Hyperparathyroidism pathophysiology
|
Hyperparathyroidism Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Hyperparathyroidism pathophysiology On the Web |
|
American Roentgen Ray Society Images of Hyperparathyroidism pathophysiology |
|
Risk calculators and risk factors for Hyperparathyroidism pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1];Associate Editor(s)-in-Chief: Anmol Pitliya, M.B.B.S. M.D.[2]
Overview
Hyperparathyroidism is an increase in serum parathyroid hormone. Normally, parathyroid hormone increases serum calcium and magnesium concentration, and decreases serum phosphate concentration. Secretion of parathyroid hormone from parathyroid gland is stimulated by low serum calcium. Parathyroid glands have calcium-sensing receptors responsible for sensing extracellular ionized calcium. Calcium and magnesium provides a negative feedback for secretion of parathyroid hormone. Primary hyperparathyroidism is due to increase in secretion of parathyroid hormone from a primary process in parathyroid gland. Majority of times, increase in secretion of parathyroid hormone is the result of parathyroid adenoma (85%). Calcium-sensing receptor expression in reduced in parathyroid adenoma resulting in an increase in calcium sensing set point. In minority of cases, development of primary hyperparathyroidism is the result of multiple genetic mutations. Genes involved in the pathogenesis of primary hyperparathyroidism include calcium-sensing receptor gene, HRPT2 gene (CDC73 gene), Cyclin D1 gene (CCND1)/PRAD1 gene, MEN1 gene, and RET gene. Secondary hyperparathyroidism is due to increase in secretion of parathyroid hormone from a secondary process, most commonly due chronic renal failure. Fibroblast growth factor 23 (FGF-23) concentration increases in chronic renal failure which plays a central role in regulation of phosphate vitamin D homeostasis and pathogenesis of secondary hyperparathyroidism. Majority of times, tertiary hyperparathyroidism occurs in patients after renal transplantation.Patients with secondary hyperparathyroidism continues to have elevated parathyroid hormone even after renal transplantation. Classically, there is hyperplasia of all four of parathyroid gland. On gross pathology, parathyroid adenoma is a soft, tan nodule which is well-circumscribed by a delicate capsule. Typically, cut surface of parathyroid adenoma is smooth, soft, and reddish brown in color. It should be differentiated from normal parathyroid gland tissue which is yellow-brown color. Parathyroid hyperplasia usually involves multiple glands. Bones and kidney are also commonly involved in hyperparathyroidism. Hypercalcemia due to hyperparathyroidism may cause metastatic calcification in many organs including lungs, heart, blood vessels, stomach. Chief cells are predominant in parathyroid adenoma on microcopy. Adenoma is seperated from a rim of non-neoplastic tissue on the edge by a fibrous capsule. Endocrine atypia (cells with bizarre and pleomorphic nuclei) is often seen in parathyroid adenoma. It should not be mistaken as a sign of malignancy. Majority of times, hyperplasia of chief cells is observed in parathyroid hyperplasia. It may be diffuse or multinodular. Cytologic details are unreliable for diagnosis of parathyroid carcinoma.
Pathophysiology
Parathyroid, Vitamin D, and Mineral Homeostasis
The effect of parathyroid hormone on mineral metabolism is as follows:[1][2]
- Effect of parathyroid hormone on inorganic phosphate metabolism:
- Increases excretion of inorganic phosphate from kidney resulting in decreased serum concentration of phosphate.
- Effect on parathyroid hormone on calcium metabolism:
- Direct effect:
- Increased resorption of bones.
- Decreases excretion from kidney.
- Indirect effect:
- Increases conversion of inactive 25-hydroxy vitamin D to the active 1,25-dihydroxy vitamin D which increases absorption of calcium from gut. Decreased phosphate concentration also increases this conversion process. Vitamin D shows synergism with parathyroid hormone action on bone.
- Decreased serum inorganic phosphate concentration prevents precipitation of calcium phosphate in bones.
- Both these direct and indirect mechanism results in an increased serum calcium concentration.
- Direct effect:
- Effect of parathyroid hormone on magnesium concentration:
Effect of minerals and vitamin D on parathyroid hormone:
- Decrease in serum calcium concentration stimulates parathyroid hormone.
- Calcium provides negative feedback on parathyroid hormone.
- Magnesium provides negative feedback on parathyroid hormone.
- Vitamin D decreases the concentration of parathyroid hormone.
| Parathyroid hormone | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kidney | Bone | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Decreased excretion of magnesium | Increasead conversion of inactive 25-hydroxy vitamin D to the active 1,25-dihydroxy vitamin D | Increase excretion of inorganic phosphate | Decrease excretion of calcium | Increased resorption of bone | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Increased serum concentration of magnesium | Increased absorption of calcium from gut | Decreased serum concentration of inorganic phosphate | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Prevents precipitation of calcium phosphate in bones | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Increased serum concentration of calcium | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Calcium-sensing receptors
- Calcium-sensing receptors are present on parathyroid glands. They are a type of 7-transmembrane receptors in G-protein coupled receptors superfamily of receptors.[3]
- Calcium-sensing receptors sense change in extracellular concentration of ionized calcium.[4]
- Calcium-sensing receptor expression in reduced in primary hyperparathyroidism (parathyroid adenoma) and secondary hyperparathyroidism.[5]
- This reduced expression of receptor causes an increases in calcium sensing set point.[6]
- This in turn leads to increase in secretion of parathyroid hormone in presence on normal serum concentration of extracellular ionized calcium.
Pathogenesis of primary hyperparathyroidism
- Primary hyperparathyroidism is due to increase in secretion of parathyroid hormone from a primary process in parathyroid gland.
- Majority of times, increase in secretion of parathyroid hormone is the result of parathyroid adenoma (85%). Other causes of increase in secretion of parathyroid hormone includes parathyroid hyperplasia (15%) and parathyroid carcinoma (5%).[7]
- Calcium-sensing receptor expression in reduced in parathyroid adenoma resulting in an increase in calcium sensing set point.[5][6]
- In parathyroid hyperplasia, an increase in cell number causes increased secretion of parathyroid hormone.
Pathogenesis of secondary hyperparathyroidism
- Secondary hyperparathyroidism is due to increase in secretion of parathyroid hormone from a secondary process, most commonly due chronic renal failure. Other causes include vitamin D deficiency, severe calcium deficiency.[8]
- Chronic renal failure leads to high serum inorganic phosphate and low serum calcium and deficiency of active form of vitamin D (1,25-dihydroxy vitamin D/calcitriol)
- This leads to continuous stimulation of parathyroid glands resulting down-regulation of parathyroid vitamin D receptors and calcium-sensing receptors.
- Fibroblast growth factor 23 (FGF-23) concentration increases in chronic renal failure which plays a central role in regulation of phosphate vitamin D homeostasis.
- Elevated FGF-23 expression down-regulates remaining 25(OH)-1-hydroxylase enzyme. 25(OH)-1-hydroxylase enzyme is responsible for conversion of inactive 25-hydroxy vitamin D into active 1,25-dihydroxy vitamin D (calcitriol). This aggravates the deficiency of active vitamin D.
- These all factors leads to hyperplasia of parathyroid gland.
| Chronic renal failure | |||||||||||||||||||||||||||||||||||||||
| Elevated serum inorganic phosphate concentration | |||||||||||||||||||||||||||||||||||||||
| Elevated FGF-23 | |||||||||||||||||||||||||||||||||||||||
| Decreased calcitriol | |||||||||||||||||||||||||||||||||||||||
| Decreaed serum calcium concentration | |||||||||||||||||||||||||||||||||||||||
| Continuous stimulation of parathyroid gland | |||||||||||||||||||||||||||||||||||||||
| Downregulation of parathyroid vitamin D receptors and calcium-sensing receptors | |||||||||||||||||||||||||||||||||||||||
| Parathyroid hyperplasia | |||||||||||||||||||||||||||||||||||||||
| Increased secretion of parathyroid hormone | |||||||||||||||||||||||||||||||||||||||
Mechanism of fibroblast growth factor 23 (FGF-23)
The mechanism of fibroblast growth factor 23 (FGF-23) in chronic renal disease and development of secondary hyperparathyroidism is as follows:[8]
- FGF-23 is a hormone produced in the osteocytes and osteoblasts.
- Its production is increased due to high serum phosphate and high calcitriol.
- FGF-23 binds and activates a receptor called fibroblast growth factor receptor 1 (FGFR1).
- FGFR1 is functional when co-expressed with the Klotho transmembrane protein, as a Klotho-FGF receptor complex.
- FGF-23 reduces the expression of type II sodium phosphate co-transporters (NaPi-2a and NaPi-2c) decreasing phosphate reabsorption in proximal tubules.
- In chronic renal failure, as a result, phosphate absorption is increased in proximal tubules due to effect of FGF-23 as well as increased parathyroid hormone. This is responsible for normal serum phosphate levels in majority of patients until the glomerular filtration rate (GFR) falls below 20 ml/min.
- As chronic renal failure progresses, these negative feedback loops are impaired leading to deranged phosphate homeostasis.
- FGF-23 have direct and indirect effect on parathyroid hormone.
- Direct effect: In normal parathyroid gland, FGF-23 decreases synthesis of parathyroid hormone through the mitogen-activated protein kinase (MAPK) pathway.[9] FGF-23 increases expression of the parathyroid calcium-sensing receptor and the vitamin D receptor, and reduces cellular proliferation.[10]
- Indirect effect: Increased synthesis of parathyroid hormone by decreasing synthesis of calcitriol.
- FGF-23 fails to activate mitogen-activated protein kinase pathway in hyperplastic parathyroid gland secondary to chronic renal failure.[10]
Pathogenesis of tertiary hyperparathyroidism
- Majority of times, tertiary hyperparathyroidism occurs in patients after renal transplantation.[11]
- Patients with secondary hyperparathyroidism continues to have elevated parathyroid hormone even after renal transplantation.
- Patients with secondary hyperparathyroidism and long term hypocalcemia tends to have hyperplasia of chief cells of parathyroid gland and increased secretion of parathyroid hormone.
- The primary disorder in secondary hyperparathyroidism is chronic renal failure in majority of patients. After correction of chronic renal failure by renal transplant, the hyperplastic parathyroid gland fails to resolve and continues to secrete excess amount of parathyroid hormone.
- Clasically, there is hyperplasia of all four of parathyroid gland.
Genetics
The development of primary hyperparathyroidism is the result of multiple genetic mutations in minority of cases. Genes involved in the pathogenesis of primary hyperparathyroidism include calcium-sensing receptor gene, HRPT2 gene (CDC73 gene), Cyclin D1 gene (CCND1)/PRAD1 gene, MEN1 gene, and RET gene.
- Calcium-sensing receptor gene mutation:[12]
- Calcium-sensing receptor (CSR) gene is present on chromosome 3q.
- Few individuals carries an inherited mutation in the extracellular calcium-sensing receptor gene.
- The first identified mutation in CSR gene is a point mutation in which phenylalanine is replaced with leucine at codon 881 of CSR gene.[13]
- This mutation reduces the activity of calcium-sensing receptor.
- This mutation can be heterozygous or homozygous.
- Individuals carrying heterozygous mutation have familial hypocalciuric hypercalcemia (FHH) or familial benign hypercalcemia. FHH is characterized by parathyroid dependent hypercalcemia and decreased responsiveness of parathyroid and kidney to hypercalcemia.
- Individuals carrying homozygous mutation have neonatal severe hyperparathyroidism. Neonatal severe hyperparathyroidism is characterized by marked parathyroid hyperplasia.
- Familial hypocalciuric hypercalcemia (FHH) and neonatal severe hyperparathyroidism are transmitted in autosomal dominant pattern.
- HRPT2 gene(CDC73 gene) mutations:[14]
- HRPT2 gene code for parafibromin protein.
- HRPT2 gene mutations are found in a type of familial hyperparathyroidism, hyperparathyroidism-jaw tumor (HPT-JT) syndrome.
- HRTP2 gene mutations increases risk of parathyroid carcinoma.
- Cyclin D1 gene (CCND1)/PRAD1 gene:[15][16]
- PRAD1 (parathyroid adenoma 1) is a protooncogene located on chromosome 11q13.
- Cyclin D1 gene translocation and oncogene action observed in 8% of adenomas.
- Cyclin D1 gene overexpression is observed in 20% to 40% of parathyroid adenomas.
- MEN1 gene:[15][17]
- MEN 1 ics a tumor supressor gene on chromosome 11q13.
- Somatic loss of single MEN1 allele is observed in 25% to 40% of sporadic parathyroid adenomas.
- RET gene:[18]
- RET gene is a proto-oncogene.
- RET proto-oncogene is associated with multiple endocrine neoplasia type 2 (MEN 2).
- MEN2A caries increased risk of parathyroid adenoma and/or parathyroid hyperplasia.
- CDNK1B gene:[19]
- CDNK1B mutation causes Multiple endocrine neoplasia type 4 (MEN 4).
- Parathyroid tumors are found along with anterior pituitary, gonadal, adrenal, and renal tumors in MEN 4 syndrome.
- CDNK1B encodes for the cyclin-dependent kinase inhibitor p27kip1.
Associated Conditions
The conditions associated with hyperparathyroidism include:[20][21][22][12][14][17][23][24][25][26][27][28]
- Brown tumor
- Chronic renal failure
- Depression
- Familial hypocalciuric hypercalcemia
- Hyperparathyroid-jaw tumor syndrome
- Hypertension
- Multiple endocrine neoplasia type 1
- Multiple endocrine neoplasia type 2A
- Multiple endocrine neoplasia type 4
- Neonatal severe hyperparathyroidism
- Osteitis fibrosa cystica
- Osteoporosis
- Osteomalacia
- Osteoarthritis
- Pancreatitis
Gross Pathology
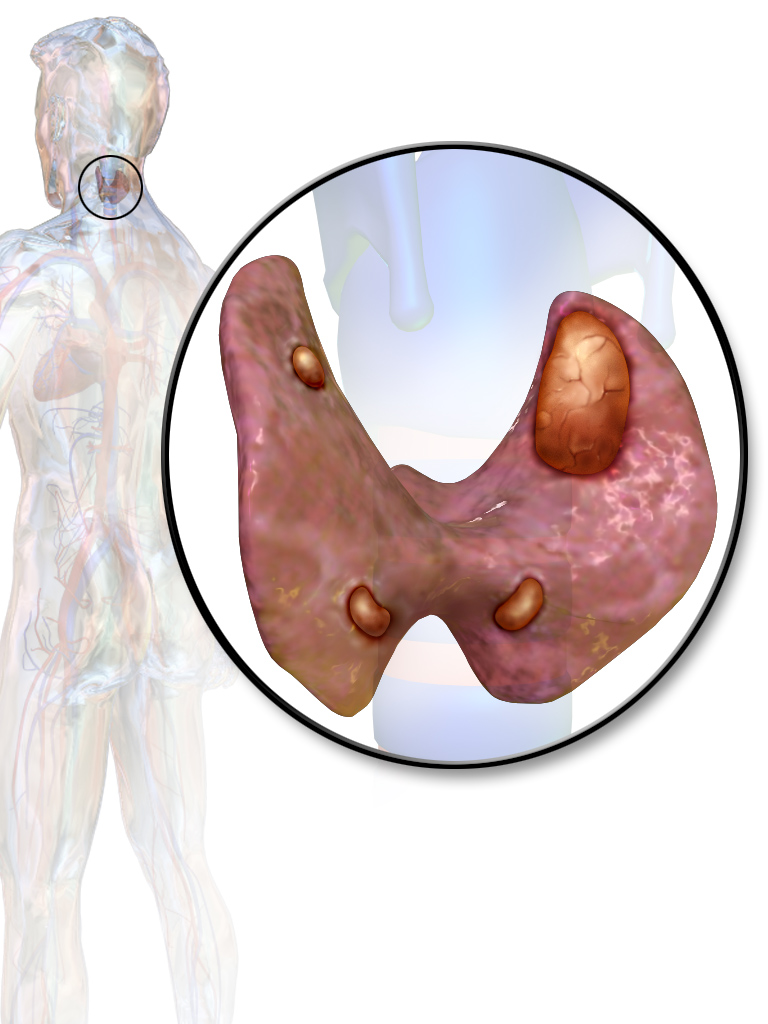
Parathyroid glands
Parathyroid adenoma
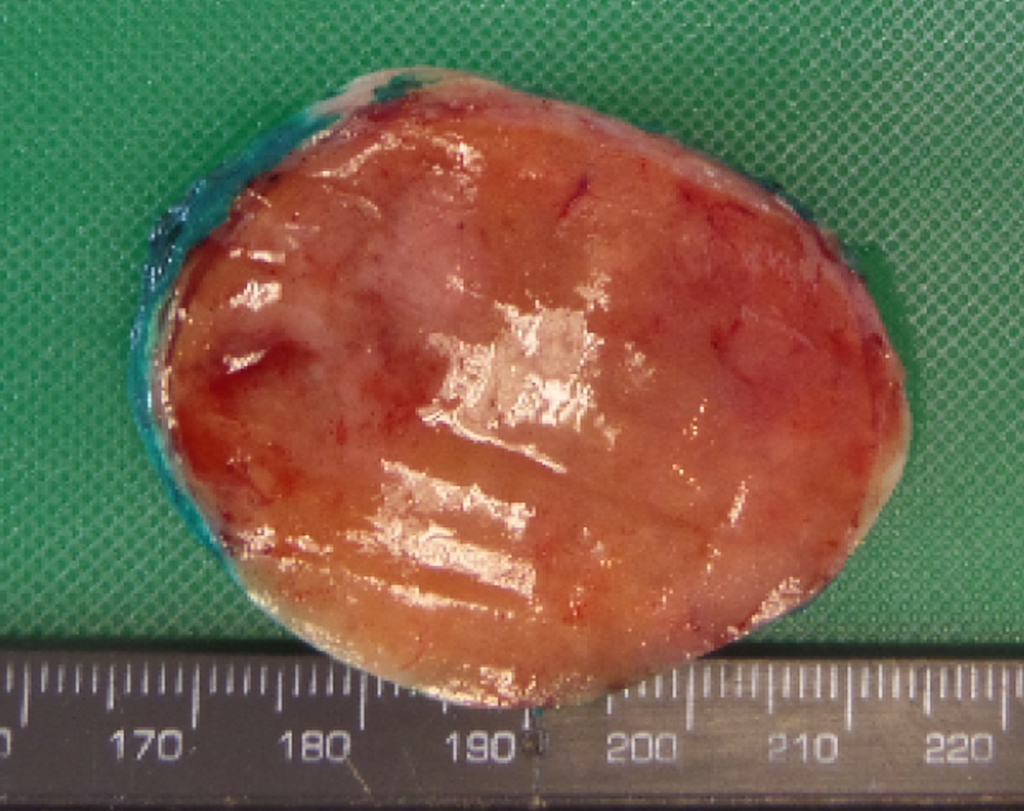
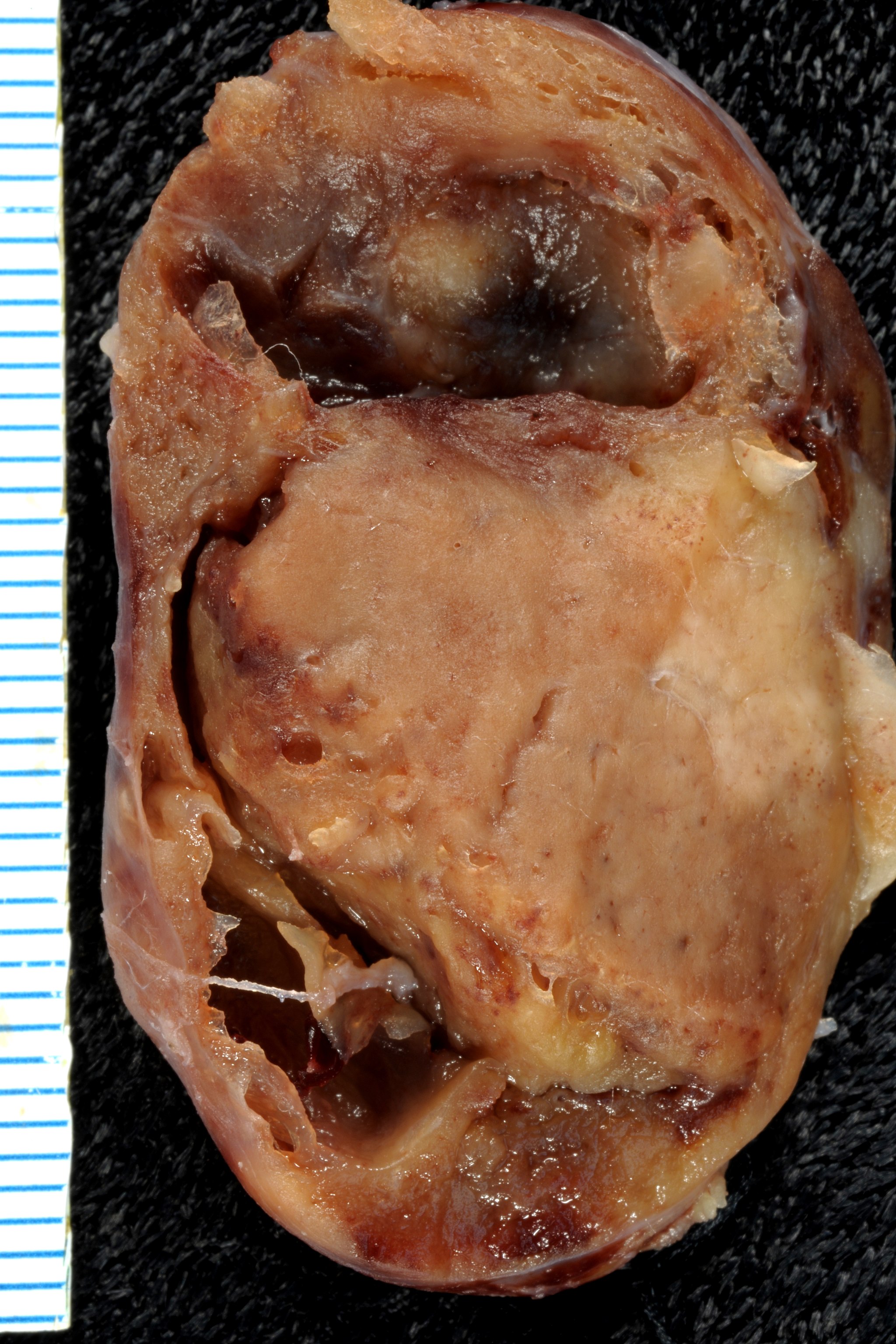
- On gross pathology, parathyroid adenoma is a soft, tan nodule which is well-circumscribed by a delicate capsule.[29]
- Most commonly, parathyroid adenoma is present in single gland. Some times multiple glands are involved.
- If single gland is involved, the other glands may shrink due to negative feedback.
- Majority of times, parathyroid adenoma weight ranges between 0.5 gram to 5 gram.
- Typically, cut surface of parathyroid adenoma is smooth, soft, and reddish brown in color. It should be differentiated from normal parathyroid gland tissue which is yellow-brown color.[30]
- The tissue of parathyroid gland that is not involved in parathyroid adenoma is typically atrophied and compressed. Fat component of normal parathyroid tissue is also observed.
- On rare occasion, parathyroid adenoma may be cystic.
 |
 |
 |
Parathyroid hyperplasia
- Parathyroid hyperplasia usually involves multiple glands.[29]
- The combine weight of all hyperplastic gland is usually less than 1 gram.
Parathyroid carcinoma
- On gross pathology, parathyroid carcinoma may range from a well circumscribed lesion to invasive neoplasm.[29]
- Sometimes, it may be difficult to differentiate from parathyroid adenoma.
- One parathyroid gland enlarges in parathyroid carcinoma, consisting gery-white and irregular mass.
- Sometimes, mass of single parathyroid carcinoma becomes more than 10 gram in weight.
Other organs
Bones
- There is erosion of bone martix and bone resorption due to increase in osteoclastic activity.[29][31]
- Most common site is metaphysis of long tubular bones.
- There is increased osteoblastic activity along with bone resorption leading to formation of new bone trabeculae.
- As severity increases, there is gross thinning of cortex and increase in amount of fibrous tissue in marrow along with multiple foci of hemorrhage and cysts. The condition is called osteitis fibrosa cystica.
- Sometimes there is development of masses due to aggregation of osteoclasts, reactive giant cells and hemorrhagic debris. This may be confused with neoplasm. This is called brown tumor of hyperparathyroidism.
Kidneys
| |
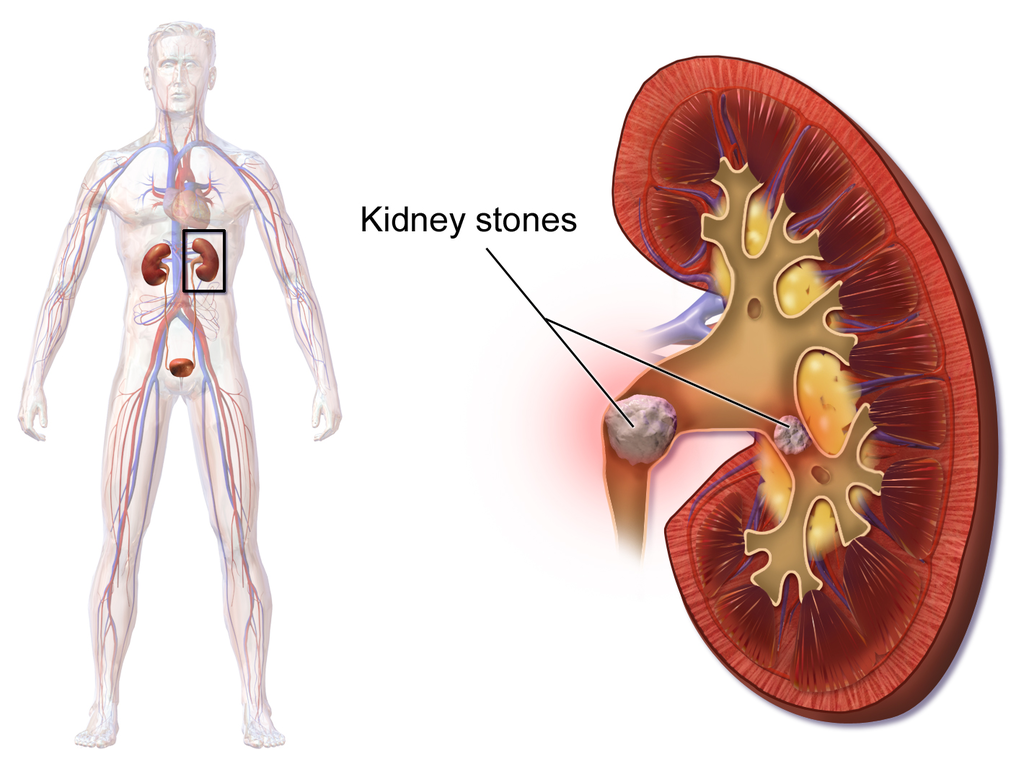 |
 |
Other organs
- Hypercalcemia due to hyperparathyroidism may cause metastatic calcification in many organs including lungs, heart, blood vessels, stomach.[29][33]
Microscopic Pathology
Parathyroid adenoma
- Chief cells are predominant in parathyroid adenoma on microcopy.
- In majority of cases, a few nests of larger oxyphil cells are also present.
- Adenoma is seperated from a rim of non-neoplastic tissue on the edge by a fibrous capsule.
- Chief cells present in adenoma larger than normal chief cells and shows greater variability on nuclear size.
- Endocrine atypia (cells with bizarre and pleomorphic nuclei) is often seen in parathyroid adenoma. It should not be mistaken as a sign of malignancy.
- Mitotic figures are rarely present.
- Parathyroid adenoma has incospicuous adipose tissue when compared with normal parathyroid gland.
-
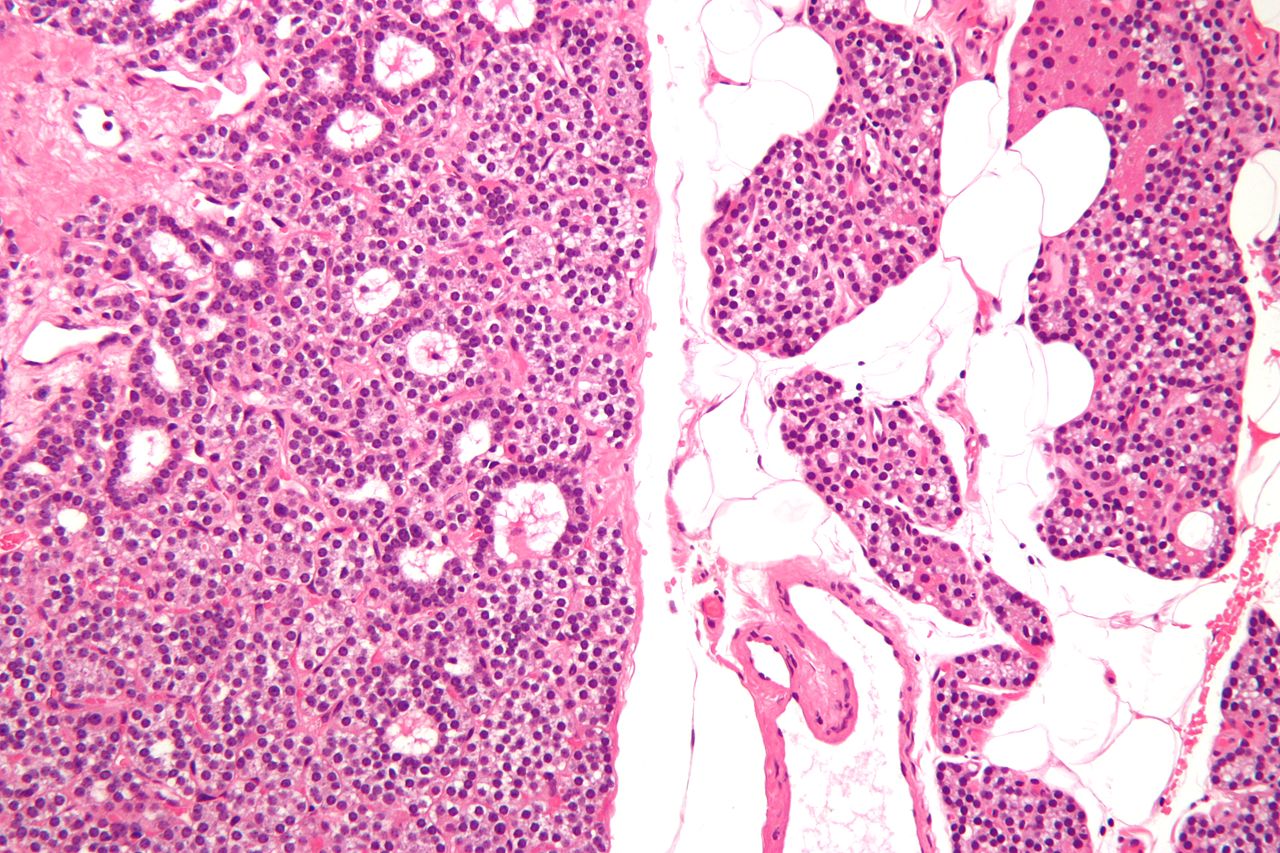
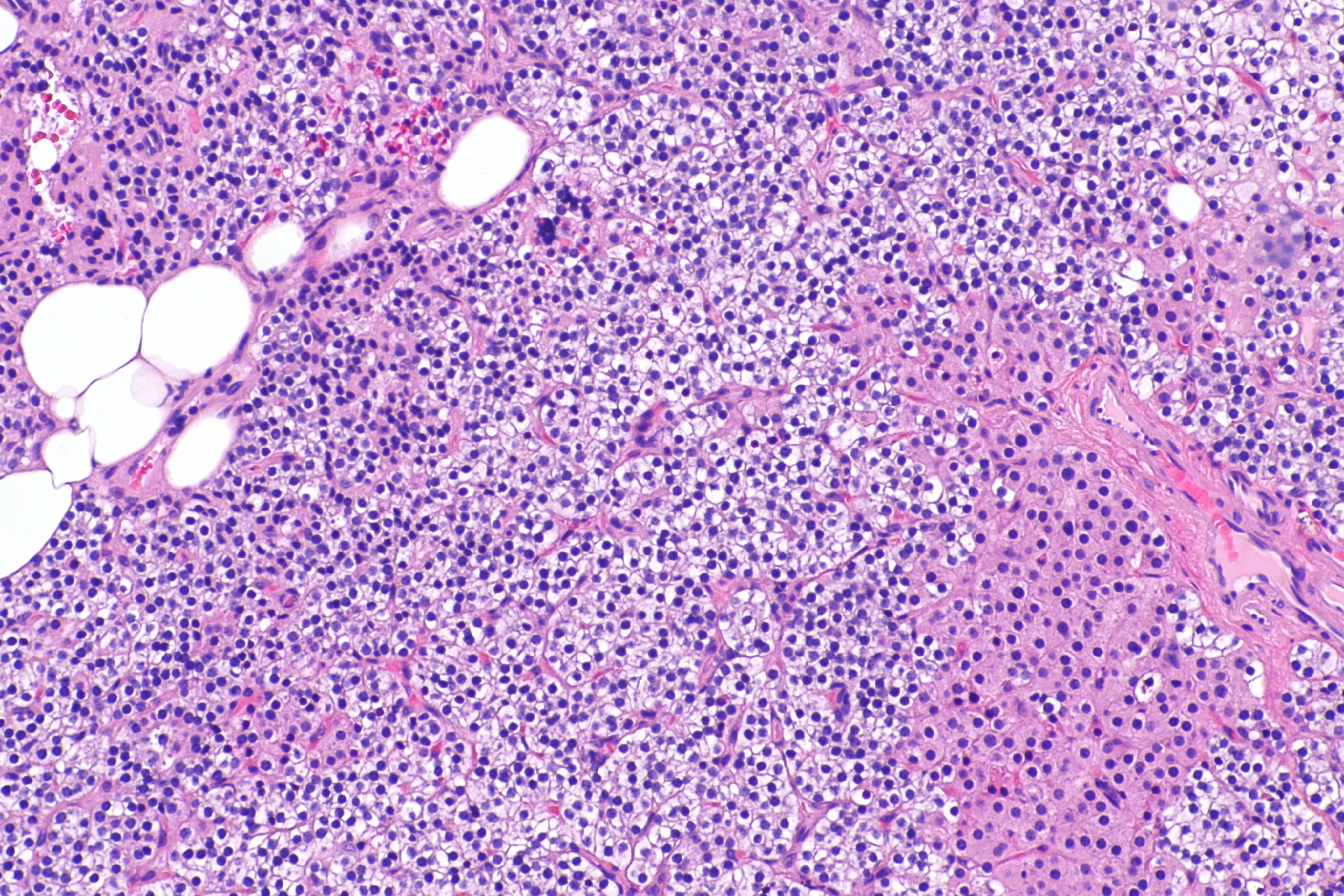
Intermediate/Low magnification micrograph of parathyroid adenoma. H&E stain. Features: Single cell population forming a single mass. Thin capsule. No adipose tissue. +/-Glandular architecture (which may lead to confusion with thyroid tissue). Normal parathyroid gland with prominent adipose tissue is seen on the right of the image. - Source: Wikipedia
-
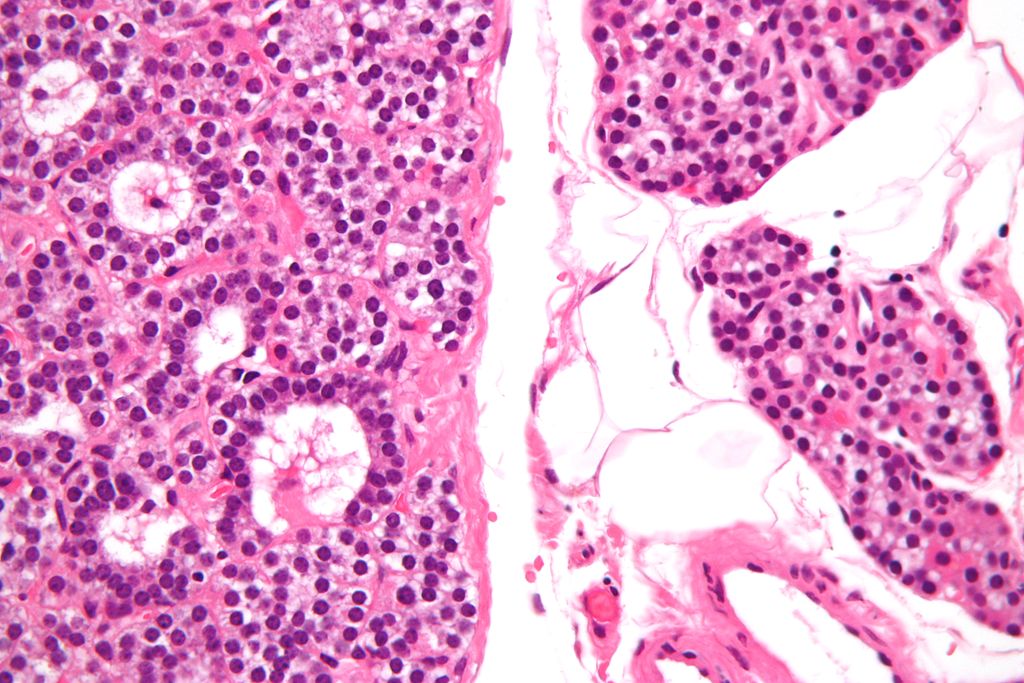
High magnification micrograph of a parathyroid adenoma. H&E stain. Features: Single cell population forming a single mass. Thin capsule. No adipose tissue. +/-Glandular architecture (which may lead to confusion with thyroid tissue). Normal parathyroid gland with prominent adipose tissue is seen on the right of the image. - Source:wikipeida
-
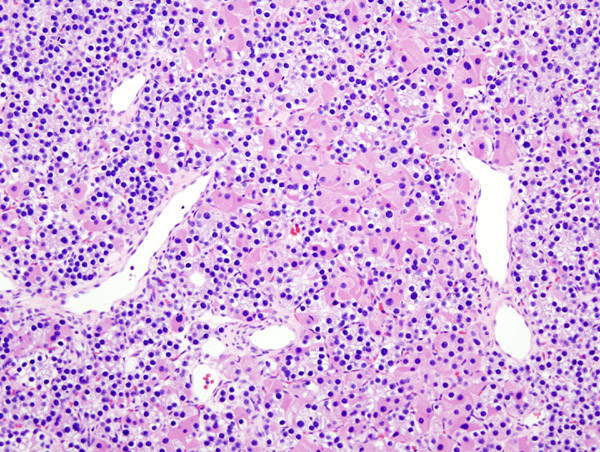
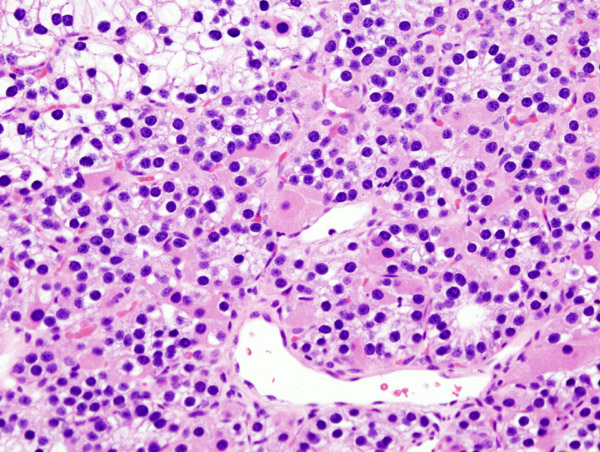
Histopatholgical image of parathyroid adenoma in a patient with primary hyperparathyroidism. Hematoxylin and eosin stain. - Source: Wikipedia
-
Histopatholgical image of parathyroid adenoma in a patient with primary hyperparathyroidism. Hematoxylin and eosin stain. - Source: Wikipedia
-
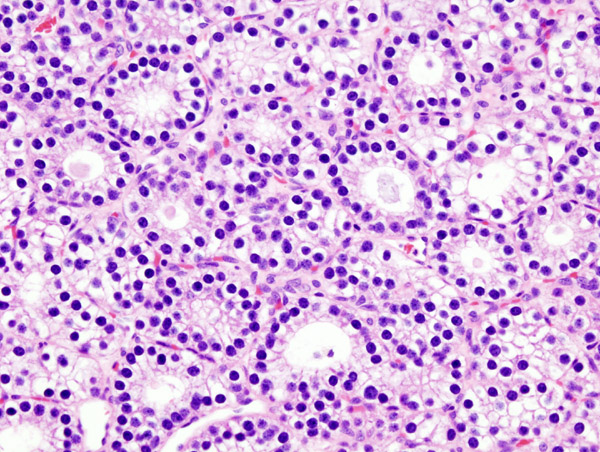
Histopatholgical image of parathyroid adenoma in a patient with primary hyperparathyroidism. - Source: Wikipedia
Parathyroid hyperplasia
- Majority of times, hyperplasia of chief cells is observed. It may be diffuse or multinodular.
- In minority of cases, there is accumulation of glycogen in cytoplasm resulting in abundant clear cytoplasm. This condition is called "water-clear cell hyperplasia".
- Adipose tissue is in incospicuous in hyperplastic foci, same as adenoma.
-
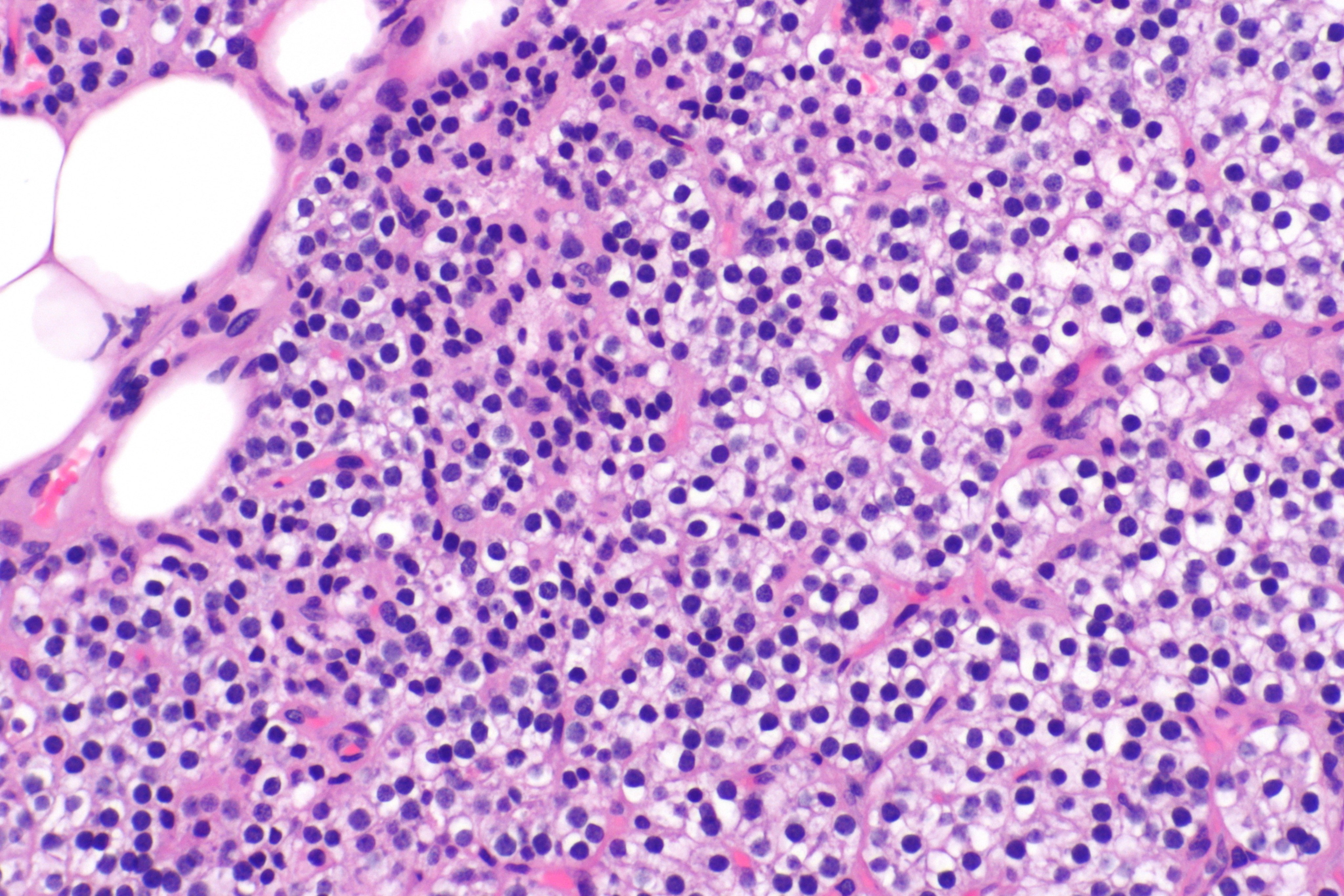
Micrograph showing a parathyroid hyperplasia (H&E stain) on intermediate magnification.Parathyroid adenoma is a clinicopathologic diagnosis. The histology is nonspecific. -Source: Wikimedia commons
-
Micrograph showing a parathyroid hyperplasia (H&E stain) on high magnification.Parathyroid adenoma is a clinicopathologic diagnosis. The histology is nonspecific. - Source: Wikimedia commons
-
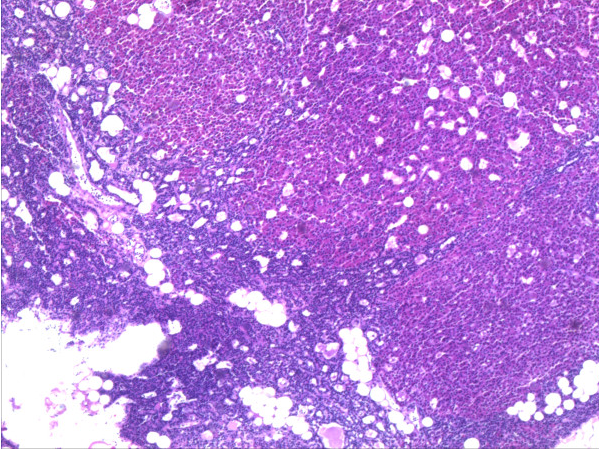
Hyperplasia of parathyroid gland HE-staining, 20× magnification. Source: Wikipedia. Biomedcentral For original file click here.Creative Commons lisence
Parathyroid carcinoma
- Cytologic details are unreliable for diagnosis of parathyroid carcinoma.
- Definitive diagnostic criteria include invasion of surrounding tissue and metastasis.
- About one third of cases have local recurrence and another one third have distant metastasis.
References
- ↑ HARRISON MT (1964). "INTERRELATIONSHIPS OF VITAMIN D AND PARATHYROID HORMONE IN CALCIUM HOMEOSTASIS". Postgrad Med J. 40: 497–505. PMC 2482768. PMID 14184232.
- ↑ Nussey, Stephen (2001). Endocrinology : an integrated approach. Oxford, UK Bethesda, Md: Bios NCBI. ISBN 1-85996-252-1.
- ↑ Brown EM, Gamba G, Riccardi D, Lombardi M, Butters R, Kifor O; et al. (1993). "Cloning and characterization of an extracellular Ca(2+)-sensing receptor from bovine parathyroid". Nature. 366 (6455): 575–80. doi:10.1038/366575a0. PMID 8255296.
- ↑ Brown EM, Pollak M, Seidman CE, Seidman JG, Chou YH, Riccardi D; et al. (1995). "Calcium-ion-sensing cell-surface receptors". N Engl J Med. 333 (4): 234–40. doi:10.1056/NEJM199507273330407. PMID 7791841.
- ↑ 5.0 5.1 Gogusev J, Duchambon P, Hory B, Giovannini M, Goureau Y, Sarfati E; et al. (1997). "Depressed expression of calcium receptor in parathyroid gland tissue of patients with hyperparathyroidism". Kidney Int. 51 (1): 328–36. PMID 8995751.
- ↑ 6.0 6.1 Kifor O, Moore FD, Wang P, Goldstein M, Vassilev P, Kifor I; et al. (1996). "Reduced immunostaining for the extracellular Ca2+-sensing receptor in primary and uremic secondary hyperparathyroidism". J Clin Endocrinol Metab. 81 (4): 1598–606. doi:10.1210/jcem.81.4.8636374. PMID 8636374.
- ↑ Wieneke JA, Smith A (2008). "Parathyroid adenoma". Head Neck Pathol. 2 (4): 305–8. doi:10.1007/s12105-008-0088-8. PMC 2807581. PMID 20614300.
- ↑ 8.0 8.1 Cunningham J, Locatelli F, Rodriguez M (2011). "Secondary hyperparathyroidism: pathogenesis, disease progression, and therapeutic options". Clin J Am Soc Nephrol. 6 (4): 913–21. doi:10.2215/CJN.06040710. PMID 21454719.
- ↑ Ben-Dov IZ, Galitzer H, Lavi-Moshayoff V, Goetz R, Kuro-o M, Mohammadi M, Sirkis R, Naveh-Many T, Silver J (2007). "The parathyroid is a target organ for FGF23 in rats". J. Clin. Invest. 117 (12): 4003–8. doi:10.1172/JCI32409. PMC 2066196. PMID 17992255.
- ↑ 10.0 10.1 Canalejo R, Canalejo A, Martinez-Moreno JM, Rodriguez-Ortiz ME, Estepa JC, Mendoza FJ, Munoz-Castaneda JR, Shalhoub V, Almaden Y, Rodriguez M (2010). "FGF23 fails to inhibit uremic parathyroid glands". J. Am. Soc. Nephrol. 21 (7): 1125–35. doi:10.1681/ASN.2009040427. PMC 3152229. PMID 20431039.
- ↑ Pitt SC, Sippel RS, Chen H (2009). "Secondary and tertiary hyperparathyroidism, state of the art surgical management". Surg. Clin. North Am. 89 (5): 1227–39. doi:10.1016/j.suc.2009.06.011. PMC 2905047. PMID 19836494.
- ↑ 12.0 12.1 Hosokawa Y, Pollak MR, Brown EM, Arnold A (1995). "Mutational analysis of the extracellular Ca(2+)-sensing receptor gene in human parathyroid tumors". J. Clin. Endocrinol. Metab. 80 (11): 3107–10. doi:10.1210/jcem.80.11.7593409. PMID 7593409.
- ↑ Carling T, Szabo E, Bai M, Ridefelt P, Westin G, Gustavsson P, Trivedi S, Hellman P, Brown EM, Dahl N, Rastad J (2000). "Familial hypercalcemia and hypercalciuria caused by a novel mutation in the cytoplasmic tail of the calcium receptor". J. Clin. Endocrinol. Metab. 85 (5): 2042–7. doi:10.1210/jcem.85.5.6477. PMID 10843194.
- ↑ 14.0 14.1 Shattuck TM, Välimäki S, Obara T, Gaz RD, Clark OH, Shoback D; et al. (2003). "Somatic and germ-line mutations of the HRPT2 gene in sporadic parathyroid carcinoma". N Engl J Med. 349 (18): 1722–9. doi:10.1056/NEJMoa031237. PMID 14585940.
- ↑ 15.0 15.1 Westin G, Björklund P, Akerström G (2009). "Molecular genetics of parathyroid disease". World J Surg. 33 (11): 2224–33. doi:10.1007/s00268-009-0022-6. PMID 19373510.
- ↑ Hsi ED, Zukerberg LR, Yang WI, Arnold A (1996). "Cyclin D1/PRAD1 expression in parathyroid adenomas: an immunohistochemical study". J Clin Endocrinol Metab. 81 (5): 1736–9. doi:10.1210/jcem.81.5.8626826. PMID 8626826.
- ↑ 17.0 17.1 Agarwal SK, Kester MB, Debelenko LV, Heppner C, Emmert-Buck MR, Skarulis MC; et al. (1997). "Germline mutations of the MEN1 gene in familial multiple endocrine neoplasia type 1 and related states". Hum Mol Genet. 6 (7): 1169–75. PMID 9215689.
- ↑ Marquard, Jessica; Eng, Charis (September 27, 1999). "Multiple Endocrine Neoplasia Type 2". GeneReviews® [Internet].
- ↑ Bilezikian JP (January 15, 2017). De Groot LJ, Chrousos G, Dungan K, et al., eds. Primary Hyperparathyroidism. Endotext [Internet]: South Dartmouth (MA): MDText.com, Inc.
- ↑ Bandeira F, Cusano NE, Silva BC, Cassibba S, Almeida CB, Machado VC, Bilezikian JP (2014). "Bone disease in primary hyperparathyroidism". Arq Bras Endocrinol Metabol. 58 (5): 553–61. PMC 4315357. PMID 25166047.
- ↑ Rodriguez M, Nemeth E, Martin D (2005). "The calcium-sensing receptor: a key factor in the pathogenesis of secondary hyperparathyroidism". Am J Physiol Renal Physiol. 288 (2): F253–64. doi:10.1152/ajprenal.00302.2004. PMID 15507543.
- ↑ Espiritu RP, Kearns AE, Vickers KS, Grant C, Ryu E, Wermers RA (2011). "Depression in primary hyperparathyroidism: prevalence and benefit of surgery". J. Clin. Endocrinol. Metab. 96 (11): E1737–45. doi:10.1210/jc.2011-1486. PMID 21917870.
- ↑ Marquard, Jessica; Eng, Charis (September 27, 1999). "Multiple Endocrine Neoplasia Type 2". GeneReviews® [Internet].
- ↑ Bilezikian JP (January 15, 2017). De Groot LJ, Chrousos G, Dungan K, et al., eds. Primary Hyperparathyroidism. Endotext [Internet]: South Dartmouth (MA): MDText.com, Inc.
- ↑ Mazzuoli GF, D'Erasmo E, Pisani D (1998). "Primary hyperparathyroidism and osteoporosis". Aging (Milano). 10 (3): 225–31. PMID 9801732.
- ↑ Lips P (2001). "Vitamin D deficiency and secondary hyperparathyroidism in the elderly: consequences for bone loss and fractures and therapeutic implications". Endocr Rev. 22 (4): 477–501. doi:10.1210/edrv.22.4.0437. PMID 11493580.
- ↑ Michael JW, Schlüter-Brust KU, Eysel P (2010). "The epidemiology, etiology, diagnosis, and treatment of osteoarthritis of the knee". Dtsch Arztebl Int. 107 (9): 152–62. doi:10.3238/arztebl.2010.0152. PMC 2841860. PMID 20305774.
- ↑ Bai HX, Giefer M, Patel M, Orabi AI, Husain SZ (2012). "The association of primary hyperparathyroidism with pancreatitis". J. Clin. Gastroenterol. 46 (8): 656–61. doi:10.1097/MCG.0b013e31825c446c. PMC 4428665. PMID 22874807.
- ↑ 29.0 29.1 29.2 29.3 29.4 Kumar, Vinay (2013). Robbins basic pathology. Philadelphia, PA: Elsevier/Saunders. p. 736-737. ISBN 9781437717815.
- ↑ Wieneke JA, Smith A (2008). "Parathyroid adenoma". Head Neck Pathol. 2 (4): 305–8. doi:10.1007/s12105-008-0088-8. PMC 2807581. PMID 20614300.
- ↑ Goeddel DV, Yansura DG, Caruthers MH (1977). "Binding of synthetic lactose operator DNAs to lactose represessors". Proc. Natl. Acad. Sci. U.S.A. 74 (8): 3292–6. PMC 431535. PMID 333432.
- ↑ Lila AR, Sarathi V, Jagtap V, Bandgar T, Menon PS, Shah NS (2012). "Renal manifestations of primary hyperparathyroidism". Indian J Endocrinol Metab. 16 (2): 258–62. doi:10.4103/2230-8210.93745. PMC 3313745. PMID 22470864.
- ↑ Gui X, Miao L, Cai H, Xiao Y, Zhang D, Wang J, Meng F (2014). "[Primary hyperparathyroidism with metastatic pulmonary calcification: a case report and review of literature]". Zhonghua Jie He He Hu Xi Za Zhi (in Chinese). 37 (5): 343–6. PMID 25011508.