SLE resident survival guide: Difference between revisions
Iqra Qamar (talk | contribs) |
Iqra Qamar (talk | contribs) |
||
| Line 453: | Line 453: | ||
* Moderate cases are defined as more than 2 organ involvement during disease flares with low grade of involvement and complications or one or two organ involvement with more extensive involvements. | * Moderate cases are defined as more than 2 organ involvement during disease flares with low grade of involvement and complications or one or two organ involvement with more extensive involvements. | ||
* Severe cases are defined as presentation of the disease with life threatening complications and multiple (more than 2) organ involvements. | * Severe cases are defined as presentation of the disease with life threatening complications and multiple (more than 2) organ involvements. | ||
== Severe disease == | |||
* Preferred regimen (1): [[Hydroxychloroquine]] PO 200 to 400 mg daily as a single daily dose or in 2 divided doses '''AND''' [[methylprednisolone]] as [[intravenous]] "pulse"; 0.5 to 1 g/day for three days in acutely ill patients, or 1 to 2 mg/kg/day in more stable patients | |||
* Alternative regimen(1): [[Hydroxychloroquine]] PO 200 to 400 mg daily as a single daily dose or in 2 divided doses '''AND''' [[prednisone]] oral; 40-60 mg/day | |||
* Alternative regimen (2): [[Mycophenolate]] | |||
** For induction: 1 g twice daily for 6 months in combination with a [[glucocorticoid]] | |||
** For maintenance: 0.5-3 g daily or 1 g twice daily | |||
*** Initial period of intensive [[immunosuppressive therapy]] (induction therapy) to control the disease and halt tissue injury | |||
* Alternative regimen (3): [[Cyclophosphamide]] IV 500 mg once every 2 weeks for 6 doses or 500 to 1,000 mg/m2 once every month for 6 doses or 500 to 1,000 mg/m2 every month for 6 months, then every 3 months for a total of at least 2.5 years | |||
* Alternative regimen (4): [[Rituximab]] IV: 375 mg/m2 once weekly for 4 doses or 1,000 mg (flat dose) on days 0 and 15 or 500 to 1,000 mg on days 1 and 15 | |||
== Less Severe (mild and moderate) disease == | |||
* Preferred regimen (1): [[Hydroxychloroquine]] PO 200 to 400 mg daily as a single daily dose or in 2 divided doses | |||
* Preferred regimen (2): [[Prednisone]] PO low doses of 10 mg/d or less for a short term therapy | |||
** For milder SLE | |||
** For treatment of [[cutaneous]] and musculoskeletal symptoms not responding to other therapies | |||
** It should be tapered once [[hydroxychloroquine]] has taken effect | |||
* Alternative regimen (1): [[Azathioprine]] PO initial 2 mg/kg/day; may reduce to 1.5 mg/kg/day after 1 month | |||
** Can be used to control symptoms | |||
* Alternative regimen (2): [[Methotrexate]] PO initial therapy with 7.5 mg once weekly; may increase by 2.5 mg increments weekly | |||
** Can be used to control symptoms | |||
== Other organ specific treatments == | |||
===== Fever management<ref name="pmid27529058">{{cite journal |vauthors=Jordan N, D'Cruz D |title=Current and emerging treatment options in the management of lupus |journal=Immunotargets Ther |volume=5 |issue= |pages=9–20 |year=2016 |pmid=27529058 |pmc=4970629 |doi=10.2147/ITT.S40675 |url=}}</ref><ref name="pmid24830791">{{cite journal |vauthors=Cobo-Ibáñez T, Loza-Santamaría E, Pego-Reigosa JM, Marqués AO, Rúa-Figueroa I, Fernández-Nebro A, Cáliz Cáliz R, López Longo FJ, Muñoz-Fernández S |title=Efficacy and safety of rituximab in the treatment of non-renal systemic lupus erythematosus: a systematic review |journal=Semin. Arthritis Rheum. |volume=44 |issue=2 |pages=175–85 |year=2014 |pmid=24830791 |doi=10.1016/j.semarthrit.2014.04.002 |url=}}</ref> ===== | |||
* Preferred regimen: [[Celecoxib]] PO 100 to 200 mg twice daily | |||
** For [[fever]] management even in SLE patients with [[Sulfa allergy|“sulfa” allergy]] | |||
* Alternative regimen: [[Acetaminophen]] 1000 mg every 6 hours; maximum daily dose: 3000 mg daily | |||
==== Raynaud's phenomenon treatment<ref name="pmid3691593">{{cite journal |vauthors=Challenor VF, Waller DG, Francis DA, Francis JL, Mani R, Roath S |title=Nisoldipine in primary Raynaud's phenomenon |journal=Eur. J. Clin. Pharmacol. |volume=33 |issue=1 |pages=27–30 |year=1987 |pmid=3691593 |doi= |url=}}</ref> ==== | |||
* Preferred regimen (1): [[Calcium channel blocker]] ([[nifedipine]]) 10 to 30 mg 3 times daily | |||
* Preferred regimen (2): Antiplatelet therapy with low-dose [[aspirin]] (75 or 81 mg/day) in all patients with secondary [[Raynaud phenomenon]] | |||
* Alternative regimen (1): [[Phosphodiesterase inhibitors|Phosphodiesterase (PDE) inhibitor]] ([[sildenafil]]) 20 mg once or twice daily | |||
** Inadequate response to a [[CCB]] | |||
* Alternative regimen (2): Addition of [[Nitroglycerin (Topical ointment)|topical nitroglycerin (NTG)]] | |||
** Inadequate response to a [[CCB]] | |||
** A [[Sildenafil|PDE inhibitor]] is not available, effective, or well-tolerated | |||
* Alternative regimen (3): Intravenous (IV) infusions of a [[Prostaglandin|prostaglandin (PG)]] especially [[Prostacyclin|prostacyclin (PGI2) analogue]] for extremely severe patients with [[Raynaud's phenomenon|raynaud's phenomenon]]<ref name="pmid6890719">{{cite journal |vauthors=Pardy BJ, Hoare MC, Eastcott HH, Miles CC, Needham TN, Harbourne T, Ellis BW |title=Prostaglandin E1 in severe Raynaud's phenomenon |journal=Surgery |volume=92 |issue=6 |pages=953–65 |year=1982 |pmid=6890719 |doi= |url=}}</ref> | |||
===== Chronic pain management<ref name="pmid24938194">{{cite journal |vauthors=Di Franco M, Guzzo MP, Spinelli FR, Atzeni F, Sarzi-Puttini P, Conti F, Iannuccelli C |title=Pain and systemic lupus erythematosus |journal=Reumatismo |volume=66 |issue=1 |pages=33–8 |year=2014 |pmid=24938194 |doi= |url=}}</ref> ===== | |||
* Moderate pain should be treated with mild prescription [[opiates]] such as: | |||
** Preferred regimen: [[Dextropropoxyphene]] 600 mg maximum daily dosage divided into 2 or 3 doses | |||
** Alternative regimen: [[Co-codamol|Co-codamol (Acetaminophene+opioid)]]: [[Acetaminophen]] (300 to 1,000 mg/dose)/[[codeine]] (15 to 60 mg/dose) every 4 hours as needed; adjust dose according to severity of pain and response of patient (maximum: [[acetaminophen]] 4,000 mg/[[codeine]] 360 mg per 24 hours) | |||
* Moderate to severe [[chronic pain]] should be treated with stronger [[Opioid|opioids]] such as: | |||
** Preferred regimen (1): [[Hydrocodone]]: Single doses >40 mg or >60 mg with a total daily dose ≥80 mg | |||
** Preferred regimen (2): [[Oxycodone]]: 5 to 15 mg every 4 to 6 hours as needed | |||
** Alternative regimen (1): [[MS Contin|MS Contin:]] Opioid naive patients can have 5 to 10 mg every 4 hours; usual dosage range between 5 to 15 mg every 4 hours | |||
*** Higher initial doses in patients with prior [[opioid]] exposure | |||
** Alternative regimen (2): [[Methadone]]: Maximum initial dose 30 mg | |||
** Alternative regimen (3): [[Fentanyl]] Duragesic Transdermal patch: A convenient treatment option for [[Systemic lupus erythematosus|lupus]] chronic pain. It has a long lasting effect as well | |||
===== Cutaneous lupus erythematosus<ref name="pmid14162995">{{cite journal |vauthors=DOEGLAS HM |title=CHRONIC DISCOID LUPUS ERYTHEMATOSUS TREATED WITH TRIAMCINOLONE AND PLASTIC OCCLUSION |journal=Dermatologica |volume=128 |issue= |pages=384–6 |year=1964 |pmid=14162995 |doi= |url=}}</ref><ref name="pmid16966017">{{cite journal |vauthors=Rothfield N, Sontheimer RD, Bernstein M |title=Lupus erythematosus: systemic and cutaneous manifestations |journal=Clin. Dermatol. |volume=24 |issue=5 |pages=348–62 |year=2006 |pmid=16966017 |doi=10.1016/j.clindermatol.2006.07.014 |url=}}</ref><ref name="pmid18797893">{{cite journal |vauthors=Sárdy M, Ruzicka T, Kuhn A |title=Topical calcineurin inhibitors in cutaneous lupus erythematosus |journal=Arch. Dermatol. Res. |volume=301 |issue=1 |pages=93–8 |year=2009 |pmid=18797893 |doi=10.1007/s00403-008-0894-6 |url=}}</ref><ref name="pmid13971327">{{cite journal |vauthors=BJORNBERG A, HELLGREN L |title=Treatment of chronic discoid lupus erythematosus with fluocinolone acetonide ointment |journal=Br. J. Dermatol. |volume=75 |issue= |pages=156–60 |year=1963 |pmid=13971327 |doi= |url=}}</ref><ref name="pmid359493">{{cite journal |vauthors=Ritschel WA, Hammer GV, Thompson GA |title=Pharmacokinetics of antimalarials and proposals for dosage regimens |journal=Int J Clin Pharmacol Biopharm |volume=16 |issue=9 |pages=395–401 |year=1978 |pmid=359493 |doi= |url=}}</ref> ===== | |||
* Preferred regimen (1): Super high potency or high potency [[Steroid|topical steroid]] twice daily for patients with DLE or SCLE | |||
** [[Hydrocortisone]] 1% or 2.5% for facial involvement | |||
** [[Triamcinolone acetonide]] 0.1% cream or [[fluocinonide]] 0.05% cream: [[trunk]], extremity, or scalp disease | |||
** [[Clobetasol propionate]] for acute flares of DLE | |||
*** Discontinue treatment in the absence of disease activity | |||
* Alternative regimen (1): [[Calcineurin inhibitor|Topical calcineurin inhibitor]] such as [[tacrolimus]] 0.1% ointment or [[pimecrolimus]] 1% cream | |||
* Preferred regimen (2): Intralesional [[corticosteroid]] injections for DLE or SCLE if an acute flare of DLE or SCLE doesn't respond to [[Topical steroid|topical steroid therapy]] for two to four week | |||
* Alternative regimen (2): Systemic medications; [[hydroxychloroquine]] 200 to 400 mg/day for at least six weeks | |||
** After improvement it should be decreased to 200 mg/day for maintenance therapy | |||
** Administered in the case of failure of local therapy or extensive disease manifestation | |||
* Alternative regimen (3): [[Quinacrine]] 100 mg/day | |||
** In case of [[Antimalarial drug|antimalarial drugs]] failure | |||
===== Lupus nephritis treatment<ref name="pmid25014039">{{cite journal |vauthors=Schwartz N, Goilav B, Putterman C |title=The pathogenesis, diagnosis and treatment of lupus nephritis |journal=Curr Opin Rheumatol |volume=26 |issue=5 |pages=502–9 |year=2014 |pmid=25014039 |pmc=4221732 |doi=10.1097/BOR.0000000000000089 |url=}}</ref><ref name="pmid23328501">{{cite journal |vauthors=Hogan J, Appel GB |title=Update on the treatment of lupus nephritis |journal=Curr. Opin. Nephrol. Hypertens. |volume=22 |issue=2 |pages=224–30 |year=2013 |pmid=23328501 |doi=10.1097/MNH.0b013e32835d921c |url=}}</ref><ref name="pmid25778500">{{cite journal |vauthors=Tunnicliffe DJ, Singh-Grewal D, Kim S, Craig JC, Tong A |title=Diagnosis, Monitoring, and Treatment of Systemic Lupus Erythematosus: A Systematic Review of Clinical Practice Guidelines |journal=Arthritis Care Res (Hoboken) |volume=67 |issue=10 |pages=1440–52 |year=2015 |pmid=25778500 |doi=10.1002/acr.22591 |url=}}</ref> ===== | |||
* Aggressive [[antihypertensive therapy]] with [[blood pressure]] goal of 130/85 | |||
* In patients with [[proteinuria]], antiproteinuric therapy with blockade of the [[renin-angiotensin system]] include [[ACEIs]] and [[ARBs]]: | |||
** [[ACE inhibitor|ACE inhibitors]]; [[captopril]] PO 25 mg 3 times daily | |||
*** Antiproteinuric effect | |||
** [[ARBs]]; [[losartan]] PO initial: 50 mg once daily; can be increased to 100 mg once daily based on [[blood pressure]] response | |||
*** Slowing progression of [[GFR]] decline; | |||
* [[Lipid]] lowering with [[statin therapy]] with the goal of [[LDL]]< 130 | |||
* Diffuse or focal proliferative LN: | |||
** Preferred regimen: [[Immunosuppressive therapy]] with [[glucocorticoids]] plus either [[Intravenous therapy|intravenous]] or oral [[Mycophenolate sodium|mycophenolate mofetil]]: 0.5 g of [[Mycophenolate sodium|mycophenolate mofetil]] twice daily for the first week, then 1 g twice daily for the second week, and thereafter increase the dose to 1.5 g twice daily | |||
** Alternative regimen: [[Immunosuppressive therapy]] with [[glucocorticoids]] plus IV [[cyclophosphamide]] 500 mg every two weeks for a total of six doses | |||
* Severe active disease: | |||
** Preferred regimen: [[Glucocorticoid|Glucocorticoid therapy]] is initiated with [[Intravenous therapy|intravenous]] pulse [[methylprednisolone]] (250 mg to 1000 mg given over 30 minutes daily for three days) to induce a rapid [[immunosuppressive]] effect, followed by conventional doses | |||
** Alternative regimen: Conventional doses of oral [[glucocorticoids]] (eg, 0.5 to 1 mg/kg per day of prednisone) without a pulse. | |||
*** Oral [[prednisolone]] at a dose of 60 mg/day, tapered every two weeks by 10 mg/day until 40 mg/day is reached, then tapered by 5 mg/day until 10 mg/day is reached | |||
===== Considerations<ref name="pmid25778500" /> ===== | |||
* Appropriate adjunct therapy: | |||
** [[Vitamin D]] and [[calcium supplement|calcium supplements]]<nowiki/> for preventing [[osteoporosis]] in patients using [[corticosteroids]] | |||
** [[Antihypertensive drugs]] and [[statins]] were also recommended in patients using [[corticosteroids]] | |||
* Adverse effects: Cutaneous [[atrophy]] is a potential side effect of the long-term use of [[Topical steroid|topical steroids]] | |||
</div>}} | </div>}} | ||
Revision as of 20:13, 30 March 2018
| SLE Resident Survival Guide |
|---|
| Overview |
| Causes |
| FIRE |
| Diagnosis |
| Treatment |
| Do's |
| Don'ts |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Iqra Qamar M.D.[2]; Aditya Ganti M.B.B.S. [3]
Overview
Causes
- Genetic predisposition:[1]
- HLA class polymorphism
- Complement genes
- Female sexual gene due to high levels of estrogen and prolactin
- Auto-immune disease[2]
- Exposure to ultraviolet (UV) light[3]
- Can exacerbate or induce systemic manifestations of SLE
- Drug-induced lupus
FIRE
Diagnosis
Diagnostic criteria:
In 2012, Systemic Lupus International Collaboration Criteria (SLICC) developed a new criteria for SLE diagnosis. SLICC criteria for the classification of systemic lupus erythematosus was developed based on the old ACR criteria for the classification of systemic lupus erythematosus to address a more sensitive diagnostic criteria and also to cover weaknesses of the previous ACR criteria.[4][5]
Based on SLICC criteria, diagnosis of SLE is defined as:[6]
- Meeting at least 4 of 17 criteria, including at least 1 of the 11 clinical criteria and one of the six immunologic criteria
OR
- Biopsy-proven nephritis compatible with SLE in the presence of antinuclear antibodies (ANA) or anti-double-stranded DNA (dsDNA) antibodies
A criterion is considered positive if one or more of the observations listed in the definition for the criterion are present in the patient. A criterion should only be counted once, regardless of the number of observations in the definition that the patient presents with.
| Category | Criterion | Definition |
|---|---|---|
| Clinical | Acute cutaneous lupus |
|
| Chronic cutaneous lupus |
| |
| Nonscarring alopecia |
| |
| Oral or nasal ulcers |
| |
| Joint disease |
| |
| Serositis |
| |
| Renal |
| |
| Neurologic |
| |
| Hematologic | Hemolytic anemia |
|
| Leukopenia or lymphopenia |
| |
| Thrombocytopenia |
| |
| Immunologic | ANA |
|
| Anti-dsDNA |
| |
| Anit-SM |
| |
| Antiphospholipid |
| |
| Low complement | ||
| Direct Coombs' test |
|
Complete diagnostic approach:
SLE Presentation
Less common Presentation
| |||||||||||
Focused History
| |||||||||||
Physical ExaminationAppearance of the Patient
Vital Signs
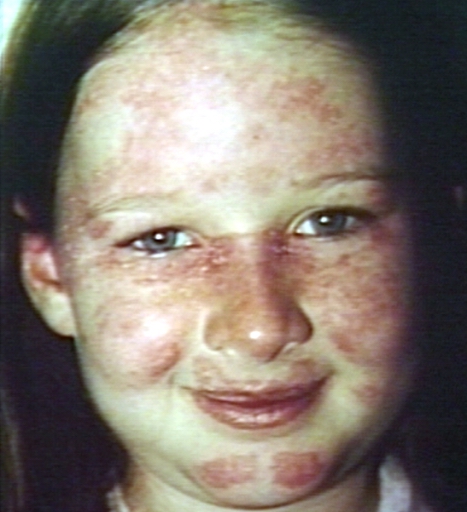
Skin[10][11][12]
HEENT
Neck[18][19]
Lungs[20][21][22]
Heart[23][19][24]
Abdomen[25][26][27][28]
Extremities[29][30][31][32][33]
Neuromuscular[34][35][36][37]
| |||||||||||
Laboratory Workup
| |||||||||||
Imaging StudyPlain radiographs of swollen joints
Ultrasonography of painful joints
Renal ultrasonography
Chest radiography
Echocardiography
Computed tomography (CT)
Magnetic resonance imaging (MRI)
| |||||||||||
Other InvestigationBronchoscopyFiberoptic bronchoscopy with bronchoalveolar lavage (BAL) and transbronchial lung biopsies:[38][39]
Barium swallow or esophagography
Biopsy
Paracentesis
Arthrocentesis | |||||||||||
Treatment
Treatment goalsTreatment goals in systemic lupus erythematosus (SLE) include:
General treatment
The treatment choice for systemic lupus erythematosus (SLE) is varied based on the severity of the disease and symptoms:
Severe disease
Less Severe (mild and moderate) disease
Other organ specific treatmentsFever management[48][49]
Raynaud's phenomenon treatment[50]
Chronic pain management[52]
Cutaneous lupus erythematosus[53][54][55][56][57]
Lupus nephritis treatment[58][59][60]
Considerations[60]
| |||||||||
Do's
Don'ts
References
- ↑ Schur PH (1995). "Genetics of systemic lupus erythematosus". Lupus. 4 (6): 425–37. doi:10.1177/096120339500400603. PMID 8749564.
- ↑ Cutolo M, Sulli A, Seriolo B, Accardo S, Masi AT (1995). "Estrogens, the immune response and autoimmunity". Clin. Exp. Rheumatol. 13 (2): 217–26. PMID 7656468.
- ↑ Cooper GS, Dooley MA, Treadwell EL, St Clair EW, Gilkeson GS (2002). "Risk factors for development of systemic lupus erythematosus: allergies, infections, and family history". J Clin Epidemiol. 55 (10): 982–9. PMID 12464374.
- ↑ Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, Schaller JG, Talal N, Winchester RJ (1982). "The 1982 revised criteria for the classification of systemic lupus erythematosus". Arthritis Rheum. 25 (11): 1271–7. PMID 7138600.
- ↑ Hochberg MC (1997). "Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus". Arthritis Rheum. 40 (9): 1725. doi:10.1002/1529-0131(199709)40:9<1725::AID-ART29>3.0.CO;2-Y. PMID 9324032.
- ↑ Petri M, Orbai AM, Alarcón GS, Gordon C, Merrill JT, Fortin PR, Bruce IN, Isenberg D, Wallace DJ, Nived O, Sturfelt G, Ramsey-Goldman R, Bae SC, Hanly JG, Sánchez-Guerrero J, Clarke A, Aranow C, Manzi S, Urowitz M, Gladman D, Kalunian K, Costner M, Werth VP, Zoma A, Bernatsky S, Ruiz-Irastorza G, Khamashta MA, Jacobsen S, Buyon JP, Maddison P, Dooley MA, van Vollenhoven RF, Ginzler E, Stoll T, Peschken C, Jorizzo JL, Callen JP, Lim SS, Fessler BJ, Inanc M, Kamen DL, Rahman A, Steinsson K, Franks AG, Sigler L, Hameed S, Fang H, Pham N, Brey R, Weisman MH, McGwin G, Magder LS (2012). "Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus". Arthritis Rheum. 64 (8): 2677–86. doi:10.1002/art.34473. PMC 3409311. PMID 22553077.
- ↑ Tench CM, McCurdie I, White PD, D'Cruz DP (2000). "The prevalence and associations of fatigue in systemic lupus erythematosus". Rheumatology (Oxford). 39 (11): 1249–54. PMID 11085805.
- ↑ McKinley PS, Ouellette SC, Winkel GH (1995). "The contributions of disease activity, sleep patterns, and depression to fatigue in systemic lupus erythematosus. A proposed model". Arthritis Rheum. 38 (6): 826–34. PMID 7779127.
- ↑ Wang B, Gladman DD, Urowitz MB (1998). "Fatigue in lupus is not correlated with disease activity". J. Rheumatol. 25 (5): 892–5. PMID 9598886.
- ↑ Parodi A, Cozzani E (2014). "Cutaneous manifestations of lupus erythematosus". G Ital Dermatol Venereol. 149 (5): 549–54. PMID 25077888.
- ↑ Szczęch J, Rutka M, Samotij D, Zalewska A, Reich A (2016). "Clinical characteristics of cutaneous lupus erythematosus". Postepy Dermatol Alergol. 33 (1): 13–7. doi:10.5114/pdia.2014.44031. PMC 4793050. PMID 26985173.
- ↑ Walling HW, Sontheimer RD (2009). "Cutaneous lupus erythematosus: issues in diagnosis and treatment". Am J Clin Dermatol. 10 (6): 365–81. doi:10.2165/11310780-000000000-00000. PMID 19824738.
- ↑ Preble JM, Silpa-archa S, Foster CS (2015). "Ocular involvement in systemic lupus erythematosus". Curr Opin Ophthalmol. 26 (6): 540–5. doi:10.1097/ICU.0000000000000209. PMID 26367085.
- ↑ Silpa-archa S, Lee JJ, Foster CS (2016). "Ocular manifestations in systemic lupus erythematosus". Br J Ophthalmol. 100 (1): 135–41. doi:10.1136/bjophthalmol-2015-306629. PMID 25904124.
- ↑ Robson AK, Burge SM, Millard PR (1992). "Nasal mucosal involvement in lupus erythematosus". Clin Otolaryngol Allied Sci. 17 (4): 341–3. PMID 1526055.
- ↑ Anyanwu CO, Ang CC, Werth VP (2013). "Oral mucosal involvement in bullous lupus". Arthritis Rheum. 65 (10): 2622. doi:10.1002/art.38051. PMC 4333153. PMID 23780804.
- ↑ Ranginwala AM, Chalishazar MM, Panja P, Buddhdev KP, Kale HM (2012). "Oral discoid lupus erythematosus: A study of twenty-one cases". J Oral Maxillofac Pathol. 16 (3): 368–73. doi:10.4103/0973-029X.102487. PMC 3519212. PMID 23248469.
- ↑ Melikoglu MA, Melikoglu M (2008). "The clinical importance of lymphadenopathy in systemic lupus erythematosus". Acta Reumatol Port. 33 (4): 402–6. PMID 19107085.
- ↑ 19.0 19.1 Sacre K, Escoubet B, Pasquet B, Chauveheid MP, Zennaro MC, Tubach F, Papo T (2014). "Increased arterial stiffness in systemic lupus erythematosus (SLE) patients at low risk for cardiovascular disease: a cross-sectional controlled study". PLoS ONE. 9 (4): e94511. doi:10.1371/journal.pone.0094511. PMC 3983200. PMID 24722263.
- ↑ Torre O, Harari S (2011). "Pleural and pulmonary involvement in systemic lupus erythematosus". Presse Med. 40 (1 Pt 2): e19–29. doi:10.1016/j.lpm.2010.11.004. PMID 21194884.
- ↑ Salvati F (2015). "[The involvement of pulmonary interstitial tissue in multisystemic lupus erythematosus: interdisciplinarity and role of the pneumologists]". Clin Ter (in Italian). 166 (5): 205–7. PMID 26550810.
- ↑ Alamoudi OS, Attar SM (2015). "Pulmonary manifestations in systemic lupus erythematosus: association with disease activity". Respirology. 20 (3): 474–80. doi:10.1111/resp.12473. PMC 4418345. PMID 25639532.
- ↑ Mak A, Kow NY (2014). "Imbalance between endothelial damage and repair: a gateway to cardiovascular disease in systemic lupus erythematosus". Biomed Res Int. 2014: 178721. doi:10.1155/2014/178721. PMC 3984775. PMID 24790989.
- ↑ Canpolat N, Kasapcopur O, Caliskan S, Gokalp S, Bor M, Tasdemir M, Sever L, Arisoy N (2013). "Ambulatory blood pressure and subclinical cardiovascular disease in patients with juvenile-onset systemic lupus erythematosus". Pediatr. Nephrol. 28 (2): 305–13. doi:10.1007/s00467-012-2317-3. PMID 23052654.
- ↑ Tian XP, Zhang X (2010). "Gastrointestinal involvement in systemic lupus erythematosus: insight into pathogenesis, diagnosis and treatment". World J. Gastroenterol. 16 (24): 2971–7. PMC 2890936. PMID 20572299.
- ↑ Alves SC, Fasano S, Isenberg DA (2016). "Autoimmune gastrointestinal complications in patients with systemic lupus erythematosus: case series and literature review". Lupus. 25 (14): 1509–1519. doi:10.1177/0961203316655210. PMID 27329649.
- ↑ Fawzy M, Edrees A, Okasha H, El Ashmaui A, Ragab G (2016). "Gastrointestinal manifestations in systemic lupus erythematosus". Lupus. 25 (13): 1456–1462. doi:10.1177/0961203316642308. PMID 27055518.
- ↑ Li Z, Xu D, Wang Z, Wang Y, Zhang S, Li M, Zeng X (2017). "Gastrointestinal system involvement in systemic lupus erythematosus". Lupus: 961203317707825. doi:10.1177/0961203317707825. PMID 28523968.
- ↑ Zoma A (2004). "Musculoskeletal involvement in systemic lupus erythematosus". Lupus. 13 (11): 851–3. doi:10.1191/0961203303lu2021oa. PMID 15580980.
- ↑ Gabba A, Piga M, Vacca A, Porru G, Garau P, Cauli A, Mathieu A (2012). "Joint and tendon involvement in systemic lupus erythematosus: an ultrasound study of hands and wrists in 108 patients". Rheumatology (Oxford). 51 (12): 2278–85. doi:10.1093/rheumatology/kes226. PMID 22956550.
- ↑ Grossman JM (2009). "Lupus arthritis". Best Pract Res Clin Rheumatol. 23 (4): 495–506. doi:10.1016/j.berh.2009.04.003. PMID 19591780.
- ↑ Zhu KK, Xu WD, Pan HF, Zhang M, Ni J, Ge FY, Ye DQ (2014). "The risk factors of avascular necrosis in patients with systemic lupus erythematosus: a meta-analysis". Inflammation. 37 (5): 1852–64. doi:10.1007/s10753-014-9917-y. PMID 24862229.
- ↑ Voulgari PV, Kosta P, Argyropoulou MI, Drosos AA (2013). "Avascular necrosis in a patient with systemic lupus erythematosus". Joint Bone Spine. 80 (6): 665. doi:10.1016/j.jbspin.2013.03.018. PMID 23731640.
- ↑ Cojocaru IM, Cojocaru M, Tănăsescu R, Burcin C, Atanasiu AN, Silosi I (2008). "Detection of autoantibodies to ribosome P in lupus patients with neurological involvement". Rom J Intern Med. 46 (3): 239–42. PMID 19366083.
- ↑ Madrane S, Ribi C (2012). "[Central neuropsychiatric involvement in systemic lupus erythematosus]". Rev Med Suisse (in French). 8 (337): 848–53. PMID 22594009.
- ↑ Sivri A, Hasçelik Z, Celiker R, Başgöze O (1995). "Early detection of neurological involvement in systemic lupus erythematosus patients". Electromyogr Clin Neurophysiol. 35 (4): 195–9. PMID 7555923.
- ↑ Juncal Gallego L, Almuíña Simón C, Muíños Esparza LF, Díaz Soto R, Ramil Fraga C, Quiroga Ordóñez E (2009). "[Systemic lupus erythematosus with fulminant neurological involvement]". An Pediatr (Barc) (in Spanish; Castilian). 70 (2): 202–4. doi:10.1016/j.anpedi.2008.09.009. PMID 19217587.
- ↑ Shen M, Wang Y, Xu WB, Zeng XJ, Zhang FC (2005). "[Pleuropulmonary manifestations of systemic lupus erythematosus]". Zhonghua Yi Xue Za Zhi (in Chinese). 85 (48): 3392–5. PMID 16409858.
- ↑ Susanto I, Peters JI (1997). "Acute lupus pneumonitis with normal chest radiograph". Chest. 111 (6): 1781–3. PMID 9187214.
- ↑ 40.0 40.1 Jiménez-Alonso J, Estev D, Vera C, Sabio JM (2003). "Dysphagia in patients with systemic lupus erythematosus". Lupus. 12 (6): 493. PMID 12873055.
- ↑ Giannico G, Fogo AB (2013). "Lupus nephritis: is the kidney biopsy currently necessary in the management of lupus nephritis?". Clin J Am Soc Nephrol. 8 (1): 138–45. doi:10.2215/CJN.03400412. PMID 22977215.
- ↑ Singh A, Ghosh R, Kaur P, Golay V, Pandey R, Roychowdhury A (2014). "Protocol renal biopsy in patients with lupus nephritis: a single center experience". Saudi J Kidney Dis Transpl. 25 (4): 801–7. PMID 24969191.
- ↑ Salomone E, Tamburino C, Bruno G, Di Paola R, Silvestri F (1989). "The role of endomyocardial biopsy in the diagnosis of cardiac involvement in systemic lupus erythematosus". Heart Vessels. 5 a (1): 52–3. PMID 2684953.
- ↑ Prasad S, Abujam B, Lawrence A, Aggarwal A (2012). "Massive ascites as a presenting feature of lupus". Int J Rheum Dis. 15 (1): e15–6. doi:10.1111/j.1756-185X.2011.01659.x. PMID 22324961.
- ↑ Palavutitotai N, Buppajarntham T, Katchamart W (2014). "Etiologies and outcomes of pleural effusions in patients with systemic lupus erythematosus". J Clin Rheumatol. 20 (8): 418–21. doi:10.1097/RHU.0000000000000179. PMID 25417677.
- ↑ Kruzliak P, Novak M, Piler P, Kovacova G (2013). "Pericardial involvement in systemic lupus erythematosus: current diagnosis and therapy". Acta Cardiol. 68 (6): 629–33. doi:10.2143/AC.68.6.8000011. PMID 24579442.
- ↑ Goldenberg DL, Cohen AS (1978). "Synovial membrane histopathology in the differential diagnosis of rheumatoid arthritis, gout, pseudogout, systemic lupus erythematosus, infectious arthritis and degenerative joint disease". Medicine (Baltimore). 57 (3): 239–52. PMID 642792.
- ↑ Jordan N, D'Cruz D (2016). "Current and emerging treatment options in the management of lupus". Immunotargets Ther. 5: 9–20. doi:10.2147/ITT.S40675. PMC 4970629. PMID 27529058.
- ↑ Cobo-Ibáñez T, Loza-Santamaría E, Pego-Reigosa JM, Marqués AO, Rúa-Figueroa I, Fernández-Nebro A, Cáliz Cáliz R, López Longo FJ, Muñoz-Fernández S (2014). "Efficacy and safety of rituximab in the treatment of non-renal systemic lupus erythematosus: a systematic review". Semin. Arthritis Rheum. 44 (2): 175–85. doi:10.1016/j.semarthrit.2014.04.002. PMID 24830791.
- ↑ Challenor VF, Waller DG, Francis DA, Francis JL, Mani R, Roath S (1987). "Nisoldipine in primary Raynaud's phenomenon". Eur. J. Clin. Pharmacol. 33 (1): 27–30. PMID 3691593.
- ↑ Pardy BJ, Hoare MC, Eastcott HH, Miles CC, Needham TN, Harbourne T, Ellis BW (1982). "Prostaglandin E1 in severe Raynaud's phenomenon". Surgery. 92 (6): 953–65. PMID 6890719.
- ↑ Di Franco M, Guzzo MP, Spinelli FR, Atzeni F, Sarzi-Puttini P, Conti F, Iannuccelli C (2014). "Pain and systemic lupus erythematosus". Reumatismo. 66 (1): 33–8. PMID 24938194.
- ↑ DOEGLAS HM (1964). "CHRONIC DISCOID LUPUS ERYTHEMATOSUS TREATED WITH TRIAMCINOLONE AND PLASTIC OCCLUSION". Dermatologica. 128: 384–6. PMID 14162995.
- ↑ Rothfield N, Sontheimer RD, Bernstein M (2006). "Lupus erythematosus: systemic and cutaneous manifestations". Clin. Dermatol. 24 (5): 348–62. doi:10.1016/j.clindermatol.2006.07.014. PMID 16966017.
- ↑ Sárdy M, Ruzicka T, Kuhn A (2009). "Topical calcineurin inhibitors in cutaneous lupus erythematosus". Arch. Dermatol. Res. 301 (1): 93–8. doi:10.1007/s00403-008-0894-6. PMID 18797893.
- ↑ BJORNBERG A, HELLGREN L (1963). "Treatment of chronic discoid lupus erythematosus with fluocinolone acetonide ointment". Br. J. Dermatol. 75: 156–60. PMID 13971327.
- ↑ Ritschel WA, Hammer GV, Thompson GA (1978). "Pharmacokinetics of antimalarials and proposals for dosage regimens". Int J Clin Pharmacol Biopharm. 16 (9): 395–401. PMID 359493.
- ↑ Schwartz N, Goilav B, Putterman C (2014). "The pathogenesis, diagnosis and treatment of lupus nephritis". Curr Opin Rheumatol. 26 (5): 502–9. doi:10.1097/BOR.0000000000000089. PMC 4221732. PMID 25014039.
- ↑ Hogan J, Appel GB (2013). "Update on the treatment of lupus nephritis". Curr. Opin. Nephrol. Hypertens. 22 (2): 224–30. doi:10.1097/MNH.0b013e32835d921c. PMID 23328501.
- ↑ 60.0 60.1 Tunnicliffe DJ, Singh-Grewal D, Kim S, Craig JC, Tong A (2015). "Diagnosis, Monitoring, and Treatment of Systemic Lupus Erythematosus: A Systematic Review of Clinical Practice Guidelines". Arthritis Care Res (Hoboken). 67 (10): 1440–52. doi:10.1002/acr.22591. PMID 25778500.