Rosuvastatin
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Alejandro Lemor, M.D. [2]
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Rosuvastatin is a HMG-CoA reductase inhibitor that is FDA approved for the treatment of hyperlipidemia and mixed dyslipidemia, hypertriglyceridemia, primary dysbetalipoproteinemia (Type III hyperlipoproteinemia), homozygous familial hypercholesterolemia, slowing of the progression of atherosclerosis, primary prevention of cardiovascular disease. Common adverse reactions include headache, myalgia, abdominal pain, asthenia, and nausea.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
General Dosing Information
- Dosing Information
- 5 to 40 mg orally once daily.
Homozygous Familial Hypercholesterolemia
- Dosing Information
- 20 mg once daily
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Rosuvastatin in adult patients.
Non–Guideline-Supported Use
Acute Coronary Syndrome
Prophylaxis for Atrial Fibrillation
Prophylaxis for Cardiovascular Event in Percutaneous Coronary Intervention (PCI)
- Dosing Information
- 40 mg preprocedure
Prophylaxis for Venous Thromboembolism
- Dosing Information
- 20 mg/day
Metabolic Syndrome
- Dosing Information
- 10 mg/day
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Heterozygous Familial Hypercholesterolemia in Pediatric Patients (10 to 17 years of age)
- Dosing Information
- 5‑20 mg/day; the maximum recommended dose is 20 mg/day
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Rosuvastatin in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Rosuvastatin in pediatric patients.
Contraindications
- Patients with a known hypersensitivity to any component of this product. Hypersensitivity reactions including rash, pruritus, urticaria, and angioedema have been reported with rosuvastatin.
- Patients with active liver disease, which may include unexplained persistent elevations of hepatic transaminase levels.
- Women who are pregnant or may become pregnant. Because HMG‑CoA reductase inhibitors decrease cholesterol synthesis and possibly the synthesis of other biologically active substances derived from cholesterol, rosuvastatin may cause fetal harm when administered to regnant women. Additionally, there is no apparent benefit to therapy during pregnancy, and safety in pregnant women has not been established. If the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the fetus and the lack of known clinical benefit with continued use during pregnancy.
- Nursing mothers. Because another drug in this class passes into breast milk, and because HMG‑CoA reductase inhibitors have the potential to cause serious adverse reactions in nursing infants, women who require rosuvastatin treatment should be advised not to nurse their infants.
Warnings
Skeletal Muscle Effects
Cases of myopathy and rhabdomyolysis with acute renal failure secondary to myoglobinuria have been reported with HMG-CoA reductase inhibitors, including rosuvastatin. These risks can occur at any dose level, but are increased at the highest dose (40 mg).
Rosuvastatin should be prescribed with caution in patients with predisposing factors for myopathy (e.g., age ≥ 65 years, inadequately treated hypothyroidism, renal impairment).
The risk of myopathy during treatment with rosuvastatin may be increased with concurrent administration of some other lipid-lowering therapies (fibrates or niacin), gemfibrozil, cyclosporine, lopinavir/ritonavir, or atazanavir/ritonavir. Cases of myopathy, including rhabdomyolysis, have been reported with HMG-CoA reductase inhibitors, including rosuvastatin, coadministered with colchicine, and caution should be exercised when prescribing rosuvastatin with colchicine.
Rosuvastatin therapy should be discontinued if markedly elevated creatine kinase levels occur or myopathy is diagnosed or suspected. Rosuvastatin therapy should also be temporarily withheld in any patient with an acute, serious condition suggestive of myopathy or predisposing to the development of renal failure secondary to rhabdomyolysis (e.g., sepsis, hypotension, dehydration, major surgery, trauma, severe metabolic, endocrine, and electrolyte disorders, or uncontrolled seizures).
There have been rare reports of immune-mediated necrotizing myopathy (IMNM), an autoimmune myopathy, associated with statin use. IMNM is characterized by: proximal muscle weakness and elevated serum creatine kinase, which persist despite discontinuation of statin treatment; muscle biopsy showing necrotizing myopathy without significant inflammation; improvement with immunosuppressive agents.
All patients should be advised to promptly report to their physician unexplained muscle pain, tenderness, or weakness, particularly if accompanied by malaise or fever or if muscle signs and symptoms persist after discontinuing rosuvastatin.
Liver Enzyme Abnormalities
It is recommended that liver enzyme tests be performed before the initiation of rosuvastatin, and if signs or symptoms of liver injury occur.
Increases in serum transaminases AST (SGOT) or ALT (SGPT) have been reported with HMG‑CoA reductase inhibitors, including rosuvastatin. In most cases, the elevations were transient and resolved or improved on continued therapy or after a brief interruption in therapy. There were two cases of jaundice, for which a relationship to rosuvastatin therapy could not be determined, which resolved after discontinuation of therapy. There were no cases of liver failure or irreversible liver disease in these trials.
In a pooled analysis of placebo-controlled trials, increases in serum transaminases to >3 times the upper limit of normal occurred in 1.1% of patients taking rosuvastatin versus 0.5% of patients treated with placebo.
There have been rare postmarketing reports of fatal and non-fatal hepatic failure in patients taking statins, including rosuvastatin. If serious liver injury with clinical symptoms and/or hyperbilirubinemia or jaundice occurs during treatment with rosuvastatin, promptly interrupt therapy. If an alternate etiology is not found, do not restart rosuvastatin.
Rosuvastatin should be used with caution in patients who consume substantial quantities of alcohol and/or have a history of chronic liver disease. Active liver disease, which may include unexplained persistent transaminase elevations, is a contraindication to the use of rosuvastatin.
Concomitant Coumarin Anticoagulants
Caution should be exercised when anticoagulants are given in conjunction with rosuvastatin because of its potentiation of the effect of coumarin-type anticoagulants in prolonging the prothrombin time/INR. In patients taking coumarin anticoagulants and rosuvastatin concomitantly, INR should be determined before starting rosuvastatin and frequently enough during early therapy to ensure that no significant alteration of INR occurs.
Proteinuria and Hematuria
In the rosuvastatin clinical trial program, dipstick-positive proteinuria and microscopic hematuria were observed among rosuvastatin treated patients. These findings were more frequent in patients taking rosuvastatin 40 mg, when compared to lower doses of rosuvastatin or comparator HMG‑CoA reductase inhibitors, though it was generally transient and was not associated with worsening renal function. Although the clinical significance of this finding is unknown, a dose reduction should be considered for patients on rosuvastatin therapy with unexplained persistent proteinuria and/or hematuria during routine urinalysis testing.
Endocrine Effects
Increases in HbA1c and fasting serum glucose levels have been reported with HMG‑CoA reductase inhibitors, including rosuvastatin. Based on clinical trial data with rosuvastatin, in some instances these increases may exceed the threshold for the diagnosis of diabetes mellitus.
Although clinical studies have shown that rosuvastatin alone does not reduce basal plasma cortisol concentration or impair adrenal reserve, caution should be exercised if rosuvastatin is administered concomitantly with drugs that may decrease the levels or activity of endogenous steroid hormones such as ketoconazole, spironolactone, and cimetidine.
Adverse Reactions
Clinical Trials Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect the rates observed in clinical practice.
Adverse reactions reported in ≥ 2% of patients in placebo-controlled clinical studies and at a rate greater than placebo are shown in Table 1. These studies had a treatment duration of up to 12 weeks.
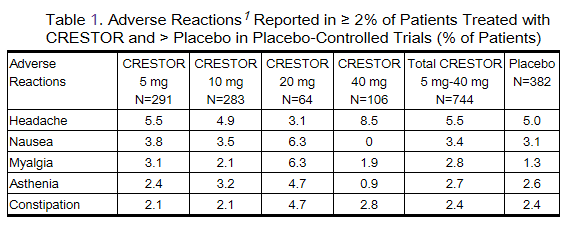
Other adverse reactions reported in clinical studies were abdominal pain, dizziness, hypersensitivity (including rash, pruritus, urticaria, and angioedema) and pancreatitis. The following laboratory abnormalities have also been reported: dipstick-positive proteinuria and microscopic hematuria; elevated creatine phosphokinase, transaminases, glucose, glutamyl transpeptidase, alkaline phosphatase, and bilirubin; and thyroid function abnormalities.
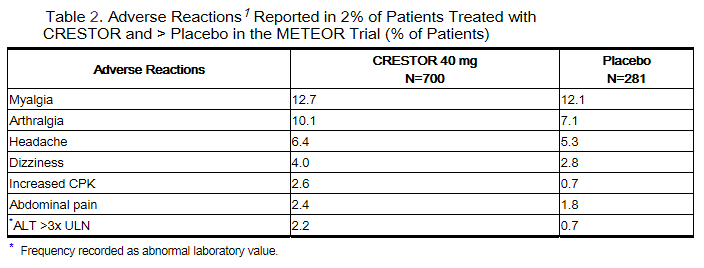
In the METEOR study, involving 981 participants treated with rosuvastatin 40 mg (n=700) or placebo (n=281) with a mean treatment duration of 1.7 years, 5.6% of subjects treated with rosuvastatin versus 2.8% of placebo-treated subjects discontinued due to adverse reactions. The most common adverse reactions that led to treatment discontinuation were: myalgia, hepatic enzyme increased, headache, and nausea.
Adverse reactions reported in ≥ 2% of patients and at a rate greater than placebo are shown in Table 2.
In the JUPITER study, 17,802 participants were treated with rosuvastatin 20 mg (n=8901) or placebo (n=8901) for a mean duration of 2 years. A higher percentage of rosuvastatin-treated patients versus placebo-treated patients, 6.6% and 6.2%, respectively, discontinued study medication due to an adverse event, irrespective of treatment causality. Myalgia was the most common adverse reaction that led to treatment discontinuation.
In JUPITER, there was a significantly higher frequency of diabetes mellitus reported in patients taking rosuvastatin (2.8%) versus patients taking placebo (2.3%). Mean HbA1c was significantly increased by 0.1% in rosuvastatin-treated patients compared to placebo-treated patients. The number of patients with a HbA1c > 6.5% at the end of the trial was significantly higher in rosuvastatin-treated versus placebo-treated patients.
Adverse reactions reported in ≥ 2% of patients and at a rate greater than placebo are shown in Table 3.
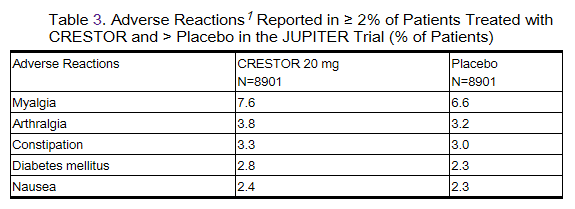
Postmarketing Experience
The following adverse reactions have been identified during postapproval use of rosuvastatin : arthralgia, fatal and non-fatal hepatic failure, hepatitis, jaundice, thrombocytopenia, depression, sleep disorders (including insomnia and nightmares) and gynecomastia. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
There have been rare reports of immune-mediated necrotizing myopathy associated with statin use.
There have been rare postmarketing reports of cognitive impairment (e.g., memory loss, forgetfulness, amnesia, memory impairment, confusion) associated with statin use. These cognitive issues have been reported for all statins. The reports are generally nonserious, and reversible upon statin discontinuation, with variable times to symptom onset (1 day to years) and symptom resolution (median of 3 weeks).
Drug Interactions
Cyclosporine
Cyclosporine increased rosuvastatin exposure (AUC) 7‑fold. Therefore, in patients taking cyclosporine, the dose of rosuvastatin should not exceed 5 mg once daily.
Gemfibrozil
Gemfibrozil significantly increased rosuvastatin exposure. Due to an observed increased risk of myopathy/rhabdomyolysis, combination therapy with rosuvastatin and gemfibrozil should be avoided. If used together, the dose of rosuvastatin should not exceed 10 mg once daily.
Protease Inhibitors
Coadministration of rosuvastatin with certain protease inhibitors given in combination with ritonavir has differing effects on rosuvastatin exposure. The protease inhibitor combinations lopinavir/ritonavir and atazanavir/ritonavir increase rosuvastatin exposure (AUC) up to threefold. For these combinations the dose of rosuvastatin should not exceed 10 mg once daily. The combinations of tipranavir/ritonavir or fosamprenavir/ritonavir produce little or no change in rosuvastatin exposure. Caution should be exercised when rosuvastatin is coadministered with protease inhibitors given in combination with ritonavir.
Coumarin Anticoagulants
Rosuvastatin significantly increased INR in patients receiving coumarin anticoagulants. Therefore, caution should be exercised when coumarin anticoagulants are given in conjunction with rosuvastatin. In patients taking coumarin anticoagulants and rosuvastatin concomitantly, INR should be determined before starting rosuvastatin and frequently enough during early therapy to ensure that no significant alteration of INR] occurs.
Niacin
The risk of skeletal muscle effects may be enhanced when rosuvastatin is used in combination with lipid-modifying doses (≥1 g/day) of niacin; caution should be used when prescribing with rosuvastatin.
Fenofibrate
When rosuvastatin was coadministered with fenofibrate, no clinically significant increase in the AUC of rosuvastatin or fenofibrate was observed. Because it is known that the risk of myopathy during treatment with HMG-CoA reductase inhibitors is increased with concomitant use of fenofibrates, caution should be used when prescribing fenofibrates with rosuvastatin.
Colchicine
Cases of myopathy, including rhabdomyolysis, have been reported with HMG‑CoA reductase inhibitors, including rosuvastatin, coadministered with colchicine, and caution should be exercised when prescribing rosuvastatin with colchicine.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): X
Rosuvastatin is contraindicated in women who are or may become pregnant. Serum cholesterol and triglycerides increase during normal pregnancy, and cholesterol products are essential for fetal development. Atherosclerosis is a chronic process and discontinuation of lipid-lowering drugs during pregnancy should have little impact on long-term outcomes of primary hyperlipidemia therapy
There are no adequate and well-controlled studies of rosuvastatin in pregnant women. There have been rare reports of congenital anomalies following intrauterine exposure to HMG CoA reductase inhibitors. In a review of about 100 prospectively followed pregnancies in women exposed to other HMG CoA reductase inhibitors, the incidences of congenital anomalies, spontaneous abortions, and fetal deaths/stillbirths did not exceed the rate expected in the general population. However, this study was only able to exclude a three-to-fourfold increased risk of congenital anomalies over background incidence. In 89% of these cases, drug treatment started before pregnancy and stopped during the first trimester when pregnancy was identified.
Rosuvastatin crosses the placenta in rats and rabbits. In rats, rosuvastatin was not teratogenic at systemic exposures equivalent to a human therapeutic dose of 40 mg/day. At 10 12 times the human dose of 40 mg/day, there was decreased pup survival, decreased fetal body weight among female pups, and delayed ossification. In rabbits, pup viability decreased and maternal mortality increased at doses equivalent to the human dose of 40 mg/day
Rosuvastatin may cause fetal harm when administered to a pregnant woman. If the patient becomes pregnant while taking rosuvastatin, the patient should be apprised of the potential risks to the fetus and the lack of known clinical benefit with continued use during pregnancy.
Pregnancy Category (AUS): D
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Rosuvastatin in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Rosuvastatin during labor and delivery.
Nursing Mothers
It is not known whether rosuvastatin is excreted in human milk, but a small amount of another drug in this class does pass into breast milk. In rats, breast milk concentrations of rosuvastatin are three times higher than plasma levels; however, animal breast milk drug levels may not accurately reflect human breast milk levels. Because another drug in this class passes into human milk and because HMG‑CoA reductase inhibitors have a potential to cause serious adverse reactions in nursing infants, women who require rosuvastatin treatment should be advised not to nurse their infants.
Pediatric Use
The safety and effectiveness of rosuvastatin in patients 10 to 17 years of age with heterozygous familial hypercholesterolemia were evaluated in a controlled clinical trial of 12 weeks duration followed by 40 weeks of open-label exposure. Patients treated with 5 mg, 10 mg, and 20 mg daily rosuvastatin had an adverse experience profile generally similar to that of patients treated with placebo. Although not all adverse reactions identified in the adult population have been observed in clinical trials of children and adolescent patients, the same warnings and precautions for adults should be considered for children and adolescents. There was no detectable effect of rosuvastatin on growth, weight, BMI (body mass index), or sexual maturation in pediatric patients (10 to 17 years of age). Adolescent females should be counseled on appropriate contraceptive methods while on rosuvastatin therapy. Rosuvastatin has not been studied in controlled clinical trials involving prepubertal patients or patients younger than 10 years of age. Doses of rosuvastatin greater than 20 mg have not been studied in the pediatric population.
In children and adolescents with homozygous familial hypercholesterolemia experience is limited to eight patients (aged 8 years and above).
In a pharmacokinetic study, 18 patients (9 boys and 9 girls) 10 to 17 years of age with heterozygous familial hypercholesterolemia received single and multiple oral doses of rosuvastatin . Both Cmax and AUC of rosuvastatin were similar to values observed in adult subjects administered the same doses.
Geriatic Use
Of the 10,275 patients in clinical studies with rosuvastatin, 3159 (31%) were 65 years and older, and 698 (6.8%) were 75 years and older. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
Elderly patients are at higher risk of myopathy and rosuvastatin should be prescribed with caution in the elderly
Gender
There is no FDA guidance on the use of Rosuvastatin with respect to specific gender populations.
Race
There is no FDA guidance on the use of Rosuvastatin with respect to specific racial populations.
Renal Impairment
Rosuvastatin exposure is not influenced by mild to moderate renal impairment (CLcr ≥ 30 mL/min/1.73 m2); however, exposure to rosuvastatin is increased to a clinically significant extent in patients with severe renal impairment who are not receiving hemodialysis. Rosuvastatin dosing should be adjusted in patients with severe renal impairment (CLcr < 30 mL/min/1.73 m2) not requiring hemodialysis.
Hepatic Impairment
Rosuvastatin is contraindicated in patients with active liver disease, which may include unexplained persistent elevations of hepatic transaminase levels. Chronic alcohol liver disease is known to increase rosuvastatin exposure; Rosuvastatin should be used with caution in these patients.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Rosuvastatin in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Rosuvastatin in patients who are immunocompromised.
Asian Patients
Pharmacokinetic studies have demonstrated an approximate 2‑fold increase in median exposure to rosuvastatin in Asian subjects when compared with Caucasian controls. Rosuvastatin dosage should be adjusted in Asian patients
Administration and Monitoring
Administration
Oral
Monitoring
Concomitant Coumarin Anticoagulants
In patients taking coumarin anticoagulants and rosuvastatin concomitantly, INR should be determined before starting rosuvastatin and frequently enough during early therapy to ensure that no significant alteration of INR occurs.
IV Compatibility
There is limited information regarding the compatibility of Rosuvastatin and IV administrations.
Overdosage
There is no specific treatment in the event of overdose. In the event of overdose, the patient should be treated symptomatically and supportive measures instituted as required. Hemodialysis does not significantly enhance clearance of rosuvastatin.
Pharmacology
Mechanism of Action
Rosuvastatin is a selective and competitive inhibitor of HMG-CoA reductase, the rate-limiting enzyme that converts 3‑hydroxy‑3‑methylglutaryl coenzyme A to mevalonate, a precursor of cholesterol. In vivo studies in animals, and in vitro studies in cultured animal and human cells have shown rosuvastatin to have a high uptake into, and selectivity for, action in the liver, the target organ for cholesterol lowering. In in vivo and in vitro studies, rosuvastatin produces its lipid-modifying effects in two ways. First, it increases the number of hepatic LDL receptors on the cell-surface to enhance uptake and catabolism of LDL. Second, rosuvastatin inhibits hepatic synthesis of VLDL, which reduces the total number of VLDL and LDL particles.
Structure
The chemical name for rosuvastatin calcium is bis[(E)-7-[4-(4-fluorophenyl)-6-isopropyl-2-[methyl(methylsulfonyl)amino] pyrimidin-5-yl](3R,5S)-3,5-dihydroxyhept-6-enoic acid] calcium salt with the following structural formula:
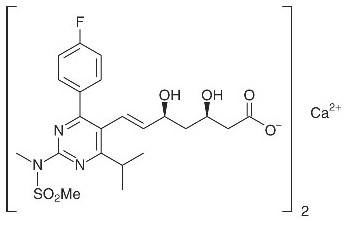
The empirical formula for rosuvastatin calcium is (C22H27FN3O6S)2Ca and the molecular weight is 1001.14. Rosuvastatin calcium is a white amorphous powder that is sparingly soluble in water and methanol, and slightly soluble in ethanol. Rosuvastatin calcium is a hydrophilic compound with a partition coefficient (octanol/water) of 0.13 at pH of 7.0.
Pharmacodynamics
There is limited information regarding Rosuvastatin Pharmacodynamics in the drug label.
Pharmacokinetics
- Absorption: In clinical pharmacology studies in man, peak plasma concentrations of rosuvastatin were reached 3 to 5 hours following oral dosing. Both Cmax and AUC increased in approximate proportion to rosuvastatin dose. The absolute bioavailability of rosuvastatin is approximately 20%. Administration of rosuvastatin with food did not affect the AUC of rosuvastatin. The AUC of rosuvastatin does not differ following evening or morning drug administration.
- Distribution: Mean volume of distribution at steady-state of rosuvastatin is approximately 134 liters. Rosuvastatin is 88% bound to plasma proteins, mostly albumin. This binding is reversible and independent of plasma concentrations.
- Metabolism: Rosuvastatin is not extensively metabolized; approximately 10% of a radiolabeled dose is recovered as metabolite. The major metabolite is N-desmethyl rosuvastatin, which is formed principally by cytochrome P450 \ 2C9, and in vitro studies have demonstrated that N-desmethyl rosuvastatin has approximately one-sixth to one-half the HMG‑CoA reductase inhibitory activity of the parent compound. Overall, greater than 90% of active plasma HMG‑CoA reductase inhibitory activity is accounted for by the parent compound.
- Excretion: Following oral administration, rosuvastatin and its metabolites are primarily excreted in the feces (90%). The elimination half-life (t1/2) of rosuvastatin is approximately 19 hours. After an intravenous dose, approximately 28% of total body clearance was via the renal route, and 72% by the hepatic route.
- Race: A population pharmacokinetic analysis revealed no clinically relevant differences in pharmacokinetics among Caucasian, Hispanic, and Black or Afro-Caribbean groups. However, pharmacokinetic studies, including one conducted in the US, have demonstrated an approximate 2‑fold elevation in median exposure (AUC and Cmax) in Asian subjects when compared with a Caucasian control group.
- Gender: There were no differences in plasma concentrations of rosuvastatin between men and women.
- Geriatric: There were no differences in plasma concentrations of rosuvastatin between the nonelderly and elderly populations (age ≥65 years).
- Renal Impairment: Mild to moderate renal impairment (CrCl ≥ 30 mL/min/1.73 m2) had no influence on plasma concentrations of rosuvastatin. However, plasma concentrations of rosuvastatin increased to a clinically significant extent (about 3‑fold) in patients with severe renal impairment (CrCl < 30 mL/min/1.73 m2) not receiving hemodialysis compared with healthy subjects (CrCl > 80 mL/min/1.73 m2).
- Hemodialysis: Steady-state plasma concentrations of rosuvastatin in patients on chronic hemodialysis were approximately 50% greater compared with healthy volunteer subjects with normal renal function.
- Hepatic Impairment: In patients with chronic alcohol liver disease, plasma concentrations of rosuvastatin were modestly increased.
- In patients with Child‑Pugh A disease, Cmax and AUC were increased by 60% and 5%, respectively, as compared with patients with normal liver function.
- In patients with Child‑Pugh B disease, Cmax and AUC were increased 100% and 21%, respectively, compared with patients with normal liver function.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
In a 104-week carcinogenicity study in rats at dose levels of 2, 20, 60, or 80 mg/kg/day by oral gavage, the incidence of uterine stromal polyps was significantly increased in females at 80 mg/kg/day at systemic exposure 20 times the human exposure at 40 mg/day based on AUC. Increased incidence of polyps was not seen at lower doses.
In a 107-week carcinogenicity study in mice given 10, 60, 200 mg/kg/day by oral gavage, an increased incidence of hepatocellular adenoma/carcinoma was observed at 200 mg/kg/day at systemic exposures 20 times the human exposure at 40 mg/day based on AUC. An increased incidence of hepatocellular tumors was not seen at lower doses.
Rosuvastatin was not mutagenic or clastogenic with or without metabolic activation in the Ames test with Salmonella typhimurium and Escherichia coli, the mouse lymphoma assay, and the chromosomal aberration assay in Chinese hamster lung cells. Rosuvastatin was negative in the in vivo mouse micronucleus test.
In rat fertility studies with oral gavage doses of 5, 15, 50 mg/kg/day, males were treated for 9 weeks prior to and throughout mating and females were treated 2 weeks prior to mating and throughout mating until gestation day 7. No adverse effect on fertility was observed at 50 mg/kg/day (systemic exposures up to 10 times the human exposure at 40 mg/day based on AUC). In testicles of dogs treated with rosuvastatin at 30 mg/kg/day for one month, spermatidic giant cells were seen. Spermatidic giant cells were observed in monkeys after 6‑month treatment at 30 mg/kg/day in addition to vacuolation of seminiferous tubular epithelium. Exposures in the dog were 20 times and in the monkey 10 times the human exposure at 40 mg/day based on body surface area. Similar findings have been seen with other drugs in this class.
Animal Toxicology and/or Pharmacology
Embryo-fetal Development
Rosuvastatin crosses the placenta and is found in fetal tissue and amniotic fluid at 3% and 20%, respectively, of the maternal plasma concentration following a single 25 mg/kg oral gavage dose on gestation day 16 in rats. A higher fetal tissue distribution (25% maternal plasma concentration) was observed in rabbits after a single oral gavage dose of 1 mg/kg on gestation day 18.
In female rats given oral gavage doses of 5, 15, 50 mg/kg/day rosuvastatin before mating and continuing through day 7 postcoitus results in decreased fetal body weight (female pups) and delayed ossification at the high dose (systemic exposures 10 times the human exposure at 40 mg/day based on AUC).
In pregnant rats given oral gavage doses of 2, 10, 50 mg/kg/day from gestation day 7 through lactation day 21 (weaning), decreased pup survival occurred in groups given 50 mg/kg/day, systemic exposures ≥ 12 times the human exposure at 40 mg/day based on body surface area.
In pregnant rabbits given oral gavage doses of 0.3, 1, 3 mg/kg/day from gestation day 6 to lactation day 18 (weaning), exposures equivalent to the human exposure at 40 mg/day based on body surface area, decreased fetal viability and maternal mortality was observed.
Rosuvastatin was not teratogenic in rats at ≤ 25 mg/kg/day or in rabbits ≤ 3 mg/kg/day (systemic exposures equivalent to the human exposure at 40 mg/day based on AUC or body surface area, respectively).
Central Nervous System Toxicity
CNS vascular lesions, characterized by perivascular hemorrhages, edema, and mononuclear cell infiltration of perivascular spaces, have been observed in dogs treated with several other members of this drug class. A chemically similar drug in this class produced dose-dependent optic nerve degeneration (Wallerian degeneration of retinogeniculate fibers) in dogs, at a dose that produced plasma drug levels about 30 times higher than the mean drug level in humans taking the highest recommended dose. Edema, hemorrhage, and partial necrosis in the interstitium of the choroid plexus was observed in a female dog sacrificed moribund at day 24 at 90 mg/kg/day by oral gavage (systemic exposures 100 times the human exposure at 40 mg/day based on AUC). Corneal opacity was seen in dogs treated for 52 weeks at 6 mg/kg/day by oral gavage (systemic exposures 20 times the human exposure at 40 mg/day based on AUC). Cataracts were seen in dogs treated for 12 weeks by oral gavage at 30 mg/kg/day (systemic exposures 60 times the human exposure at 40 mg/day based on AUC). Retinal dysplasia and retinal loss were seen in dogs treated for 4 weeks by oral gavage at 90 mg/kg/day (systemic exposures 100 times the human exposure at 40 mg/day based on AUC). Doses ≤30 mg/kg/day (systemic exposures ≤60 times the human exposure at 40 mg/day based on AUC) did not reveal retinal findings during treatment for up to one year.
Clinical Studies
Hyperlipidemia and Mixed Dyslipidemia
Rosuvastatin reduces Total‑C, LDL‑C, ApoB, nonHDL‑C, and TG, and increases HDL‑C, in adult patients with [hyperlipidemia]] and mixed dyslipidemia.
Dose-Ranging Study: In a multicenter, double-blind, placebo-controlled, dose-ranging study in patients with [hyperlipidemia]] rosuvastatin given as a single daily dose for 6 weeks significantly reduced Total‑C, LDL‑C, nonHDL‑C, and ApoB, across the dose range.
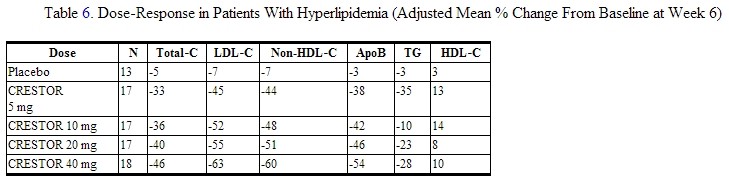
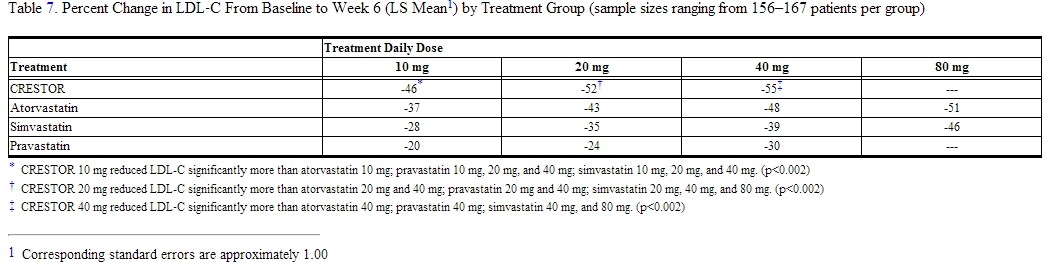
Active-Controlled Study: rosuvastatin was compared with the HMG‑CoA reductase inhibitors atorvastatin, simvastatin, and pravastatin in a multicenter, open-label, dose-ranging study of 2240 patients with hyperlipidemia or mixed dyslipidemia. After randomization, patients were treated for 6 weeks with a single daily dose of either rosuvastatin, atorvastatin, simvastatin, or pravastatin (Figure 1 and Table 7).
Figure 1. Percent LDL‑ C Change by Dose of rosuvastatin, Atorvastatin, Simvastatin, and Pravastatin at Week 6 in Patients with [hyperlipidemia]] or Mixed Dyslipidemia

Box plots are a representation of the 25th, 50th, and 75th percentile values, with whiskers representing the 10th and 90th percentile values. Mean baseline LDL‑C: 189 mg/dL
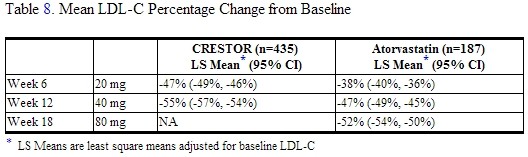
Heterozygous Familial Hypercholesterolemia
Active-Controlled Study: In a study of patients with heterozygous FH (baseline mean LDL of 291), patients were randomized to rosuvastatin 20 mg or atorvastatin 20 mg. The dose was increased by 6-week intervals. Significant LDL-C reductions from baseline were seen at each dose in both treatment groups (Table 8).
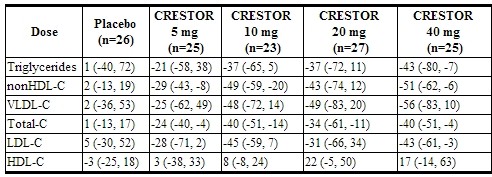
Hypertriglyceridemia
Dose-Response Study: In a double-blind, placebo-controlled dose-response study in patients with baseline TG levels from 273 to 817 mg/dL, rosuvastatin given as a single daily dose (5 to 40 mg) over 6 weeks significantly reduced serum TG levels (Table 9).
Primary Dysbetalipoproteinemia (Type III Hyperlipoproteinemia)
In a randomized, multicenter, double-blind crossover study, 32 patients (27 with є2/є2 and 4 with apo E mutation [Arg145Cys] with primary dysbetalipoproteinemia (Type III Hyperlipoproteinemia) entered a 6-week dietary lead-in period on the NCEP Therapeutic Lifestyle Change (TLC) diet. Following dietary lead-in, patients were randomized to a sequence of treatments in conjunction with the TLC diet for 6 weeks each: rosuvastatin 10 mg followed by rosuvastatin 20 mg or rosuvastatin 20 mg followed by rosuvastatin 10 mg. rosuvastatin reduced nonHDL‑C (primary end point) and circulating remnant lipoprotein levels. Results are shown in the table below.

Homozygous Familial Hypercholesterolemia
Dose-Titration Study: In an open-label, forced-titration study, homozygous FH patients (n=40, 8‑63 years) were evaluated for their response to rosuvastatin 20 to 40 mg titrated at a 6‑week interval. In the overall population, the mean LDL‑C reduction from baseline was 22%. About one-third of the patients benefited from increasing their dose from 20 mg to 40 mg with further LDL lowering of greater than 6%. In the 27 patients with at least a 15% reduction in LDL‑C, the mean LDL-C reduction was 30% (median 28% reduction). Among 13 patients with an LDL‑C reduction of <15%, 3 had no change or an increase in LDL‑C. Reductions in LDL‑C of 15% or greater were observed in 3 of 5 patients with known receptor negative status.
Pediatric Patients with Heterozygous Familial Hypercholesterolemia
In a double blind, randomized, multicenter, placebo-controlled, 12 week study, 176 (97 male and 79 female) children and adolescents with heterozygous familial Hypercholesterolemia were randomized to rosuvastatin 5, 10 or 20 mg or placebo daily. Patients ranged in age from 10 to 17 years (median age of 14 years) with approximately 30% of the patients 10 to 13 years and approximately 17%, 18%, 40%, and 25% at Tanner stages II, III, IV, and V, respectively. Females were at least 1 year postmenarche. Mean LDL C at baseline was 233 mg/dL (range of 129 to 399). The 12 week double blind phase was followed by a 40 week open label dose-titration phase, where all patients (n=173) received 5 mg, 10 mg or 20 mg rosuvastatin daily.
Rosuvastatin significantly reduced LDL-C (primary end point), total cholesterol and ApoB levels at each dose compared to placebo. Results are shown in Table 11 below.
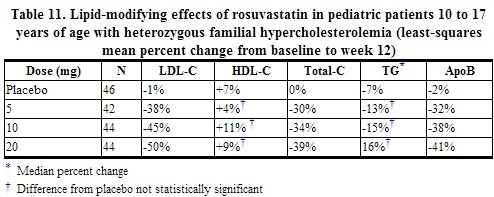
At the end of the 12 week, double blind treatment period, the percentage of patients achieving the LDL C goal of less than 110 mg/dL (2.8 mmol/L) was 0% for placebo, 12% for rosuvastatin 5 mg, 41% for rosuvastatin 10 mg and 41% for rosuvastatin 20 mg. For the 40 week, open label phase, 71% of the patients were titrated to the maximum dose of 20 mg and 41% of the patients achieved the LDL C goal of 110 mg/dL.
The long-term efficacy of rosuvastatin therapy initiated in childhood to reduce morbidity and mortality in adulthood has not been established.
Slowing of the Progression of Atherosclerosis
In the Measuring Effects on Intima Media Thickness: an Evaluation Of Rosuvastatin 40 mg (METEOR) study, the effect of therapy with rosuvastatin on carotid atherosclerosis was assessed by B-mode ultrasonography in patients with elevated LDL‑C, at low risk (Framingham risk <10% over ten years) for symptomatic coronary artery disease and with subclinical atherosclerosis as evidenced by carotid intimal-medial thickness (cIMT). In this double-blind, placebo-controlled clinical study 984 patients were randomized (of whom 876 were analyzed) in a 5:2 ratio to rosuvastatin 40 mg or placebo once daily. Ultrasonograms of the carotid walls were used to determine the annualized rate of change per patient from baseline to two years in mean maximum cIMT of 12 measured segments. The estimated difference in the rate of change in the maximum cIMT analyzed over all 12 carotid artery sites between patients treated with rosuvastatin and placebo-treated patients was -0.0145 mm/year (95% CI –0.0196, –0.0093; p<0.0001).
The annualized rate of change from baseline for the placebo group was +0.0131 mm/year (p<0.0001). The annualized rate of change from baseline for the group treated with rosuvastatin was -0.0014 mm/year (p=0.32).
At an individual patient level in the group treated with rosuvastatin, 52.1% of patients demonstrated an absence of disease progression (defined as a negative annualized rate of change), compared to 37.7% of patients in the placebo group.
Primary Prevention of Cardiovascular Disease
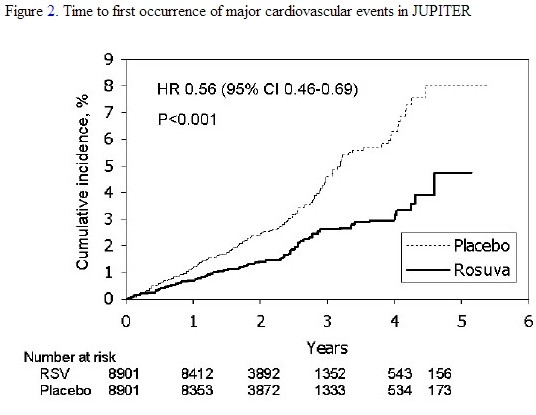
In the Justification for the Use of Statins in Primary Prevention: An Intervention Trial Evaluating Rosuvastatin (JUPITER) study, the effect of rosuvastatin (rosuvastatin calcium) on the occurrence of major cardiovascular (CV) disease events was assessed in 17,802 men (≥50 years) and women (≥60 years) who had no clinically evident cardiovascular disease, LDL‑C levels <130 mg/dL (3.3 mmol/l) and hs‑CRP levels ≥2 mg/L. The study population had an estimated baseline coronary heart disease risk of 11.6% over 10 years based on the Framingham risk criteria and included a high percentage of patients with additional risk factors such as hypertension (58%), low HDL‑C levels (23%), cigarette smoking (16%), or a family history of premature CHD (12%). Study participants had a median baseline LDL‑C of 108 mg/dL and hsCRP of 4.3 mg/L. Study participants were randomly assigned to placebo (n=8901) or rosuvastatin 20 mg once daily (n=8901) and were followed for a mean duration of 2 years. The JUPITER study was stopped early by the Data Safety Monitoring Board due to meeting predefined stopping rules for efficacy in rosuvastatin-treated subjects.
The primary end point was a composite end point consisting of the time-to-first occurrence of any of the following major CV events: CV death, nonfatal myocardial infarction, nonfatal stroke, hospitalization for unstable angina or an arterial revascularization procedure.
Rosuvastatin significantly reduced the risk of major CV events (252 events in the placebo group vs. 142 events in the rosuvastatin group) with a statistically significant (p<0.001) relative risk reduction of 44% and absolute risk reduction of 1.2% (see Figure 2). The risk reduction for the primary end point was consistent across the following predefined subgroups: age, sex, race, smoking status, family history of premature CHD, body mass index, LDL‑C, HDL‑C, and hsCRP levels.
Figure 2. Time to first occurrence of major cardiovascular events in JUPITER
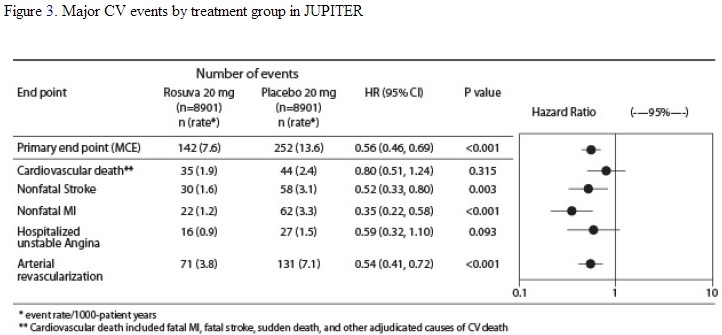
The individual components of the primary end point are presented in Figure 3. Rosuvastatin significantly reduced the risk of nonfatal myocardial infarction, nonfatal stroke, and arterial revascularization procedures. There were no significant treatment differences between the rosuvastatin and placebo groups for death due to cardiovascular causes or hospitalizations for unstable angina.
Rosuvastatin significantly reduced the risk of myocardial infarction (6 fatal events and 62 nonfatal events in placebo-treated subjects vs. 9 fatal events and 22 nonfatal events in rosuvastatin-treated subjects) and the risk of stroke (6 fatal events and 58 nonfatal events in placebo-treated subjects vs. 3 fatal events and 30 nonfatal events in rosuvastatin-treated subjects).
In a post-hoc subgroup analysis of JUPITER subjects (n=1405; rosuvastatin=725, placebo=680) with a hsCRP ≥2 mg/L and no other traditional risk factors (smoking, BP ≥140/90 or taking antihypertensives, low HDL‑C) other than age, after adjustment for high HDL‑C, there was no significant treatment benefit with rosuvastatin treatment.
At one year, rosuvastatin increased HDL‑C and reduced LDL‑C, hsCRP, total cholesterol and serum triglyceride levels (p<0.001 for all versus placebo).
How Supplied
CRESTOR® (rosuvastatin calcium) Tablets are supplied as:
- NDC 0310-0755-90: 5 mg. Yellow, round, biconvex, coated tablets. Debossed “CRESTOR” and “5” on one side; bottle of 90 tablets
- NDC 0310-0751-90: 10 mg. Pink, round, biconvex, coated tablets. Debossed “CRESTOR” and “10” on one side; bottle of 90 tablets
- NDC 0310-0751-39: 10 mg. Pink, round, biconvex, coated tablets. Debossed “CRESTOR” and “10” on one side; unit dose packages of 100
- NDC 0310-0752-90: 20 mg. Pink, round, biconvex, coated tablets. Debossed “CRESTOR” and “20” on one side; bottles of 90
- NDC 0310-0752-39: 20 mg. Pink, round, biconvex, coated tablets. Debossed “CRESTOR” and “20”on one side; unit dose packages of 100
- NDC 0310-0754-30: 40 mg. Pink, oval, biconvex, coated tablets. Debossed “CRESTOR” on one side and “40” on the other side; bottles of 30
Storage
Store at controlled room temperature, 20‑25ºC (68-77ºF). Protect from moisture.
Images
Drug Images
{{#ask: Page Name::Rosuvastatin |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Rosuvastatin |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Skeletal Muscle Effects
Patients should be advised to report promptly unexplained muscle pain, tenderness, or weakness, particularly if accompanied by malaise or fever or if these muscle signs or symptoms persist after discontinuing rosuvastatin.
Concomitant Use of Antacids
When taking rosuvastatin with an aluminum and magnesium hydroxide combination antacid, the antacid should be taken at least 2 hours after rosuvastatin administration.
Pregnancy
If the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the fetus and the lack of known clinical benefit with continued use during pregnancy.
Liver Enzymes
It is recommended that liver enzyme tests be performed before the initiation of rosuvastatin and if signs or symptoms of liver injury occur. All patients treated with rosuvastatin should be advised to promptly report any symptoms that may indicate liver injury, including fatigue, anorexia, right upper abdominal discomfort, dark urine or jaundice.
Precautions with Alcohol
Rosuvastatin should be used with caution in patients who consume substantial quantities of alcohol and/or have a history of chronic liver disease.
Brand Names
Crestor
Look-Alike Drug Names
There is limited information regarding Rosuvastatin Look-Alike Drug Names in the drug label.
Drug Shortage
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Page Name=Rosuvastatin |Pill Name=Crestor 5.jpg |Drug Name=Rosuvastatin |Pill Ingred=lactose monohydrate, cellulose, microcrystalline, hypromelloses, triacetin, titanium dioxide, ferric oxide yellow|+sep=; |Pill Imprint=5;crestor |Pill Dosage=5 mg |Pill Color=Yellow|+sep=; |Pill Shape=Round |Pill Size (mm)=7.00 |Pill Scoring=1 |Pill Image= |Drug Author=AstraZeneca Pharmaceuticals LP |NDC=0310-0755-90
}}
{{#subobject:
|Page Name=Rosuvastatin |Pill Name=Crestor 10.jpg |Drug Name=Rosuvastatin |Pill Ingred=lactose monohydrate, cellulose, microcrystalline, hypromelloses, triacetin, titanium dioxide, ferric oxide yellow|+sep=; |Pill Imprint=10;crestor |Pill Dosage=10 mg |Pill Color=Pink|+sep=; |Pill Shape=Round |Pill Size (mm)=7.00 |Pill Scoring=1 |Pill Image= |Drug Author=AstraZeneca Pharmaceuticals LP |NDC=0310-0751-90
}}
{{#subobject:
|Page Name=Rosuvastatin |Pill Name=Crestor 20.jpg |Drug Name=Rosuvastatin |Pill Ingred=lactose monohydrate, cellulose, microcrystalline, hypromelloses, triacetin, titanium dioxide, ferric oxide yellow|+sep=; |Pill Imprint=20;crestor |Pill Dosage=20 mg |Pill Color=Pink|+sep=; |Pill Shape=Round |Pill Size (mm)=9.00 |Pill Scoring=1 |Pill Image= |Drug Author=AstraZeneca Pharmaceuticals LP |NDC=0310-0752-90: 20 mg
}}
{{#subobject:
|Page Name=Rosuvastatin |Pill Name=Crestor 40.jpg |Drug Name=Rosuvastatin |Pill Ingred=lactose monohydrate, cellulose, microcrystalline, hypromelloses, triacetin, titanium dioxide, ferric oxide yellow|+sep=; |Pill Imprint=40;crestor |Pill Dosage=40 mg |Pill Color=Pink|+sep=; |Pill Shape=Oval |Pill Size (mm)=11.00 |Pill Scoring=1 |Pill Image= |Drug Author=AstraZeneca Pharmaceuticals LP |NDC=0310-0754-30
}}
{{#subobject:
|Label Page=Rosuvastatin |Label Name=Rosuvastatin17.jpg
}}
{{#subobject:
|Label Page=Rosuvastatin |Label Name=Rosuvastatin19.jpg
}}