 9 | issue= 4 | pages= 239-48 | pmid=17705563 | doi=10.2165/00148581-200709040-00005 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=17705563 }} </ref><ref name="pmid12632346">{{cite journal| author=Neville H, Corpron C, Blakely ML, Andrassy R| title=Pediatric neurofibrosarcoma. | journal=J Pediatr Surg | year= 2003 | volume= 38 | issue= 3 | pages= 343-6; discussion 343-6 | pmid=12632346 | doi=10.1053/jpsu.2003.50105 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=12632346 }} </ref><ref name="ZehouFabre2013">{{cite journal|last1=Zehou|first1=Ouidad|last2=Fabre|first2=Elizabeth|last3=Zelek|first3=Laurent|last4=Sbidian|first4=Emilie|last5=Ortonne|first5=Nicolas|last6=Banu|first6=Eugeniu|last7=Wolkenstein|first7=Pierre|last8=Valeyrie-Allanore|first8=Laurence|title=Chemotherapy for the treatment of malignant peripheral nerve sheath tumors in neurofibromatosis 1: a 10-year institutional review|journal=Orphanet Journal of Rare Diseases|volume=8|issue=1|year=2013|pages=127|issn=1750-1172|doi=10.1186/1750-1172-8-127}}</ref> | |||
| | |||
* 50% arise denovo | |||
* 50% associated with [[neurofibromatosis]] ([[loss of heterozygosity]] of [[p53]] on 17p [[chromosome]]) | |||
| | |||
* Generalized [[atypia]] | |||
* Increased [[mitotic]] [[Activity (chemistry)|activity]] | |||
* [[Diffuse]] hypercellularity | |||
* [[Infiltration (medical)|Infiltrative]] [[growth]] | |||
* [[Pleomorphic]] [[nuclei]] | |||
* [[Area|Areas]] of geographic [[necrosis]] may show [[Divergent synthesis|divergent]] [[differentiation]], with [[tumor]] palisading at edges, resembling [[glioblastoma multiforme]] | |||
* Monomorphic [[Serpentine receptor|serpentine]] [[Cells (biology)|cells]], large [[Gap phenomenon|gaping]] [[vascular]] spaces, [[Perivascular cell|perivascular]] plump [[Tumor cell|tumor cells]] | |||
* May have bizarre [[Cells (biology)|cells]] | |||
* 15% have [[Metaplasticity|metaplastic]] [[cartilage]], [[bone]], and [[muscle]] | |||
* May have [[glandular]] [[differentiation]], if so, presume [[malignant]] | |||
* May have [[melanin]] in [[Tumor cell|tumor cells]], particularly if arise from [[Roots of spinal nerves|spinal nerve roots]] (overlaps with primary [[melanoma]] of [[Nerves|nerves)]] | |||
* Some have no discernable [[Schwann cell|Schwannian]] features at any level | |||
[[Electron microscopy]] shows: | |||
* [[Cell membrane]] infoldings with [[Lamellar bodies|lamellar]] [[Configuration interaction|configuration]], discontinuous [[basal lamina]], conspicuous intercellular junctions, and occasional [[dense]]-[[Core (anatomy)|core]] [[granules]] | |||
|Positive for: | |||
* [[CD99]]/O13 (86%) | |||
* [[S100A1|S-100]] (patchy in 62% cases) | |||
* [[CD57]] (55%) | |||
* [[Collagen, type IV, alpha 1|Collagen IV]] | |||
* [[p53]] | |||
* Leu7/[[CD57]] (in [[neurofibroma]]-like [[Area|areas]]) | |||
* [[Protein]] [[gene product]] 9.5 (more sensitive than [[S100A1|S100]] but not specific) | |||
In case of [[glandular]] [[differentiation]] ([[malignant]]), positive for: | |||
* [[Keratin]] | |||
* EMA | |||
* [[CEA]] | |||
* [[Chromogranin]] | |||
Negative for: | |||
* [[CD19]] | |||
|Associated with: | |||
* [[NF1]] | |||
May be associated with: | |||
* [[Radiation|Radiations]] | |||
* [[Ganglioneuroma]] ([[Rare|rarely]]) | |||
|Bulky deep-seated [[tumor]] usually arising from major [[nerves]] in: | |||
* [[Neck]] | |||
* [[Forearm]] | |||
* [[Lower leg]] | |||
* [[Buttock]] | |||
| | |||
* Painless [[swelling]] in [[extremities]] (arms or [[legs]], aka [[peripheral edema]]) | |||
* Difficulty [[moving]] the extremity with [[tumor]] ([[Limp|limping]]) | |||
* Localized [[Sore|soreness]] in [[tumor]] [[area]] | |||
* [[Neurological]] [[symptoms]] | |||
* [[Pain]] or [[discomfort]]: [[numbness]], burning, or [[Tingling|tingling (pins and needles)]] | |||
* [[Dizziness]] | |||
* [[Loss of balance]] | |||
| | |||
* Most common frequent [[soft tissue sarcoma]] in the [[pediatrics]] [[population]] | |||
|- | |||
| style="background:#DCDCDC;" align="center" + |'''[[Dermatofibrosarcoma protuberans]] ([[DFSP]])''' | |||
| | |||
* t(17,22)(q21;q13) ([[Collagen, type I, alpha 1|collagen type 1 alpha 1(COL1A1)]] [[gene]] and [[Platelet-derived growth factor|platelet derived growth factor (PDGF)]] [[Beta-1|beta]] [[Chain (sequence)|chain]] [[gene]]), resulting [[fusion protein]] is [[Process (anatomy)|processed]] into mature [[platelet-derived growth factor]] which is a [[Potential|potent]] [[growth factor]] | |||
* Supernumerary ring [[chromosomes]] derived from t(17;22) | |||
| | |||
* Usually forms a [[mass]] | |||
* Non circumscribed, highly [[cellular]], tight storiform [[pattern]] ([[Cells (biology)|cells]] [[Radiating fibers|radiating]] in spokes at right angles around a central point that often contains a [[vessel]]) deeply [[Infiltration (medical)|infiltrating]] into [[subcutaneous tissue]] and entraping [[fat cells]] leading to characteristic honeycomb [[pattern]] | |||
* [[Area|Areas]] of [[Fascicle|fascicular]] [[growth]] (some [[tumors]]) | |||
* [[Distinctive feature|Distinct]] storiform [[pattern]] may be absent in early [[plaque]] stage | |||
* Monomorphic, thin and [[Spindle cells|spindly cells]] with scant [[eosinophilic]] [[cytoplasm]] and hyperchromatic [[nuclei]] (resembling [[neurofibroma]]) | |||
* Numerous [[mitotic]] figures (not atypical ones) | |||
* Non-polarizable and thin [[collagen]] | |||
* Only mild [[pleomorphism]] and focal [[atypia]] | |||
* May coexist with [[giant cell]] fibroblastoma | |||
* Usually no [[Significant figure|significant]] [[pleomorphism]], no / [[rare]] [[histiocytes]], no [[histiocyte]]-like [[Cells (biology)|cells]], no [[foam cells]], no [[giant cells]] or other [[inflammatory cells]] | |||
* '''Variants:''' [[Atrophic]] (depressed [[lesion]]), [[collagenous]] (with [[central]] thick [[collagen]] bundles), [[granular cell]], myxoid, palisading, [[Pigmented Lesions|pigmented]], and sclerosing | |||
|Positive for: | |||
* [[CD34]] ([[strong]] in 95%) | |||
* [[Vimentin]] | |||
* [[Actin]] (focal) | |||
* ApoD | |||
* [[Bcl-2|Bcl2]] | |||
* NKI-C3 | |||
* [[CD99]] | |||
Negative for: | |||
* [[S-100]] | |||
* Factor XIIIa (usually) | |||
* [[Keratin]] | |||
* EMA | |||
* [[S100A1|S100]] | |||
* HMB45 | |||
* [[Desmin]] | |||
* [[CD117]] | |||
|_ | |||
| | |||
* [[Head]] | |||
* Deep [[soft tissue]] of ([[posterior]]) [[neck]] | |||
* [[Trunk]] | |||
* [[Arm|Arms]] | |||
* [[Legs]] | |||
* Doesn't involve [[hands]] and [[feet]] | |||
| | |||
* Begins as a minor firm [[area]] of [[skin]] | |||
* 1 to 5 cm in [[diameter]] | |||
* Resembles a [[bruise]], [[birthmark]], or [[pimple]] | |||
* Can become a raised [[Nodule (medicine)|nodule]] after [[growth]] | |||
* May cause [[redness]], open up or [[bleed]] | |||
| | |||
* Also called intermediate ([[borderline]]) [[fibrous]] [[histiocytoma]] | |||
* More common in blacks in [[United States|US]] | |||
* Involves [[Adult|adults]] of 20 - 40 years of [[age]] | |||
|- | |||
| style="background:#DCDCDC;" align="center" + |'''[[Spindle cell]] [[lipoma]]''' | |||
| | |||
* 16q [[abnormalities]] (usually) | |||
| | |||
* Delicate [[Encapsulation (pharmacology)|encapsulation]] | |||
* Floret [[cell]] [[Formation matrix|formation]] | |||
* No [[degenerative]] [[atypia]] | |||
* [[Mixture]] of mature [[adipocytes]] and mildly [[pleomorphic]] bland [[spindle cells]] ([[Paleness|pale]] [[eosinophilic]] [[cytoplasm]] with uniform wavy [[nuclei]] similar to [[neurofibroma]]) in [[mucinous]] / myxoid or [[fibrous]] [[background]] with thick [[collagen]] bundles | |||
* [[Spindle cells]] arranged in short [[fascicles]] with occasional [[nuclear]] palisading | |||
* Hemangiopericytic or angiomatous [[vascular]] [[pattern]] may be seen | |||
* Minimal or no [[fat]] | |||
* Variable [[mast cells]] and [[lymphocytes]] | |||
* No storiform [[pattern]], no lipoblasts, no/[[rare]] [[mitotic]] [[Activity (chemistry)|activity]] | |||
|Positive for: | |||
* [[CD34]] ([[Strong|strongly]], [[spindle cells]]) | |||
* [[Androgen receptors]] in [[men]] and usually women ([[spindle cells]]) | |||
* [[S-100]]([[Stain|stains]] only [[adipocytes]]) | |||
[[Spindle cells]] are negative for: | |||
* [[S100A1|S100]] | |||
* [[Desmin]] | |||
|_ | |||
| | |||
* [[Neck]] | |||
* [[Posterior]] upper [[back]] | |||
* [[Shoulder]] | |||
| | |||
* Multiple well-circumscribed painless [[nodules]] involving several [[body]] parts | |||
|_ | |||
|- | |||
| style="background:#DCDCDC;" align="center" + |'''[[Ganglioneuroma]]'''<ref name="VasiliadisPapavasiliou2012">{{cite journal|last1=Vasiliadis|first1=K.|last2=Papavasiliou|first2=C.|last3=Fachiridis|first3=D.|last4=Pervana|first4=S.|last5=Michaelides|first5=M.|last6=Kiranou|first6=M.|last7=Makridis|first7=C.|title=Retroperitoneal extra-adrenal ganglioneuroma involving the infrahepatic inferior vena cava, celiac axis and superior mesenteric artery: A case report|journal=International Journal of Surgery Case Reports|volume=3|issue=11|year=2012|pages=541–543|issn=22102612|doi=10.1016/j.ijscr.2012.07.008}}</ref><ref>https://radiopaedia.org/articles/ganglioneuroma</ref> | |||
|[[Genes]] involved in the [[pathogenesis]] of [[ganglioneuroma]] include: | |||
* ''MYCN'' [[oncogene]] | |||
* [[Chromosome]] 1p36 | |||
*[[Activating group|Activating]] [[RET proto-oncogene|RET protooncogene]] [[mutation]] ([[adrenal]] [[Ganglioneuroma|ganglioneuromas]]) | |||
| | |||
* Derived from the [[Primordial elements|primordial]] [[neural crest cells]] ([[undifferentiated]] [[Cells (biology)|cells]] of the [[sympathetic nervous system]]) | |||
* Admixture of [[ganglion cells]], [[schwann cells]], and [[fibrous tissue]] | |||
* Doesn't contain [[neuroblasts]], intermediate [[Cells (biology)|cells]], or [[Mitotic|mitotic figures]] | |||
* Characterized by [[spindle]]-shaped [[Cells (biology)|cells]] with [[cell]] borders in a [[Fibrillarin|fibrillar]] [[matrix]] containing [[ganglion cells]] with large round [[nuclei]], prominent [[nucleoli]], and abundant [[eosinophilic]] [[cytoplasm]] | |||
* No [[Significant figure|significant]] [[atypia]], [[necrosis]] or [[mitotic]] [[Activity (chemistry)|activity]] is present | |||
* Well [[Differentiate|differentiated]] [[neuronal]] [[tumors]] that do not contain immature [[Element|elements]] | |||
*[[Ganglion cells]] are mature to mildly [[dysmorphic]]: | |||
**Mature: [[Compact tissue|compact]], [[eosinophilic]] [[cytoplasm]] with [[Distinctive feature|distinct]] [[Cell (biology)|cell]] borders, single [[Eccentricity (mathematics)|eccentric]] [[nucleus]], prominent [[nucleolus]] | |||
**[[Dysmorphic]]: single or multiple [[Pyknosis|pyknotic]] [[nuclei]] | |||
**Vary in [[Distribution (pharmacology)|distribution]] and [[number]], may be quite [[Sparse coding|sparse]] | |||
**May contain finely [[Granular cell|granular]], [[gold]] to [[brown]] [[pigment]] ([[lipofuscin]] or [[neuromelanin]]) | |||
*[[Schwann cells]]: | |||
**Ensheath neuritic [[Process (anatomy)|processes]] | |||
**Arranged in small intersecting [[fascicles]], separated by loose myxoid [[stroma]] | |||
Two [[histologic]] subtypes: | |||
*Mature = every [[ganglion cell]] is mature | |||
*Maturing = minor component of scattered collections of [[Differentiating (disease name) from other diseases page|differentiating]] [[neuroblasts]] or maturing [[ganglion cells]] (unlike intermixed subtype of ganglioneuroblastoma, these immature foci do not form [[Distinctive feature|distinct]] [[microscopic]] nests) | |||
*[[Background]] may include [[lobules]] of mature [[adipose tissue]] (especially at [[periphery]] of [[lesion]]), [[mast cells]], [[Chronic (medicine)|chronic]] [[inflammation]], [[dense]] [[Collagen|collagenized]] [[stroma]] | |||
*Mild variation in [[Cellular|cellularity]] may be present | |||
*[[Masculinization|Masculinizing]] [[ganglioneuroma]] is an admixture of [[ganglioneuroma]] and [[Leydig cells]] with crystalloids of [[Reinke's edema|Reinke]] or strands/[[Cluster (epidemiology)|clusters]] of [[Cells (biology)|cells]] resembling [[Adrenal cortex|adrenal cortical]] [[Cells (biology)|cells]] | |||
*[[Electron microscopy]]: | |||
**[[Mixture]] of [[neural]] bundles and normal [[Appearance|appearing]] [[ganglion cells]] with [[Eccentricity (mathematics)|eccentric]] [[nuclei]] and large [[Number|numbers]] of [[cytoplasmic]] [[organelles]] | |||
|Positive for: | |||
*[[Schwann cells]]/[[stroma]]: | |||
**[[S100A1|S100]] | |||
**[[Synaptophysin]] | |||
**[[Neurofilament protein|Neurofilament (NF) protein]] | |||
*[[Ganglion cells]]: | |||
**[[S100A1|S100]] | |||
**[[Synaptophysin]] | |||
**[[Chromogranin A]] | |||
**[[Neurofilament protein|NF protein]] | |||
**[[Glial fibrillary acidic protein]] ([[GFAP]]) | |||
**PGP 9.5 | |||
**[[Type IV collagen]] | |||
**[[Vasoactive intestinal peptide]] ([[VIP]]) | |||
Negative for: | |||
*EMA | |||
*[[Cytokeratin]] | |||
*HMB45 | |||
*[[WT1]] | |||
*[[CD99]] | |||
*[[CD45]] | |||
*[[Desmin]] | |||
*[[Myogenic]] [[Marker|markers]] ([[myogenin]], MyoD1) | |||
| | |||
[[Ganglioneuroma|Ganglioneuromas]] may be associated with: | |||
* [[Multiple endocrine neoplasia]] type IIb ([[mucosal]] [[Ganglioneuroma|ganglioneuromas]]) | |||
* [[Turner syndrome]] | |||
* [[Neurofibromatosis type 1]] | |||
| | |||
Located along [[Distribution (pharmacology)|distribution]] of [[sympathetic nervous system]]: | |||
* [[Posterior]] paraspinal [[mediastinum]] (most common) | |||
* [[Adrenal gland]] (~20-30% of cases) | |||
* Paraspinal [[retroperitoneum]] (especially [[presacral space]]) | |||
*[[Cervical]] and [[Parapharyngeal space infection|parapharyngeal area]] in [[neck]] | |||
*[[Urinary bladder]] | |||
*[[Prostate]] | |||
*[[Bone]] | |||
*[[Pancreas]] | |||
*[[Skin]] | |||
*[[Orbit (anatomy)|Orbit]] | |||
*Paratesticular [[area]] | |||
*[[Appendix]] | |||
*[[Gastrointestinal tract]] | |||
|[[Symptoms]] of [[ganglioneuroma]] vary depending on the [[Location parameter|location]] of [[tumor]], and include the following: | |||
* [[Mediastinum]]: | |||
** [[Dyspnea]] | |||
** [[Chest pain]] | |||
**[[Trachea]] compression | |||
* [[Retroperitoneum]]: | |||
** [[Abdominal pain]] | |||
** [[Bloating]] | |||
* [[Spinal cord]]: | |||
** [[Paresis]] | |||
** [[Pain]] and [[numbness]]/[[loss of sensation]] in [[limbs]] | |||
[[Patients]] with [[ganglioneuroma]] may also have [[paraneoplastic syndrome]], which may manifest with: | |||
* [[Diarrhea]] | |||
* [[Diaphoresis]] | |||
* [[Hirsuitism|Hirsutism]] | |||
*[[Clitoris enlargement|Enlarged clitoris]] (in [[females]]) | |||
*[[High blood pressure]] | |||
*[[Sweating]] | |||
| | |||
[[Ganglioneuroma|Ganglioneuromas]] are included in the ''neuroblastic [[tumors]]'' [[Group (sociology)|group]], which includes: | |||
* [[Ganglioneuroma]] ([[benign]]) | |||
* Ganglioneuroblastoma (intermediate) | |||
* [[Neuroblastoma]] (aggressive) | |||
|- | |||
| style="background:#DCDCDC;" align="center" + |'''Myxoid [[liposarcoma]]'''<ref>{{Cite journal | |||
| author = [[Khin Thway]], [[Rashpal Flora]], [[Chirag Shah]], [[David Olmos]] & [[Cyril Fisher]] | |||
| title = Diagnostic utility of p16, CDK4, and MDM2 as an immunohistochemical panel in distinguishing well-differentiated and dedifferentiated liposarcomas from other adipocytic tumors | |||
| journal = [[The American journal of surgical pathology]] | |||
| volume = 36 | |||
| issue = 3 | |||
| pages = 462–469 | |||
| year = 2012 | |||
| month = March | |||
| doi = 10.1097/PAS.0b013e3182417330 | |||
| pmid = 22301498 | |||
}}</ref><ref>{{Cite journal | |||
| author = [[J. Rosai]], [[M. Akerman]], [[P. Dal Cin]], [[I. DeWever]], [[C. D. Fletcher]], [[N. Mandahl]], [[F. Mertens]], [[F. Mitelman]], [[A. Rydholm]], [[R. Sciot]], [[G. Tallini]], [[H. Van den Berghe]], [[W. Van de Ven]], [[R. Vanni]] & [[H. Willen]] | |||
| title = Combined morphologic and karyotypic study of 59 atypical lipomatous tumors. Evaluation of their relationship and differential diagnosis with other adipose tissue tumors (a report of the CHAMP Study Group) | |||
| journal = [[The American journal of surgical pathology]] | |||
| volume = 20 | |||
| issue = 10 | |||
| pages = 1182–1189 | |||
| year = 1996 | |||
| month = October | |||
| pmid = 8827023 | |||
}}</ref><ref name="Dal CinKools1993">{{cite journal|last1=Dal Cin|first1=Paola|last2=Kools|first2=Patrick|last3=Sciot|first3=Raf|last4=De Wever|first4=Ivo|last5=Van Damme|first5=Boudewijn|last6=Van de Ven|first6=Wim|last7=Van Den Berghe|first7=Herman|title=Cytogenetic and fluorescence in situ hybridization investigation of ring chromosomes characterizing a specific pathologic subgroup of adipose tissue tumors|journal=Cancer Genetics and Cytogenetics|volume=68|issue=2|year=1993|pages=85–90|issn=01654608|doi=10.1016/0165-4608(93)90001-3}}</ref><ref name="Dei TosDoglioni2000">{{cite journal|last1=Dei Tos|first1=Angelo P.|last2=Doglioni|first2=Claudio|last3=Piccinin|first3=Sara|last4=Sciot|first4=Raf|last5=Furlanetto|first5=Alberto|last6=Boiocchi|first6=Mauro|last7=Dal Cin|first7=Paola|last8=Maestro|first8=Roberta|last9=Fletcher|first9=Christopher D. M.|last10=Tallini|first10=Giovanni|title=Coordinated expression and amplification of theMDM2,CDK4, andHMGI-C genes in atypical lipomatous tumours|journal=The Journal of Pathology|volume=190|issue=5|year=2000|pages=531–536|issn=0022-3417|doi=10.1002/(SICI)1096-9896(200004)190:5<531::AID-PATH579>3.0.CO;2-W}}</ref><ref name="Dei Tos2000">{{cite journal|last1=Dei Tos|first1=A|title=Liposarcoma: New entities and evolving concepts|journal=Annals of Diagnostic Pathology|volume=4|issue=4|year=2000|pages=252–266|issn=10929134|doi=10.1053/adpa.2000.8133}}</ref><ref>{{Cite journal | |||
| author = [[M. D. Kraus]], [[L. Guillou]] & [[C. D. Fletcher]] | |||
| title = Well-differentiated inflammatory liposarcoma: an uncommon and easily overlooked variant of a common sarcoma | |||
| journal = [[The American journal of surgical pathology]] | |||
| volume = 21 | |||
| issue = 5 | |||
| pages = 518–527 | |||
| year = 1997 | |||
| month = May | |||
| pmid = 9158675 | |||
}}</ref><ref>{{Cite journal | |||
| author = [[P. Argani]], [[F. Facchetti]], [[G. Inghirami]] & [[J. Rosai]] | |||
| title = Lymphocyte-rich well-differentiated liposarcoma: report of nine cases | |||
| journal = [[The American journal of surgical pathology]] | |||
| volume = 21 | |||
| issue = 8 | |||
| pages = 884–895 | |||
| year = 1997 | |||
| month = August | |||
| pmid = 9255251 | |||
}}</ref><ref>{{Cite journal | |||
| author = [[H. L. Evans]] | |||
| title = Liposarcoma: a study of 55 cases with a reassessment of its classification | |||
| journal = [[The American journal of surgical pathology]] | |||
| volume = 3 | |||
| issue = 6 | |||
| pages = 507–523 | |||
| year = 1979 | |||
| month = December | |||
| pmid = 534388 | |||
}}</ref><ref>{{Cite journal | |||
| author = [[A. P. Dei Tos]], [[T. Mentzel]], [[P. L. Newman]] & [[C. D. Fletcher]] | |||
| title = Spindle cell liposarcoma, a hitherto unrecognized variant of liposarcoma. Analysis of six cases | |||
| journal = [[The American journal of surgical pathology]] | |||
| volume = 18 | |||
| issue = 9 | |||
| pages = 913–921 | |||
| year = 1994 | |||
| month = September | |||
| pmid = 8067512 | |||
}}</ref><ref>{{Cite journal | |||
| author = [[D. C. Dahlin]], [[K. K. Unni]] & [[T. Matsuno]] | |||
| title = Malignant (fibrous) histiocytoma of bone--fact or fancy? | |||
| journal = [[Cancer]] | |||
| volume = 39 | |||
| issue = 4 | |||
| pages = 1508–1516 | |||
| year = 1977 | |||
| month = April | |||
| pmid = 192432 | |||
}}</ref> | |||
| | |||
Atypical [[Lipomatous neoplasm|lipomatous tumor]]/well differentiated [[liposarcoma]] and dedifferentiated [[liposarcoma]] are associated with: | |||
* Presence of a large/giant [[marker]][[chromosome]] and/or [[Ring chromosome|ring chromosomes]] at 12q13-15 region | |||
* [[Amplification]] of this [[chromosome]] region rich in [[Protooncogene|protooncogenes]], including ''[[CHOP]]'', ''[[Cyclin-dependent kinase 4|CDK4]]'', ''[[MDM2]]'', ''HMGI-C'', ''[[GLI1|GLI]]'', ''[[SASS6|SAS]]'', ''OS1'', [[HMGA2]] and''[[OS9 (gene)|OS9]]'' | |||
Myxoid [[liposarcoma]] is associated with: | |||
* t(12:16)(q13;p11) - [[CHOP]]([[DDIT3 gene|DDIT3]]) / [[FUS]]<nowiki/>or t(12;22)(q13;q22) - [[CHOP]]([[DDIT3 gene|DDIT3]]) / [[EWSR1 gene|EWS]] | |||
[[Pleomorphic]][[liposarcoma]] is associated with: | |||
* [[Complex (chemistry)|Complex]] [[Karyotype|karyotypic]]<nowiki/>aberrations | |||
| | |||
'''Well-differentiated liposarcoma''': | |||
* '''Sclerosing [[liposarcoma]] ('''distinctive [[stromal]] [[Cells (biology)|cells]] distributed across the [[Tissue (biology)|tissue]], associated with lipoblasts filled with multiple [[vacuoles]], and [[collagenous|collageno]]<nowiki/>[[collagenous|us]] background of fibrillary appearance) | |||
* '''[[Adipocyte|Adipocytic]][[liposarcoma]]''' ([[adipocytes]] with different [[Cell (biology)|cell]] sizes, hyperchromasia, and [[nuclear]] atypia. [[Fibrous]] [[septa|sept]]<nowiki/>[[septa|a]]<nowiki/>containing hyperchromatic [[stromal cells|stromal cel]]<nowiki/>[[stromal cells|ls]] surrounding [[adipocytes]]) | |||
* '''[[Inflammatory]][[liposarcoma]]''' (heavy [[Chronic (medical)|chronic]] [[inflammatory]]<nowiki/>infiltrate composed of different lympho-plasmacytic aggregates) | |||
* '''[[Spindle cells|Spindle cell]][[liposarcoma]]'''([[proliferation]] of [[neural]]-like [[spindle cells]] organized in a [[fibrous]] [[Structure factor|structure]]<nowiki/>containing lipoblasts) | |||
'''De-differentiated [[liposarcoma]]''': | |||
* '''Myxoid [[liposarcoma]]''' ( non-homogenous [[appearance]] with [[cystic]] and [[solid|soli]]<nowiki/>[[solid|d]] components) | |||
* '''Round [[cell]][[liposarcoma]]''' (small, round, or [[spindle cells]] with sparse [[Eosinophilia|eosinophilic]] and [[Granular cell|granular]] [[cytoplasm]]<nowiki/>a<nowiki/>nd large [[nuclei]],scattered lipoblasts and [[Area|areas]] <nowiki/>of [[necrosis]]) | |||
* '''[[Pleiomorphic|Pleomorphic]][[liposarcoma]]''' ([[pleiomorphic|pleomorphic]] [[Cells (biology)|cells]] <nowiki/>with enlarged round to bizarre [[nuclei]]) | |||
| | |||
Atypical [[Lipomatous neoplasm|lipomatous tumor]]/well differentiated [[liposarcoma]] is positive for: | |||
* [[MDM2]] | |||
* [[Cyclin-dependent kinase 4|CDK4]] | |||
* [[p16]] | |||
* [[S100A1|S100]] ([[Stain|stains]][[adipocytes]] and lipoblasts) | |||
| | |||
* [[Chemical]] [[carcinogens]] | |||
** Phenoxyacetic [[herbicides]] | |||
** Chlorophenols | |||
** [[Dioxin]][[Contamination|contaminations]] | |||
** [[Arsenic]] | |||
** [[Thorium dioxide]] ([[Thorotrast]]) | |||
* [[Radiation]] ([[dose]] of 50 GY) | |||
* [[Immunodeficiency]](regional [[acquired]][[immunodeficiency]]) | |||
* [[Genetic]] susceptibility | |||
*** [[Li-Fraumeni syndrome]] | |||
*** [[Neurofibromatosis]]([[NF1]]; [[von Recklinghausen disease]]) | |||
*** [[Gardner syndrome]] ([[Familial adenomatous polyposis]]) | |||
*** [[Retinoblastoma]] | |||
*** [[Werner syndrome]] | |||
*** [[Basal cell carcinoma|Nevoid basal cell carcinoma]] ([[Gorlin syndrome]]) | |||
** [[Viral infection|Viral infections]] | |||
| | |||
* [[Retroperitoneum]] | |||
* [[Esophagus]] | |||
* [[Bowel]] | |||
* [[Mediastinum]] | |||
| | |||
* '''[[Retroperitoneal]][[liposarcoma]]''' maybe [[asymptomatic]] or [[causes]]: | |||
** [[Weight loss]] | |||
** [[Abdominal pain]] | |||
** [[Oliguria]] | |||
** [[renal failure]] (due to [[ureters]] or [[kidneys]]' compression) | |||
** [[Palpable]] [[abdominal]][[mass]] | |||
** [[Abdominal tenderness]] | |||
** [[Abdominal distention]] | |||
* '''[[Esophageal]] [[liposarcoma]]''' may [[Causes|cause]]: | |||
** [[Dysphagia]] | |||
** [[Vomiting]] | |||
** [[Cough]] | |||
** [[Gastrointestinal bleeding]] | |||
** [[Hoarseness]] | |||
* '''[[Bowel]] [[liposarcoma]]''' may cause: | |||
** [[Gastrointestinal tract|Gastrointestinal]][[bleeding]] | |||
* '''[[Mediastinal]] [[liposarcoma]]''' may [[Causes|cause]]: | |||
** [[Dyspnea]] | |||
** [[Cough]] | |||
** [[Chest pain]] | |||
** [[Weight loss]] | |||
|_ | |||
|- | |||
| style="background:#DCDCDC;" align="center" + |'''[[Leiomyoma]]'''<ref name="pmid7611533">{{cite journal| author=Coffin CM, Watterson J, Priest JR, Dehner LP| title=Extrapulmonary inflammatory myofibroblastic tumor (inflammatory pseudotumor). A clinicopathologic and immunohistochemical study of 84 cases. | journal=Am J Surg Pathol | year= 1995 | volume= 19 | issue= 8 | pages= 859-72 | pmid=7611533 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=7611533 }} </ref><ref name="pmid8635024">{{cite journal| author=Wenig BM, Devaney K, Bisceglia M| title=Inflammatory myofibroblastic tumor of the larynx. A clinicopathologic study of eight cases simulating a malignant spindle cell neoplasm. | journal=Cancer | year= 1995 | volume= 76 | issue= 11 | pages= 2217-29 | pmid=8635024 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=8635024 }} </ref><ref name="pmid8847061">{{cite journal| author=Ramachandra S, Hollowood K, Bisceglia M, Fletcher CD| title=Inflammatory pseudotumour of soft tissues: a clinicopathological and immunohistochemical analysis of 18 cases. | journal=Histopathology | year= 1995 | volume= 27 | issue= 4 | pages= 313-23 | pmid=8847061 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=8847061 }} </ref><ref name="pmid12673560">{{cite journal| author=Häusler M, Schaade L, Ramaekers VT, Doenges M, Heimann G, Sellhaus B| title=Inflammatory pseudotumors of the central nervous system: report of 3 cases and a literature review. | journal=Hum Pathol | year= 2003 | volume= 34 | issue= 3 | pages= 253-62 | pmid=12673560 | doi=10.1053/hupa.2003.35 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=12673560 }} </ref><ref name="pmid16160478">{{cite journal| author=Rabban JT, Zaloudek CJ, Shekitka KM, Tavassoli FA| title=Inflammatory myofibroblastic tumor of the uterus: a clinicopathologic study of 6 cases emphasizing distinction from aggressive mesenchymal tumors. | journal=Am J Surg Pathol | year= 2005 | volume= 29 | issue= 10 | pages= 1348-55 | pmid=16160478 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=16160478 }} </ref><ref name="pmid16967468">{{cite journal| author=Kovach SJ, Fischer AC, Katzman PJ, Salloum RM, Ettinghausen SE, Madeb R et al.| title=Inflammatory myofibroblastic tumors. | journal=J Surg Oncol | year= 2006 | volume= 94 | issue= 5 | pages= 385-91 | pmid=16967468 | doi=10.1002/jso.20516 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=16967468 }} </ref><ref name="pmid9606802">{{cite journal| author=Coffin CM, Dehner LP, Meis-Kindblom JM| title=Inflammatory myofibroblastic tumor, inflammatory fibrosarcoma, and related lesions: an historical review with differential diagnostic considerations. | journal=Semin Diagn Pathol | year= 1998 | volume= 15 | issue= 2 | pages= 102-10 | pmid=9606802 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=9606802 }} </ref><ref name="pmid6336632">{{cite journal| author=Berardi RS, Lee SS, Chen HP, Stines GJ| title=Inflammatory pseudotumors of the lung. | journal=Surg Gynecol Obstet | year= 1983 | volume= 156 | issue= 1 | pages= 89-96 | pmid=6336632 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=6336632 }} </ref><ref name="pmid8635024">{{cite journal| author=Wenig BM, Devaney K, Bisceglia M| title=Inflammatory myofibroblastic tumor of the larynx. A clinicopathologic study of eight cases simulating a malignant spindle cell neoplasm. | journal=Cancer | year= 1995 | volume= 76 | issue= 11 | pages= 2217-29 | pmid=8635024 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=8635024 }} </ref><ref name="pmid16160478">{{cite journal| author=Rabban JT, Zaloudek CJ, Shekitka KM, Tavassoli FA| title=Inflammatory myofibroblastic tumor of the uterus: a clinicopathologic study of 6 cases emphasizing distinction from aggressive mesenchymal tumors. | journal=Am J Surg Pathol | year= 2005 | volume= 29 | issue= 10 | pages= 1348-55 | pmid=16160478 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=16160478 }} </ref><ref name="pmid17414097">{{cite journal| author=Coffin CM, Hornick JL, Fletcher CD| title=Inflammatory myofibroblastic tumor: comparison of clinicopathologic, histologic, and immunohistochemical features including ALK expression in atypical and aggressive cases. | journal=Am J Surg Pathol | year= 2007 | volume= 31 | issue= 4 | pages= 509-20 | pmid=17414097 | doi=10.1097/01.pas.0000213393.57322.c7 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=17414097 }} </ref> | |||
| | |||
* Loss of normal [[chromosome]] 1q ([[hereditary]][[leiomyomatosis]]) | |||
* [[Renal cell cancer]](HLRCC) [[gene mutation]] | |||
| | |||
* Prominent [[cellular]] [[atypia]] | |||
* [[Nuclear]] [[atypia]], including [[nuclear]] [[pleomorphism]], hyperchromatism, irregularity in [[nuclear|nucle]]<nowiki/>[[nuclear|ar]] [[Membrane|membranes]], high [[nuclear]] size, and prominent [[nucleoli]] | |||
* [[Cigar]]-shaped [[nuclei]] | |||
* Abundant [[mitoses]], [[mitotic index]] higher than 10 or more per 10 <nowiki/>high-power fields | |||
* [[Area]]<nowiki/>s of [[coagulative necrosis]] ([[tumor cell]][[necrosis]]) | |||
* Pali<nowiki/>sading and extensive [[degenerative]] [[Change|changes]] in the form of hyalinization, [[calcification]], and myxoid changes | |||
* Elon<nowiki/>gated [[Cells (biology)|cells]] with [[eosinophilic]] or occasional [[Fibrillarin|fibrillar]] [[cytoplasm]] with [[Distinctive feature|distinct]] [[cell membranes]] | |||
| | |||
Positive for: | |||
* HHF35 (90%) | |||
* Alpha-[[smooth muscle]] [[actin]] (90%) | |||
* [[Vimentin]] | |||
* [[Desmin]] (75%) | |||
* H-[[caldesmon]] | |||
* [[Phosphotungstic acid hematoxylin|PTAH]] ([[Stain|stains]][[myofibrils]]) | |||
* [[Keratin]] (30%) | |||
* [[ER]] (usually in [[uterine]] and [[female]][[retroperitoneal]][[tumors]]) | |||
* [[S100A1|S100]] (occasionally weak [[staining]]) | |||
* EMA (may be focal) | |||
* [[CD34]] | |||
Negative for: | |||
* [[CD117]] | |||
| | |||
* Immundeficiency ([[HIV]]) | |||
* [[Dioxin|Digoxin]] [[Exposure assessment|exposure]] | |||
* [[HHV-8|Human herpes virus type-8 (HHV-8)]] | |||
* [[Epstein barr virus mononucleosis|Epstein barr virus]] | |||
* Long term [[tamoxifen]] use | |||
* [[History and Physical examination|History]] of [[pelvic]][[Radiation|radiations]] | |||
* [[Hereditary]] [[breast carcinoma]] with [[BRCA1]][[mutation]] | |||
* [[Hereditary nonpolyposis colorectal cancer|Hereditary nonpolyposis colorectal carcinoma]] with [[MSH2]] [[mutation]] | |||
* [[Li-Fraumeni syndrome]] | |||
* [[Malignant]] [[melanoma]] | |||
* [[Retinoblastoma]] | |||
| | |||
* [[Uterus]] | |||
* [[Abdomen]] | |||
* [[Esophagus]] | |||
* [[Rectum]] | |||
* [[Skin]] / [[subcutis]] | |||
* [[Retroperitoneum]] | |||
* [[Extremities]] | |||
* Large [[vessels]] ([[inferior vena cava]], [[saphenous vein]], [[femoral vein]], [[pulmonary artery]], [[femoral artery]]) | |||
* [[Superficial]] or deep [[Soft tissue|soft tissues]] | |||
* [[Bone]] | |||
* [[Breast]] | |||
* [[Colon]] | |||
* [[Epididymis]] | |||
* [[Mediastinum]] | |||
* [[Lungs]] | |||
| | |||
* [[Asymptomatic]] | |||
* [[Uterine|(uterine]] [[leiomyosarcoma]]<nowiki/>may be associated with: | |||
** Irregular [[vaginal bleeding]](intermenstrual or [[postmenopausal]]) | |||
** New [[lump]] or a [[mass]]<nowiki/>protruding into [[vagina]]<nowiki/>or growing [[mass]] in [[abdomen]] or [[pelvis]] | |||
** [[Abdominal pain]] | |||
** [[Abdominal distension]] | |||
** [[Pelvic pain]] | |||
** [[Urinary system|Urinary]] [[symptoms]] | |||
* [[Esophageal]][[leiomyosarcoma]] may cause: | |||
** [[Dysphagia]] | |||
** [[Hematemesis]] | |||
* [[rectal]] [[leiomyosarcoma]]<nowiki/>may cause: | |||
** [[Black]], [[tarry stools]] | |||
** [[Rectal bleeding]] | |||
|_ | |||
|- | |||
| style="background:#DCDCDC;" align="center" + |'''[[Inflammatory]] myofibroblastic [[tumor]](IMT)'''<ref name="pmid7611533">{{cite journal| author=Coffin CM, Watterson J, Priest JR, Dehner LP| title=Extrapulmonary inflammatory myofibroblastic tumor (inflammatory pseudotumor). A clinicopathologic and immunohistochemical study of 84 cases. | journal=Am J Surg Pathol | year= 1995 | volume= 19 | issue= 8 | pages= 859-72 | pmid=7611533 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=7611533 }} </ref><ref name="pmid8635024">{{cite journal| author=Wenig BM, Devaney K, Bisceglia M| title=Inflammatory myofibroblastic tumor of the larynx. A clinicopathologic study of eight cases simulating a malignant spindle cell neoplasm. | journal=Cancer | year= 1995 | volume= 76 | issue= 11 | pages= 2217-29 | pmid=8635024 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=8635024 }} </ref><ref name="pmid8847061">{{cite journal| author=Ramachandra S, Hollowood K, Bisceglia M, Fletcher CD| title=Inflammatory pseudotumour of soft tissues: a clinicopathological and immunohistochemical analysis of 18 cases. | journal=Histopathology | year= 1995 | volume= 27 | issue= 4 | pages= 313-23 | pmid=8847061 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=8847061 }} </ref><ref name="pmid12673560">{{cite journal| author=Häusler M, Schaade L, Ramaekers VT, Doenges M, Heimann G, Sellhaus B| title=Inflammatory pseudotumors of the central nervous system: report of 3 cases and a literature review. | journal=Hum Pathol | year= 2003 | volume= 34 | issue= 3 | pages= 253-62 | pmid=12673560 | doi=10.1053/hupa.2003.35 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=12673560 }} </ref><ref name="pmid16160478">{{cite journal| author=Rabban JT, Zaloudek CJ, Shekitka KM, Tavassoli FA| title=Inflammatory myofibroblastic tumor of the uterus: a clinicopathologic study of 6 cases emphasizing distinction from aggressive mesenchymal tumors. | journal=Am J Surg Pathol | year= 2005 | volume= 29 | issue= 10 | pages= 1348-55 | pmid=16160478 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=16160478 }} </ref><ref name="pmid16967468">{{cite journal| author=Kovach SJ, Fischer AC, Katzman PJ, Salloum RM, Ettinghausen SE, Madeb R et al.| title=Inflammatory myofibroblastic tumors. | journal=J Surg Oncol | year= 2006 | volume= 94 | issue= 5 | pages= 385-91 | pmid=16967468 | doi=10.1002/jso.20516 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=16967468 }} </ref><ref name="pmid9606802">{{cite journal| author=Coffin CM, Dehner LP, Meis-Kindblom JM| title=Inflammatory myofibroblastic tumor, inflammatory fibrosarcoma, and related lesions: an historical review with differential diagnostic considerations. | journal=Semin Diagn Pathol | year= 1998 | volume= 15 | issue= 2 | pages= 102-10 | pmid=9606802 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=9606802 }} </ref><ref name="pmid6336632">{{cite journal| author=Berardi RS, Lee SS, Chen HP, Stines GJ| title=Inflammatory pseudotumors of the lung. | journal=Surg Gynecol Obstet | year= 1983 | volume= 156 | issue= 1 | pages= 89-96 | pmid=6336632 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=6336632 }} </ref><ref name="pmid8635024">{{cite journal| author=Wenig BM, Devaney K, Bisceglia M| title=Inflammatory myofibroblastic tumor of the larynx. A clinicopathologic study of eight cases simulating a malignant spindle cell neoplasm. | journal=Cancer | year= 1995 | volume= 76 | issue= 11 | pages= 2217-29 | pmid=8635024 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=8635024 }} </ref><ref name="pmid16160478">{{cite journal| author=Rabban JT, Zaloudek CJ, Shekitka KM, Tavassoli FA| title=Inflammatory myofibroblastic tumor of the uterus: a clinicopathologic study of 6 cases emphasizing distinction from aggressive mesenchymal tumors. | journal=Am J Surg Pathol | year= 2005 | volume= 29 | issue= 10 | pages= 1348-55 | pmid=16160478 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=16160478 }} </ref><ref name="pmid17414097">{{cite journal| author=Coffin CM, Hornick JL, Fletcher CD| title=Inflammatory myofibroblastic tumor: comparison of clinicopathologic, histologic, and immunohistochemical features including ALK expression in atypical and aggressive cases. | journal=Am J Surg Pathol | year= 2007 | volume= 31 | issue= 4 | pages= 509-20 | pmid=17414097 | doi=10.1097/01.pas.0000213393.57322.c7 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=17414097 }} </ref> | |||
| | |||
Unknown underlying [[etiology]], may be due to [[inflammatory]] reaction to: | |||
* [[Infection]] | |||
* Underlying low grade [[malignancy]] | |||
[[Mutations]] such as: | |||
* [[Anaplastic large cell lymphoma, ALK positive|ALK (anaplastic lymphoma kinase)]][[gene]] [[mutations]] in the [[tyrosine kinase]][[locus]] at band 2p23 | |||
| | |||
* [[Spindle]] to [[Stellate cell|stellate]]-shaped [[Cell (biology)|cells]] | |||
* [[Spindle cells]]<nowiki/>arranged in short [[fascicles]] with a focal storiform (whorled or cartwheel-like) architecture | |||
* [[Spindle cells]] show features of [[fibroblasts]]<nowiki/>and [[myofibroblasts]] | |||
* Variably dense, chronic, mixed [[polymorphic]] infiltrateof [[Mononuclear cells|mononuclear]][[inflammatory]] [[Cells (biology)|cells]] ([[plasma cells]] and [[lymphocytes]], [[histiocytes]], [[neutrophils]], and occasional [[eosinophils]]) | |||
* [[Histiocytes]] have [[multinucleated]] forms with finely vacuolated [[cytoplasmic]] [[lipid]]<nowiki/>droplets | |||
* [[Plasma cells]] with [[cytoplasmic]] [[Russell bodies]] ([[Globular protein|globular]][[cytoplasmic]][[inclusions]] of [[immunoglobulin]]) and [[polyclonal]] pattern of [[light chain]] [[expression]] | |||
* Absent hyperchromasia and atypical [[mitoses]] | |||
| | |||
Positive for: | |||
* IG+ ([[plasma cells]]) | |||
* [[IL-1]] | |||
* [[Interleukin 6|IL-6]] | |||
* [[Smooth muscle]] [[actin]] | |||
* [[Desmin]] | |||
* [[Calponin]] | |||
* [[Activin]]-like [[kinase]] 1 | |||
Negative for: | |||
* [[Beta-catenin]] | |||
| | |||
* [[Multiple organ dysfunction syndrome|Multiorgan disease]] in association with [[Chronic (medical)|chronic]]<nowiki/>persistent [[Eikenella corrodens]] [[infection]] | |||
* [[Epstein-Barr Virus|Epstein Barr virus]][[infection]] | |||
* [[HHV-8|Human herpes virus-8(HHV-8)]] [[infection]]([[Kaposi's sarcoma]], multicentric [[Castleman's disease]]) | |||
| | |||
* [[Lungs]] | |||
* [[Gastrointestinal system]] | |||
* [[Pelvic]] region | |||
** [[Urinary bladder|Bladder]] | |||
** [[Uterus]] | |||
* [[Retroperitoneum]] | |||
* [[Skin]] | |||
* [[Bone]] ([[femur]], [[temporal bone]], [[jaw]][[bone]]) | |||
* [[CNS]] | |||
* [[Soft tissue|Soft tissues]] | |||
* [[Larynx]] | |||
* [[Heart]] ([[right ventricle]]<nowiki/>is most commonly involved) | |||
* [[Pancreas]] (rarely) | |||
| | |||
* [[Asymptomatic]] (70%) | |||
* Painless [[asymptomatic]][[mass]]/[[lump]]/[[swelling]] | |||
* [[Pulmonary]] IMT presents as: | |||
** [[Chest pain]] | |||
** [[Cough]] | |||
** [[Dyspnea]] | |||
** [[Hemoptysis]] (recurrent) | |||
** [[Fever]] | |||
** [[Fatigue]] | |||
** [[Weight loss]] | |||
** [[Appetite loss]] | |||
* [[Bone]] [[IMT]] presents with: | |||
** Mild [[bone pain]] | |||
** Easy [[fractures]] | |||
** [[Headache]] | |||
** [[Dizziness]] | |||
** [[Numbness]] at [[tumor]]<nowiki/>site | |||
** [[Bone marrow]]<nowiki/>involvement in some cases | |||
* [[Heart]] [[IMT]] presents with: | |||
** [[Chest pain]] | |||
** [[Difficulty breathing]] | |||
** [[Palpitations]] | |||
** [[Fainting]] | |||
** [[Obstruction]] of [[blood flow]] in the [[heart]] (large [[tumors]]) | |||
* [[Urinary bladder|Bladder]] [[IMT]] presents with: | |||
** Painless [[hematuria]] | |||
** [[Chronic (medical)|Chronic]] [[pelvic pain]] | |||
** [[Difficulty passing urine|Difficulty in urinating]] | |||
** Presence of [[Burning sensation throughout the urethra|burning sensation]] | |||
* [[CNS]] [[IMT]] presents with: | |||
** Presence of [[solitary]] or multiple [[tumors]] at various [[Location parameter|location]]<nowiki/>s in the [[brain]] | |||
** Recurrent [[headaches]] | |||
** [[Headache]] | |||
** [[Nausea and vomiting]] | |||
** [[Blurred vision]] | |||
** [[Double vision]] | |||
** [[Ptosis|Drooping of the eyelid]] | |||
** [[Dizziness]] | |||
** [[Back pain]] (if [[spine]]<nowiki/>involved) | |||
** [[Seizures]] | |||
| | |||
Also known as: | |||
* Pseudo-[[inflammatory]][[tumors]] | |||
* [[Inflammatory]] pseudotumor | |||
* [[Plasma cell]] [[granuloma]] | |||
* [[Inflammatory]] pseudotumor | |||
* [[Fibrous histiocytoma]] | |||
* [[Fibroxanthoma]] | |||
* [[Xanthogranuloma]] | |||
* [[Inflammatory]]<nowiki/>pseudosarcoma | |||
* Atypical fibromyxoid [[tumor]] | |||
* Atypical myfibroblastic [[tumor]] | |||
|- | |||
| style="background:#DCDCDC;" align="center" + |'''[[Fibroepithelial polyp]]/[[Acrochordon]]'''<ref name="pmid30997841">{{cite journal| author=Cukic O, Jovanovic MB| title=Large Fibroepithelial Polyp of the Palatine Tonsil. | journal=Ear Nose Throat J | year= 2019 | volume= | issue= | pages= 145561319841203 | pmid=30997841 | doi=10.1177/0145561319841203 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=30997841 }} </ref><ref name="pmid30916216">{{cite journal| author=Vatansever M, Dinç E, Dursun Ö, Oktay ÖÖ, Arpaci R| title=Atypical presentation of fibroepithelial polyp: a report of two cases. | journal=Arq Bras Oftalmol | year= 2019 | volume= | issue= | pages= | pmid=30916216 | doi=10.5935/0004-2749.20190050 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=30916216 }} </ref><ref name="pmid30864355">{{cite journal| author=Rexhepi M, Trajkovska E, Besimi F, Rufati N| title=Giant Fibroepithelial Polyp of Vulva: A Case Report and Review of Literature. | journal=Pril (Makedon Akad Nauk Umet Odd Med Nauki) | year= 2018 | volume= 39 | issue= 2-3 | pages= 127-130 | pmid=30864355 | doi=10.2478/prilozi-2018-0051 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=30864355 }} </ref><ref name="pmid30778021">{{cite journal| author=Jabbour J, Chappell JR, Busby M, McCubbery NW, Brown DF, Park SJK et al.| title=Glottic Obstruction from Fibroepithelial Polyp. | journal=Am J Case Rep | year= 2019 | volume= 20 | issue= | pages= 219-223 | pmid=30778021 | doi=10.12659/AJCR.914907 | pmc=6388646 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=30778021 }} </ref><ref name="pmid30448831">{{cite journal| author=Hong P, Cai Y, Li Z, Fan S, Yang K, Hao H et al.| title=Modified Laparoscopic Partial Ureterectomy for Adult Ureteral Fibroepithelial Polyp: Technique and Initial Experience. | journal=Urol Int | year= 2019 | volume= 102 | issue= 1 | pages= 13-19 | pmid=30448831 | doi=10.1159/000494804 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=30448831 }} </ref><ref name="pmid30426076">{{cite journal| author=Uçar M, Baş E, Akkoç A, Topçuoğlu M| title=Fibroepithelial Polyp of the Ureter: A Rare Cause of Hydronephrosis. | journal=J Endourol Case Rep | year= 2018 | volume= 4 | issue= 1 | pages= 166-168 | pmid=30426076 | doi=10.1089/cren.2018.0031 | pmc=6225073 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=30426076 }} </ref><ref name="pmid30425926">{{cite journal| author=Chaker K, Rhouma SB, Daly KM, Zehani A, Bibi M, Chehida MAB et al.| title=Benign fibroepithelial polyp of the ureter: A case report. | journal=Urol Case Rep | year= 2019 | volume= 22 | issue= | pages= 52-53 | pmid=30425926 | doi=10.1016/j.eucr.2018.10.019 | pmc=6226574 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=30425926 }} </ref><ref name="pmid30421620">{{cite journal| author=Hajji F, Moufid K, Ghoundale O, Touiti D| title=A rare case of successful endoscopic management of a fibroepithelial polyp with intussusception of the ureter and periodic prolapse into bladder. | journal=Ann R Coll Surg Engl | year= 2019 | volume= 101 | issue= 2 | pages= e66-e70 | pmid=30421620 | doi=10.1308/rcsann.2018.0198 | pmc=6351868 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=30421620 }} </ref><ref name="pmid30401014">{{cite journal| author=Lee H, Sade I, Gilani S, Zhong M, Lombardo G| title=A Giant Fibroepithelial Polyp of the Small Bowel Associated with High-Grade Obstruction. | journal=Am Surg | year= 2018 | volume= 84 | issue= 7 | pages= e210-e211 | pmid=30401014 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=30401014 }} </ref><ref name="pmid30319938">{{cite journal| author=Chaker K| title=Benign fibroepithelial polyp of the ureter: A case report. | journal=Urol Case Rep | year= 2019 | volume= 22 | issue= | pages= 15-16 | pmid=30319938 | doi=10.1016/j.eucr.2018.09.021 | pmc=6180234 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=30319938 }} </ref><ref name="pmid30009441">{{cite journal| author=Lozano-Peña AK, Lamadrid-Zertuche AC, Ocampo-Candiani J| title=Giant fibroepithelial polyp of the vulva. | journal=Australas J Dermatol | year= 2019 | volume= 60 | issue= 1 | pages= 70-71 | pmid=30009441 | doi=10.1111/ajd.12886 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=30009441 }} </ref><ref name="pmid29883781">{{cite journal| author=Eckstein M, Agaimy A, Woenckhaus J, Winter A, Bittmann I, Janzen J et al.| title=DICER1 mutation-positive giant botryoid fibroepithelial polyp of the urinary bladder mimicking embryonal rhabdomyosarcoma. | journal=Hum Pathol | year= 2019 | volume= 84 | issue= | pages= 1-7 | pmid=29883781 | doi=10.1016/j.humpath.2018.05.015 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=29883781 }} </ref><ref name="pmid29843497">{{cite journal| author=Akdere H, Çevik G| title=Rare Fibroepithelial Polyp Extending Along the Ureter: A Case Report | journal=Balkan Med J | year= 2018 | volume= 35 | issue= 3 | pages= 275-277 | pmid=29843497 | doi=10.4274/balkanmedj.2017.1537 | pmc=5981127 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=29843497 }} </ref><ref name="pmid29800931">{{cite journal| author=Ballard DH, Rove KO, Coplen DE, Chen TY, Hulett Bowling RL| title=Fibroepithelial polyp causing urethral obstruction: Diagnosis by cystourethrogram. | journal=Clin Imaging | year= 2018 | volume= 51 | issue= | pages= 164-167 | pmid=29800931 | doi=10.1016/j.clinimag.2018.05.009 | pmc=6404776 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=29800931 }} </ref><ref name="pmid29574427">{{cite journal| author=Amin A, Amin Z, Al Farsi AR| title=Septic presentation of a giant fibroepithelial polyp of the vulva. | journal=BMJ Case Rep | year= 2018 | volume= 2018 | issue= | pages= | pmid=29574427 | doi=10.1136/bcr-2017-222789 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=29574427 }} </ref><ref name="pmid29487969">{{cite journal| author=Gupta R, Smita S, Sinha R, Sinha N, Sinha L| title=Giant fibroepithelial polyp of the thigh and retroperitoneal fibromatosis in a young woman: a rare case. | journal=Skeletal Radiol | year= 2018 | volume= 47 | issue= 9 | pages= 1299-1304 | pmid=29487969 | doi=10.1007/s00256-018-2904-x | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=29487969 }} </ref><ref name="pmid29200704">{{cite journal| author=Rajeesh Mohammed PK, Choudhury BK, Dalai RP, Rana V| title=Fibroepithelial Polyp with Sebaceous Hyperplasia: A Case Report. | journal=Indian J Med Paediatr Oncol | year= 2017 | volume= 38 | issue= 3 | pages= 404-406 | pmid=29200704 | doi=10.4103/ijmpo.ijmpo_124_17 | pmc=5686997 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=29200704 }} </ref><ref name="pmid28791276">{{cite journal| author=Lee MH, Hwang JY, Lee JH, Kim DH, Song SH| title=Fibroepithelial polyp of the vulva accompanied by lymphangioma circumscriptum. | journal=Obstet Gynecol Sci | year= 2017 | volume= 60 | issue= 4 | pages= 401-404 | pmid=28791276 | doi=10.5468/ogs.2017.60.4.401 | pmc=5547092 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=28791276 }} </ref><ref name="pmid28693464">{{cite journal| author=Ten Donkelaar CS, Houwert AC, Ten Kate FJW, Lock MTWT| title=Polypoid arteriovenous malformation of the ureter mimicking a fibroepithelial polyp, a case report. | journal=BMC Urol | year= 2017 | volume= 17 | issue= 1 | pages= 55 | pmid=28693464 | doi=10.1186/s12894-017-0237-z | pmc=5504856 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=28693464 }} </ref><ref name="pmid28603622">{{cite journal| author=Saito N, Yamasaki M, Daido W, Ishiyama S, Deguchi N, Taniwaki M| title=A bronchial fibroepithelial polyp with abnormal findings on auto-fluorescence imaging. | journal=Respirol Case Rep | year= 2017 | volume= 5 | issue= 5 | pages= e00244 | pmid=28603622 | doi=10.1002/rcr2.244 | pmc=5465754 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=28603622 }} </ref> | |||
|Associated with: | |||
* [[Human papillomavirus|HPV 6]] (low risk) | |||
* [[Human papillomavirus|HPV 11]] | |||
| | |||
* Fibrovascular [[Core (anatomy)|cores]] covered by [[squamous epithelium]] | |||
* Larger [[lesions]] may have a [[Flat affect|flattened]] [[Epidermis (skin)|epidermis]] | |||
* Smaller [[lesions]] can have [[epidermal]] [[hyperplasia]] or [[seborrheic keratosis]]-like changes | |||
* [[Central]] [[Core (anatomy)|core]] composed of [[Loose connective tissue|loose]] [[collagen]] with increased [[blood vessels]] | |||
* In larger [[lesion]], may have a [[central]] [[Core (anatomy)|core]] of [[adipose tissue]] | |||
* [[Pagetoid]] [[Dyskeratosis congenita|dyskeratosis]] is sometimes present as an [[incidental finding]] | |||
* May have [[ischemic necrosis]] due to [[torsion]] | |||
|Positive for: | |||
* [[Desmin]] | |||
* [[Vimentin]] | |||
* [[ER]] | |||
* [[PR]] | |||
Negative for: | |||
* [[Actin]] | |||
|Associated with: | |||
* [[Diabetes]] (elevated [[blood sugar]] and [[insulin]]) | |||
* [[Abnormal]] [[lipid profile]] | |||
* Other components of [[metabolic syndrome]] | |||
* [[Birt-Hogg-Dubé syndrome|Birt-Hogg-Dube syndrome]] | |||
* [[Acromegaly]] | |||
* [[Polycystic ovary syndrome]] | |||
* May increase in [[number]] during [[pregnancy]] | |||
| | |||
* Occurs usually in intertriginous [[Area|areas]] (i.e. [[axilla]], [[groin]]) | |||
* [[Face]] | |||
* [[Neck]] | |||
* [[Eyelids]] | |||
* [[Vulva]] | |||
* [[Tonsils]] | |||
* [[Ureter]] | |||
* [[Bowel]] | |||
* [[Urinary bladder]] | |||
* [[Bronchi]] | |||
| | |||
* [[Soft tissue|Soft]] [[papilloma]], [[flesh]] [[Color|colored]] to dark [[brown]], [[sessile]] to [[pedunculated]] | |||
* A few [[millimeters]] to multiple [[Centimeter|centimeters]] in size | |||
* Larger [[lesions]] often [[Attachment theory|attach]] to [[skin]] by slender stalks | |||
| | |||
* [[Benign]] [[skin]] [[lesions]] in [[Adult|adults]], excised for cosmetic [[Reasoning|reasons]] | |||
Also known as: | |||
* [[Skin tags|Skin tag]] | |||
* [[Soft tissue|Soft]] [[fibroma]] | |||
* [[Cutaneous]] [[papilloma]] | |||
* [[Cutaneous]] tag | |||
* [[Fibroma]] pendulum | |||
* [[Fibroma]] [[molluscum]] | |||
|} | |||
==References== | ==References== | ||
Latest revision as of 04:00, 28 August 2020
|
Dermatofibrosarcoma protuberans Microchapters |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1] Associate Editor(s)-in-Chief: Sara Mohsin, M.D.[2], Kiran Singh, M.D. [3], Faizan Sheraz, M.D. [4]
Synonyms and keywords: Darier-Ferrand tumor, Darier-Hoffmann tumor, Dermatofibrosarcoma, DFSP, Intermediate fibrous histiocytoma, Borderline fibrous histiocytoma
Overview
Dermatofibrosarcoma protuberans (DFSP) is a rare non-hereditary neoplasm of the dermis layer of the skin which is sometimes described as having the tentacles growing into the surrounding fat, muscle and even bone and is therefore, classified as a soft tissue sarcoma. In many respects, the disease behaves as a benign tumor, but in 2-5% of cases it can metastasize, so it should be considered to have a malignant potential. Over 95% of DFSP tumors have the chromosomal translocation t(17;22). The translocation fuses the collagen gene (COL1A1) with the platelet-derived growth factor gene. The fibroblast, the cell of origin of this tumor, expresses the fusion gene in the belief that it is collagen. However, the resulting fusion protein is processed into mature platelet-derived growth factor which is a potent growth factor. Fibroblasts contain the receptor for this growth factor. Thus, the cell "thinks" it is producing a structural protein, but in fact produces a self-stimulatory growth signal. The cell divides rapidly and a tumor forms. In dermatofibrosarcoma protuberans, the tumor has a tendency to return after being removed. However, it does not often metastasize to other parts of the body.
Classification
There are several variants of dermatofibrosarcoma protuberans in which different cell types are involved in the tumor. [1][2][3]
| Variant subtype | Details |
|---|---|
| Pigmented dermatofibrosarcoma protuberans (Bednar tumor) | |
| Myxoid dermatofibrosarcoma protuberans tumor |
|
| Juvenile dermatofibrosarcoma protuberans (Giant cell fibroblastoma) |
|
| Fibrosarcomatous (FS) Dermatofibrosarcoma protuberans |
|
Pathophysiology
Epigenetics
- Dermatofibrosarcoma protuberans is associated with the genetic rearrangement (i.e. translocation) between chromosomes 17 and 22[4][5][6][7][8][9][10][11][12][13][14]
- t(17;22)(q21;q13.1) leads to fusion of a part of COL1A1 gene from chromosome 17 with a part of the PDGFB gene from chromosome 22[15][16][2][16][12][17][18][19][20][21][22]
- The translocation is found on one or more extra chromosomes that can be either the normal linear or circular in shape
- Circular extra chromosomes are known as supernumerary ring chromosomes (which form after a chromosome breaks in two places and the ends of the chromosomal arms fuse together to form a circular structure)
- Other genes from chromosomes 17 and 22 can be found on the extra chromosomes, but the role of these genes in the development of this condition is unclear
- Translocation is acquired during a person's lifetime and the chromosomes containing the translocation are present only in the tumor cells, this type of genetic change is known as somatic mutation
- In normal cells, the COL1A1 gene provides instructions for making part of a large molecule called type I collagen, which strengthens and supports many tissues in the body
- The PDGFB gene provides instructions for making one version (isoform) of the platelet derived growth factor (PDGF) protein which by attaching to its receptor, becomes activated and stimulates many cellular processes, including cell growth and division (proliferation) and maturation (differentiation)
- The abnormally fused COL1A1-PDGFB gene provides instructions for making an abnormal combined (fusion) protein that ultimately functions like the PDGFB protein
- This gene fusion leads to the production of an excessive amount of protein that functions like the PDGFB protein
- In excess, this fusion protein stimulates cells to proliferate and differentiate abnormally, leading to the tumor formation as seen in dermatofibrosarcoma protuberans
- COL1A1-PDGFB fusion gene is found in more than 90% of the dermatofibrosarcoma protuberans cases
- In the remaining 10% of the cases, changes in some other unidentified genes may be associated with this condition such as complex t(5;8) involving the CSPG2 and PTK2B genes[23]
Gross Pathology
Gross features of dermatofibrosarcoma protuberans include:
- Nodular, polypoid or plaque-like mass
- Centered in the dermis
- Can also occur in deep soft tissue
- Mean size is 5 cm
- Gray-white, or brown/black in color (if melanocytes are present)
- May appear to be circumscribed
- Rarely shows:
Histopathology
Microscopic features include:
- Non-circumscribed
- Highly cellular with cells having following characteristics:
- Monomorphic
- Thin
- Spindly
- Scant eosinophilic cytoplasm
- Hyperchromatic nuclei (resembling neurofibroma)
- Tight storiform pattern (cells radiating in spokes at right angles around a central point that often contains a vessel) infiltrating deeply into subcutaneous tissue and entraping fat cells to form a characteristic honeycomb pattern
- Areas of fascicular growth (seen in some cases)
- The early plaque stage may lack the particular storiform pattern
- Many non-atypical mitotic figures may be present
- Non-polarized, thin collagen
- Mild pleomorphism (not significant)
- Focal atypia
- May coexist with giant cell fibroblastoma
- Absent or rare histiocytes
- Following cell types are absent:
- Histiocyte-like cells
- Foam cells
- Giant cells
- Other inflammatory cells
- Different variants include:
- Atrophic (depressed lesion)
- Collagenous (with central thick collagen bundles)
- Granular cell (S100 negative)
- Myxoid
- Palisading
- Pigmented
- Sclerosing
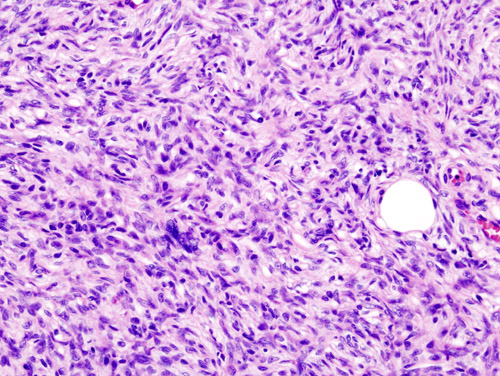 |
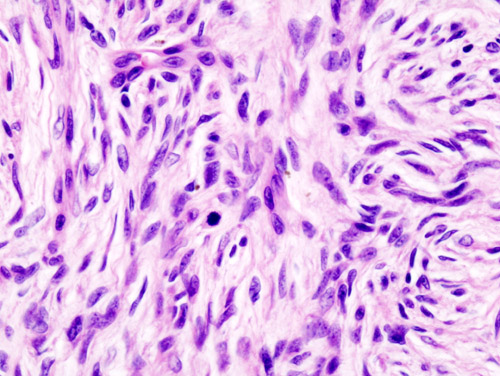 |
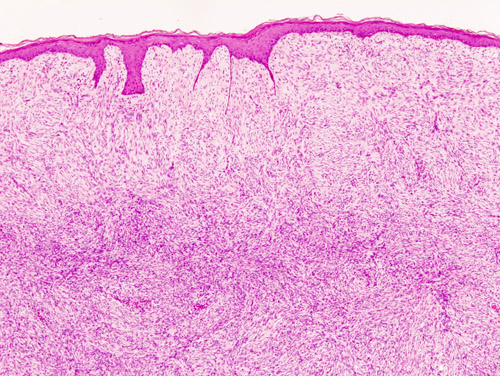 |
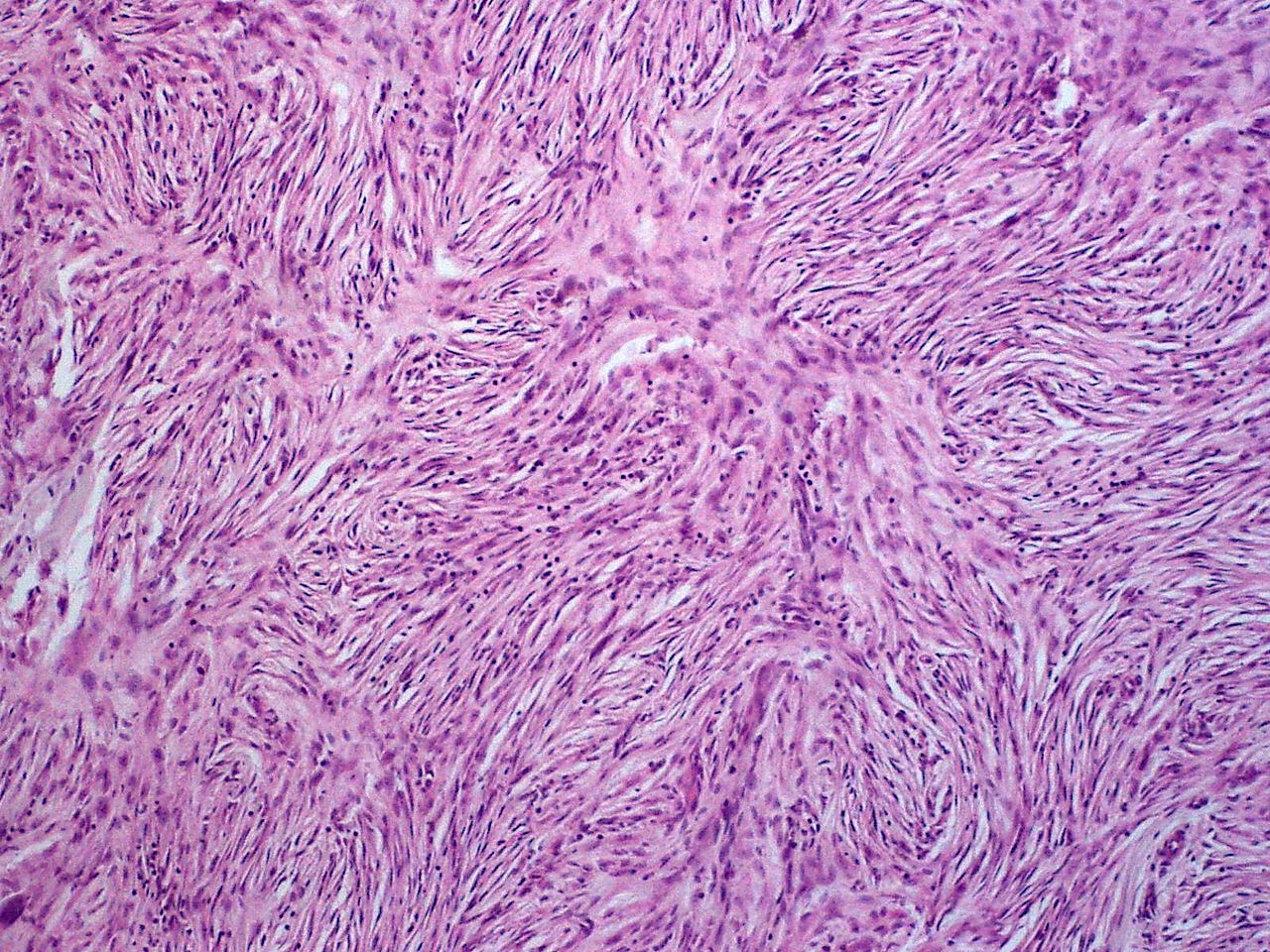 |
Cytology
Cytology of dermatofibrosarcoma has following characteristics:[24][25]
- Homogeneous
- Isolated spindle cells
- Tissue fragments often present with storiform pattern
- Fibrillary stromal fragments
- Naked nuclei
- Slight to moderate atypia (occasionally)[26]
Electron microscopy
- Stellate or spindle cells are present which have long, slender, ramified cell processes which are joined by primitive junctions, often with subplasmalemmal densities[27][28]
- Multivesicular buds are also present commonly[29][30]
Immunohistochemistry
- Immunhistochemical staining of dermatofibrosarcoma protuberans shows:
Epidemiology and Demographics
- Dermatofibrosarcoma protuberans is estimated to occur in 1 in 100,000 to 1-5 in 1 million people per year
- It usually occurs in adults of 20 - 40 years of age
- It affects twice more commonly the blacks than whites in US[37]
- It can also occur in infants and children[38][39][40]
- It has a stable incidence which is highest among women[41][42]
- Worse survival is associated with:
Risk Factors
- Common risk factors for the development of dermatofibrosarcoma include:
Diagnosis
History and symptoms
- Symptoms include:[43]
- Flat or a slightly raised skin patch (first sign)
- 1 to 5 centimeters in diameter
- Rubbery (hard to touch)
- Gives the appearance of a scar or a wrinkled skin patch
- Skin-colored, violet or reddish-brown
- Soft, depressed skin area (rarely, makes it difficult to diagnose)
- Grows very slowly over the period of years
Common sites of involvement
Physical examination
- On physical examination, it can be felt as a small, purplish, reddish, or flesh-colored, flat or raised skin patch or nodule almost 1-5 centimeters in diameter
Skin
Extremities
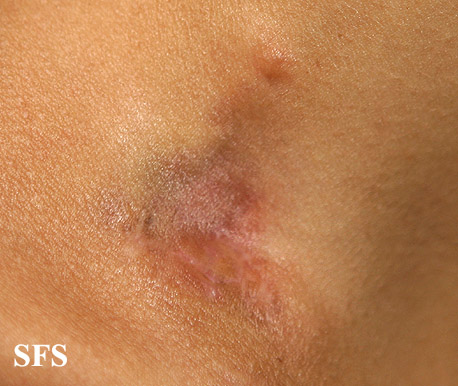 |
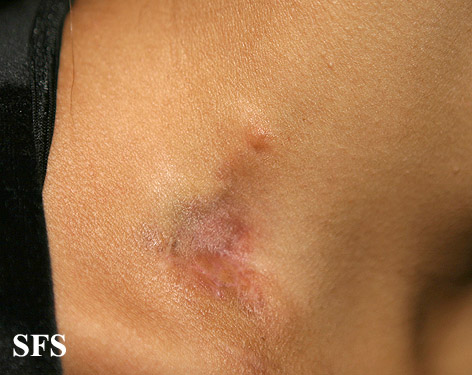 |
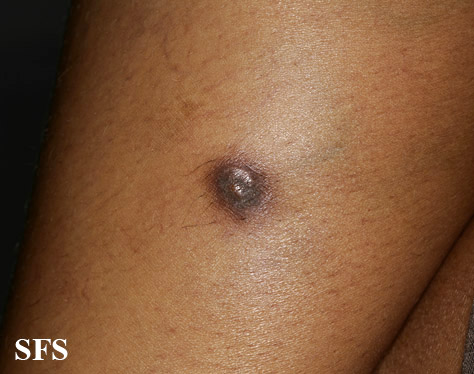 |
Trunk
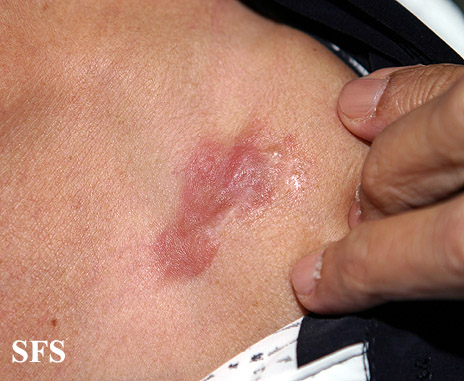 |
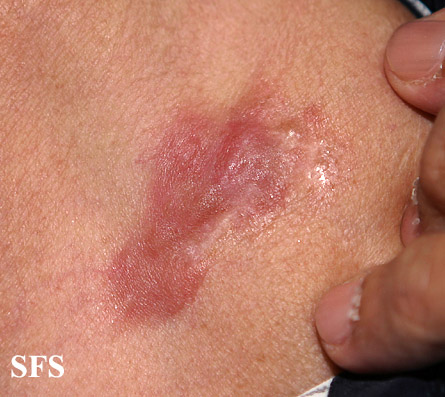 |
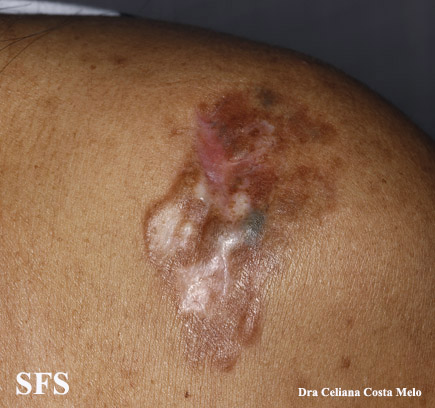 |
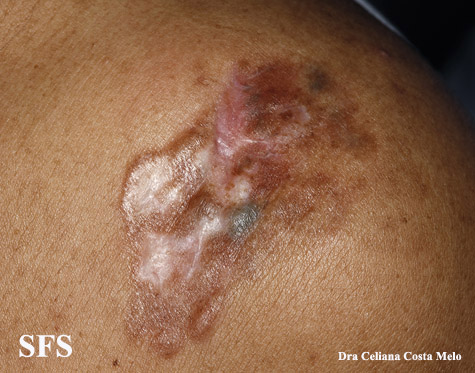 |
Diagnostic studies
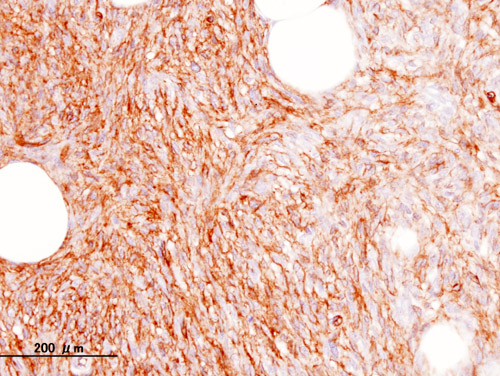 |
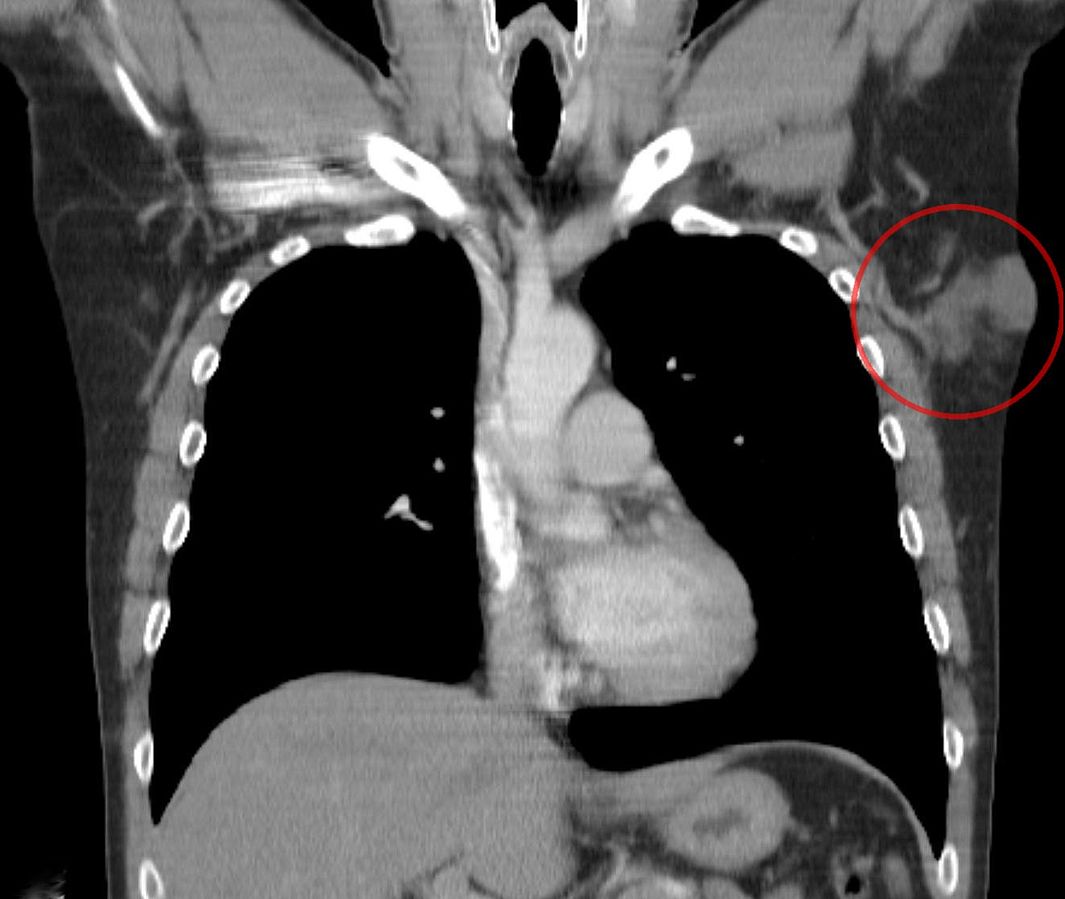 |
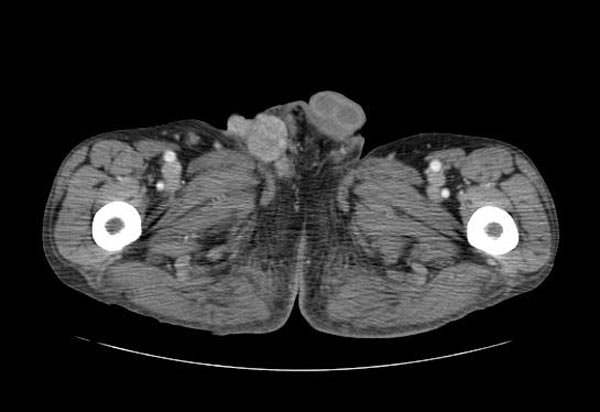 |
Treatment
| Treatment option | Details |
|---|---|
| Surgery |
|
| Targeted therapy |
|
| Chemotherapy |
|
| Radiotherapy |
|
Differential Diagnosis
Dermatofibrosarcoma protuberans must be differentiated must be differentiated from the following:[54][55][56]
- Neurofibroma
- Schwannoma
- Ganglioneuroma
- Dermal neurotized melanocytic nevus
- Myxoid liposarcoma
- Solitary circumscribed neuroma/palisaded encapsulated neuroma
- Traumatic neuroma
- Superficial angiomyxoma
- Nerve sheath myxoma
- Malignant peripheral nerve sheath tumor (MPNST)/malignant schwannoma
- Spindle cell lipoma
- Leiomyoma
- Inflammatory myofibroblastic tumor
- Fibroepithelial polyp/acrochordon (aka skin tag or soft fibroma)
References
- ↑ Lee SW, Zaesim A, Jackson A, Borkat M (2018). "Fibrosarcomatous dermatofibrosarcoma protuberans from scar following trauma". Autops Case Rep. 8 (4): e2018039. doi:10.4322/acr.2018.039. PMC 6360829. PMID 30775318.
- ↑ 2.0 2.1 Mentzel T, Schärer L, Kazakov DV, Michal M (2007). "Myxoid dermatofibrosarcoma protuberans: clinicopathologic, immunohistochemical, and molecular analysis of eight cases". Am J Dermatopathol. 29 (5): 443–8. doi:10.1097/DAD.0b013e318145413c. PMID 17890911.
- ↑ Reimann JD, Fletcher CD (2007). "Myxoid dermatofibrosarcoma protuberans: a rare variant analyzed in a series of 23 cases". Am J Surg Pathol. 31 (9): 1371–7. doi:10.1097/PAS.0b013e31802ff7e7. PMID 17721193.
- ↑ http://ghr.nlm.nih.gov/condition/dermatofibrosarcoma-protuberans
- ↑ Yokoyama D, Kunisada M, Nakamura K, Takemori C, Tajima S, Sudo T; et al. (2019). "Case of two lesions of dermatofibrosarcoma protuberans revealing identical COL1A1-PDGFB fusion gene: Skin metastasis or multicentric lesions?". J Dermatol. doi:10.1111/1346-8138.15028. PMID 31353504.
- ↑ Abbott JJ, Erickson-Johnson M, Wang X, Nascimento AG, Oliveira AM (2006). "Gains of COL1A1-PDGFB genomic copies occur in fibrosarcomatous transformation of dermatofibrosarcoma protuberans". Mod Pathol. 19 (11): 1512–8. doi:10.1038/modpathol.3800695. PMID 16980946.
- ↑ Jahanseir K, Xing D, Greipp PT, Sukov WR, Keeney GL, Howitt BE; et al. (2018). "PDGFB Rearrangements in Dermatofibrosarcoma Protuberans of the Vulva: A Study of 11 Cases Including Myxoid and Fibrosarcomatous Variants". Int J Gynecol Pathol. 37 (6): 537–546. doi:10.1097/PGP.0000000000000472. PMC 5951727. PMID 29140881.
- ↑ Ha SY, Lee SE, Kwon MJ, Kim YJ, Lee EH, Seo J; et al. (2013). "PDGFB rearrangement in dermatofibrosarcoma protuberans: correlation with clinicopathologic characteristics and clinical implications". Hum Pathol. 44 (7): 1300–9. doi:10.1016/j.humpath.2012.09.021. PMID 23347652.
- ↑ Segura S, Salgado R, Toll A, Martín-Ezquerra G, Yébenes M, Sáez A; et al. (2011). "Identification of t(17;22)(q22;q13) (COL1A1/PDGFB) in dermatofibrosarcoma protuberans by fluorescence in situ hybridization in paraffin-embedded tissue microarrays". Hum Pathol. 42 (2): 176–84. doi:10.1016/j.humpath.2010.07.015. PMID 21111450.
- ↑ Wang J, Hisaoka M, Shimajiri S, Morimitsu Y, Hashimoto H (1999). "Detection of COL1A1-PDGFB fusion transcripts in dermatofibrosarcoma protuberans by reverse transcription-polymerase chain reaction using archival formalin-fixed, paraffin-embedded tissues". Diagn Mol Pathol. 8 (3): 113–9. PMID 10565681.
- ↑ Gökden N, Dehner LP, Zhu X, Pfeifer JD (2003). "Dermatofibrosarcoma protuberans of the vulva and groin: detection of COL1A1-PDGFB fusion transcripts by RT-PCR". J Cutan Pathol. 30 (3): 190–5. PMID 12641779.
- ↑ 12.0 12.1 Patel KU, Szabo SS, Hernandez VS, Prieto VG, Abruzzo LV, Lazar AJ; et al. (2008). "Dermatofibrosarcoma protuberans COL1A1-PDGFB fusion is identified in virtually all dermatofibrosarcoma protuberans cases when investigated by newly developed multiplex reverse transcription polymerase chain reaction and fluorescence in situ hybridization assays". Hum Pathol. 39 (2): 184–93. doi:10.1016/j.humpath.2007.06.009. PMID 17950782.
- ↑ 13.0 13.1 Kutzner H, Mentzel T, Palmedo G, Hantschke M, Rütten A, Paredes BE; et al. (2010). "Plaque-like CD34-positive dermal fibroma ("medallion-like dermal dendrocyte hamartoma"): clinicopathologic, immunohistochemical, and molecular analysis of 5 cases emphasizing its distinction from superficial, plaque-like dermatofibrosarcoma protuberans". Am J Surg Pathol. 34 (2): 190–201. doi:10.1097/PAS.0b013e3181c7cf11. PMID 20061935.
- ↑ Mentzel T, Beham A, Katenkamp D, Dei Tos AP, Fletcher CD (1998). "Fibrosarcomatous ("high-grade") dermatofibrosarcoma protuberans: clinicopathologic and immunohistochemical study of a series of 41 cases with emphasis on prognostic significance". Am J Surg Pathol. 22 (5): 576–87. PMID 9591728.
- ↑ Abbott JJ, Oliveira AM, Nascimento AG (2006). "The prognostic significance of fibrosarcomatous transformation in dermatofibrosarcoma protuberans". Am J Surg Pathol. 30 (4): 436–43. PMID 16625088.
- ↑ 16.0 16.1 Terrier-Lacombe MJ, Guillou L, Maire G, Terrier P, Vince DR, de Saint Aubain Somerhausen N; et al. (2003). "Dermatofibrosarcoma protuberans, giant cell fibroblastoma, and hybrid lesions in children: clinicopathologic comparative analysis of 28 cases with molecular data--a study from the French Federation of Cancer Centers Sarcoma Group". Am J Surg Pathol. 27 (1): 27–39. PMID 12502925.
- ↑ Simon MP, Navarro M, Roux D, Pouysségur J (2001). "Structural and functional analysis of a chimeric protein COL1A1-PDGFB generated by the translocation t(17;22)(q22;q13.1) in Dermatofibrosarcoma protuberans (DP)". Oncogene. 20 (23): 2965–75. doi:10.1038/sj.onc.1204426. PMID 11420709.
- ↑ Simon MP, Pedeutour F, Sirvent N, Grosgeorge J, Minoletti F, Coindre JM; et al. (1997). "Deregulation of the platelet-derived growth factor B-chain gene via fusion with collagen gene COL1A1 in dermatofibrosarcoma protuberans and giant-cell fibroblastoma". Nat Genet. 15 (1): 95–8. doi:10.1038/ng0197-95. PMID 8988177.
- ↑ O'Brien KP, Seroussi E, Dal Cin P, Sciot R, Mandahl N, Fletcher JA; et al. (1998). "Various regions within the alpha-helical domain of the COL1A1 gene are fused to the second exon of the PDGFB gene in dermatofibrosarcomas and giant-cell fibroblastomas". Genes Chromosomes Cancer. 23 (2): 187–93. PMID 9739023.
- ↑ Maire G, Martin L, Michalak-Provost S, Gattas GJ, Turc-Carel C, Lorette G; et al. (2002). "Fusion of COL1A1 exon 29 with PDGFB exon 2 in a der(22)t(17;22) in a pediatric giant cell fibroblastoma with a pigmented Bednar tumor component. Evidence for age-related chromosomal pattern in dermatofibrosarcoma protuberans and related tumors". Cancer Genet Cytogenet. 134 (2): 156–61. PMID 12034531.
- ↑ Vanni R, Faa G, Dettori T, Melis GB, Dumanski JP, O'Brien KP (2000). "A case of dermatofibrosarcoma protuberans of the vulva with a COL1A1/PDGFB fusion identical to a case of giant cell fibroblastoma". Virchows Arch. 437 (1): 95–100. PMID 10963386.
- ↑ Macarenco RS, Zamolyi R, Franco MF, Nascimento AG, Abott JJ, Wang X; et al. (2008). "Genomic gains of COL1A1-PDFGB occur in the histologic evolution of giant cell fibroblastoma into dermatofibrosarcoma protuberans". Genes Chromosomes Cancer. 47 (3): 260–5. doi:10.1002/gcc.20530. PMID 18069662.
- ↑ Bianchini L, Maire G, Guillot B, Joujoux JM, Follana P, Simon MP; et al. (2008). "Complex t(5;8) involving the CSPG2 and PTK2B genes in a case of dermatofibrosarcoma protuberans without the COL1A1-PDGFB fusion". Virchows Arch. 452 (6): 689–96. doi:10.1007/s00428-008-0580-2. PMID 18253748.
- ↑ 24.0 24.1 Klijanienko J, Caillaud JM, Lagacé R (2004). "Fine-needle aspiration of primary and recurrent dermatofibrosarcoma protuberans". Diagn Cytopathol. 30 (4): 261–5. doi:10.1002/dc.20024. PMID 15048962.
- ↑ 25.0 25.1 Domanski HA, Gustafson P (2002). "Cytologic features of primary, recurrent, and metastatic dermatofibrosarcoma protuberans". Cancer. 96 (6): 351–61. doi:10.1002/cncr.10760. PMID 12478683.
- ↑ 26.0 26.1 Domanski HA (2005). "FNA diagnosis of dermatofibrosarcoma protuberans". Diagn Cytopathol. 32 (5): 299–302. doi:10.1002/dc.20238. PMID 15830369.
- ↑ Li N, McNiff J, Hui P, Manfioletti G, Tallini G (2004). "Differential expression of HMGA1 and HMGA2 in dermatofibroma and dermatofibrosarcoma protuberans: potential diagnostic applications, and comparison with histologic findings, CD34, and factor XIIIa immunoreactivity". Am J Dermatopathol. 26 (4): 267–72. PMID 15249855.
- ↑ Mori T, Misago N, Yamamoto O, Toda S, Narisawa Y (2008). "Expression of nestin in dermatofibrosarcoma protuberans in comparison to dermatofibroma". J Dermatol. 35 (7): 419–25. doi:10.1111/j.1346-8138.2008.00496.x. PMID 18705829.
- ↑ Dominguez-Malagon H, Valdez-Carrillo Mdel C, Cano-Valdez AM (2006). "Dermatofibroma and dermatofibrosarcoma protuberans: a comparative ultrastructural study". Ultrastruct Pathol. 30 (4): 283–91. doi:10.1080/01913120600820468. PMID 16971353.
- ↑ Kahn HJ, Fekete E, From L (2001). "Tenascin differentiates dermatofibroma from dermatofibrosarcoma protuberans: comparison with CD34 and factor XIIIa". Hum Pathol. 32 (1): 50–6. doi:10.1053/hupa.2001.21137. PMID 11172295.
- ↑ West RB, Harvell J, Linn SC, Liu CL, Prapong W, Hernandez-Boussard T; et al. (2004). "Apo D in soft tissue tumors: a novel marker for dermatofibrosarcoma protuberans". Am J Surg Pathol. 28 (8): 1063–9. PMID 15252314.
- ↑ Lisovsky M, Hoang MP, Dresser KA, Kapur P, Bhawan J, Mahalingam M (2008). "Apolipoprotein D in CD34-positive and CD34-negative cutaneous neoplasms: a useful marker in differentiating superficial acral fibromyxoma from dermatofibrosarcoma protuberans". Mod Pathol. 21 (1): 31–8. doi:10.1038/modpathol.3800971. PMID 17885669.
- ↑ Bandarchi B, Ma L, Marginean C, Hafezi S, Zubovits J, Rasty G (2010). "D2-40, a novel immunohistochemical marker in differentiating dermatofibroma from dermatofibrosarcoma protuberans". Mod Pathol. 23 (3): 434–8. doi:10.1038/modpathol.2009.176. PMID 20062007.
- ↑ Ma CK, Zarbo RJ, Gown AM (1992). "Immunohistochemical characterization of atypical fibroxanthoma and dermatofibrosarcoma protuberans". Am J Clin Pathol. 97 (4): 478–83. doi:10.1093/ajcp/97.4.478. PMID 1553911.
- ↑ Diwan AH, Skelton HG, Horenstein MG, Kelly DR, Barrett TL, Bussian AH; et al. (2008). "Dermatofibrosarcoma protuberans and giant cell fibroblastoma exhibit CD99 positivity". J Cutan Pathol. 35 (7): 647–50. doi:10.1111/j.1600-0560.2007.00872.x. PMID 18201229.
- ↑ Labonte S, Hanna W, Bandarchi-Chamkhaleh B (2007). "A study of CD117 expression in dermatofibrosarcoma protuberans and cellular dermatofibroma". J Cutan Pathol. 34 (11): 857–60. doi:10.1111/j.1600-0560.2007.00731.x. PMID 17944726.
- ↑ Criscione VD, Weinstock MA (2007). "Descriptive epidemiology of dermatofibrosarcoma protuberans in the United States, 1973 to 2002". J Am Acad Dermatol. 56 (6): 968–73. doi:10.1016/j.jaad.2006.09.006. PMID 17141362.
- ↑ Reddy C, Hayward P, Thompson P, Kan A (2009). "Dermatofibrosarcoma protuberans in children". J Plast Reconstr Aesthet Surg. 62 (6): 819–23. doi:10.1016/j.bjps.2007.11.009. PMID 18096453.
- ↑ Zaraa I, Ben abdallah M, Driss M, Trojjet S, Ben Sassi M, El Euch D; et al. (2011). "[Dermatofibrosarcoma protuberans in children]". Arch Pediatr. 18 (1): 23–7. doi:10.1016/j.arcped.2010.09.010. PMID 20952167.
- ↑ Maire G, Fraitag S, Galmiche L, Keslair F, Ebran N, Terrier-Lacombe MJ; et al. (2007). "A clinical, histologic, and molecular study of 9 cases of congenital dermatofibrosarcoma protuberans". Arch Dermatol. 143 (2): 203–10. doi:10.1001/archderm.143.2.203. PMID 17310000.
- ↑ Kreicher KL, Kurlander DE, Gittleman HR, Barnholtz-Sloan JS, Bordeaux JS (2016). "Incidence and Survival of Primary Dermatofibrosarcoma Protuberans in the United States". Dermatol Surg. 42 Suppl 1: S24–31. doi:10.1097/DSS.0000000000000300. PMID 26730971.
- ↑ Kuzel P, Metelitsa AI, Dover DC, Salopek TG (2012). "Epidemiology of dermatofibrosarcoma protuberans in Alberta, Canada, from 1988 to 2007". Dermatol Surg. 38 (9): 1461–8. doi:10.1111/j.1524-4725.2012.02482.x. PMID 22691126.
- ↑ Maji S, Paul MJ, Sen S (2018). "Dermatofibrosarcoma Protuberans of the Breast-a Rare Entity". Indian J Surg Oncol. 9 (3): 351–354. doi:10.1007/s13193-017-0684-8. PMC 6154368. PMID 30287997.
- ↑ Zee SY, Wang Q, Jones CM, Abadi MA (2002). "Fine needle aspiration cytology of dermatofibrosarcoma protuberans presenting as a breast mass. A case report". Acta Cytol. 46 (4): 741–3. doi:10.1159/000326988. PMID 12146044.
- ↑ Filipowicz EA, Ventura KC, Pou AM, Logrono R (1999). "FNAC in the diagnosis of recurrent dermatofibrosarcoma protuberans of the forehead. A case report". Acta Cytol. 43 (6): 1177–80. doi:10.1159/000331376. PMID 10579001.
- ↑ Zamecnik M (2001). "Fibrosarcomatous dermatofibrosarcoma protuberans with giant rosettes". Am J Dermatopathol. 23 (1): 41–5. PMID 11176051.
- ↑ Harvell JD, Kilpatrick SE, White WL (1998). "Histogenetic relations between giant cell fibroblastoma and dermatofibrosarcoma protuberans. CD34 staining showing the spectrum and a simulator". Am J Dermatopathol. 20 (4): 339–45. PMID 9700370.
- ↑ Huis In 't Veld EA, van Houdt WJ (2019). "Reply to Follow-up after treatment of dermatofibrosarcoma protuberans". Cancer. doi:10.1002/cncr.32341. PMID 31251395.
- ↑ Loss L, Zeitouni NC (2005). "Management of scalp dermatofibrosarcoma protuberans". Dermatol Surg. 31 (11 Pt 1): 1428–33. PMID 16416612.
- ↑ Dawes KW, Hanke CW (1996). "Dermatofibrosarcoma protuberans treated with Mohs micrographic surgery: cure rates and surgical margins". Dermatol Surg. 22 (6): 530–4. PMID 8646467.
- ↑ Bowne WB, Antonescu CR, Leung DH, Katz SC, Hawkins WG, Woodruff JM; et al. (2000). "Dermatofibrosarcoma protuberans: A clinicopathologic analysis of patients treated and followed at a single institution". Cancer. 88 (12): 2711–20. PMID 10870053.
- ↑ DuBay D, Cimmino V, Lowe L, Johnson TM, Sondak VK (2004). "Low recurrence rate after surgery for dermatofibrosarcoma protuberans: a multidisciplinary approach from a single institution". Cancer. 100 (5): 1008–16. doi:10.1002/cncr.20051. PMID 14983497.
- ↑ Snow SN, Gordon EM, Larson PO, Bagheri MM, Bentz ML, Sable DB (2004). "Dermatofibrosarcoma protuberans: a report on 29 patients treated by Mohs micrographic surgery with long-term follow-up and review of the literature". Cancer. 101 (1): 28–38. doi:10.1002/cncr.20316. PMID 15221986.
- ↑ Neurofibroma. Libre Pathology 2015. http://librepathology.org/wiki/index.php/Neurofibroma#cite_note-pmid15486243-2 Accessed on November 17, 2015
- ↑ http://surgpathcriteria.stanford.edu/peripheral-nerve/neurofibroma/
- ↑ http://surgpathcriteria.stanford.edu/peripheral-nerve/neurofibroma/
- ↑ Rodriguez, Fausto J.; Folpe, Andrew L.; Giannini, Caterina; Perry, Arie (2012). "Pathology of peripheral nerve sheath tumors: diagnostic overview and update on selected diagnostic problems". Acta Neuropathologica. 123 (3): 295–319. doi:10.1007/s00401-012-0954-z. ISSN 0001-6322.
- ↑ Choi, Kwangmin; Komurov, Kakajan; Fletcher, Jonathan S.; Jousma, Edwin; Cancelas, Jose A.; Wu, Jianqiang; Ratner, Nancy (2017). "An inflammatory gene signature distinguishes neurofibroma Schwann cells and macrophages from cells in the normal peripheral nervous system". Scientific Reports. 7 (1). doi:10.1038/srep43315. ISSN 2045-2322.
- ↑ Liao, Chung-Ping; Booker, Reid C.; Brosseau, Jean-Philippe; Chen, Zhiguo; Mo, Juan; Tchegnon, Edem; Wang, Yong; Clapp, D. Wade; Le, Lu Q. (2018). "Contributions of inflammation and tumor microenvironment to neurofibroma tumorigenesis". Journal of Clinical Investigation. 128 (7): 2848–2861. doi:10.1172/JCI99424. ISSN 0021-9738.
- ↑ 60.0 60.1 Staser, K.; Yang, F.-C.; Clapp, D. W. (2010). "Mast cells and the neurofibroma microenvironment". Blood. 116 (2): 157–164. doi:10.1182/blood-2009-09-242875. ISSN 0006-4971.
- ↑ Muir, David; Neubauer, Debbie; Lim, Ingrid T.; Yachnis, Anthony T.; Wallace, Margaret R. (2001). "Tumorigenic Properties of Neurofibromin-Deficient Neurofibroma Schwann Cells". The American Journal of Pathology. 158 (2): 501–513. doi:10.1016/S0002-9440(10)63992-2. ISSN 0002-9440.
- ↑ Wilkinson, Lana M.; Manson, David; Smith, Charles R. (2004). "Best Cases from the AFIP". RadioGraphics. 24 (suppl_1): S237–S242. doi:10.1148/rg.24si035170. ISSN 0271-5333.
- ↑ Bernthal, Nicholas; Jones, Kevin; Monument, Michael; Liu, Ting; Viskochil, David; Randall, R. (2013). "Lost in Translation: Ambiguity in Nerve Sheath Tumor Nomenclature and Its Resultant Treatment Effect". Cancers. 5 (4): 519–528. doi:10.3390/cancers5020519. ISSN 2072-6694.
- ↑ Mautner, V. F.; Friedrich, R. E.; von Deimling, A.; Hagel, C.; Korf, B.; Knöfel, M. T.; Wenzel, R.; Fünsterer, C. (2003). "Malignant peripheral nerve sheath tumours in neurofibromatosis type 1: MRI supports the diagnosis of malignant plexiform neurofibroma". Neuroradiology. 45 (9): 618–625. doi:10.1007/s00234-003-0964-6. ISSN 0028-3940.
- ↑ Shen, M H; Harper, P S; Upadhyaya, M (1996). "Molecular genetics of neurofibromatosis type 1 (NF1)". Journal of Medical Genetics. 33 (1): 2–17. doi:10.1136/jmg.33.1.2. ISSN 1468-6244.
- ↑ Rubin, Joshua B.; Gutmann, David H. (2005). "Neurofibromatosis type 1 — a model for nervous system tumour formation?". Nature Reviews Cancer. 5 (7): 557–564. doi:10.1038/nrc1653. ISSN 1474-175X.
- ↑ Gray, Mark H. (1990). "Immunohistochemical Demonstration of Factor XIIIa Expression in Neurofibromas". Archives of Dermatology. 126 (4): 472. doi:10.1001/archderm.1990.01670280056009. ISSN 0003-987X.
- ↑ Schwannoma. Dr Tim Luijkx and Dr Sara Wein et al. http://radiopaedia.org/articles/schwannoma
- ↑ Vestibular Schwannoma. Wikipedia(2015) https://en.wikipedia.org/wiki/Vestibular_schwannoma Accessed on October 2 2015
- ↑ Giordano J, Rogers LV (1989). "Peripherally administered serotonin 5-HT3 receptor antagonists reduce inflammatory pain in rats". European Journal of Pharmacology. 170 (1–2): 83–6. PMID 2612565.
|access-date=requires|url=(help) - ↑ Kolvenbach H, Lauven PM, Schneider B, Kunath U (1989). "Repetitive intercostal nerve block via catheter for postoperative pain relief after thoracotomy". The Thoracic and Cardiovascular Surgeon. 37 (5): 273–6. doi:10.1055/s-2007-1020331. PMID 2588243. Retrieved 2015-11-20.
- ↑ Opaleva-Stegantseva VA, Ivanov AG, Gavrilina IA, Khar'kov EI, Ratovskaia VI (1986). "[Incidence of sudden death cases in acute coronary insufficiency and acute myocardial infarction at the pre-hospital stage in Krasnoyarsk]". Kardiologiia (in Russian). 26 (5): 23–6. PMID 3735913.
|access-date=requires|url=(help) - ↑ Misago N, Inoue T, Narisawa Y (2007). "Unusual benign myxoid nerve sheath lesion: myxoid palisaded encapsulated neuroma (PEN) or nerve sheath myxoma with PEN/PEN-like features?". Am J Dermatopathol. 29 (2): 160–4. doi:10.1097/01.dad.0000256688.91974.09. PMID 17414438.
- ↑ Lee EJ, Calcaterra TC, Zuckerbraun L (1998). "Traumatic neuromas of the head and neck". Ear Nose Throat J. 77 (8): 670–4, 676. PMID 9745184.
- ↑ Hanna SA, Catapano J, Borschel GH (2016). "Painful pediatric traumatic neuroma: surgical management and clinical outcomes". Childs Nerv Syst. 32 (7): 1191–4. doi:10.1007/s00381-016-3109-z. PMID 27179535.
- ↑ Foltán R, Klíma K, Spacková J, Sedý J (2008). "Mechanism of traumatic neuroma development". Med Hypotheses. 71 (4): 572–6. doi:10.1016/j.mehy.2008.05.010. PMID 18599222.
- ↑ Yao C, Zhou X, Zhao B, Sun C, Poonit K, Yan H (2017). "Treatments of traumatic neuropathic pain: a systematic review". Oncotarget. 8 (34): 57670–57679. doi:10.18632/oncotarget.16917. PMC 5593675. PMID 28915703.
- ↑ Gray MH, Smoller BR, McNutt NS, Hsu A (1990). "Neurofibromas and neurotized melanocytic nevi are immunohistochemically distinct neoplasms". Am J Dermatopathol. 12 (3): 234–41. PMID 1693815.
- ↑ Chen Y, Klonowski PW, Lind AC, Lu D (2012). "Differentiating neurotized melanocytic nevi from neurofibromas using Melan-A (MART-1) immunohistochemical stain". Arch Pathol Lab Med. 136 (7): 810–5. doi:10.5858/arpa.2011-0335-OA. PMID 22742554.
- ↑ Singh N, Chandrashekar L, Kar R, Sylvia MT, Thappa DM (2015). "Neurotized congenital melanocytic nevus resembling a pigmented neurofibroma". Indian J Dermatol. 60 (1): 46–50. doi:10.4103/0019-5154.147789. PMC 4318062. PMID 25657396.
- ↑ Gray MH, Smoller BR, McNutt NS, Hsu A (1990). "Immunohistochemical demonstration of factor XIIIa expression in neurofibromas. A practical means of differentiating these tumors from neurotized melanocytic nevi and schwannomas". Arch Dermatol. 126 (4): 472–6. PMID 1690969.
- ↑ https://www.sciencedirect.com/topics/medicine-and-dentistry/cutaneous-myxoma
- ↑ Alaiti, Samer; Nelson, Fern P.; Ryoo, Jei W. (2000). "Solitary cutaneous myxoma". Journal of the American Academy of Dermatology. 43 (2): 377–379. doi:10.1067/mjd.2000.101878. ISSN 0190-9622.
- ↑ Carney, J. Aidan (1986). "Cutaneous Myxomas". Archives of Dermatology. 122 (7): 790. doi:10.1001/archderm.1986.01660190068018. ISSN 0003-987X.
- ↑ Iida, Ken; Egi, Takeshi; Shigi, Masato; Sogabe, Yusuke; Ohashi, Hirotsugu (2019). "Cutaneous Myxoma of Multiple Lesions". Plastic and Reconstructive Surgery - Global Open. 7 (2): e2040. doi:10.1097/GOX.0000000000002040. ISSN 2169-7574.
- ↑ Fetsch JF, Laskin WB, Miettinen M (2005). "Nerve sheath myxoma: a clinicopathologic and immunohistochemical analysis of 57 morphologically distinctive, S-100 protein- and GFAP-positive, myxoid peripheral nerve sheath tumors with a predilection for the extremities and a high local recurrence rate". Am J Surg Pathol. 29 (12): 1615–24. PMID 16327434.
- ↑ Yadav SK, Singh S, Sarin N, Naeem R, Pruthi SK (2019). "Nerve Sheath Myxoma of Scalp: A Rare Site of Presentation". Int J Trichology. 11 (1): 34–37. doi:10.4103/ijt.ijt_45_18. PMC 6385516. PMID 30820132.
- ↑ Bhat A, Narasimha A, C V, Vk S (2015). "Nerve sheath myxoma: report of a rare case". J Clin Diagn Res. 9 (4): ED07–9. doi:10.7860/JCDR/2015/10911.5810. PMC 4437072. PMID 26023558.
- ↑ Avninder S, Ramesh V, Vermani S (2007). "Benign nerve sheath myxoma (myxoid neurothekeoma) in the leg". Dermatol Online J. 13 (2): 14. PMID 17498433.
- ↑ Kim BW, Won CH, Chang SE, Lee MW (2014). "A case of nerve sheath myxoma on finger". Indian J Dermatol. 59 (1): 99–101. doi:10.4103/0019-5154.123526. PMC 3884944. PMID 24470676.
- ↑ Pulitzer DR, Reed RJ (1985). "Nerve-sheath myxoma (perineurial myxoma)". Am J Dermatopathol. 7 (5): 409–21. PMID 4091218.
- ↑ Valeyrie-Allanore, L.; Ismaili, N.; Bastuji-Garin, S.; Zeller, J.; Wechsler, J.; Revuz, J.; Wolkenstein, P. (2005). "Symptoms associated with malignancy of peripheral nerve sheath tumours: a retrospective study of 69 patients with neurofibromatosis 1". British Journal of Dermatology. 153 (1): 79–82. doi:10.1111/j.1365-2133.2005.06558.x. ISSN 0007-0963.
- ↑ Evans DG, Baser ME, McGaughran J, Sharif S, Howard E, Moran A (2002). "Malignant peripheral nerve sheath tumours in neurofibromatosis 1". J Med Genet. 39 (5): 311–4. PMC 1735122. PMID 12011145.
- ↑ Panigrahi S, Mishra SS, Das S, Dhir MK (2013). "Primary malignant peripheral nerve sheath tumor at unusual location". J Neurosci Rural Pract. 4 (Suppl 1): S83–6. doi:10.4103/0976-3147.116480. PMC 3808069. PMID 24174807.
- ↑ Ferrari A, Bisogno G, Carli M (2007). "Management of childhood malignant peripheral nerve sheath tumor". Paediatr Drugs. 9 (4): 239–48. doi:10.2165/00148581-200709040-00005. PMID 17705563.
- ↑ Neville H, Corpron C, Blakely ML, Andrassy R (2003). "Pediatric neurofibrosarcoma". J Pediatr Surg. 38 (3): 343–6, discussion 343-6. doi:10.1053/jpsu.2003.50105. PMID 12632346.
- ↑ Zehou, Ouidad; Fabre, Elizabeth; Zelek, Laurent; Sbidian, Emilie; Ortonne, Nicolas; Banu, Eugeniu; Wolkenstein, Pierre; Valeyrie-Allanore, Laurence (2013). "Chemotherapy for the treatment of malignant peripheral nerve sheath tumors in neurofibromatosis 1: a 10-year institutional review". Orphanet Journal of Rare Diseases. 8 (1): 127. doi:10.1186/1750-1172-8-127. ISSN 1750-1172.
- ↑ Vasiliadis, K.; Papavasiliou, C.; Fachiridis, D.; Pervana, S.; Michaelides, M.; Kiranou, M.; Makridis, C. (2012). "Retroperitoneal extra-adrenal ganglioneuroma involving the infrahepatic inferior vena cava, celiac axis and superior mesenteric artery: A case report". International Journal of Surgery Case Reports. 3 (11): 541–543. doi:10.1016/j.ijscr.2012.07.008. ISSN 2210-2612.
- ↑ https://radiopaedia.org/articles/ganglioneuroma
- ↑ Khin Thway, Rashpal Flora, Chirag Shah, David Olmos & Cyril Fisher (2012). "Diagnostic utility of p16, CDK4, and MDM2 as an immunohistochemical panel in distinguishing well-differentiated and dedifferentiated liposarcomas from other adipocytic tumors". The American journal of surgical pathology. 36 (3): 462–469. doi:10.1097/PAS.0b013e3182417330. PMID 22301498. Unknown parameter
|month=ignored (help) - ↑ J. Rosai, M. Akerman, P. Dal Cin, I. DeWever, C. D. Fletcher, N. Mandahl, F. Mertens, F. Mitelman, A. Rydholm, R. Sciot, G. Tallini, H. Van den Berghe, W. Van de Ven, R. Vanni & H. Willen (1996). "Combined morphologic and karyotypic study of 59 atypical lipomatous tumors. Evaluation of their relationship and differential diagnosis with other adipose tissue tumors (a report of the CHAMP Study Group)". The American journal of surgical pathology. 20 (10): 1182–1189. PMID 8827023. Unknown parameter
|month=ignored (help) - ↑ Dal Cin, Paola; Kools, Patrick; Sciot, Raf; De Wever, Ivo; Van Damme, Boudewijn; Van de Ven, Wim; Van Den Berghe, Herman (1993). "Cytogenetic and fluorescence in situ hybridization investigation of ring chromosomes characterizing a specific pathologic subgroup of adipose tissue tumors". Cancer Genetics and Cytogenetics. 68 (2): 85–90. doi:10.1016/0165-4608(93)90001-3. ISSN 0165-4608.
- ↑ Dei Tos, Angelo P.; Doglioni, Claudio; Piccinin, Sara; Sciot, Raf; Furlanetto, Alberto; Boiocchi, Mauro; Dal Cin, Paola; Maestro, Roberta; Fletcher, Christopher D. M.; Tallini, Giovanni (2000). "Coordinated expression and amplification of theMDM2,CDK4, andHMGI-C genes in atypical lipomatous tumours". The Journal of Pathology. 190 (5): 531–536. doi:10.1002/(SICI)1096-9896(200004)190:5<531::AID-PATH579>3.0.CO;2-W. ISSN 0022-3417.
- ↑ Dei Tos, A (2000). "Liposarcoma: New entities and evolving concepts". Annals of Diagnostic Pathology. 4 (4): 252–266. doi:10.1053/adpa.2000.8133. ISSN 1092-9134.
- ↑ M. D. Kraus, L. Guillou & C. D. Fletcher (1997). "Well-differentiated inflammatory liposarcoma: an uncommon and easily overlooked variant of a common sarcoma". The American journal of surgical pathology. 21 (5): 518–527. PMID 9158675. Unknown parameter
|month=ignored (help) - ↑ P. Argani, F. Facchetti, G. Inghirami & J. Rosai (1997). "Lymphocyte-rich well-differentiated liposarcoma: report of nine cases". The American journal of surgical pathology. 21 (8): 884–895. PMID 9255251. Unknown parameter
|month=ignored (help) - ↑ H. L. Evans (1979). "Liposarcoma: a study of 55 cases with a reassessment of its classification". The American journal of surgical pathology. 3 (6): 507–523. PMID 534388. Unknown parameter
|month=ignored (help) - ↑ A. P. Dei Tos, T. Mentzel, P. L. Newman & C. D. Fletcher (1994). "Spindle cell liposarcoma, a hitherto unrecognized variant of liposarcoma. Analysis of six cases". The American journal of surgical pathology. 18 (9): 913–921. PMID 8067512. Unknown parameter
|month=ignored (help) - ↑ D. C. Dahlin, K. K. Unni & T. Matsuno (1977). "Malignant (fibrous) histiocytoma of bone--fact or fancy?". Cancer. 39 (4): 1508–1516. PMID 192432. Unknown parameter
|month=ignored (help) - ↑ 110.0 110.1 Coffin CM, Watterson J, Priest JR, Dehner LP (1995). "Extrapulmonary inflammatory myofibroblastic tumor (inflammatory pseudotumor). A clinicopathologic and immunohistochemical study of 84 cases". Am J Surg Pathol. 19 (8): 859–72. PMID 7611533.
- ↑ 111.0 111.1 111.2 111.3 Wenig BM, Devaney K, Bisceglia M (1995). "Inflammatory myofibroblastic tumor of the larynx. A clinicopathologic study of eight cases simulating a malignant spindle cell neoplasm". Cancer. 76 (11): 2217–29. PMID 8635024.
- ↑ 112.0 112.1 Ramachandra S, Hollowood K, Bisceglia M, Fletcher CD (1995). "Inflammatory pseudotumour of soft tissues: a clinicopathological and immunohistochemical analysis of 18 cases". Histopathology. 27 (4): 313–23. PMID 8847061.
- ↑ 113.0 113.1 Häusler M, Schaade L, Ramaekers VT, Doenges M, Heimann G, Sellhaus B (2003). "Inflammatory pseudotumors of the central nervous system: report of 3 cases and a literature review". Hum Pathol. 34 (3): 253–62. doi:10.1053/hupa.2003.35. PMID 12673560.
- ↑ 114.0 114.1 114.2 114.3 Rabban JT, Zaloudek CJ, Shekitka KM, Tavassoli FA (2005). "Inflammatory myofibroblastic tumor of the uterus: a clinicopathologic study of 6 cases emphasizing distinction from aggressive mesenchymal tumors". Am J Surg Pathol. 29 (10): 1348–55. PMID 16160478.
- ↑ 115.0 115.1 Kovach SJ, Fischer AC, Katzman PJ, Salloum RM, Ettinghausen SE, Madeb R; et al. (2006). "Inflammatory myofibroblastic tumors". J Surg Oncol. 94 (5): 385–91. doi:10.1002/jso.20516. PMID 16967468.
- ↑ 116.0 116.1 Coffin CM, Dehner LP, Meis-Kindblom JM (1998). "Inflammatory myofibroblastic tumor, inflammatory fibrosarcoma, and related lesions: an historical review with differential diagnostic considerations". Semin Diagn Pathol. 15 (2): 102–10. PMID 9606802.
- ↑ 117.0 117.1 Berardi RS, Lee SS, Chen HP, Stines GJ (1983). "Inflammatory pseudotumors of the lung". Surg Gynecol Obstet. 156 (1): 89–96. PMID 6336632.
- ↑ 118.0 118.1 Coffin CM, Hornick JL, Fletcher CD (2007). "Inflammatory myofibroblastic tumor: comparison of clinicopathologic, histologic, and immunohistochemical features including ALK expression in atypical and aggressive cases". Am J Surg Pathol. 31 (4): 509–20. doi:10.1097/01.pas.0000213393.57322.c7. PMID 17414097.
- ↑ Cukic O, Jovanovic MB (2019). "Large Fibroepithelial Polyp of the Palatine Tonsil". Ear Nose Throat J: 145561319841203. doi:10.1177/0145561319841203. PMID 30997841.
- ↑ Vatansever M, Dinç E, Dursun Ö, Oktay ÖÖ, Arpaci R (2019). "Atypical presentation of fibroepithelial polyp: a report of two cases". Arq Bras Oftalmol. doi:10.5935/0004-2749.20190050. PMID 30916216.
- ↑ Rexhepi M, Trajkovska E, Besimi F, Rufati N (2018). "Giant Fibroepithelial Polyp of Vulva: A Case Report and Review of Literature". Pril (Makedon Akad Nauk Umet Odd Med Nauki). 39 (2–3): 127–130. doi:10.2478/prilozi-2018-0051. PMID 30864355.
- ↑ Jabbour J, Chappell JR, Busby M, McCubbery NW, Brown DF, Park SJK; et al. (2019). "Glottic Obstruction from Fibroepithelial Polyp". Am J Case Rep. 20: 219–223. doi:10.12659/AJCR.914907. PMC 6388646. PMID 30778021.
- ↑ Hong P, Cai Y, Li Z, Fan S, Yang K, Hao H; et al. (2019). "Modified Laparoscopic Partial Ureterectomy for Adult Ureteral Fibroepithelial Polyp: Technique and Initial Experience". Urol Int. 102 (1): 13–19. doi:10.1159/000494804. PMID 30448831.
- ↑ Uçar M, Baş E, Akkoç A, Topçuoğlu M (2018). "Fibroepithelial Polyp of the Ureter: A Rare Cause of Hydronephrosis". J Endourol Case Rep. 4 (1): 166–168. doi:10.1089/cren.2018.0031. PMC 6225073. PMID 30426076.
- ↑ Chaker K, Rhouma SB, Daly KM, Zehani A, Bibi M, Chehida MAB; et al. (2019). "Benign fibroepithelial polyp of the ureter: A case report". Urol Case Rep. 22: 52–53. doi:10.1016/j.eucr.2018.10.019. PMC 6226574. PMID 30425926.
- ↑ Hajji F, Moufid K, Ghoundale O, Touiti D (2019). "A rare case of successful endoscopic management of a fibroepithelial polyp with intussusception of the ureter and periodic prolapse into bladder". Ann R Coll Surg Engl. 101 (2): e66–e70. doi:10.1308/rcsann.2018.0198. PMC 6351868. PMID 30421620.
- ↑ Lee H, Sade I, Gilani S, Zhong M, Lombardo G (2018). "A Giant Fibroepithelial Polyp of the Small Bowel Associated with High-Grade Obstruction". Am Surg. 84 (7): e210–e211. PMID 30401014.
- ↑ Chaker K (2019). "Benign fibroepithelial polyp of the ureter: A case report". Urol Case Rep. 22: 15–16. doi:10.1016/j.eucr.2018.09.021. PMC 6180234. PMID 30319938.
- ↑ Lozano-Peña AK, Lamadrid-Zertuche AC, Ocampo-Candiani J (2019). "Giant fibroepithelial polyp of the vulva". Australas J Dermatol. 60 (1): 70–71. doi:10.1111/ajd.12886. PMID 30009441.
- ↑ Eckstein M, Agaimy A, Woenckhaus J, Winter A, Bittmann I, Janzen J; et al. (2019). "DICER1 mutation-positive giant botryoid fibroepithelial polyp of the urinary bladder mimicking embryonal rhabdomyosarcoma". Hum Pathol. 84: 1–7. doi:10.1016/j.humpath.2018.05.015. PMID 29883781.
- ↑ Akdere H, Çevik G (2018). "Rare Fibroepithelial Polyp Extending Along the Ureter: A Case Report". Balkan Med J. 35 (3): 275–277. doi:10.4274/balkanmedj.2017.1537. PMC 5981127. PMID 29843497.
- ↑ Ballard DH, Rove KO, Coplen DE, Chen TY, Hulett Bowling RL (2018). "Fibroepithelial polyp causing urethral obstruction: Diagnosis by cystourethrogram". Clin Imaging. 51: 164–167. doi:10.1016/j.clinimag.2018.05.009. PMC 6404776. PMID 29800931.
- ↑ Amin A, Amin Z, Al Farsi AR (2018). "Septic presentation of a giant fibroepithelial polyp of the vulva". BMJ Case Rep. 2018. doi:10.1136/bcr-2017-222789. PMID 29574427.
- ↑ Gupta R, Smita S, Sinha R, Sinha N, Sinha L (2018). "Giant fibroepithelial polyp of the thigh and retroperitoneal fibromatosis in a young woman: a rare case". Skeletal Radiol. 47 (9): 1299–1304. doi:10.1007/s00256-018-2904-x. PMID 29487969.
- ↑ Rajeesh Mohammed PK, Choudhury BK, Dalai RP, Rana V (2017). "Fibroepithelial Polyp with Sebaceous Hyperplasia: A Case Report". Indian J Med Paediatr Oncol. 38 (3): 404–406. doi:10.4103/ijmpo.ijmpo_124_17. PMC 5686997. PMID 29200704.
- ↑ Lee MH, Hwang JY, Lee JH, Kim DH, Song SH (2017). "Fibroepithelial polyp of the vulva accompanied by lymphangioma circumscriptum". Obstet Gynecol Sci. 60 (4): 401–404. doi:10.5468/ogs.2017.60.4.401. PMC 5547092. PMID 28791276.
- ↑ Ten Donkelaar CS, Houwert AC, Ten Kate FJW, Lock MTWT (2017). "Polypoid arteriovenous malformation of the ureter mimicking a fibroepithelial polyp, a case report". BMC Urol. 17 (1): 55. doi:10.1186/s12894-017-0237-z. PMC 5504856. PMID 28693464.
- ↑ Saito N, Yamasaki M, Daido W, Ishiyama S, Deguchi N, Taniwaki M (2017). "A bronchial fibroepithelial polyp with abnormal findings on auto-fluorescence imaging". Respirol Case Rep. 5 (5): e00244. doi:10.1002/rcr2.244. PMC 5465754. PMID 28603622.