Phosphate
|
WikiDoc Resources for Phosphate |
|
Articles |
|---|
|
Most recent articles on Phosphate |
|
Media |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Phosphate at Clinical Trials.gov Clinical Trials on Phosphate at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Phosphate
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Phosphate Discussion groups on Phosphate Directions to Hospitals Treating Phosphate Risk calculators and risk factors for Phosphate
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Phosphate |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: Henry A. Hoff
Overview
A phosphate, in inorganic chemistry, is a salt of phosphoric acid. In organic chemistry, a phosphate, or organophosphate, is an ester of phosphoric acid. Phosphates are important in biochemistry and biogeochemistry or ecology.
Chemical properties
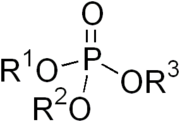
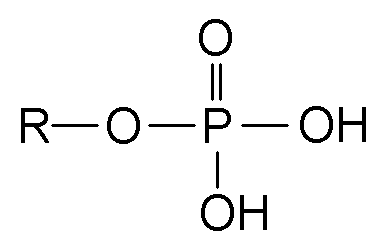
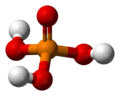
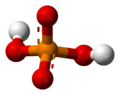
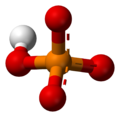
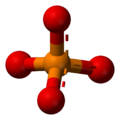
The phosphate ion is a polyatomic ion with the empirical formula PO43− and a molar mass of 94.97 g/mol; it consists of one central phosphorus atom surrounded by four identical oxygen atoms in a tetrahedral arrangement. The phosphate ion carries a negative three formal charge and is the conjugate base of the hydrogenphosphate ion, HPO42−, which is the conjugate base of H2PO4−, the dihydrogen phosphate ion, which in turn is the conjugate base of H3PO4, phosphoric acid. It is a hypervalent molecule (the phosphorus atom has 10 electrons in its valence shell). Phosphate is also an organophosphorus compound with the formula OP(OR)3.
A phosphate salt forms when a positively charged ion attaches to the negatively charged oxygen atoms of the ion, forming an ionic compound. Many phosphates are insoluble in water at standard temperature and pressure, except for the alkali metal salts.
In dilute aqueous solution, phosphate exists in four forms. In strongly basic conditions, the phosphate ion (PO43−) predominates, while in weakly basic conditions, the hydrogen phosphate ion (HPO42−) is prevalent. In weakly acid conditions, the dihydrogen phosphate ion (H2PO4−) is most common. In strongly acid conditions, aqueous phosphoric acid (H3PO4) is the main form.
-
H3PO4 -
H2PO4− -
HPO42− -
PO43−
More precisely, considering the following three equilibrium reactions:
- H3PO4 ⇌ H+ + H2PO4−
- H2PO4− ⇌ H+ + HPO42−
- HPO42− ⇌ H+ + PO43−
the corresponding constants at 25°C (in mol/L) are (see phosphoric acid):
<math> K_{a1}=\frac{[\mbox{H}^+][\mbox{H}_2\mbox{PO}_4^-]}{[\mbox{H}_3\mbox{PO}_4]}\simeq 7.5\times10^{-3}</math>
<math>K_{a2}=\frac{[\mbox{H}^+][\mbox{HPO}_4^{2-}]}{[\mbox{H}_2\mbox{PO}_4^-]}\simeq 6.2\times10^{-8}</math>
<math> K_{a3}=\frac{[\mbox{H}^+][\mbox{PO}_4^{3-}]}{[\mbox{HPO}_4^{2-}]}\simeq 2.14\times10^{-13}</math>
For a strongly basic pH (pH=13), we find
<math>\frac{[\mbox{H}_2\mbox{PO}_4^-]}{[\mbox{H}_3\mbox{PO}_4]}\simeq 7.5\times10^{10} \mbox{ , }\frac{[\mbox{HPO}_4^{2-}]}{[\mbox{H}_2\mbox{PO}_4^-]}\simeq 6.2\times10^5 \mbox{ , } \frac{[\mbox{PO}_4^{3-}]}{[\mbox{HPO}_4^{2-}]}\simeq 2.14</math>
showing that only PO43− and HPO42− are in significant amounts.
For a neutral pH (for example the cytosol pH=7.0), we find
<math> \frac{[\mbox{H}_2\mbox{PO}_4^-]}{[\mbox{H}_3\mbox{PO}_4]}\simeq 7.5\times10^4 \mbox{ , }\frac{[\mbox{HPO}_4^{2-}]}{[\mbox{H}_2\mbox{PO}_4^-]}\simeq 0.62 \mbox{ , } \frac{[\mbox{PO}_4^{3-}]}{[\mbox{HPO}_4^{2-}]}\simeq 2.14\times10^{-6}</math>
so that only H2PO4− and HPO42− ions are in significant amounts (62% H2PO4−, 38% HPO42−). Note that in the extracellular fluid (pH=7.4), this proportion is inverted (61% HPO42−, 39% H2PO4−).
For a strongly acid pH (pH=1), we find
<math>\frac{[\mbox{H}_2\mbox{PO}_4^-]}{[\mbox{H}_3\mbox{PO}_4]}\simeq 0.075 \mbox{ , }\frac{[\mbox{HPO}_4^{2-}]}{[\mbox{H}_2\mbox{PO}_4^-]}\simeq 6.2\times10^{-7} \mbox{ , } \frac{[\mbox{PO}_4^{3-}]}{[\mbox{HPO}_4^{2-}]}\simeq 2.14\times10^{-12}</math>
showing that H3PO4 is dominant with respect to H2PO4−. HPO42− and PO43− are practically absent.
Phosphate can form many polymeric ions, diphosphate (also pyrophosphate), P2O74−, triphosphate, P3O105−, et cetera. The various metaphosphate ions have an empirical formula of PO3− and are found in many compounds.
Phosphate deposits can contain significant amounts of naturally occurring uranium. Subsequent uptake of such soil amendments can lead to crops containing uranium concentrations.
Occurrence
Elemental phosphorus and phosphides are not found (rare phosphide minerals may be found in meteorites).
Geochemistry
Phosphates are the naturally occurring form of the element phosphorus, found in many phosphate minerals. In mineralogy and geology, phosphate refers to a rock or ore containing phosphate ions.
The largest rock phosphate deposits in North America lie in the Bone Valley region of central Florida, United States, the Soda Springs region of Idaho, and the coast of North Carolina. Smaller deposits are located in Montana, Tennessee, Georgia and South Carolina near Charleston along Ashley Phosphate road. The small island nation of Nauru and its neighbor Banaba Island, which used to have massive phosphate deposits of the best quality, have been mined excessively. Rock phosphate can also be found on Egypt, Israel, Morocco, Navassa Island, Tunisia, Togo and Jordan have large phosphate mining industries as well.
Biochemistry
In biological systems, phosphorus is found as a free phosphate ion in solution and is called inorganic phosphate, to distinguish it from phosphates bound in various phosphate esters. Inorganic phosphate is generally denoted Pi and can be created by the hydrolysis of pyrophosphate, which is denoted PPi:
- P2O74− + H2O → 2HPO42−
However, phosphates are most commonly found in the form of adenosine phosphates, (AMP, ADP and ATP) and in DNA and RNA and can be released by the hydrolysis of ATP or ADP. Similar reactions exist for the other nucleoside diphosphates and triphosphates. Phosphoanhydride bonds in ADP and ATP, or other nucleoside diphosphates and triphosphates, contain high amounts of energy which give them their vital role in all living organisms. They are generally referred to as high energy phosphate, as are the phosphagens in muscle tissue. Compounds such as substituted phosphines, have uses in organic chemistry but do not seem to have any natural counterparts.
Ecology of phosphates
In ecological terms, because of its important role in biological systems, phosphate is a highly sought after resource. Consequently, it is often a limiting reagent in environments, and its availability may govern the rate of growth of organisms. Addition of high levels of phosphate to environments and to micro-environments in which it is typically rare can have significant ecological consequences. For example, booms in the populations of some organisms at the expense of others, and the collapse of populations deprived of resources such as oxygen (see eutrophication). In the context of pollution, phosphates are a principal component of total dissolved solids, a major indicator of water quality.
Calcium hydroxyapatite and calcite precipitates can be found around bacteria in alluvial topsoil.[2] As clay minerals promote biomineralization, the presence of bacteria and clay minerals resulted in calcium hydroxyapatite and calcite precipitates.[2]
Phosphate deposits can contain significant amounts of naturally occurring heavy metals such as uranium. Mining operations processing phosphate rock can leave tailings piles containing elevated levels of cadmium, lead, nickel, copper, chromium, and uranium. Unless carefully managed, these waste products can leach heavy metals into groundwater or nearby estuaries. Uptake of these substances by plants and marine life can lead to concentration of toxic heavy metals in food products.[3]
Uses
Phosphates were once commonly used in laundry detergent in the form trisodium phosphate (TSP), but because of algae boom-bust cycles tied to emission of phosphates into watersheds, phosphate detergent sale or usage is restricted in some areas.
In agriculture phosphate is one of the three primary plant nutrients, and it is a component of fertilizers. Rock phosphate is quarried from phosphate beds in sedimentary rocks. In former times it was simply crushed and used as is, but the crude form is now used only in organic farming. Normally it is chemically treated to make superphosphate, triple superphosphate, or ammonium phosphates, which have higher concentration of phosphate and are also more soluble, therefore more quickly usable by plants.
Fertilizer grades normally have three numbers; the first is the available nitrogen, the second is the available phosphate (expressed on a P2O5 basis), and the third is the available potash (expressed on a K2O basis). Thus a 10-10-10 fertilizer would contain ten percent of each, with the remainder being filler.
Surface runoff of phosphates from excessively fertilized farmland can be a cause of phosphate pollution leading to eutrophication (nutrient enrichment), algal bloom and consequent oxygen deficit. This can lead to anoxia for fish and other aquatic organisms in the same manner as phosphate-based detergents.
Phosphate compounds are occasionally added to the public drinking water supply to counter plumbosolvency.
The food industry uses phosphates to perform several different functions. For example, in meat products, it solubilizes the protein. This improves its water-holding ability and increases its moistness and succulence. In baked products, such as cookies and crackers, phosphate compounds can act as part of the leavening system when it reacts with an alkalai, usually sodium bicarbonate (baking soda).[4][5]
See also
- organophosphorus compounds
- Phosphine - PR3
- Phosphine oxide - OPR3
- Phosphinite - P(OR)R2
- Phosphonite - P(OR)2R
- Phosphite - P(OR)3
- Phosphinate - OP(OR)R2
- Phosphonate - OP(OR)2R
- Phosphate - OP(OR)3, such as triphenyl phosphate
- Phosphorus oxoacids
References
- ↑ Campbell Neil A, Reece, Jane B (2005). Biology (Seventh Edition ed.). San Francisco, California: Benjamin Cummings. p. 65. ISBN 0-8053-7171-0.
- ↑ 2.0 2.1 Schmittner KE, Giresse P (1999). "Micro-environmental controls on biomineralization: superficial processes of apatite and calcite precipitation in Quaternary soils, Roussillon, France". Sedimentology. 46 (3). doi:10.1046/j.1365-3091.1999.00224.x.
- ↑ Gnandil K, Tchangbedjil G, Killil K, Babal G, Abbel E (2006). "The Impact of Phosphate Mine Tailings on the Bioaccumulation of Heavy Metals in Marine Fish and Crustaceans from the Coastal Zone of Togo". Mine Water Environ. 25 (1): 56–62. Unknown parameter
|month=ignored (help) - ↑ Lynn A. Kuntz. "Figuring Out Phosphates". Unknown parameter
|month=ignored (help); Unknown parameter|Year=ignored (|year=suggested) (help) - ↑ Lynn A. Kuntz (2006). "Food Product Design". Unknown parameter
|month=ignored (help)